
Chlorine and Chlorinated Compounds Removal from Industrial Wastewater Discharges: A Review
Mohammad Al-Hwaiti, Hamidi Abdul Aziz*, Mohd Azmier Ahmad, and Reyad Al-ShawabkehPublished Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.047
Journal Issues : Number 3, July-September 2021
Abstract Adsorption techniques for industrial wastewater treatment rich in heavy metals and aqueous solutions of water-soluble such as Cl−, F−, HCO3−, NO3−, SO2−4, and PO3−, often include technologies for toxicity removals. The recent advancement and technical applicability in the treatment of chlorine and chlorinated compounds from industrial wastewater are reviewed in this article. Chlorine and chlorinated compounds are among the common discharged constituents from numerous industries. They can be carcinogenic or naturally toxic and can pose issues to aquatic ecosystems and human beings. Thus, elimination of chlorides and chlorinated compounds from water or wastewater is inevitable to get rid of the problem. Several techniques are being applied for the reduction of chlorine and chlorinated compounds in water. These include biodegradation, photochemical, adsorption, chemical, electrochemical, photo-electrochemical, membrane, supercritical extraction and catalytic method. Chlorine can react with various organic and inorganic micro-pollutants. However, the potential reactivity of chlorine for specific compounds is small, and only minor variations in the structure of the parent compound are anticipated in the water treatment process under typical conditions. This paper reviews different techniques and aspects related to chlorine removal, the types of chlorine species in solution and their catalyst, chlorine fate and transport into the environment, electrochemical techniques for de-chlorination of water, kinetics, mechanisms of reduction of chlorinated compounds, and kinetics of the electrochemical reaction of chlorine compounds.
Keywords: Industrial waste, Kinetics, Wastewater, Water purification
Citation: Al-Hwaiti, M., Aziz, H.A., Mohd Azmier Ahmad, M.A., and Al-Shawabkeh, R. 2021.Chlorine and chlorinated compounds removal from industrial wastewater discharges: A review. CMUJ. Nat. Sci. 20(3): e2021047.
INTRODUCTION
Enormous industrial effluent pollutants contaminated wastewater, bearing heavy metals and aqueous solutions of water-soluble, such as Cl-, F−, HCO3−, NO3−, SO2−4, and PO3−4, may be entered and accumulated in the food chain and then human body, therefore, they can cause serious health disorders as well as unsuitable for human consumption or irrigation (Tilley et al. 2016; Xiong et al. 2019; Eunice et al., 2020). Chlorinated compounds, for example, have an adverse effect on human and animals. Agency for Toxic substances and Disease Registry (ATSDR, 2019) listed these compounds among the top 20 substances that need urgent remediation. Human consumption of chlorinated water results in several organs cancer such as the bladder, kidney, and rectal (Mahvi, et al. 2009; Ghoochani et al., 2017). Also, they affect reproduction outcome.
The treatment of certain industrial mine wastewater rich in soluble chloride salts leads to increased chlorine concentrations as well as an enrichment of undesirable elements such as F−, Ca2+, HCO3−, NO3−, SO2−4, and PO3−4 and certain heavy metals (Al-Hwaiti et al. 2015; Al-Hwaiti et al. 2016; Al-Hwaiti et al. 2018; Masixole et al. 2019). The conventional treatment processes have a significant faced problem due to incomplete removal anion or metal from inorganic effluent mine wastewater (Eccles, 1999). These treatment techniques are chemical precipitation, ion exchange, and electrochemical.
Nowadays, the adsorption technique can be applied for wastewater treatment in order to improve mine water effluent. However, this technique has shown significant advantage, which includes low-cost treatment, and high efficiency and convenient operation (Flávia et al., 2018). Kurniawan et al. (2005) reported that most solid adsorbents such as industrial wastes, zeolites, biomass materials, agricultural wastes, and polymeric materials might be used for the reduction of inorganic effluent mine water. Among various water and wastewater treatment technologies, process techniques including physicochemical treatment techniques for wastewater (Solimana and Moustafa, 2020), electrodialytic process by various fly ashes for treating heavy metals (Pedersen et al., 2003), photocatalytic (Skubal et al., 2002), coagulation- flocculation process (Hakizimana et al., 2017; Emad et al., 2020), water and wastewater treatment by flotation(George et al., 2018), biological filtration process (Shuokr et al., 2016), ion exchange process for wastewater treatment (Nirali et al. 2016), biodegradable processes of sewage by an aerobic and anaerobic process (Anijiofor et al., 2017; Wang et al., 2020), water and wastewater purification using photochemical Advanced Oxidation Processes (Marta and Natalia, 2010), solvent extraction and adsorption for wastewater effluent (Aswathy et al. 2016), water treatment and purification by adsorption processes(Adrián et al. 2017; Alshammari et al., 2020), electrolytic treatment of wastewater (Vinod Kumar, 2017; Jannatul et al., 2020), activated microbial sludge treatment for wastewater (Yongkui, 2020), chemical reduction of chlorinated organic compounds (Rodrigues et al., 2020), a catalyzed by laccase in aqueous solution (Asadgol et al., 2014); green synthesis iron oxide nanoparticles (Ehrampoush et al., 2015); Magnetic heterogeneous catalytic ozonation (Shahamat et al. 2014); ultrasound waves and ultraviolet irradiation; carbon nanotubes: kinetic and equilibrium studie (Mahvi et al., 2009); magnetic adsorption separation process (Dehghani et al. 2015); electro-coagulation process using iron and aluminum electrodes (Azari et al. 2017).
Recent research reviewed by (Adeola, 2018), suggests that chloride and other compounds released from industrial waste effluent are harmful to aquatic ecosystems and human health. This is because they form a stable compound, especially when they linked to alkali and alkali earth elements such as Na, K, Ca and Mg (Malcolm et al. 2017).
Although numerous reports showed that chloride enriched in municipal wastes and other industrial activities might be increased the chloride content in fresh and wastewater (Sarzamin and Jawad, 2018). Vít and Petr (2019) have investigated different chlorine species initiated in saline solution such as hypochlorites HOCl/OCl¯, chlorites ClO2-, chlorates ClO3-, chlorine dioxide ClO2. Chlorine is among the highest commonly applied disinfectants in water purification; therefore, chlorine can be applied in gas form or in solution as sodium hypochlorite or calcium hypochlorite to remove or reduce microorganism in water treatment (Enric et al., 2015). Chlorine species have subjected under regulation to low allowable concentration levels. The ion ClO2- (chlorite) comes from the decomposition of ClO2, being the most common DBP from ClO2. Current research reports indicate that chloride mostly flows into wastewater treatment facilities from industry effluents. According to Scott et al. (2018), there are three softening processes such as scrutinized, reverse-osmosis, and lime softening are used for treating chloride at Wastewater Treatment Plants. György et al. (2018) Deborde and Von Gunten (2008) have used kinetics and mechanisms process in order to examine chlorine interactions in organic and inorganic mixtures. The results revealed that possible pathways of reaction are electrophilic substitution reactions with organic compounds.
The effects of structural and thermodynamic on process kinetics are adopted by Wolfgang et al. (2016). They used the Butler-Volmer equation for the reduction of chlorinated species reaction rate constant by mass transfer. The main objective of this paper is reviewing chloride and chlorinated compounds removal from water and wastewater, with emphasized on the reactivity kinetic and mechanisms on chloride and chlorinated compounds through water and wastewater effluent.
Types of chlorine species in solution and their catalyst
The aqueous chemistry of chlorine with respect to chlorination of water and wastewater has been reviewed by several investigators (Abarnou and Miosse, 1992; EPA, 2013; Cristina et al., 2012). The following review summarizes the chemistry of chlorine relevant to the discharge of chlorinated waters and wastewaters to the environment. Thorben et al. (2019) used the cathodic electrode processes included in metallic corrosion in chlorinated saline seawater for reduction of chlorine species in the chlorinated water system. Adna et al. (2017) and Wang et al. (2018) reported that chlorinated species have controlled by reactant concentrations, pH, temperature, reaction rates and equilibria. Bromide and iodide, if present even at low concentrations, are oxidized by chlorine forming oxidation products such as hypobromous acid (HOBr) and iodine (I2). Hypobromous acid may, in turn, react with ammonia, if present, to form bromamines analogous to chloramines. Bromine and iodine-containing oxidants become increasingly significant with increasing bromide and iodide concentrations in estuarine and marine waters (Guanghui et al. 2006). Although generally of little consequence in waters at near-neutral pH values, molecular chlorine and nitrogen trichloride are also usually analyzed as free chlorine. Thus, chlorine has a higher oxidation potential than chlorine compounds such as (NH2Cl), (NHCl2), Nitrogen trichloride (NCl3), and other organic compounds. Hypobromous acid and hypoiodous acid are distinguished by the usual analytical methods as free chlorine. HOBr reacts with ammonia to produce bromamines that are also detected as free chlorine. Thus the terminologies “free oxidant” and “combined oxidant” are more apt than free chlorine and combined chlorine when solutions containing bromide or iodide (e.g., seawater) are the subject of analysis. Nonetheless, present analytical approaches may not differentiate between free and combined bromine species. EU (2017) classified chlorine into different forms, such as chlorine gas, calcium hypochlorite, sodium hypochlorite. Table 1 shows the oxidation state of various inorganic oxidized forms of chloride and their stability in water.
Table 1. The oxidation state, free energy and stability of chloride forms in water.
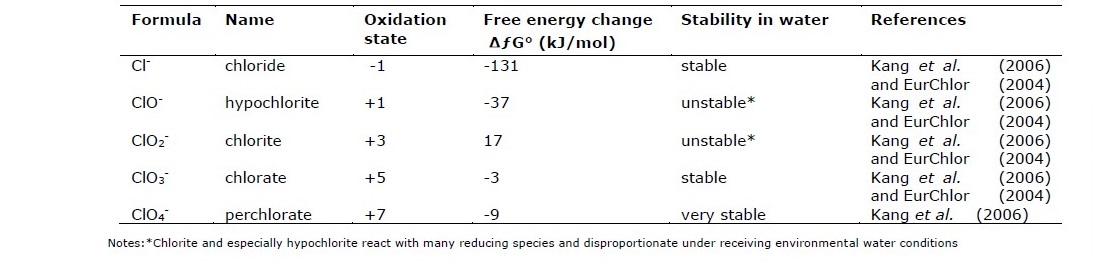
Daniel and Vallero (2019) have reported that in the gas phase, Vinyl chloride gas may be absorbed into particles rather than into the aerosol phase as the particle phase is expected to be relatively small. Most delicate of chlorine is main source cause of toxicity, with a mean critical acute concentration (LC50) of about 2,500 mg / L and a mean critical chronic concentration (IC25) of around 720 mg/L. Chloride levels may always be required to verify whether chloride is toxic in the wastewater effluent (William et al., 2000; Mount et al., 1997).
