
A Preliminary Study on Morphological Variations from Wet and Dry Microhabitats of Hyophila involuta (Pottiaceae, Bryophyta): A Case Study from Chiang Mai Province, Northern Thailand
Narin Printarakul* and Arunothai JampeetongPublished Date : 2020-12-17
DOI : https://doi.org/10.12982/CMUJNS.2021.020
Journal Issues : Number 1, January-March 2021
Abstract The morphological variations of a cosmopolitan moss, Hyophila involuta (Hook.) A. Jaeger, are reported here using t-test to distinguish 2 distinct forms of ecotypes (humid and arid forms). Three replicates each from 145 collections from 27 places in Doi Inthanon National Park, Doi Suthep-Pui National Park, and Chiang Dao Wildlife Sanctuary, Chiang Mai province, northern Thailand were examined. Eleven gametophytic characters were measured such as stem height, branching, stem diameter, leaf size, leaf blade thickness, size of median and basal laminal cells, length of innermost perichaetial leaf, and length of archegonia. Of these, 10 characters, viz. stem height, leaf size, stem diameter, branching, length of basal laminal cells, length of innermost perichaetial leaf, and length of archegonia were significantly different among populations. Other additional features of the two different ecotypes of H. involuta were recorded and discussed including leaf apices, innermost perichaetial leaf apices, marginal teeth, hyaline nodules, and number of cortical and medullary central strand cells.
Keywords: Bryophytes, Chiang Mai, Morphometry, Pottiaceous moss, Wet and dry microhabitats
Funding: This research was supported by a grant from the Faculty of Science, Chiang Mai University. This work was also partly supported by the Thailand Research Fund (no. DBG6080015).
Citation: Printarakul, N. and Jampeetong, A. 2021. A preliminary study on morphological variations from wet and dry microhabitats of Hyophila involuta (Pottiaceae, Bryophyta): A case study from Chiang Mai province, northern Thailand. CMUJ. Nat. Sci. 20(1): e2021020.
INTRODUCTION
Hyophila involuta (Hook.) A. Jaeger (cement moss), an acrocarpous moss in the family Pottiaceae, is a common species which is widely distributed throughout tropical and subtropical regions of the world (Zander, 1993). It can be found at various elevations and in different habitats such as deserts, humid soil, wet rocks, and stream banks of waterfalls and is also found on manmade concrete structures in urban habitats (Eddy, 1990; Li et al., 2001; and Deora & Deora, 2017).
The distinctive characters of the species described in several taxonomic treatments and floras include spathulate-lanceolate leaves with serrulate to denticulate leaf margins that are strongly involute when dry, bulging-mamillose upper laminal cells, well-differentiated leaf bases, costae with two well-developed stereid bands in cross section, stems with a well-differentiated central strand, and cylindrical eperistomate capsules (Gangulee, 1972; Eddy, 1990; Zander 1993; Li et al., 2001; Virendra & Reesa, 2009; Costa, 2015). During our excursions in Doi Suthep-Pui National Park, we found an interesting humid ecotype of cement moss with large stems, up to 5 cm height including 4–5 (8) branches, and vegetative leaf length up to 4 mm. In contrast, the plants growing in dry areas had smaller stems (about 1 cm height, usually unbranched and leaf length up to 2.5 mm). After carefully examination with many literatures reported this species from different part of the world and also comparison with several identified herbarium specimens deposited at the CMUB Herbarium collected from various places in Chiang Mai province, northern Thailand, we did not find any one who described arid and humid form of H. involuta. Many literatures cites here including the taxonomic monographs, revisions, and floras such as Gangulee (1972), Saito (1975), Noguchi (1988), Eddy (1990), Zander (1993), Li et al. (2001), Allen (2002) Costa (2016) and etc., usually reported only common morphology of the local plants of H. involuta but did not compare size of gametophytes and their sexual structures from wet and dry areas. So, this study aims to preliminarily investigate morphological differences of the cosmopolitan moss, H. involuta from dry and wet areas using a morphometric method. This study will provide a new insight on plant structures particularly from the different ecotypes.
MATERIALS AND METHODS
Plant collection
Samples of cement moss (145 packets) were collected from two distinct microhabitats including aquatic microhabitats (shaded-wet rocks in streams or waterfalls, Figure 1C) and terrestrial microhabitats (open-dry soils or rocks, Figure 1H) (see Appendix). The specimens were identified using the taxonomic keys in Gangulee (1972), Eddy (1990), Zander (1993), Li et al. (2001), Virendra & Reesa (2009) Costa (2015), and Deora & Deora (2017). All specimens were then collected in paper bags and dried at room temperature. The vouchers were deposited in the CMUB herbarium of the Biology Department, Faculty of Science, Chiang Mai University, Thailand.
Morphometric study
Three replicate gametophytes and sporophytes of H. involuta from the 145 collections from 27 populations were selected and redehydrated in the laboratory. Morphological characters of each gametophyte (150 gametophytes from 7 wet areas and 200 gametophytes with sporophytes from 20 dry areas, see Supplement) were examined. Stem height, branching (number of branches and the length of branches) were measured. Stem diameter, leaf size, leaf blade thickness, size of median and basal laminal cells were determined using a light-transmission microscope, and the number of sporophytes was also recorded. We also considered other gametophytic characters such as leaf apices (apiculus, apical leaf shaped, and number of apical and marginal teeth), hyaline nodules (a group of swollen, color less, thin-walled cells, well differentiate in the epidermis of stem near the leaf insertion) and number of cortical and medullary cells in cross section of stems, which were noted as additional characters.
Data analysis
Statistical analysis was performed using SPSS statistic program version 17 (SPSS Inc., Chicago, USA). Significantly different means of each character between dry and wet areas were identified by the T-test at the 99.99% confidence level.