Chlorine fate and transport into the environment
Several investigators have reported the ecological fate of chlorine in water are understood, and it not remain in the environmental system for a long time (Truhlar et al. 2006; Zheng et al., 2015; Cotton et al., 1999). Researchers have indicated that chlorine photolysis is naturally present in seawater through fate and transport of aerosols (Knipping and Dabdub, 2003; Saeed et al., 2015; Amy et al., 2017). When the chlorine gas reacts with water, it is immediately changed into different forms such as hypochlorous acid, chloride ion, and hypochlorite; however, hypochlorite is formed when pH falls below 4 (Farr et al., 2003). Furthermore, chlorine also occurs mostly as the chloride anion (Cl‾), and then Cl‾can fate and transport into the soil through water flows. The fate of chlorite stabilities and persistent or non-persistent in the aquatic environment is indicated in several literature sources (Table 2).
Table 2. The literature reviewed for chlorate (ClO3-) stability in the aquatic environment.
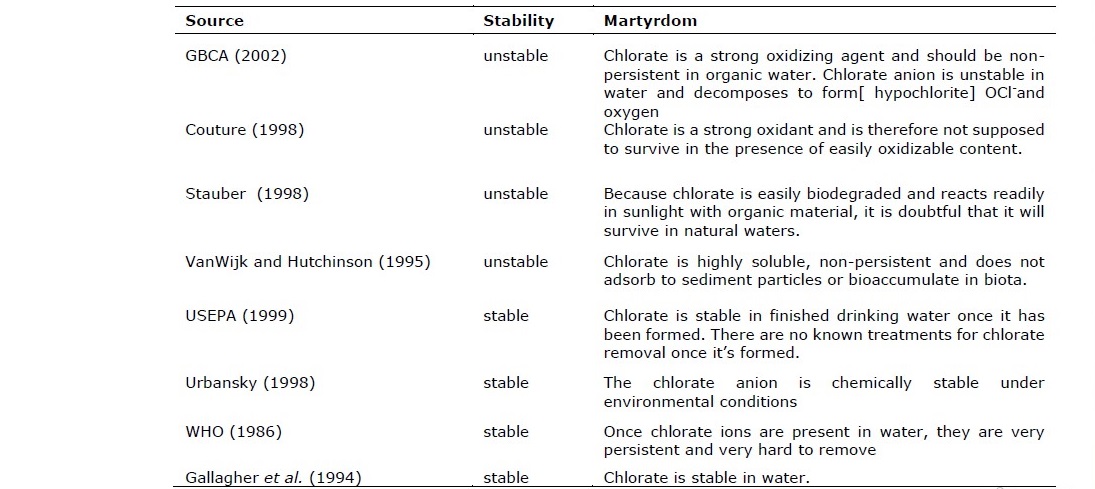
The study made by Zoeteman (1980) assessed the level of NaCl and CaCl2 concentrated in water ranged between 200–300 mg/L. The study revealed that chloride ion showed high mobility moved into water bodies, this is due to intensive weathering process for rocks rich chlorides content, and then chloride leached into the soil. Hu et al. (2013) determined the transfer of Cl- from the soil to other ecosystem is controlled by several complex processes such as hydrogeochemical process, besides that, contaminated soil by Cl- can be a significant source for the groundwater contamination, subsurface soil gas, atmospheric air, surface water, and sediments (Lu, 2010).
The fate of contaminants introduced into the environment by chlorine compounds is the result of various chemical industries and pharmaceutical industry activities discharged (Michałowicz and Duda, 2007; Mahendran et al. 2015; Atashgahi et al., 2018). reported that chlorophenols have a serious challenge in human healths. The transformation and fate of chlorophenols compounds may lead to increase toxicity or carcinogenic, damage gene products or DNA (Michałowicz and Duda, 2007; Etinosa et al. 2013). However, these chlorophenols fates adsorption in soils is governed by acidic conditions (pH) and vice versa. Chlorophenols have long been known released from industrial waste and landfills into surface water or atmosphere through volatilization (Adeola, 2018; Philip et al., 2001). Chlorophenols fates and transports to the environment are influenced by several processes such as chemical, physical, biological transformations, volatilization, sorption, degradation, and leaching (Schummer et al., 2006). Federica et al. (2015) stated that chlorinated solvents have faced several problems in terms of fate and transport these chemicals into the environment. In the pure chemical state, most chlorinated solvent mixtures having densities greater than water, and aren’t speciation into cations and anions in aqueous solution but dissolve as neutral species. Phenol is considered to be one of the organic industrial effluents that release water from various organic chemical industries. The general specifications of different industrial organic industrial effluent waters are described in (Table 3).
Table 3. The parameter of organic industrial effluent water.

Technologies used for de-chlorination of water
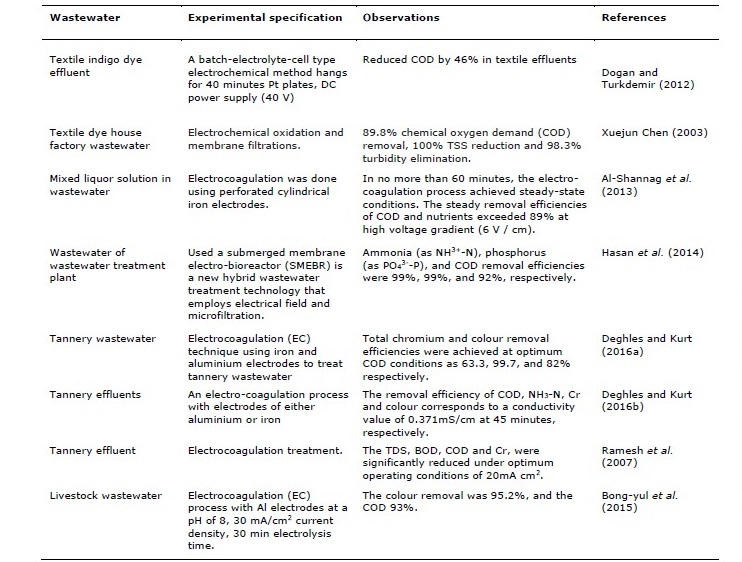
Several techniques are used for treating chlorinated compounds from water. These include biodegradation (Baskaran and Rajamanickam, 2019; Frascari et al., 2019; Khan et al., 2019; Papazi et al., 2019; Pathiraja et al., 2019; Nivorlis et al., 2019; Serbent et al., 2019), photochemical (Liu et al., 2020; Ding et al., 2020; Li et al., 2019), adsorption (Dontriros et al., 2020; Du et al., 2020; Liang et al., 2020; Lo et al., 2020; Qian et al., 2020), chemical (Kim et al., 2020; Qian et al., 2020; Song et al., 2020; Wang et al., 2020), electrochemical (Du et al., 2020; McQuillan et al., 2020; Wang et al., 2020; Xu et al., 2020), photo-electrochemical (Liu et al., 2020) membrane (Abdel-Shafy et al., 2017; Du et al., 2020; Vlotman et al., 2019; Wan et al., 2020), supercritical extraction (Zhang and Zhang, 2020), electron selectivity (Wang et al., 2020), neural network modeling ( Baskaran et al., 2019), and catalytic (Nieto-Sandoval et al., 2019; Ruan et al., 2019; Zheng et al., 2020) method. Related literature on the electrolytic treatment methods application for different types of wastewater is shown in Table 4.
Chlorinated compounds are readily degraded using several bioactive species from several strains such as Pseudomonas guguanensis, Desulfomonile tiedjei, Methylobacterium, Methylophilus, Hyphomicrobium, Nitrosomonas europea, Pseudomonas cepacia, Escherichia coli, Alcaligenes denitrificans and Rhodococcus erythropolis. The role of these strains is divided into two categories; co-metabolic conversions or conversions. The chlorinated hydrocarbons have a major carbon source to these strains where they act as electron acceptors in the respiratory process insolutions lack of oxygen. For example, trichloroethylene is readily degraded to CO2 and lighter hydrocarbons using Pseudomonas guguanensis at a rate of degradation of 0.41 mg/L.h with 90% of removal efficiency (Han and Yan, 2016; Quan et al., 2017). Several methods are used for the degradation of trichloroethylene from aqueous water such as groundwater. These include dehalococcoides-containing microbial communities, polyethyleneimine-modified zero-valent iron nanoparticles, bysonophotolytic-activated persulfate processes, and polyethyleneimine-modified zero-valent iron nanoparticles (Mao et al., 2017; Lin et al., 2018; Bahrami et al., 2018; Mdlovu et al., 2019).
Table 4. Different electrolytic treatment methods using various types of wastewater.
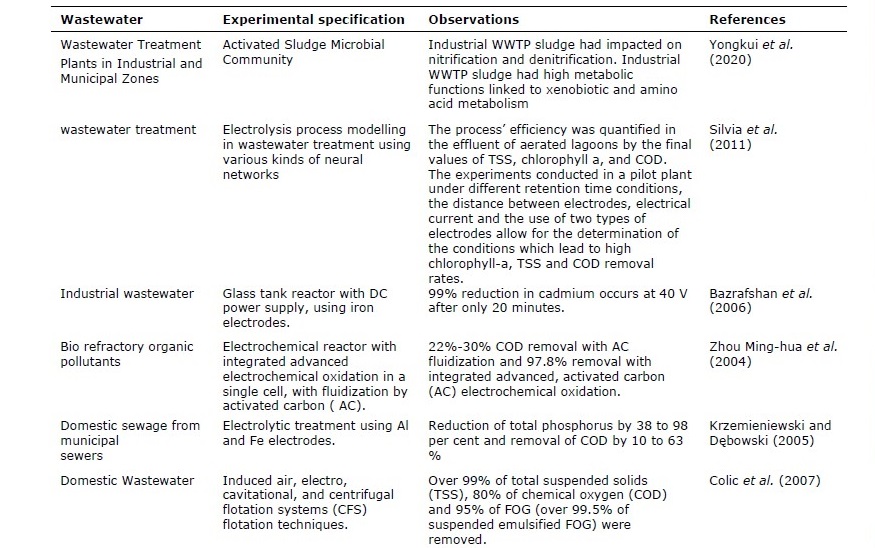
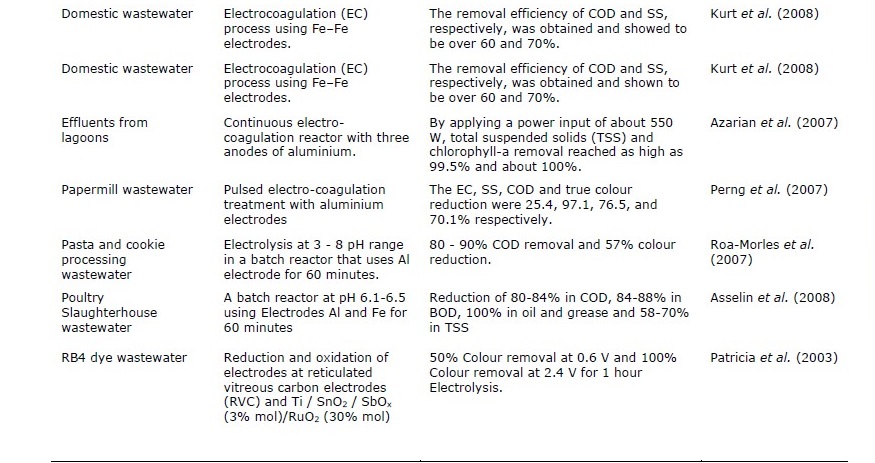
Table 4. Continued.
Pesticides such as DDT, DDE, Endrin and Lindane were totally diminished using P. cepacia at low concentration (0.05 ppm) and after 24 h exposure.
Zero-valent iron is used in chemical, photo-electrochemical and catalyst degradation of chlorinated compounds in the adsorption and degradation of chlorinated acid, where this rate decreases with increasing solution acidity (Mdlovu et al. 2018; Kim et al., 2020; Qian et al., 2020; Song et al., 2020; Wang et al., 2020). Furthermore, Pd/Al2O3 catalyst was used to remove chlorinated micropollutants from the water with a reduction rate constants 0.32–1.56 L/g.min(Nieto-Sandoval et al. 2019). Also, Pd/CeO2 catalysts were applied to degrade bromochloroacetic acid from the liquid phase through the hydrogenation process (Zheng et al., 2020). Furthermore, 2,4- dichlorophenol can be de-chlorinated using by Fe/Ni nanoparticles (Ruan et al., 2019). Photocatalytic and electrochemical methods were also adopted to catalyze chlorinated compounds to produce less harmful products such as carbon dioxide. Several photo-catalyst such as C3N4/NiO are used to degrade 70.4% of 2-chlorodibenzo- p-dioxin in the presence UV–vis light illumination (Ding et al., 2020). Also, tungsten oxides nano-film anode is used for chlorinated compounds. The removal efficiency of 4- chlorophenol is 85.5%, and 48% is obtained with tungsten photoanode electrochemical (Liu et al., 2020). The biocharcoal-based electrode is also used to achieve 86.7% removal rates of Cl− from leather processing wastewater (Kim et al., 2020). Different carbon- supported iron was used for removal of chloramphenicol (Stryer 1988). Pd-TiO2 catalyst is used as electrocatalyst for hydrodechlorination reaction for 2,4- dichlorophenol (Wang et al., 2020).
Mining wastewater treatment with a focus on a natural substrate or sorbent adsorption can be used to remove contaminants such as heavy metals and chlorinated compounds. A description of the most adsorption for mining wastewaters treatment is shown in Table 5.
Table 5. Adsorption process for mining wastewaters treatment
The effect of support of these catalysts is investigated by studying the adsorption effect of several adsorbents on the uptake of these compounds. A CuOx/Na2CO3 porous sorbent is used to remove HCl from CO2- rich mixture gases. The results show that 400,000 m3/h of hydrochloric acid can be removed from flue gas using 500 tons sorbents (Marschner, 1995). Chloro-organic in synthetic and site groundwater was successfully remediated by Palladium/Fe nanoparticle integrated membranes (Wan et al., 2020). Immobilized clostridium butyricum in silica gel is also employed for de- chlorination of trichloroethene (Lo et al., 2020).
Electrochemical Techniques for de-chlorination of water
Different electrochemical techniques were used in electro redox reaction of chlorinated compounds (García-Armada, 1996; Fritz et al., 2013; Eduardo et al., 2014). Electrochemical method type Cyclic Voltammetry (CV) is used to measure the current adsorbed to an electrode in an electrochemical cell under conditions and to measure the current produced. The electrochemical potential is scanned linearly from initial to a final value, and the corresponding current is measured (Joshi and Sutrave, 2018). The measure upper and lower peaks of the current-potential curve represent the redox reaction of the existing species in solution. This technique is useful to provide information about thermodynamic and kinetics of the redox reactions (Florica, 2014). This technique was used for de-chlorination in solution depends on different types of electrodes where the optimum condition for its electrochemical behaviour is monitored at pH 8. When boron-doped diamond (BDD) electrodes are used, the chloride ions exists for the anodic reaction as ClO‾ (Michio et al., 2008). The coupled amperometric technique was employed to estimate chlorine concentration at a low concentration of 0.1-2 mg/L.
Linear sweep voltammetry technique was used graphite electrodes in order to determine chlorine concentrations with a range between (1.0-300.0 mg/dm3), and ClO- concentration less than (3) (Pathiratne et al., 2008). The differential pulse voltammetric technique was used as a gold electrode to determine chlorine concentration ranged between 1 and 5 mg Cl/dm3 (Sulistyo et al., 2010). The voltammetric potential sweep technique was also used different electrodes (e.g. platinum, gold, and glassy carbon disk) to measure chlorine concentration with a range of about 4-400mgCl/L, whereas a sensitivity concentration is 1.0mgCl/L (Fumihiro et al., 2005). Fumihiro et al. (2004) used Pt for electrode linear sweep-voltammetry in order to determine the hypochlorite ion. They also investigated physical, chemical factors, pH, temperature, sweep rate, dissolved oxygen and metal might be controlled electrochemical reaction. Cyclic voltammetry technique was used a pencil graphite electrode (PGE) to determine Polyvinyl chloride stability (Melih et al., 2019). The differential pulse polarography (DDP) technique was used to determine the electrochemical reduction of chlorite. The reaction condition investigated that concentrations of chlorite ranged from 19 μg/L-19 mg/L, pH ranged from 3.7 to 14, ionic strength range from 0.05–3.0 M (Nakareseisoon et al., 1988).
Mechanisms of reduction of chlorinated compounds
The most common reaction pathways for the electrochemical reaction of halogen ions and their compounds in aqueous solution and at the surface of electrodes are proposed by many researchers (Kraft, 2008; Radjenovic and Sedlak, 2015; Katsaounis and Souentie, 2014; Exner et al., 2016; Exner et al., 2018). These halogen ions have several oxidation states based on existence in their compounds. For example, chlorine has seven oxidation numbers that have values of -1, 0, +1, +3, +4, +5, or +7. When choline presents as free chlorine gas it has a 0 oxidation number; however, the most common oxidation number is -1 when reacts with hydrogen and mono- or divalent metals to produce hydrochloric acid and metal chlorides as shown:
H+ + Cl- → HCl
M+ + Cl- → MCl
M2+ + Cl- → MCl2
In the presence of oxygen, chloride ion can have positive oxidation number, and its value increases with increasing oxygen in the compound according:
The oxidation number of +1: Na+ + ClO- → NaClO
The oxidation number of +3: Na+ + ClO2- → NaClO2
The oxidation number of +4: ClO2
The oxidation number of +5: Na+ + ClO3- → NaClO3
The oxidation number of +7: Na+ + ClO4– → NaClO4
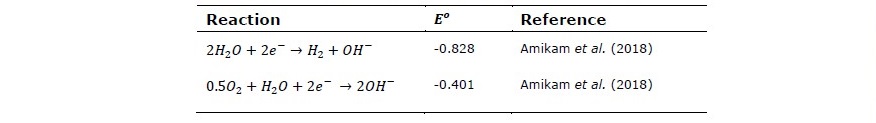
In electrochemical cell reaction, these compounds are either oxidized to produce free chlorine gas at the surface of cell anode or reduced to produce chloride ions on cathode according to the reactions shown in Table 6 and Table 7. The values of standard oxidation potential
Table 6. Standard reduction potential for various chlorinated compounds vs SHE.
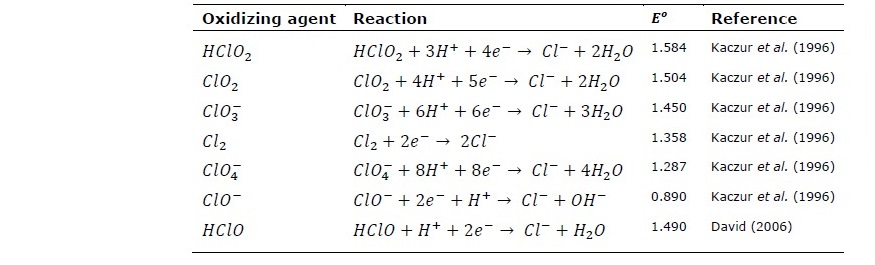
Table 7. The standard reduction potential of water vs SHE.
On the surface of the catalytic electrodes chloride ions undergoes adsorption by the surface of the anode as per Figure 1 (Liang et al., 2020). First chloride ions first diffuse to the surface of the electrode and get adsorbed as
Cl‾ → Cl*
Then the adsorbed chloride ion undergoes electrochemical oxidation reaction to form chlorine gas as
2Cl- → Cl2 + 2e-
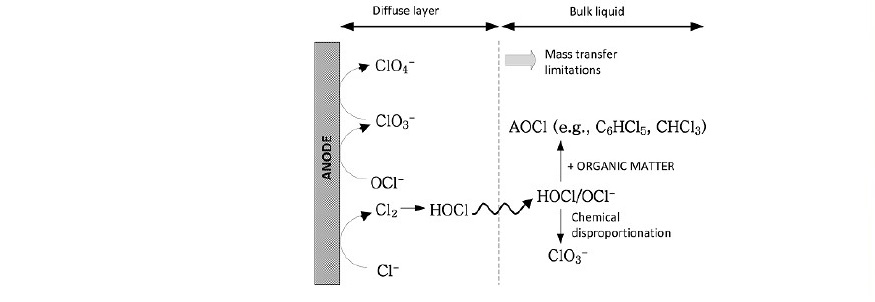
Figure 1. Adsorption/electrochemical reaction of chloride ions and related compounds on the electrode surface.