RESULTS
Independent samples T-test of gametophytic characters of humid and arid forms of H. involuta
T-test of 10 gametophytic characters of H. involuta grown on wet and dry habitats showed significantly different among populations (Table 1). Generally, gametophytes from the dry habitats were mostly small with average stem height and stem diameter of 6.5 mm (Figures 1E, 1D af, 1G, and 3#1–20) and 180.0 µm (Figure 2B), respectively. Most plants were unbranched and also had small leaves (approximately 0.7×2.2 mm). Gametophyte stems usually produced sporophytes (Figure 1E, 1G, and 1H, and Figures 3#1–20). In contrast, most gametophytes from the wet habitats were larger, with average stem height and stem diameter values of about 28.5 mm (Figures 1A, 1B, 1D hf, and Figures 5#21–27) and 323.3 µm (Figure 2A), respectively. Up to 6 branches (average 2.2) per stem were found (Figures 1A, 1B, and Figures 5#21–27). The plants also had bigger leaves up to 4 mm long (Figures 1F hf, 4#27, and 5#21–27), but they rarely produced sporophytes. The length of basal laminal cells of the humid form were longer than those of the arid form (Figure 2I), approximately 13.3×56.1 µm (vs 13.6×38.6 µm in the arid form, Figure 2J). Further, innermost perichaetial leaves of humid forms were different from arid forms by having larger size (approximately 2.1–2.6 mm long), ovate-lanceolate with abruptly narrow acuminate leaf apices and shortly excurrent costae (Figures 6G-K), whereas smaller size of innermost perichaetial leaves (approximately 1.2–1.4 mm long), lingulate with obtuse-rounded leaf apices and the costae ending below leaf apex in several cells were found in arid form (Figures 6A-F). The length of archegonia of humid form were longer than the archegonia of arid form (Figure 2D), approximately 0.8–1.0 mm long (vs 0.45–0.56 mm long in arid form, Figure 2E).
Table 1. Independent samples T-test of 10 characters of H. involuta grown on wet and dry habitats from Chiang Mai province, northern Thailand (*, P ˂0.0001).
|
Characters |
Habitats |
N |
Mean ± SD |
t |
|
1. Stem height (mm) |
wet dry |
150 200 |
28.5 ± 7.3 6.5 ± 3.3 |
34.2* |
|
2. Width of leaves (mm) |
wet dry |
150 200 |
0.8 ± 0.2 0.7 ± 0.1 |
6.6* |
|
3. Length of leaves (mm) |
wet dry |
150 200 |
3.0 ± 0.4 2.2 ± 0.4 |
17.8* |
|
4. Branching number |
wet dry |
150 200 |
2.2 ± 1.3 0.4 ± 0.8 |
14.9* |
|
5. Length of branches (mm) |
wet dry |
150 200 |
8.9 ± 5.7 0.7 ± 1.8 |
16.9* |
|
6. Length of innermost perichaetial leaves (mm) |
wet dry |
30 50 |
2.4 ± 0.2 1.4 ± 0.2 |
19.7* |
|
7. Length of archegonia (mm) |
wet dry |
150 150 |
0.89 ± 0.09 0.52 ± 0.06 |
38.3* |
|
8. Stem diameter (µm) |
wet dry |
150 200 |
323.3 ± 44.4 180.0 ± 38.3 |
31.7* |
|
9. Width of basal cells (µm) |
wet dry |
150 200 |
13.3 ± 2.1 13.6 ± 2.4 |
-1.2 |
|
10. Length of basal laminal cells (µm) |
wet dry |
150 200 |
56.1 ± 12.4 38.6 ± 9.7 |
14.4* |
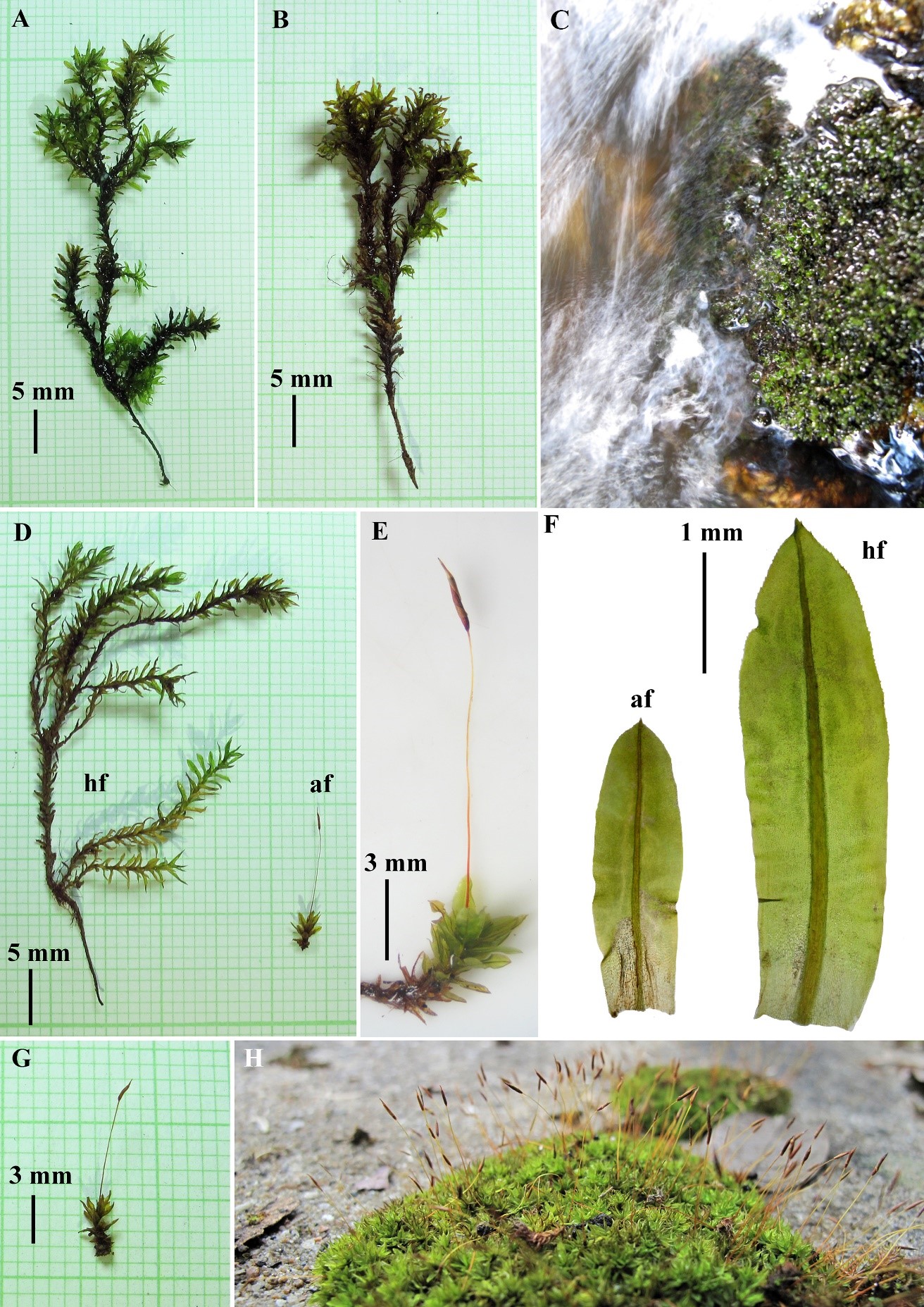
Figure 1. Ecotypes of Hyophila involuta (Hook.) A. Jaeger (Pottiaceae), humid form (hf) and arid form (af). A and B: Humid form habits. C: Wet habitat at Huay Kaew Falls, ca. 415 m, Doi Suthep-Pui National Park. D: Comparison of habit size from different habitats. E: and G: Arid form habits. F: Comparison of leaf size from different habitat. H: Dry habitat (opened soil) at Sirindhorn Observatory area, ca. 850 m, Doi Suthep-Pui National Park.
Additional characters on morphological variations of H. involuta
Among additional characters, the leaf apices of H. involuta from humid areas were narrowly acute and cuspidate with strongly serrate margins, cusps multicellar, 2–3 cells (Figures 2F, H) while those of the plants from dry areas were broadly acute to obtuse and remotely denticulate (Figures 2C, G).
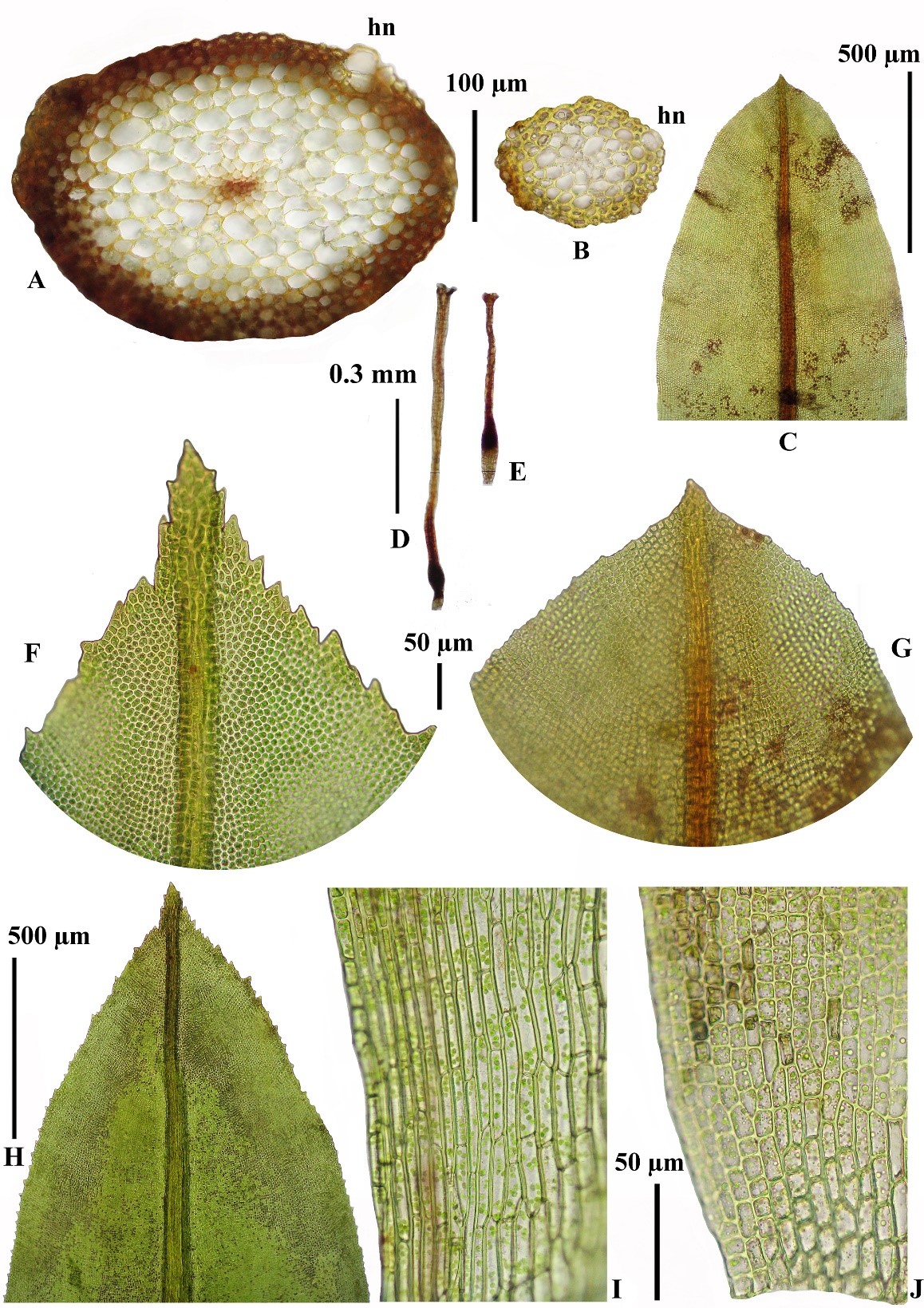
Figure 2. Character variations of H. involuta grown on different microhabitats. A: Stem cross sections showing broader cortical layer and well-differentiated central strand and hyaline nodules (hn) in humid form. B: Stem cross section of arid form. C and H: Leaf apices of arid form (C) and humid form (H). D: Archegonia of humid form (D) and arid form (E). F and G: Apical laminal cells and their denticulae variations of a humid form (F) and an arid form (G). I and J: Comparison of basal laminal cells of humid form (I) and arid form (J).