Depending on solution acidity (Figure 2) chlorine gas evolved from solution at low pH; however, it undergoes oxidation reaction to form HClO which in turn its ions ClO‾ is further undergoes adsorption/electrochemical reactions to form ClO3- and ClO4. Some of the produced HClO diffuses back into solution with its base to disproportionate to O3.
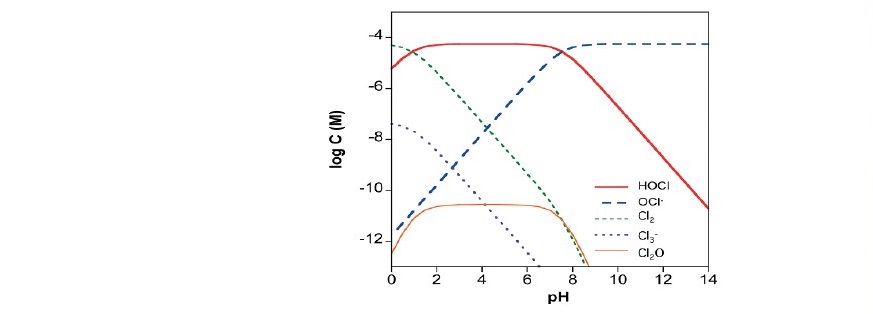
Figure 2. Speciation of Chlorine and its species at various pH values (Remucal and Manley 2016)
Kinetics of Electrochemical Reactions of chlorine compounds
While there are many chlorine species exists in the electrochemical cell such as Cl‾, ClO‾, ClO2-, ClO3- , ClO4- , and HClO , and other counter species such as OH‾ and H+, their existence affect the electrochemical reaction to liberate chlorine from solution. The rate of the electrochemical reaction of chloride ions on the surface electrode has therefore regulated the reaction rate between chloride ions and their solution types, as well as the electrical potential gap between electrode surface and solution. Exner et al. (2016) used DFT to study the kinetics reaction of chlorine at aRuO2(110) Model Electrode. They found that Volmer–Heyrovsky mechanism significantly influenced the adsorption of chloride ions on the active oxygen atoms of RuO2 lead to further reaction to liberate chlorine gas from solution. The rate of development of the produced chlorine is linearly proportional with current density.
Dickinson and Wynne-Jones (Dickinson et al., 2016) studied the rate of chlorine gas formation at the platinum electrode. They proved that the rate is inhibited by the oxide that makes on the electrode surface where the order of reaction was unity at low chlorine concentration. Isai et al. (2013) performed Tafel analysis on chloride ions oxidation on the platinum electrode and showed that the mechanism of the reaction is influenced by the dissociative electrochemical adsorption of chlorine species and chemical recombination of the adsorbed Pt–Cl.
Nicoson et al. (2002) studied the mechanism and the kinetics of the electrochemical reaction of chlorine gas and hypochlorite ions produced in acetate buffer solution. The reaction was first order in [Cl2] and [ClO2-], with a rate constant of k1 = (5.7 ± 0.2) × 105 M-1 s-1 at 25.0 °
Therefore, the target electrochemical reaction of chloride ions on the surface of the electrode is governed by
O + ne- ↔ R
Where O represent the free chlorine in solution, Cl2, R is the chloride ions, Cl- in solution, and ne- are the number of electrons transfer in the electrochemical cell.
Some of the produced chlorine can future reacts with oxygen to produce hypochlorous acid (HClO) in solution according to the general equation
R → Y
Hypochlorous acid may dissociate on the surface of the electrode to produce hypochlorite ions (ClO‾) as
Y → P + H+
Further series reactions on the surface of the electrode such as
ClO‾ → ClO3- → ClO4-
On a planner electrode, the rate of oxidation of chloride ions is related to the rate of change of these ions within a distance, x, from the electrode surface according
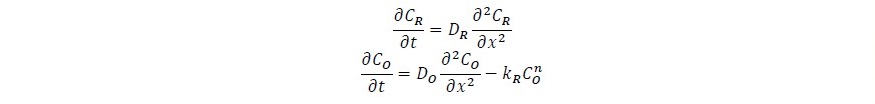
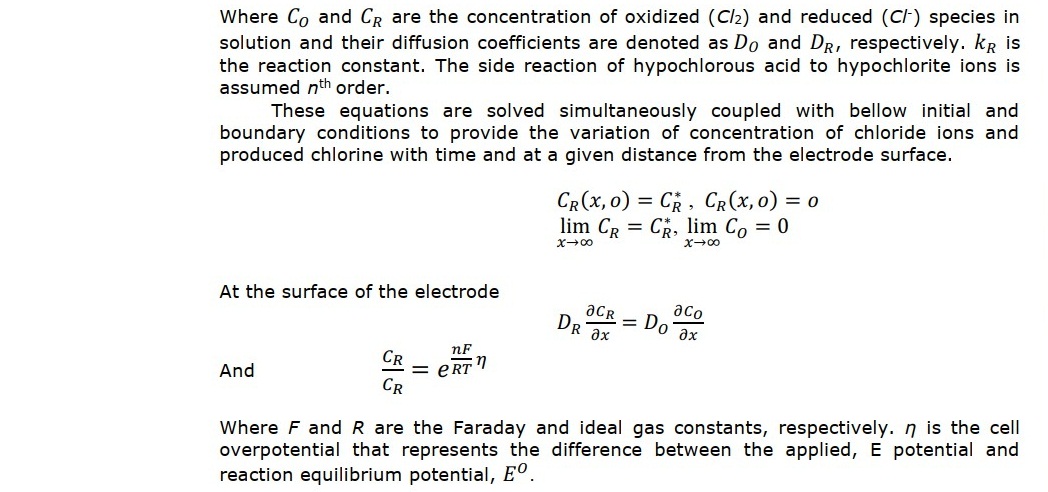
CONCLUSION
In this review, adsorption techniques have been reviewed for the removal of chlorine and chlorinated compounds in water and wastewater, and biodegradation, photochemical, adsorption, chemical, electrochemical, photo-electrochemical, membrane, supercritical extraction and catalytic methods may be used. In addition, types of chlorine species in solution such as chlorine (Cl2), hypochlorous acid (HOCl), hypochlorite ion (OCl-), monochloramine (NH2Cl), dichloramine (NHCl2), nitrogen- trichloride (NCl3), monochloramine (NH2Cl), dichloramine (NHCl2), nitrogen-trichloride (NCl3), and chlorophenol. Numerous industrial usages and production of chlorine and chlorinated compounds has resulted in the discharge of different types of chemicals into the environment and have a serious challenge in the health sectors.
The fate and transport of chlorophenol compounds may lead to an increase in toxicity that may damage DNA or gene products as well as that may mutagenic and carcinogenic for living organisms. The primary processes involving transport, mobility and distribution of these chemicals in the environment at various environmental parameters such as chemical, physical, and biological transformations, sorption, volatilization, degradation, and leaching were discussed. However, further study is necessary to comprehend the mechanisms of reduction of chlorinated compounds, which has electrochemical cell reaction, chlorine compounds are either oxidized to produce free chlorine gas at the surface of cell anode or reduced to produce chloride ions on cathode according to the reactions. Moreover, the kinetics of the electrochemical reaction of chlorine compounds, which has many chlorine species exists in the electrochemical cell such as Cl‾, ClO‾, ClO2-, ClO3- , ClO4- , and HClO and other counter species such as OH- and H+, their existence affect the electrochemical reaction to liberate chlorine from solution.
The scientific research to develop novel technology for solid adsorbent processes, either for the rate of the electrochemical reaction of chloride ions on the surface electrode is controlled the rate of reaction between chloride ions and their species in solution, as well as, the electrical potential difference between electrode surface and solution.
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
Abarnou, A. and Miossec, L. 1992. Chlorinated waters discharged to the marine environment chemistry and environmental impact: An overview. Science of the Total Environment. 126: 173-197.
Abdel-Shafy H.I. and Abdel-Shafy, S.H. 2017. Membrane technology for water and wastewater management and application in Egypt, Egypt. J. Chem. 60: 347– 360.
Adeola, A.O., 2018. Fate and toxicity of chlorinated phenols of environmental implications: A Review. Medicinal and Analytical Chemistry International Journal. 2: 000126.
Adna, B., Zarema, O., Adisa, D., and Amina, O. 2017. The effect of temperature and chlorine residual on the presence of Legionella spp. in water systems of public and tourist facilities. Adna Bešić et al. Journal of Health Sciences. 7: 50-58
Adrián, B.P., Mendoza, C., Didilia, I., Reynel, Á., and Hilda, E. 2017. Adsorption Processes for Water Treatment and Purification. ISBN 978-3-319-58135-4 ISBN 978-3-319-58136-1 (eBook).
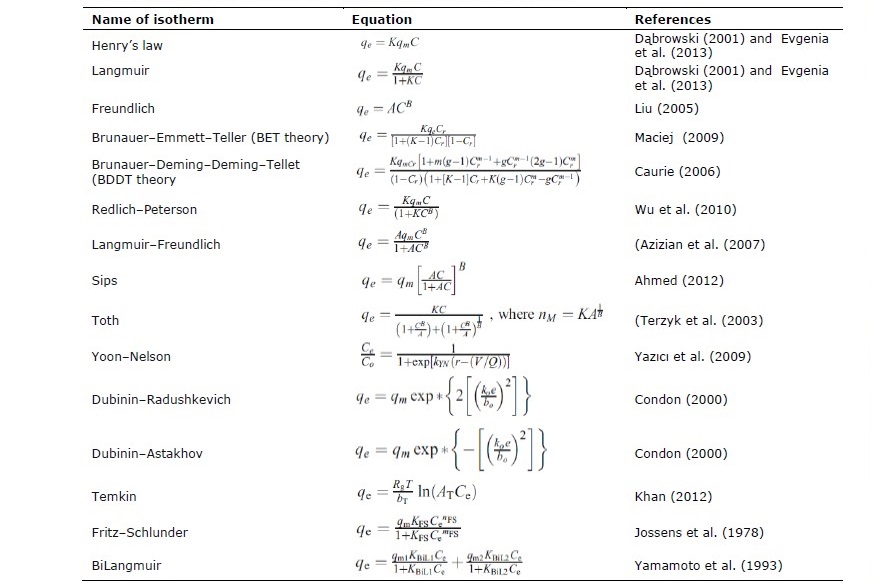
Ahmed, M.J. 2012. Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilibria. 317: 9–14.
Al-Hwaiti, M., Brumsack, H., and Schnetger, B. 2018. Heavy metal contamination and health risk assessment in waste mine water dewatering using phosphate beneficiation processes in Jordan. Environmental Earth Sciences. 77:6617845-0.
Al-Hwaiti, M., Brumsack, H., and Schnetger, B. 2016. Suitability assessment of phosphate mine waste water for agricultural irrigation, an example from Eshidiya Mines, South Jordan. Environmental Earth Sciences. 75:276.
Al-Hwaiti, M., Brumsack, H., and Schnetger, B. 2015. Fraction distribution and risk assessment of heavy metal in waste clay sediment discharged through phosphate beneficiation process in Jordan. Environmental Monitoring and Assessment. 187: 401.
Amikam, G., Nativ, P., and Gendel, Y. 2018. Chlorine-free alkaline seawater electrolysis for hydrogen production. International Journal of Hydrogen Energy. 43: 6504- 6514.
Amy, L., Bondy, B.W., Alexander, L., Rebecca, L.C., Manelisi V.N., Steven B.B., Kerri A.P., Paul, B.S., and Andrew P.A.O. 2017. Inland sea spray aerosol transport and incomplete chloride depletion: varying degrees of reactive processing observed during SOAS. Environmental Science & Technology. 51: 9533–9542
Anijiofor, S.C., Nor, A.M.J., Sarah, J., Saad, S., and Chandima, G. 2017. Aerobic and anaerobic sewage biodegradable processes: the gap analysis. International Journal of Research in Environmental Science. 3: 9-19.
Alshammari, M., Maad, F.A., Laith, A.N., Ayad, A.H.F., Hongshan, Z., Nadhir, A., and Mu, N. 2020. Synthesis of a novel composite sorbent coated with siderite nanoparticles and its application for remediation of water contaminated with congo red dye. International Journal of Environmental Research. 14:177–191.
Al-Shannag M., Bani-Melhem K., Al-Anber Z.,and Al-Qodah Z. 2013. Enhancement of COD-Nutrients removals and filterability of secondary clarifier municipal wastewater influent using electrocoagulation technique. Separation Science and Technology. 48: 673-680.
Asadgol, Z., Forootanfar, H., Rezaei, S., Mahvi, A.H., and Faramarzi, M.A. 2014. Removal of phenol and bisphenol-A catalyzed by laccase in aqueous solution. Journal of Environmental Health Science and Engineering. 12:93.
Aswathy, R., Sanju, S., and Babu, V. 2016. Solvent extraction and adsorption techniqes for the treatment of pesticide effluent. Civil Engineering and Urban Planning: An International Journal. 3:155-165.
Asselin, M., Drogui, P., Benmoussa, H., and Blais, J. 2008. Effectiveness of electro- coagulation process in removing organic compounds from slaughterhouse waste water using monopolar and bipolar electrolytic cells. Chemosphere. 72: 1727- 1733.
Atashgahi, S., Liebensteiner, M.G., Janssen, D.B., Smidt, H., Stams, A.J., and Sipkema, D. 2018. Microbial synthesis and transformation of inorganic and organic chlorine compounds. Frontiers in Microbiology. 9: 3079.
Azari, A., Gharibi, H. Kakavandi, B., Ghanizadeh, G., Javid, A., Mahvi, A.H., Sharafia, K., and Khosravia, B.T. 2017. Magnetic adsorption separation process: an alternative method of mercury extracting from aqueous solution using modified chitosancoated Fe3O4 nanocomposites. Journal of Chemical Technology & Biotechnology. 92: 188–200
Azarian, G.H., Mesdaghinia, A.R., Vaezi, F., Nabizadeh, R., and Nematollahi, D. 2007. Algae removal by electro-coagulation process, application for treatment of the effluent from an industrial wastewater treatment plant. Iranian Journal of Public Health. 36: 57-64.
Azizian, S., Haerifar, M., and Basiri-Parsa, J. 2007. Extended geometric method: a simple approach to derive adsorption rate constants of Langmuir–Freundlich kinetics. Chemosphere 68:2040–2046.
Bahrami, H., Eslami, A., Nabizadeh, R., Mohseni-Bandpi, A., Asadi, A., Sillanpää, M. 2018. Degradation of trichloroethylene by sonophotolytic-activated persulfate processes: Optimization using response surface methodology. Journal of Cleaner Production. 198: 1210-1218.
Baskaran, D., Rajamanickam, R., Pakshirajan, K. 2019. Experimental studies and neural network modeling of the removal of trichloroethylene vapor in a biofilter. Journal of Environmental Management. 250: 109385.
Baskaran, D. and Rajamanickam, R. 2019. Aerobic biodegradation of trichloroethylene by consortium microorganism from turkey litter compost. Journal of Environmental Chemical Engineering. 7: 103260.
Bazrafshan, E., Mahvi, A.H., Nasseri, S., Mesdaghinia, A.R., Vaezi, F., and Nazmara, S.H. 2006. Removal of cadmium from industrial effluents by electro-coagulation process using iron electrodes. Iranian Journal of Environmental Health, Science and Engineering. 3: 261-266.
Bej, S., Mondal, A., and Banerjee, P. 2020. Effluent water treatment: a potential way out towards conservation of fresh water in India. Recent Trends in Waste Water Treatment and Water Resource Management.
Bong-yul, T., Bong-sik, T., Young-ju, K., Yong-jin, P., Young-hun, Y., and Gil-ho, M. 2015. Optimization of colour and COD removal from livestock wastewater by electro-coagulation process: Application of Box–Behnken design (BBD). Journal of Industrial and Engineering Chemistry. 28: 307-315.
Caurie, M. 2006. The derivation of the GAB adsorption equation from the BDDT adsorption theory. Int J Food Sci Tech 41:173–179
Colic, M., Morse, D.E., Morse, W.O., Matherly, T.G., Carty, S., and Miller J.D. 2001. From Air-sparged Hydrocyclone to bubble accelerated flotation: mineral industry technology sets stage for development of new wastewater treatment flotation. Proceedings Engineering Foundation Conference, Froth Flotation/Dissolved Air Flotation: Bridging the Gap, Tahoe City California.
Condon, J.B. 2000. Equivalency of the Dubinin–Polanyi equations and the QM based sorption isotherm equation. A. Mathematical derivation. Microporous Mesoporous Mater. 38:359–376.
Cotton, F.A., Wilkinson, G., and Mullo, C.A. 1999. Advanced inorganic chemistry. New York, NY. John Wiley and Sons, Inc. 550-566.
Couture, E. 1998. Chlorate and chlorite analysis in seawater, chlorate sinks, and toxicity to phytoplankton. Master of Science Thesis submitted to Dalhousie University, Halifax, Nova Scotia, Canada.
Cristina, P., Elisabetta, F., Renato, R., Raffaella, D., Eugenio, L., Margherita, D.C., Tiziana, S. and Giorgio, G. 2012. Chlorination in a wastewater treatment plant: acute toxicity effects of the effluent and of the recipient water body. Environmental Monitoring and Assessment Volume. 184: 2091–2103.
Csekö, G., Pan, C., Gao, Q., and Horváth, A.K. 2018. Kinetics of the two-stage oxidation of sulfide by chlorine dioxide. Inorganic Chemistry. 57: 10189–10198.
Dąbrowski, A. 2001. Adsorption—from theory to practice. Advances in Colloid and Interface Science. 93:135–224.
Daniel, A.V. 2019. Waste management accountability: risk, reliability, and resilience. Waste, (second edition).
David, R. 2006. CRC Handbook of Chemistry and Physics, CRC Press., Boca Raton, FL, 2006.
Deborde, M., von Gunten, U. 2008. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: a critical review. Water Research. 42:13-51
Deghles, A. and Kurt, U. 2016a. Treatment of raw tannery wastewater by electro- coagulation technique: optimization of effective parameters using Taguchi method. Desalination and Water Treatment. 57: 14798-14809.
Deghles, A. and Kurt, U. 2016b. Treatment of tannery wastewater by a hybrid electro- coagulation/electrodialysis process. Chemical Engineering and Processing: Process Intensification. 104: 43-50.
Dehghani, M.H., Mahvi, A.H., Rastkari, N., Saeedi, R., Nazmara, S., and Iravani, E. 2015. Adsorption of bisphenol A (BPA) from aqueous solutions by carbon nanotubes: kinetic and equilibrium studies. Desalination and Water Treatment. 54: 1.
Dogan, D. and Turkdemir, H. 2012. Electrochemical treatment of actual textile indigo dye effluent. Polish Journal of Environmental Study. 21: 1185-1190.
Dickinson, T., Greef, R., and Wynne-Jones, L. 1969. The kinetics of the chlorine electrode reaction at a platinum electrode, Electrochimica Acta. 14: 467-489.
Ding, J., Lu, S., Shen, L., Yan, R., Zhang, Y., and Zhang, H. 2020. Enhanced photocatalytic reduction for the de-chlorination of 2-chlorodibenzo-p-dioxin by high-performance g-C3N4/NiO heterojunction composites under ultraviolet- visible light illumination. Journal of Hazardous Materials. 384: 121255.
Dontriros, S., Likitlersuang, S., and Janjaroen, D. 2020. Mechanisms of chloride and sulfate removal from municipal-solid-waste-incineration fly ash (MSWI FA): Effect of acid-base solutions. Waste Management. 101: 44-53.
Du, Z., Tian, W., Qiao, K., Zhao, J., Wang, L., Xie, W., Chu, M., and Song, T. 2020 Improved chlorine and chromium ion removal from leather processing wastewater by biocharcoal-based capacitive deionization. Separation and Purification Technology. 233: 116024.
Eccles, H. 1999. Treatment of metal-contaminated wastes: why select a biological process? Trends in Biotechnology. 17: 462–465.
Eduardo, L., González, J., and Molina, Á. 2014. Recent advances on the theory of pulse techniques: A mini review. Electrochemistry Communications. 43: 25–30.