Figure 3. Arid form habits of H. involuta collected from several places in Chiang Mai Province; numbering codes of locations are listed in supplement.

Figure 4. Morphological variations of leaves in H. involuta collected from several places in Chiang Mai Province, arid form (af), humid form (hf); numbering codes of locations are listed in supplement.

Figure 5. Humid form habits and their leaves of H. involuta collected from several places in Chiang Mai Province; the numbering codes of locations are listed in supplement.
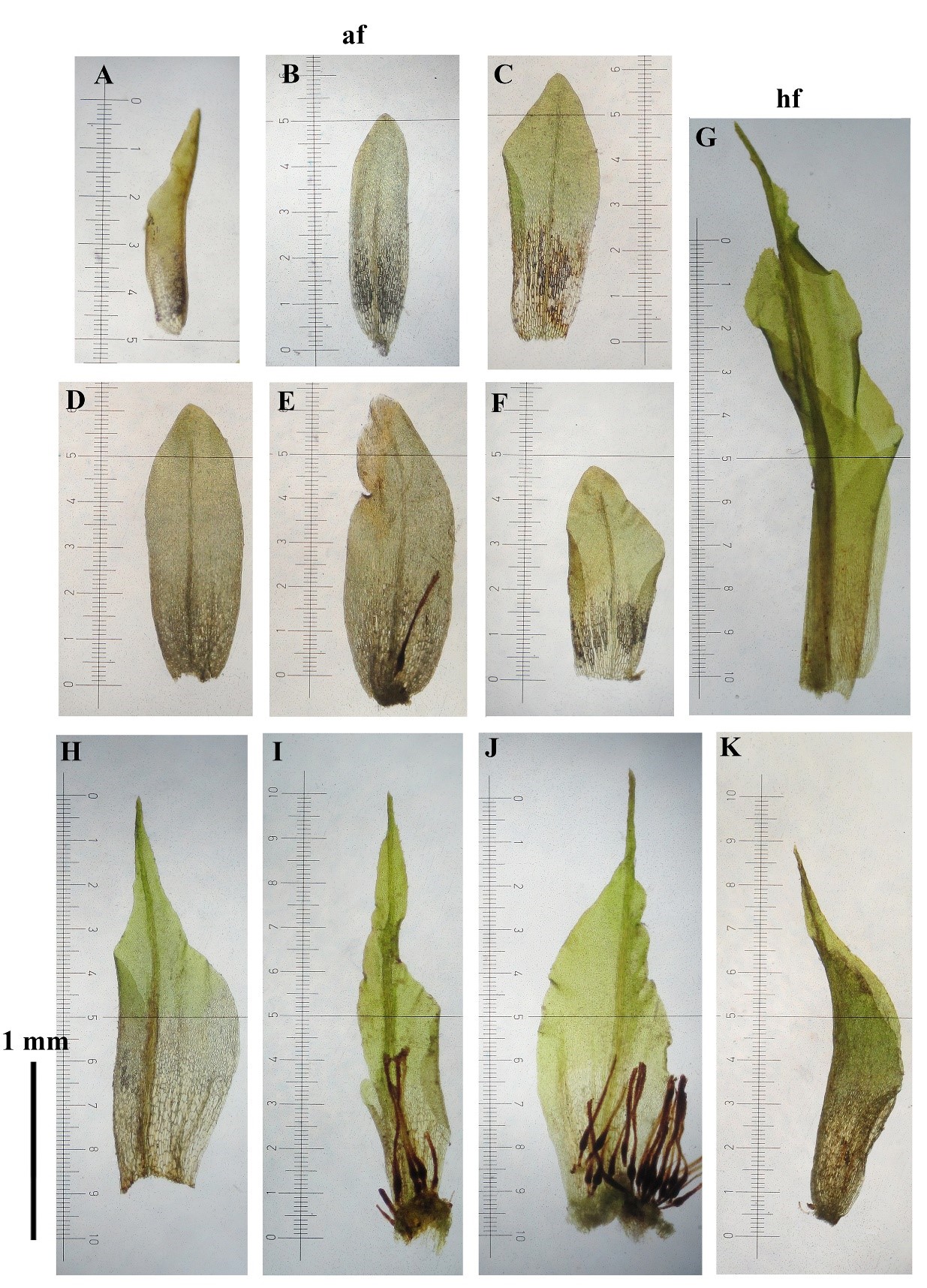
Figure 6. Innermost perichaetial leaves of H. involuta grown on different microhabitats. A-F: Innermost perichaetia of arid form (af). G-K: Innermost perichaetia of humid form (hf).
Belonging to the species of genus Hyophila that have involute leaves, bulging mamillose cells, and entire leaf margins. Hyophila involuta can be easily separated by its remotely denticulate to serrate upper leaf margins. However, this worldwide species can be confused only with H. propagulifera Broth., which has finely serrulate to entire leaf margins and produced numerous gemmae. Hyophila propagulifera differs from H. involuta by its leaf cross sections with bulging mamillose on both surfaces, gemmae obovoid, smooth on surfaces, and was known from Japan and China (Brotherus, 1899; Saito, 1975; Noguchi, 1988; Li et al., 2001), whereas bulging mamillose only on adaxial surfaces and smooth on abaxial surfaces of laminal cells in cross section (Figures 7A-D), and tuberculose to echinate gemmae were found in H. involuta (Figure 7E).