Ehrampoush, M.H., Miria, M., Salmani, M.H., and Mahvi, A.H. 2015. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract Ehrampoush et al. Journal of Environmental Health Science & Engineering. 13: 84.
Emad, S.E., Walid, H., Sohier, M., and Boktor, M. 2020. Natural products for surface water Coagulation: an alternative sustainable solution for rural areas. International Journal of Environmental Research.
Enric, P.D. and Aleix, M.C. 2015. Pedro Javier Miranda Luján 2015. chlorine dioxide as disinfectant for pre-treatment in seawater desalination plant. Conference Paper. American Water Works Association AMTA/AWWA Membrane Technology Conference Proceedings.
Etinosa O.I., Emmanuel, E.O., Vincent, N.C., Isoken, H.I., Alexander, O.E., Fredrick, O.E., Nicholas, O.I., and Omoruyi, G.I. 2013. Review Article Toxicological Profile of Chlorophenols and Their Derivatives in the Environment: The Public Health Perspective. Hindawi Publishing Corporation The Scientific World Journal Volume 2013. Article ID 460215: 11.
Eunice, I., Olusola, O.O., Henry, Ogola, J.O., and Ramganesh, S. 2020. Investigating industrial effluent impact on municipal wastewater treatment plant in Vaal, South Africa. International Journal of Environmental Research. Public Health. 17: 1096.
EuroChlor, 2004. Science Dossier. EuroChlor workshop on soil chlorine chemistry: Workshop Proceedings. November, 2004.
EU 2017. Active chlorine released from sodium hypochlorite Product-type 1 (Human hygiene). Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products.
Evgenia, I. and Mika, S. 2013. The use of low-cost adsorbents for wastewater purification in mining industries. Environmental Science and Pollution Research. 20: 7878–7899.
Exner, K.S., Anton, J., Jacob, T., and Over, H. 2016. Full kinetics from first principles of the chlorine evolution reaction over a RuO2(110) model electrode. Angewandte Chemie International Edition. 55: 7501-7504.
Exner, K.S., Iman, S.E., and Herbert, O. 2018. A universal approach to determine the free energy diagram of an electro catal Reac. ACS Catal. 8: 1864-1879.
Farr, J.P., Smith, W.L., and Steichen, D.S. 2003. Bleaching agents. In: Kirk-Othmer encyclopedia of chemical toxicology. John Wiley & Sons, Inc., 4: 43-81.
Flávia, R., Pereira, S., Reynaldo, B., Galvão, S., Gesivaldo, J., Alves, D.F., Paulo, H., Almeida, D.H., Antonio, and Cícero, D.S. 2018. Wastewater treatment using adsorption process in column for agricultural purposes. Rev. Rev. Ambiente & Água - An Interdisciplinary Journal of Applied Science. 14: 1, e2178.
Florica, M. 2014. Electrochemical techniques for characterization and detection application of nanostructured carbon composite. The World’s Leading Publisher of Open Access Books.
Frascari, D., Pinelli, D., Ciavarelli, R., Nocentini, M., and Zama, F. 2019. Chloroform aerobic cometabolic biodegradation in a continuous-flow reactor: model calibration by means of the gauss-newton method. Canadian Journal of Chemical Engineering. 97: 1771-1784.
Fritz, S. 2013. Electroanalytical Methods: Guide to Experiments and Applications. Springer. pp. 109. ISBN 978-3-662-04757-6.
Fumihiro, K., Minoru, U., and Akifumi, Y. 2005. Determination of free chlorine based on anodic voltammetry using platinum, gold, and glassy carbon electrodes. Analytica Chimica Acta. 537: 293-298.
Fumihiro, K., Minoru, U., and Akifumi, Y. 2004. Determination of hypochlorite ion by anodic voltammetry. Bunseki Kagaku. 53: 905-910.
Gallagher, M. W., Choularton, T. W., Bower, K. N., Stromberg, M., Beswick, K. M., Fowler, D. and Hargreaves, K. J. 1994. Measurements of methane fluxes on the landscape scale from a wetland area in North Scotland. Atmospheric Environment. 28: 242
García-Armada, P., Losada, J., and de Vicente-Pérez, S. 1996. Cation analysis scheme by differential pulse polarography. Journal of Chemical Education. 73: 544.
George, Z.K. and Kostas, A.M. 2018. Flotation in water and wastewater treatment. processes. 6: 116.
Ghoochani, M., Rastkari, N., Heibati, B., Ghozikali, M.G., Jeddi, M.Z; Fawell, J., Nazmara, S., and Mahvi, A.H. 2017. Risk assessment of haloacetic acids in the water supply of Tehran, Iran. Water Supply. 17: 958–965.
Government of British Columbia, 2002. Water quality: ambient water quality guidelines for chlorate. Technical Background Report. Water, Air and Climate Change Branch, Ministry of Environment, Government of British Columbia, February, 2002.
Hua, G., Reckhow, D.A., and Kim, J. 2006. Effect of Bromide and Iodide Ions on the Formation and Speciation of Disinfection Byproducts during Chlorination. Environmental Science & Technology. 40: 3050–3056.
Hakizimana J.N., 2017. Electrocoagulation process in water treatment: a review of electro-coagulation modeling approaches, Desalination 404: 1–21.
Han, Y. and Yan, W. 2016. Reductive de-chlorination of trichloroethene by zero-valent iron nanoparticles: reactivity enhancement through sulfidation treatment. Environmental Science & Technology. 50: 12992-13001.
Hasan, S.W., Elektorowicz, M., and Oleszkiewicz, J.A. 2014. Start-up period investigation of pilot-scale submerged membrane electro-bioreactor (SMEBR) treating raw municipal wastewater. Chemosphere. 97: 71-77.
Hu, Y., Zhang, C., Wang, D.Z., Wen, J.Y., Chen, M.H., and Li, Y. 2013. Chloride ion transport and fate in oilfield wastewater reuse by interval dynamic multimedia aquivalence model. Water Science and Technology. 628-634.
Isai, G.M., Tanja, V.K., Rafael, K., Ulrich, K., Thomas, T., and Kai, S. 2013. The kinetics of the hydrogen chloride oxidation. Journal of the Serbian Chemical Society. 78: 2115-2130.
Jannatul, R., Walter, Z., Tang, M., and Sillanpää, S., 2020. Analysis of anode efficiency in electrochemical treatment of wastewater and sludge. Environmental Processes. 7: 1041–1064
Joshi, P.S. and Sutrave, D.S. 2018. A brief study of cyclic voltammetry and electrochemical analysis. International Journal of ChemTech Research. 11: 77- 88, 2018.
Jossens, L., Prausnitz, J.M., Fritz, W., Schlünder, E.U., and Myers, A.L. 1978. Thermodynamics of multi-solute adsorption from dilute aqueous solutions. Chemical Engineering Science. 33: 1097–1106.
Kaczur, J.J. 1996. Oxidation chemistry of chloric acid in NOx/SO2 and air toxic metal removal from gas streams. Environmental Progress. 15: 245-254
Kang, N., Anderson, T.A., and Jackson, W.A. 2006. Photochemical formation of perchlorate from aqueous oxychlorine anions. Analytica Chimica Acta. 567: 48– 56.
Katsaounis, A. and Souentie, S. 2014. Organic pollutants in water using DSA electrodes, in-cell mediated (via active chlorine) electrochemical oxidation. in: G. Kreysa, K.-i. Ota, R.F. Savinell (Eds.). Encyclopedia of Applied Electrochemistry, New York: Springer. p. 1407-1416.
Khan, N., Khan, M.D., Ansari, M.Y., Ahmad, A., and Khan, M.Z. 2019. Bio- electrodegradation of 2,4,6-Trichlorophenol by mixed microbial culture in dual chambered microbial fuel cells. Journal of Bioscience and Bioengineering. 127: 353-359.
Khan, A.S.A. 2012. Evaluation of thermodynamic parameters of cadmium adsorption on sand from Temkin adsorption isotherm. Turkish Journal of Chemistry. 36: 437–443.
Kim, M.S., Piggott, E., Zrinyi, N., Lee, C., and Pham, A.L.T. 2020. Reduction of chlorendic acid by zero-valent iron: Kinetics, products, and pathways, Journal of Hazardous Materials, 384.
Knipping, E.M. and Dabdub, D. 2003. Impact of chlorine emissions from sea-salt aerosol on coastal urban ozone. Environmental Science and Technology. 37: 275-284.
Kraft, A. 2008. Electrochemical water disinfection: a short review. Platinum Metals Review. 52: 177-185.
Krzemieniewski, M. and Dębowski, M. 2005. The influence of a constant electromagnetic field on phosphorus removal from wastewater in metal packing systems. Environment Protect Ion Engineering. 31: 23-37.
Kumar, V. 2017. A review on the feasibility of electrolytic treatment of wastewater: Prospective and constraints. Archives of Agriculture and Environmental Science. 2: 52-62.
Kurniawan, T.A., Chan, G.Y.S., Lo, W.H., and Babel, S. 2005. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Science of the Total Environment. 366: 409–426.
Kurt, U., Talha, G.M., Fatih, I., and Kamil, V. 2008. Treatment of domestic wastewater by electro-coagulation in a cell with Fe–Fe electrodes. Environmental Engineering Science. 25: 153-162.
Liang, S., Liu, S., Fan, Z., Zhang, W., Guo, M., Cheng, F., and Zhang, M. 2020. Enhanced HCl removal from CO2-rich mixture gases by CuOx/Na2CO3 porous sorbent at low temperature: Kinetics and forecasting, Chemical Engineering Journal. 381:122738.
Li, K., Yang, Y., Bacha, A.U.R., Feng, Y., Ajmal, S., Nabi, I., Zhang, L. 2019. Efficiently complete degradation of 2,4-DCP using sustainable photo-electrochemical reduction and sequential oxidation method. Chemical Engineering Journal. 378: 122191.
Lin, K. S., Mdlovu, N. V., Chen, C. Y., Chiang, C. L., and Dehvari, K. 2018. Degradation of TCE, PCE, and 1, 2–DCE DNAPLs in contaminated groundwater using polyethylenimine-modified zero-valent iron nanoparticles. Journal of Cleaner Production. 175: 456-466.
Liu, Y. 2005. Derivation of a general adsorption isotherm model. J Environ Eng 131:1466–1468.
Liu, Z., Lin, Y.L., Xu, B., Hu, C.Y., Zhang, T.Y., Cao, T.C., Pan, Y., and Gao, N.Y. 2020.
Degradation of diiodoacetamide in water by UV/chlorination: Kinetics, efficiency, influence factors and toxicity evaluation. Chemosphere. 240: 124761.
Lo, K.H., Lu, C.W., Lin, W.H., Chien, C.C., Chen, S.C., and Kao, C.M. 2020. Enhanced reductive de-chlorination of trichloroethene with immobilized Clostridium butyricum in silica gel. Chemosphere. 238: 124596.
Lu, H.J. 2010. Study on the environmental impact of constructed wetlands treatment of oil-field wastewater. Master’s Thesis, Jilin University, Jilin, China.
Liu, X., Zhou, H., Pei, S., Xie, S., and You, S. 2020. Oxygen-deficient WO3−x nanoplate array film photoanode for efficient photoelectrocatalytic water decontamination. Chemical Engineering Journal. 381: 122740.
Maciej, J. 2009. BET-type adsorption isotherms for gaseous mixtures. BET-type Adsorption Isotherms for Gaseous Mixtures. 23:487–495.
Malcolm, J., Brandt, K., Michael, J., Andrew, J.E., and Don, D.R. 2017. Chemistry, Microbiology and Biology of Water”. Elsevier BV. p. 235-321.
Mahendran, B., Bondili, J.S., and Pardhasaradhu, M. 2015. Water chlorination and its relevance to human health Mahendran. Asian Journal of Pharmaceutical and Clinical Research. 8: 20-24
Mahvi, A., Maleki, A., Rezaee, R., and Safari, M. 2009. Reduction of humic substances in water by application of ultrasound waves and ultraviolet irradiation September 2009. Iranian Journal of Environmental Health Science Engineering. 6: 233-240
Mao, X., Polasko, A., and Alvarez-Cohen, L. 2017. Effects of sulfate reduction on trichloroethene de-chlorination by Dehalococcoides-containing microbial communities. Applied and Environmental Microbiology. 83.
Marschner, H.1995. Mineral nutrition of higher plants, 2nd edn. London: Academic Press. Marta, I.L. and Natalia, Q. 2010. Photochemical Advanced Oxidation Processes for Water and Wastewater Treatment. : Recent Patents on Engineering. 4: 217-241.
Masixole, S., Hassina, M., and Philiswa, N.N. 2019. Uptake of trace elements by vegetable plants grown on agricultural soils: evaluation of trace metal accumulation and potential health risk. Journal of African Earth Sciences. 160: 103635.
Matteucci, F., Ercole, C., and de Gallo, M. 2015. A study of chlorinated solvent contamination of the aquifers of an industrial area in central Italy: a possibility of bioremediation. Front Microbiology. 2015; 6: 924.
McQuillan, R.V., Stevens, G.W., and Mumford, K.A. 2020. Electrochemical removal of naphthalene from contaminated waters using carbon electrodes, and viability for environmental deployment. Journal of Hazardous Materials. 383: 121244
Mdlovu, N.V., Lin, K.S., Chen, C.Y., Mavuso, F.A., Kunene, S.C., and Espinoza, M.J.C. 2019. In-situ reductive degradation of chlorinated DNAPLs in contaminated groundwater using polyethyleneimine-modified zero-valent iron nanoparticles. Chemosphere. 224: 816-826.
Mdlovu, N.V., Lin, K.S., Dwitya, S.S., Chen, C.Y., and Chiang, C.L. 2018. Decontamination of 1, 2-dichloroethane DNAPL in contaminated groundwater by polymer-modified zero-valent iron nanoparticles. Topics in Catalysis. 61: 1653- 1664.
Melih, B.A., Ozge, G., Metin, G., and Yucel, S. 2019. Preparation of a novel electrochemical sensor for phosphate detection based on a molybdenum blue modified poly(vinyl chloride) coated pencil graphite electrode. Analytical Methods. 11: 3874-3881.
Michio, M., Tribidasari, A.I., Mamoru, S., Satoshi, N., Akira, F., and Yasuaki, E. 2008 Electrochemical detection of free chlorine at highly boron-doped diamond electrodes. Journal of Electroanalytical Chemistry. 612: 29-36.
Michałowicz, J. and Duda, W. 2007. Phenols—sources and toxicity. Polish Journal of Environmental Studies. 16: 347–362.
Mount, D.R., Gulley, D.D., Hockett, J.R., Garrison, T.D., and Evans, J.M. 1997. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna, and Pimephales promelas (fathead minnows). Environmental Toxicology and Chemistry. 16: 2009-2019.
Nakareseisoon, S., Tachiyashiki, S.J., Benga, G., Pacey, E., and Gordon, G. 1988. Determination of chlorite at very low levels by using differential pulse polarography. Analytica Chimica Acta. 204: 169-177.
Nicoson, J.S., Margerum, D.W. 2002. Kinetics and Mechanisms of Aqueous Chlorine Reactions with Chlorite Ion in the Presence of Chloride Ion and Acetic Acid/Acetate Buffer. Inorganic Chemistry. 41: 342-347.
Nieto-Sandoval, J., Munoz, M., de Pedro, Z.M., and Casas, J.A. 2019. Catalytic hydrodechlorination as polishing step in drinking water treatment for the removal of chlorinated micropollutants. Separation and Purification Technology. 227: 115717.
Nirali, K., Lavleen, B., Mansi, N., and Vaishnavi, R. 2016. Wastewater treatment by ion exchange method: a review of past and recent researches. Environmental Science: An Indian Journal. 12: 143-150.
Nivorlis, A., Dahlin, T., Rossi, M., Höglund, N., and Sparrenbom, C. 2019. Multidisciplinary characterization of chlorinated solvents contamination and in- situ remediation with the use of the direct current resistivity and time-domain induced polarization tomography. Geosciences. 9: 487.
Quan, Y., Wu, H., Yin, Z., Fang, Y., and Yin, C. 2017. Effect of static magnetic field on trichloroethylene removal in a biotrickling filter. Bioresource Technology. 239: 7-16.
Patricia, A.C., Cecílio, S.F., Raquel, F.P.N., Nivaldo, B., and Maria, V.B.Z. 2003. A comparative study on chemical and electrochemical degradation of reactive Blue 4 Dye. Portugaliae Electrochimica Acta. 21: 49-67.
Pedersen, A.J.1., Ottosen, L.M., and Villumsen, A. 2003. Electrodialytic removal of heavy metals from different fly ashes. Influence of heavy metal speciation in the ashes. Journal of Hazardous Materials. 100: 65-78.
Perng, Y.S., Wang, I.C., Yu, S.T., Hsieh, Y.C., Chen, P.R., and Chi, H.Y. 2007. Treatment of a specialty paper mill wastewater using a pilot-scale pulsed electro- coagulation unit. Taiwan Journal for Science. 22: 355-366.
Papazi, A., Karamanli, M., and Kotzabasis, K. 2019. Comparative biodegradation of all chlorinated phenols by the microalga Scenedesmus obliquus — The biodegradation strategy of microalgae. Journal of Biotechnology. 296: 61-68.
Pathiraja, G., Egodawatta, P., Goonetilleke, A., and Te’o, V.S.J. 2019. Effective degradation of polychlorinated biphenyls by a facultative anaerobic bacterial consortium using alternating anaerobic aerobic treatments. Science of the Total Environment. 659: 507-514.
Pathiratne, K.A.S., Skandaraja S.S., and Jayasena, E.M.C.M. 2008. Linear sweep voltammetric determination of free chlorine in waters using graphite working electrodes. National Science Foundation Sri Lanka. 36:25-31.
Philip, J.W., Martin, R.B. 2001. Chloride in Soils and its Uptake and Movement within the Plant: A Review. Annals of Botany. 88: 967-988.
Qian, L., Chen, Y., Ouyang, D., Zhang, W., Han, L., Yan, J., vapil, P.K., and Chen, M. 2020. Field demonstration of enhanced removal of chlorinated solvents in groundwater using biochar-supported nanoscale zero-valent iron. Science of the Total Environment. 698: 134215.
Radjenovic, J. and Sedlak, D.L. 2015. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environmental Science and Technology. 49: 11292-11302.
Ramesh, B.R., Bhadrinarayana, N.S., Meera, K.M., Sheriffa, B., and Anantharaman, N. 2007. Treatment of tannery wastewater by electro-coagulation. Journal of the University of Chemical Technology and Metallurgy. 42: 201-206.
Roa-Morles, G., Campos-Medina, E., Aguilera-Cotero, J., Bilyeu, B., Barrera-Diaz, C. 2007. Aluminium electro-coagulation with peroxide applied to waste water pasta and cookie processing. Separation and Purification Technology. 54: 124-129.
Remucal, C.K. and Manley D., 2016. Emerging investigators series: the efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environmental Science: Water Research & Technology. 2: 565-579
Rodrigues, R., Betelu, S., Colombano, S., Tzedakis, T., Masselot, G., and Ignatiadis, I. 2020. In situ chemical reduction of chlorinated organic compounds. In Environmental Soil Remediation and Rehabilitation. p. 283-398. Springer, Cham.
Ruan, X., Liu, H., Wang, J., Zhao, D., and Fan, X. 2019. A new insight into the main mechanism of 2,4-dichlorophenol de-chlorination by Fe/Ni nanoparticles. Science of the Total Environment. 697: 133996.
Saeed, S., Shwet, P., Nandita, D., Ross, C., Venkat, K., Eric, F. and Jennifer, D. 2015. Development of a Site-Specific Kinetic Model for Chlorine Decay and the Formation of Chlorination By-Products in Seawater. Journal of Marine Science and Engineering. 3: 772-792.
Sarzamin, K. and Jawad, A. 2018. Chemical analysis of air and water. Advanced Methods and Applications. p. 21-39.
Schummer, C., Sadiki, M., Mirabel, P., and Millet, M. 2006. Analysis of t- butyldimethylsilyl derivatives of chlorophenols in the atmosphere of urban and rural areas in east of France. Chromatographia. 63: 189-195.
Scott, K., MPCCA, and Elise, D. 2018. Alternatives for addressing chloride in wastewater effluent. Book, published by Minnesota Pollution Control Agency. PP 37.