The plants described as H. angustiuscula Baumgartner & Dixon, which had been treated as synonym of H. involuta (Eddy, 1990: 199). Hyophila angustiuscula differs from normal H. involuta by only slightly larger laminal cells than usual and the involute leaves were found even in the fresh state; the enlarged laminal cells might be the habitat modification found in submerged (Eddy, 1990: 199). This is probably being the early evidence for the humid ecotype of H. involuta, which had been reported from other wet places. Unfortunately, other characters of humid form did not discuss in his hand book of Malesian mosses. By the way, our study showed insignificantly on the size and thickness of upper laminal cells, which were about 5–7 µm diameter in both humid and arid ecotypes (Figures 7A-D); but we found the difference of leaf cross sections, humid form showed slightly wider costae about 50–75 µm whereas narrower costae about 25–35 µm were found in arid form (Figures 7C-D). Other distinctive different characters of humid form found in our study were significantly larger inner perichaetial leaves with narrowly acuminate apices and excurrent costae of inner perichaetia, whereas smaller inner perichaetial leaves with obtuse rounded and costae vanishing below apex in inner perichaetia of arid form; archegonia of humid forms were longer neck cells than arid form; basal laminal cells of humid form were different longer than arid form; stems in cross section of humid form showed more cell layers both in cortex and central strands (4–7 cortically and 6–8 medullary central strand, Figure 2A) than those arid-form stems that had 2–3 cortical layers and 3–5 layers of central strand (Figure 2B); the water conducting hyaline nodules were well differentiated on the epidermis and outer cortex of humid-form stems with 2–3 groups of large hyaline cells (usually 1–3 cell layers, Figure 2A, hn), comparing in the arid-form stems, which had only 1 smaller hyaline cell (Figure 2B, hn).
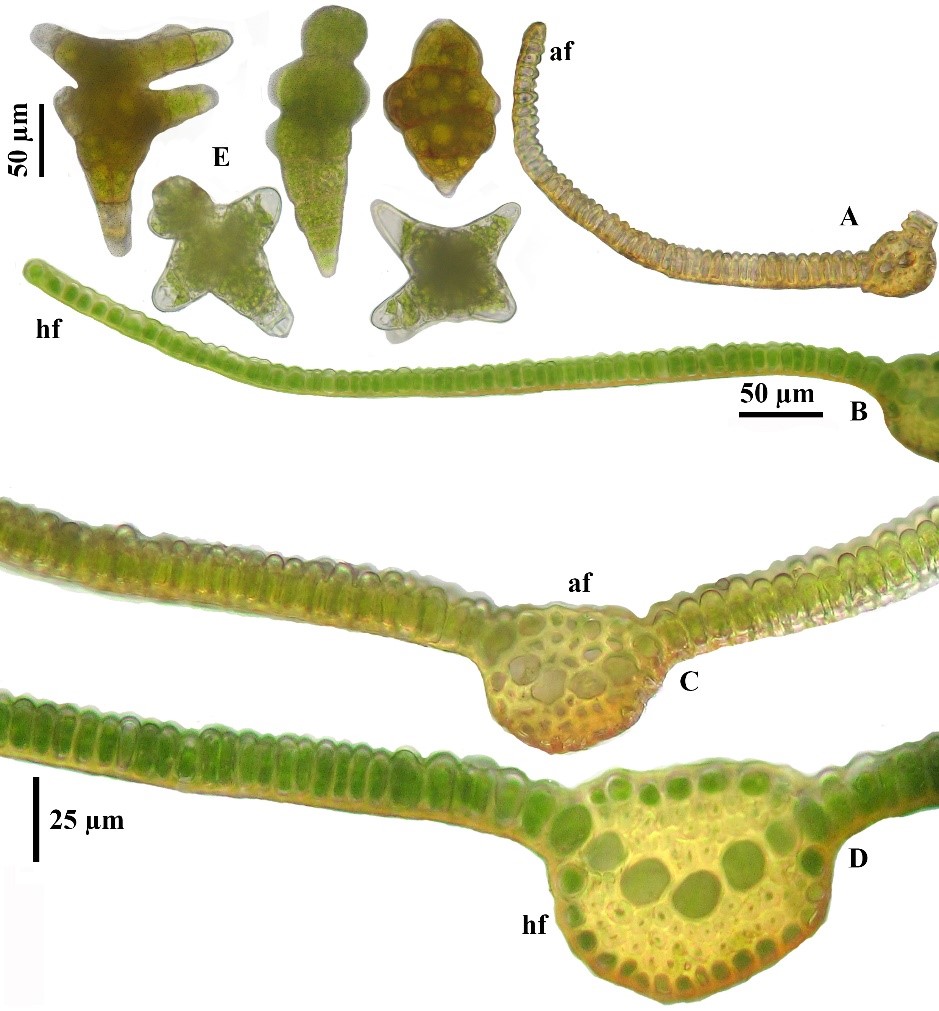
Figure 7. Hyophila involuta, leaves in cross section. A and C: Leaves in cross section of arid form (af). B and D. Leaves in cross section of humid form (hf). E. Gemmae of humid form.
DISCUSSIONS
Results from this study showed distinct characters of H. involuta growing on dry and wet areas. Stem length, stem diameter and length of basal laminal cells are major characters distinguishing between the plants from dry and wet areas while other characters such as branching, leaf size, innermost perichaetial leaves, and length of archegonia can be used as supporting evidences. Our results are different from a previous study on a pottiaceous moss, Syntrichia laevipila complex in which sporophyte characters (sporophyte size) were suggested as a main factor indicating variation among populations (Gallego et al., 2005).
The innermost perichaetial leaves are smaller than normal leaves, highly unstable lingulate to lanceolate, and having obtuse-rounded to acute apices found in many species of Hyophila, e.g. H. apiculata M. Fleisch., H. javanica (Nees) Brid., and H. involuta (Li et al., 2001; Eddy, 1990: 199–201). These are also variable in the costae of the innermost perichaetia, which are vanishing far below apices in several row of cells in H. javanica but lacking a costa in H. apiculata (Eddy, 1990: 199–201). It is interesting to note that H. involuta in wet habitats having ovate-lanceolate innermost perichaetia (up to 2.5 mm long) with abruptly narrow acuminate leaf apices, and having shortly excurrent costae, were recorded at first time for this study.