Serbent, M.P., Rebelo, A.M., Pinheiro, A., Giongo, A., and Tavares, L.B.B. 2019. Biological agents for 2,4-dichlorophenoxyacetic acid herbicide degradation. Applied Microbiology and Biotechnology. 103:5065-5078.
Shahamat, Y.D., Farzadkia, M., Nasseri, S., Mahvi, A.H., Gholami, M., and Esrafili, A. 2014. Magnetic heterogeneous catalytic ozonation: a new removal method for phenol in industrial wastewater. Environmental Health Science and Engineering. 12: 50.
Silvia, C., Ciprian, G., Piuleaca, K.m, Godinib, and Ghasem, A. 2011. Modeling of electrolysis process in wastewater treatment using different types of neural networks. Chemical Engineering Journal. 172: 267– 276.
Skubal, L.R. and Meshkov, N.K. 2002. Reduction and removal of mercury from water using arginine-modified TiO2. Journal of Photochemistry and Photobiology A Chemistry. 148: 211–214.
Solimana N.K. and Moustafa A.F. 2020. Industrial solid waste for heavy metals adsorption features and challenges; a review. Materials Research and Technology. 9: 10235-10253
Song, H., Tsang, D.C.W., Kwon, G., Kwon, E.E., and Cho, D.W. 2019. Coupling carbon dioxide and magnetite for the enhanced thermolysis of polyvinyl chloride. Science of the Total Environment. 696: 133951.
Stauber, J.L. 1998. Toxicity of chlorate to marine microalgae. Aquatic Toxicology. 41: 213–227.
Stryer, L. 1988. Biochemistry. W. H. Freeman and Company, New York.
Shuokr, Q.A. and Sazan, M.A. 2016. Performance of Biological Filtration Process for Wastewater Treatment: A review. ZANCO Journal of Pure and Applied Sciences. 28: s554-563.
Sulistyo, S., Kô, T., Kazuhisa, Y., Shiro, M., and Narsito, 2010. Differential pulse voltammetric determination of free chlorine for water disinfection process. Electroanalysis. 22: 2765 – 2768.
Urbansky E.T. 1998. Perchlorate chemistry: implications for analysis and remediation. Biorem J. 2: 81–95
USEPA. 1999. Compendium of Methods for the Determination of Inorganic Compounds in Ambient Air EPA/625/R96/010a. United States Environmental Protection Agency, Cincinnati Ohio, 21. Available from: http://www.epa.gov/ttnamti1/files/ambient/inorganic/iocompen.pdf
Terzyk, A.P., Chatłas, J., Gauden, P.A., Rychlicki, G., and Kowalczyk, P. 2003. Developing the solution analogue of the Toth adsorption isotherm equation. Colloid and Interface Science. 266: 473–476.
Tilley, E., Ulrich, L., Lüthi, C., Reymond, Ph., and Zurbrügg C. 2016. Compendium of sanitation systems and technologies (2nd Revised Edition). Swiss Federal Institute of Aquatic Science and Technology (Eawag), Duebendorf, Switzerland, p. 175. ISBN 978-3-906484-57-0. Archived from the original on 8 April.
Thorben Muddemann, Dennis Haupt, Michael Sievers, Ulrich Kun 2019. Electrochemical Reactors for Wastewater Treatment. Chem Bio Eng Reviews. 6: 142–156
Truhlar D.G., Cramer, C.J., Gao, J., Garrett, B.C., Dupuis, M., Straatsma, T.P., Morokuma, K., Dunning, Jr. T.H., Borisov, Y.A. , Arcia, E.E. , Thompson, J. 2006. Chemical Fate of Contaminants in the Environment: Chlorinated Hydrocarbons in the Groundwater. Pacific Northwest National Laboratory United States Department of Energy under Contract DE-AC05-76RL01830, PNNL-16064.
Van Wijk, D.L. and Hutchinson, T.H. 1995. The ecotoxicity of chlorate to aquatic organisms: A critical review. Ecotoxicology and Environmental Safety. 32: 244– 253.
Vinod, K. 2017. A review on the feasibility of electrolytic treatment of wastewater: prospective and constraints. Archives of Agriculture and Environmental Science. 2: 52-62.
Vít, J. and Petr, L. 2019. Formation of reactive chlorine species in saline solution treated by non-equilibrium atmospheric pressure He/O2 plasma jet. Plasma Sources Science and Technology. 28.
Vlotman, D.E., Ngila, J.C., Ndlovu, T., Doyle, B., Carleschi, E., and Malinga, S.P. 2019. Hyperbranched polymer membrane for catalytic degradation of polychlorinated biphenyl-153 (PCB-153) in water. Reactive and Functional Polymers. 136: 44- 57.
Wang, B., Dong, H., Li, L., Wang, Y., Ning, Q., Tang, L., and Zeng, G. 2020. Influence of different co-contaminants on trichloroethylene removal by sulfide-modified nanoscale zero-valent iron. Chemical Engineering Journal. 381: 122773.
Wang, K., Shu, S., Chen, M., Li, J., Zhou, K., Pan, J., Wang, X., Li, X., Sheng, J., Dong, F., et al. 2020. Pd-TiO2 Schottky heterojunction catalyst boost the electrocatalytic hydrodechlorination reaction. Chemical Engineering Journal. 381: 122673.
Wang, X., Xin, J., Yuan, M., and Zhao, F. 2020. Electron competition and electron selectivity in abiotic, biotic, and coupled systems for de-chlorinating chlorinated aliphatic hydrocarbons in groundwater: A review. Water Research. 116060.
Wang, Q., Song, X., Tang, S., and Yu, L. 2020. Enhanced removal of tetrachloroethylene from aqueous solutions by biodegradation coupled with nZVI modified by layered double hydroxide. Chemosphere. 243: 125260.
Wang, L.P., Lee, W.H., Tseng, S.M., and Cheng, T.W. 2018. Removal of Chloride Ions from an Aqueous Solution Containing a High Chloride Concentration through the Chemical Precipitation of Friedelʼs Salt. Materials Transactions. 59: 297-302.
Wan, H., Islam, M.S., Briot, N.J., Schnobrich, M., Pacholik, L., Ormsbee, L., and Bhattacharyya, D. 2020. Pd/Fe nanoparticle integrated PMAA-PVDF membranes for chloro-organic remediation from synthetic and site groundwater. Journal of Membrane Science. 594: 117454.
WHO. 1986. Chlorite and chlorate in drinking water. Background document for development of WHO Guidelines for Drinking Water Quality. World Health Organisation. report No. WHO/SDE/WSH/05.08/86. Available from: http://www.who.int/water_sanitation_health/dwq/chemicals/chlorateandchlorit e0505. pdf.
William, G., Lawrence, W.A., Dennis, T.B., Debra, L.D., Philip, B.D., Donald, R.G., Margarete, A.H., Teresa, J.N.K., and John, R. 2000. Major ion toxicity in effluents 2000. A review with permitting recommendations. Environmental Toxicology and Chemistry. 19: 175–182.
Wolfgang Dreyer,a Clemens Guhlkea and Rüdiger Müller 2016. A new perspective on the electron transfer: recovering the Butler–Volmer equation in non-equilibrium thermodynamics. Physical Chemistry Chemical Physics. 18.
Wu, F., Liu, B., Wu, K., and Tseng, R. 2010. A new linear form analysis of Redlich– Peterson isotherm equation for the adsorptions of dyes. Chemical Engineering Journal. 162: 21–27.
Xuejun, C., Zhemin, S., Xiaolong, Z., Yaobo, F., and Wenhua, W. 2003. Advanced treatment of textile wastewater for reuse using electrochemical oxidation and membrane filtration. Water SA. 31: 127-132.
Xu, J., Liu, X., Cao, Z., Bai, W., Shi, Q., and Yang, Y. 2020. Fast degradation, large capacity, and high electron efficiency of chloramphenicol removal by different carbon-supported nanoscale zero-valent iron. Journal of Hazardous Materials. 384: 121253.
Yamamoto, A., Matsunaga, A., Mizukami, E., Hayakawa, K., and Miyazaki, M. 1993. Adsorption isotherm of undissociated eluent acid and its relation to the retention of system peaks in non-suppressed ion chromatography. Journal of Chromatography A. 644: 183–187.
Yazıcı, E.Y., Deveci, H., and Alp, İ. 2009. Treatment of cyanide effluents by oxidation and adsorption in batch and column studies. Journal of Hazardous Materials. 166: 1362–1366.
Yongkui, Y., Longfei, W., Feng, X., Lin, Z., and Zhi, Q. 2020. Activated sludge microbial community and treatment performance of wastewater treatment plants in industrial and municipal zones. International Journal of Environmental Research and Public Health. 17: 436.
Zhang, C.C. and Zhang, F.S. 2020. Enhanced dehalogenation and coupled recovery of complex electronic display housing plastics by sub/supercritical CO2. Journal of Hazardous Materials. 382: 121140.
Zheng, C., Li. M, Liu, H., and Xu, Z. 2020. Complete dehalogenation of bromochloroacetic acid by liquid phase catalytic hydrogenation over Pd/CeO2 catalysts. Chemosphere. 239: 124740.
Zheng, M., Chunguang, H., and Qiang, H. 2015. Fate of free chlorine in drinking water during distribution in premise plumbing. Ecotoxicology. 24: 2151–2155
Zhou, M., Dai, Q., Lei, L., and Wang, D. 2004. Synergetic effects for p-nitrophenol abatement using a combined activated carbon adsorption-electrooxidation process. Journal of Zhejiang University SCIENCE. 5: 1512-1516.
Zoeteman, B.C.J. 1980. Sensory assessment of water quality. 1st Edition, eBook ISBN: 9781483150307. New York: Pergamon Press.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Mohammad Al-Hwaiti1, Hamidi Abdul Aziz1, 2,*, Mohd Azmier Ahmad2, 3, and Reyad Al-Shawabkeh4
1 School of Civil Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Pulau Pinang, Malaysia
2 Solid Waste Management Cluster, Science and Technology Research Centre, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Pulau Pinang, Malaysia
3 School of Chemical Engineering, Universiti Sains Malaysia, Pulau Pinang, Malaysia
4 Faculty of Engineering, Chemical Engineering Department, University of Jordan, Amman, Jordan
Corresponding author: Hamidi Abdul Aziz, E-mail: cehamidi@usm.my
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: July 27, 2020;
Revised: November 4, 2020;
Accepted: November 25, 2020;
Published online: March 5, 2021