Different characters found in this species reflect its ability to grown in various habitats. Hyophila involuta can produce both spores and asexual propagules. This might be being a good strategy for this cosmopolitan species, resulting in its worldwide distribution everywhere, especially in lowland habitats. In this study, we found an abundance of stalks producing claviform-stellate gemmae clustered on the leaf axils of the humid form, but stalks were reduced in the arid form, while sporophytes were abundantly produced in the arid form. Even though morphological adaptations in bryophytes have been little documented, reduced or minute gametophytic forms without branching could be an important strategy to cope with dryness (Proctor et al., 2007; Stark et al., 2007). In a study of Deora & Deora (2017), only 5 species of mosses including H. involuta could be found in the Thar Desert, the hottest and driest region on the Indian subcontinent. The plants of H. involuta in this area were very small (only 2 mm height). In our study, the smallest size (2–4 mm height) arid forms can be found on rocks and soils in open-dry habitat at Sirindhorn Observatory area, Doi Suthep-Pui National Park in deciduous, dipterocarp-oak, seasonal, hardwood forest, ca. 850 m elevation. Forming crowed colonies with short and dense stems could help this moss survive in arid areas (Mägdefrau, 1982; Grace, 1995). Moreover, leaves that are always involute when dry, is considered to be another adaptation to prevent water loss from the plants. In this study, we also found hyaline cells which are probably involved in the poikilohydric property of H. involuta, to enhance water absorption and maintain moisture inside stems when exposed to dry air. The combination of desiccation tolerance and poikilohydry in several mosses is a highly evolved strategy of morphological adaptation to minute size, which enables growth on limited moisture substrates (Proctor & Tuba, 2002).
In contrast, in humid forms, H. involuta increased stiffness of the stems by forming a hypodermal sterome that can support long stems that stand upright or resist rapid water flow. This adaptation has been observed in giant terrestrial mosses for example Dendroligotrichum dendroides (Brid. ex Hedw.) Broth., Polytrichaceae (Frenzke et al., 2011). Aquatic mosses like Fontinalis dalecarlica Bruch & Schimp., Fontinalaceae also have stiff stems to resist rapid currents, while F. flaccida Renauld & Cardot has flaccid stems that cannot withstand strong water flow, resulting in this species occurring only in lakes and pools (Glime, 2017). Furthermore, many studies found two kinds of stems in mosses according to their water conducting pathways (Buch, 1947; Schofield, 1981; Huttunen et al., 2018). Ectohydric stems rely mainly on water conduction along the cortex or external surface of the plants whereas endohydric mosses conduct water inside the central strand. Our results revealed that stems of humid forms matched well with endohydric stems by having well differentiated 6–8 medullary central strand cells unlike forms in the dry places that have less differentiate central strand cells with shorter stems. Moreover, the stems of humid forms of H. involuta also had clustered hyaline nodules. It could help water conduction and act as branch primordia (buds) to produce more branches. This structure can help other mosses adapt to their habitats such as Fissidens spp., Fissidentaceae, (Iwatsuki & Pursell, 1980; Pursell, 2007). It is interesting to note that Thai specimens found on wet rocks in the waterfalls have more differentiate axillary hyaline nodules than those of H. involuta from other parts of the world, which were reported absent or weakly differentiate hyalodermis (Zander, 1993; Eddy, 1990, Li et al., 2001; Saito, 1975; etc.). In our field works, we found H. involuta on wet rocks along waterfalls at elevation between 350–1,240 m, growing intermingled with Fissidens spp. that have well differentiated hyaline nodules such as F. crispulus Brid. var. crispulus, F. geminiflorus Dozy & Molk. F. subangustus M. Fleisch., and F. javanicus Dozy & Molk., while in dry places H. involuta can be found on soils, rocks, and concretes in lowland, deciduous forest, near ridged mountains, and manmade habitats at elevation between 350 m to 2,500 m of disturbed places near summit of Doi Inthanon (Mt.) along with F. zollingeri Mont., F. biformis Mitt., F. ceylonensis Dozy & Molk., and F. wichurae Broth. & M. Fleisch., which are smaller size, have well differentiate hyaline nodules and shared habitats with H. involuta.
Evidence for cryptic speciation has been reported in other mosses (Shaw, 2001). In this case, we found that the two forms from different microhabitats are morphologically differentiated enough to separate H. involuta into two forms of ecotypes (humid and arid forms). However, molecular information is still needed to confirm separation of the two forms as distinct species.
CONCLUSION
The morphological variations of H. involuta grown on wet habitats were larger gametophytic characters than dry habitats such as stem heigh, leaf size, stem diameter, innermost perichaetia, and archegonia. Number of branching and length of branches in wet habitats were up to 6 branches and 15 mm long (vs unbranched in dry habitats). Innermost perichaetial leaves in wet habitats were ovate-lanceolate with abruptly narrow acuminate leaf apices and shortly excurrent costae (vs lingulate with obtuse-rounded leaf apices and the costae ending below leaf apex in several cells found in dry habitats). T-test of independent samples separated H. involuta populations into 2 groups according to wet and dry habitats and distinguished 2 distinct forms of ecotypes (humid and arid forms).
ACKNOWLEDGEMENTS
We would like to thank the staffs of Doi Inthanon National Park, Doi Suthep-Pui National Park, and Chiang Dao Wildlife Sanctuary for field assistance. Thanks are also given to the Department of National Parks, Wildlife and Plant Conservation of Thailand for permission to collect specimens. We also thank Dr. Sahut Chantanaorrapint, Ms. Kanonrat Adulkittichai, and Mrs. Priwan Printarakul for their kind help in field and herbarium database assistance. The first author is deeply grateful to the many years of encouragement given by Dr. Kanya Santanachote, the late Dr. B.C. Tan, and the late J.F. Maxwell. Finally, we wish to thanks Mr. Alvin Yoshinaga and Dr. Mashuri Waite for their kind help to improve the English text.
REFERENCES
Allen, B. 2002. Moss flora of central america part 2. Encalyptaceae-Orthotrichaceae. Monographs in Systematic Botany from the Missouri Botanical Garden 90: 1–699.
Brotherus, V.F. 1899. Neue beitrage zur moosflora japans. Hedwigia. 38: 204–247.
Buch, H. 1947. Ueber die wasser- and mineralstoffversorgung der moose. Societas Scientiarum Fennica, Commentationes Biologicae IX. 20: 1–66.
Costa, D.P.D. 2015. Diversity and conservation of Pottiaceae (Pottiales) in the Atlantic Rainforest. Acta Botanica Brasilica. 29: 354–374.
Costa, D.P.D. 2016. A synopsis of the family Pottiaceae in Brazil. Phytotaxa. 251: 1–66.
Deora, V. and Deora, G.S. 2017. Morphotaxonomical studies on some mosses of Indian Thar Desert. Annales of Plant Sciences. 6: 1893–1897.
Eddy, A. 1990. A handbook of Malesian Mosses, 2. Leucobryaceae to Buxbaumiaceae. British Museum (Natural History), London, UK. 256 pp.
Frenzke, L., Wanke, S., Isnard, S., Stoll, A., Neinhuis, C., and Rowe, N.P. 2011. Stem biomechanics of the giant moss Dendroligotrichum dendroides s.l. and its significance for growth form diversity in mosses. Journal of Bryology. 33: 229–236.
Gangulee, H.C. 1972. Mosses of Eastern India and adjacent regions. Volume 1, Fascicle 3. University of Calcutta, Calcutta, India. 567–830.
Gallego, M.T., Werner, O., Sérgio, C., and Guerra, J. 2005. A morphological and molecular study of the Syntrichia laevipila complex (Pottiaceae) in Portugal. Nova Hedwigia. 80: 301–322.
Glime, J.M. 2017. Adaptive strategies: growth and life forms. Chapters 4–5. In: Glime, J.M. Bryophyte Ecology. Volume 1. 4–5–1 Physiological Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. p. 1–26.
Grace, M. 1995. A key to the growth forms of mosses and liverworts and guide to their educational value. Journal of Biological Education. 29: 272–278.
Huttunen, S., Bell, N., & Hedenäs, L. 2018. The evolutionary diversity of mosses–taxonomic heterogeneity and its ecological drivers. Critical reviews in plant sciences. 37: 128–174.
Iwatsuki, Z. and Pursell, R. 1980. Axillary hyaline nodules in Fissidens (Fissidentaceae). Journal of the Hattori Botanical Laboratory. 48: 329–335.
Li, X.J., He, S., and Iwatsuki, Z. 2001. Pottiaceae. In: He, S., editor. Moss Flora of China, English version, Volume 2. Science Press, Beijing, China, and Missouri Botanical Garden Press, St. Louis, U.S.A. pp.114–249.
Mägdefrau, K. 1982. Life-forms of bryophytes. In: Smith, A.J.E. Bryophyte Ecology. Chapman and Hall, London. pp. 45–58.
Noguchi, A., (supplemented by Iwatsuki, Z. & Yamaguchi, T.) 1988. Illustrated mosses flora of Japan, Part 2. The Hattori botanical laboratory, Nichinan-shi, Japan. 243–492.
Proctor, M.C.F. and Tuba, Z. 2002. Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytologist. 156: 327–349.
Proctor, M.C., Oliver, M.J., Wood, A.J., Alpert, P., Stark, L.R., Cleavitt, N.L., and Mishler, B.D. 2007. Desiccation-tolerance in bryophytes: a review. The bryologist. 110: 595–621.
Pursell, R.A. 2007. Flora Neotropica, Volume 101: Fissidentaceae. New York: New York Botanical Garden Press.
Saito, K. 1975. A monograph of Japanese Pottiaceae (Musci). Journal of the Hattori botanical laboratory. 39: 373–537.
Schofield, W.B. 1981. Ecological significance of morphological characters in the moss gametophyte. Bryologist. 84: 149–165.
Shaw, A.J. 2001. Biogeographic patterns and cryptic speciation in bryophytes. Journal Biogeography. 28: 253–261.
Stark, L.R., Oliver, M.J., Mishler, B.D., and McLetchie, D.N. 2007. Generational differences in response to desiccation stress in the desert moss Tortula inermis. Annals of Botany. 99: 53–60.
Virendra, N. and Reesa, G. 2009. Hyophila involuta (Hook.) Jaeg.-new addition to the bryoflora of Pachmarhi Biosphere Reserve. Indian Journal of Forestry. 32: 297–299.
Zander, R.H. 1993. Genera of the Pottiaceae: mosses of harsh environments. Bulletin of the Buffalo Society of Natural Sciences. 32: 1–378.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Supplement
Two hundred arid form gametophytes and sporophytes of H. involuta (100 pockets belonging to 20 populations of dry areas) grown on soils, rocks, in forests and urban concretes in Chiang Mai Province, Northern Thailand were listed below:
|
Code No. |
Places |
Elevation (m) |
Specimens examined (CMUB Herbarium) |
No. of gametophytes examined (plants) |
|
|
1 |
Chiang Mai University |
350 |
28 August 2009: Printarakul 2171; 6 January 2010: Printarakul 2580; 5 January 2012: Printarakul 5214 (3 pockets) |
10 |
|
|
2 |
Wachirathan Falls, Doi Inthanon National Park |
660 |
11 April 2020: Printarakul 11042020_34, 11042020_35, 11042020_36 (3 pockets) |
10 |
|
|
3 |
Siriphum Falls, |
1,240 |
11 April 2020: Printarakul 11042020_10, 11042020_11, 11042020_12, 11042020_16, 11042020_17 (5 pockets) |
10 |
|
|
4 |
Kew Mae Pan, |
2,160 |
23 July 2007; Nathi 194; 22 November 2019: Jampeetong 32; 11 April 2020: Printarakul 11042020_20, 11042020_21, 11042020_22, 11042020_23 (6 pockets) |
10 |
|
|
5 |
Summit of Doi Inthanon, |
2,565 |
11 April 2020: Printarakul 11042020_23A, 11042020_24, 11042020_25 (3 pockets) |
10 |
|
|
6 |
Huay Kaew Falls, |
410 |
12 May 2012: Printarakul 5293, 5342; 20 August 2012: Printarakul 5477; |
10 |
|
|
7 |
Mae Sa Falls, |
420 |
23 November 2012: Printarakul 5717, 5723; 29 November 2012: Printarakul 5755 |
10 |
|
|
8 |
Tad Mork Falls, |
500 |
5 December 2012: Printarakul 5821, 5841, 5856 (3 pockets) |
10 |
|
|
9 |
Mork Fa Falls, |
560 |
12 December 2012: Printarakul 5905, 5936, 5984 (3 pockets) |
10 |
|
|
10 |
Huay Pha Lad, |
620-750 |
6 January 2011: Printarakul 3415; 14 June 2011: Printarakul 3917, 3937; 22 June 2011: Printarakul 4026, 4068 (5 pockets) |
10 |
|
|
11 |
Doi Suthep, |
480-1,050 |
1 December 2005: Printarakul 14; 3 June 2009: Printarakul 1220; 6 September 2011: Printarakul 4660, 4712; 14 September 2011: Printarakul 4731, 4738; 21 September 2011: Printarakul 4783; 13 March 2020: Printarakul 13032020_5, 13032020_6, 13032020_7 (10 pockets) |
10 |
|
|
12 |
Monthathan Falls, |
730 |
27 November 1997: Rattanayan & Santanachote 207; 15 June 2006: Polboonsri 32; |
10 |
|
|
13 |
Sirindhorn Observatory areas, Doi Suthep-Pui National Park |
850 |
17 September 2005: Manachit 163, 164; 12 December 2005: Manachit 263; |
10 |
|
|
14 |
Ru See (hermit) Cave, |
1,150 |
13 March 2020: Printarakul 13032020_8, 13032020_9, 13032020_10 (3 pockets) |
10 |
|
|
15 |
Puping Palace, |
1,300 |
8 August 2012: Printarakul 5431; 1 August 2017: Printarakul 7166; 13 March 2020: Printarakul 13032020_11, 13032020_12, 13032020_13 (5 pockets) |
10 |
|
|
Code No. |
Places |
Elevation (m) |
Specimens examined (CMUB Herbarium) |
No. of gametophytes examined (plants) |
|
|
16 |
Bahn Mohng (village) Doi Pui, Doi Suthep-Pui National Park |
1,400 |
13 March 2020: Printarakul 13032020_14, 13032020_15, 13032020_16 (3 pockets) |
10 |
|
|
17 |
Summit of Doi Pui, |
1,685 |
13 March 2020: Printarakul 13032020_18, 13032020_19, 13032020_23, 13032020_24, 13032020_25, 13032020_26, 13032020_27, 13032020_28, 13032020_29, 13032020_30 (10 pockets) |
10 |
|
|
18 |
Doi Mon Long, |
1,430 |
6 November 2012: Printarakul 5554; 13 November 2012 : Printarakul 5632 ; |
10 |
|
|
19 |
Nature trail to Doi Luang Chiang Dao, Chiang Dao Wildlife Sanctuary |
1,100-2,100 |
11 February 1995: Allen Y77, Y78; 11 August 1995: Allen Y100; 20 October 1995: Gardner Y40; 21 October 1995: Gardner Y59; 27 October 2017: Printarakul 7113, 7116; 9 February 2020: Printarakul 09022020_7; 10 February 2020: Printarakul 10022020_6, 10022020_7 (10 pockets) |
10 |
|
|
20 |
Doi Sam Pee Nong, Chiang Dao Wildlife Sanctuary |
1,800 |
11 March 1995: Allen Y91, Y92, Y96 (3 pockets) |
10 |
|
|
One hundred and fifty humid form gametophytes of H. involuta (45 pockets belonging to 7 populations of wet areas) grown on wet rocks in streams and water falls in Chiang Mai Province, Northern Thailand were listed below: |
|
||||
|
Code No. |
Places |
Elevation (m) |
Specimens examined (CMUB Herbarium) |
No. of gametophytes examined (plants) |
|
|
21 |
Wachirathan Falls, |
687 |
11 April 2020: Printarakul 11042020_26,11042020_27, 11042020_28,11042020_29, 11042020_30,11042020_31, 11042020_33 (7 pockets) |
20 |
|
|
22 |
Sirithan Falls, |
812 |
11 April 2020: Printarakul 11042020_1, 11042020_2, 11042020_3, 11042020_4 |
20 |
|
|
23 |
Pha Dok Seaw Falls, |
1,128 |
11 April 2020: Printarakul 11042020_6, 11042020_7, 11042020_8 (3 pockets) |
20 |
|
|
24 |
Siriphum Falls, Doi Inthanon National Park |
1,279 |
11 April 2020: Printarakul 11042020_13,11042020_14, 11042020_15, 11042020_18 |
20 |
|
|
25 |
Huay Kaew Falls, |
417 |
5 February 2020: Printarakul 05022020_9, 05022020_10, 05022020_11, 05022020_12 (4 pockets) |
21 |
|
|
26 |
Monthathan Falls, |
730 |
16 May 2006: Phrompa 6; 24 January 2011: Printarakul 3456; 5 February 2020: Printarakul 05022020_5, 05022020_6, 05022020_7, 05022020_8 (6 pockets) |
28 |
|
|
27 |
Mahidol Falls, |
1,100 |
20 August 2009: Printarakul 1947, 1949; 25 August 2009: Printarakul 2004, 2005, 2012, 2030; 31 March 2020: Printarakul 31032020_1, 31032020_2, 31032020_3. 31032020_4, 31032020_5, 31032020_6, 31032020_7, 31032020_8, 31032020_9, 31032020_10, 31032020_11 (17 pockets) |
21 |
|
Narin Printarakul* and Arunothai Jampeetong
Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand
Corresponding author: Narin Printarakul, E-mail: kame_cobtor@hotmail.com
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: August 19, 2020;
Revised: October 5, 2020;
Accepted: October 22, 2020

