
Azolla Pinnata as Phytoremediation Agent of Iron (Fe) in Ex Sand Mining Waters
Qudar Hasani*, Niken T.M. Pratiwi, Hefni Effendi, Yusli Wardiatno, Jupendi A. Raja Guk Guk, Henni Wijayanti Maharani, and Miftahur RahmanPublished Date : 2020-12-17
DOI : https://doi.org/10.12982/CMUJNS.2021.017
Journal Issues : Number 1, January-March 2021
Abstract Phytoremediation is one effective method used for reducing the iron (Fe) from waters. Azolla pinnata is a plant that has potential as an agent of phytoremediation of Fe in waters. This study aims to verify the ability of Azolla pinnata to reduce Fe from sand excavated water in Pasir Sakti District, East Lampung Regency, Indonesia. The study was conducted with three treatments. The treatments of 25% cover area, 50% cover area, and 75% cover of Azolla pinnata, with three replications each. The experiments were carried out until the water quality became suitable for aquaculture. The results showed that the area cover of Azolla pinnata had a different effect (P <0.05) on the decrease in iron concentration in the water. The treatment with 75% cover area of Azolla pinnata, showed a reduction percentage of iron concentration of 98.10%. It is the most significant reduction percentage of iron. Whereas the treatment with 25% and 50% Azolla pinnata cover area of succeeded in reducing the concentration of Fe in water 92.5% and 93.3% respectively.
Keywords: Aquaculture, Iron, Phytoremediation, Removal efficiency
Funding: This research was funded by the Deputy for Research and Development Strengthening, Ministry of Research and Technology-Research and Innovation Agency. The authors thank the Integrated Laboratory, and Center for Technological Innovation, Lampung University for providing the instrument.
Citation: Hasani, Q., Pratiwi, N.T.M., Effendi, H., Wardiatno, Y., Raja Guk Guk, J.A., Maharani, H.W., and Rahman, M. 2021. Azolla pinnata as phytoremediation agent of iron (Fe) in ex sand mining waters. CMUJ. Nat. Sci. 20(1): e2021017.
INTRODUCTION
Large-scale sand mining activities for industrial and building needs often do not heed the ecological aspects so that they can adversely affect to environmental conditions and ecosystems (Rahmadian and Dharmawan, 2014; Rizqan et al., 2016). The impact can be in the form of physical, chemical and biological damages (Siswanto et al., 2012). The Physical impact of sand mining can result in erosion and changes in soil structure (Asabonga et al., 2017). Large scale erosion of topsoil can form large holes. Furthermore, it is filled with rainwater and groundwater recharge to create large lakes (Saviour and Stalin, 2012; Asabonga et al., 2017; Gavriletea, 2017).
Identification of chemical compounds in the sand in Aceh and Cilacap-Indonesia- by Darmayanti et al., (2000), showed that the highest metal content was iron (Fe) reaching 30%. Fe minerals that dominate the sand content are Fe2O3 and Fe3O4, causing sand to be black coloured (Rusianto et al., 2012). According to Schumann et al., (2015), some sulfide minerals generally interact significantly with pyrite (FeS2) Robertson et al., (2015); Schumann et al., (2015). Iron (Fe) and Sulfur (S) are metals and acids that are indicators of mining areas. The mining area can producing iron-based iron acids including pyrite (FeS2), pyrhotite (FeS), chalcopyrite (CuFeS) and arsenopyrite (FeAsS) (Commonwealt of Australia, 2016; Runtti et al., 2017). The high metal content of Fe hurts aquatic organisms such as low growth fish and can cause the death of aquatic organisms (Baby et al., 2010; Rajeshkumar and Li. 2018).
Pasir Sakti Subdistrict (Lampung Province, Indonesia) is a vast sand mining area that forms a large basin. The process of rain and groundwater input causes the holes filled with water and form large lakes (Malik, 2017). The ex-sand dugouts are thought to contain high concentrations of iron, so they cannot be used for aquaculture activities. Therefore, some efforts are needed to reduce the Fe concentration in waters on ex sand mining.
Physical reduction of Fe from water can be made with activated carbon (Syauqiah et al., 2011; Abdi et al., 2016), filtration (Giwa et al., 2017), zeolites (Runtti et al., 2017; Nur'Aini and Wilopo, 2017). Chemically, it can be done using chitosan (Burke et al., 2002; Lapo et al., 2019), and Chelating Agent (EDTA) (Aziz et al., 2016). Biological treatment can use bacterial isolates (Leung et al., 2010; Irawati et al., 2017) or phytoremediation by aquatic plants (Bharti and Banerjee, 2012; Singh et al., 2012; Ajibade et al., 2013). Phytoremediation has economic advantages, is environmentally friendly, and is applicable so that it is easily implemented by the community (Lee, 2013; Ajibade et al., 2013).
Some species of aquatic plants are known as phytoremediation agents. According to Arimby (2014), A. pinnata can grow fast, can adapt to acidity, infertile soil, temperature and pollutants. A. pinnata can be found on the surface of rice fields, lakes, ponds and swamps (Hidayati, 2013). Fast biomass production in a short time causes A. pinnata to be an ideal plant as a phytoremediation agent (Anjuli et al., 2004; Sood et al., 2012). A. pinnata is a weed in rice plants that can be used as a phytoremediation agent to reduce iron (Fe) (Thayaparan et al., 2013); and other metal such as Hg and Cd (Sood et al., 2012), Cu, Cr, Cd, and Zn (Shafi et al., 2015; Noorjahan and Jamuna, 2015).
Based on research by Vaceem and Banerjee (2012), Azolla pinnata has reduced Fe concentrations in water by up to 70%. Other studies have verified that Azolla pinnata can reduce Fe to 95.4% (Bharti and Banerjee, 2012). Akinbile et al., (2016) have stated that Azolla pinnata has been proven effective in improving the quality of domestic wastewater, reaching the highest value of 99.55%. This study tries to verify the ability of A. pinnata to reduce iron in ex-sand mining water in Pasir Sakti District, East Lampung, Indonesia. Furthermore, ex-sand mining waters can be used for aquaculture activities.
MATERIALS AND METHODS
Research design
This study uses A. pinnata as a phytoremediation agent to reduce iron in the water of ex-sand mining area of Pasir Sakti District, East Lampung Regency, Indonesia. The treatment has been constructed using different percentages of A. pinnata cover area in the experimental tanks, i.e.:
K: Azolla pinnata with 0% covered area (Control)
A: Azolla pinnata with 25% covered area
B: Azolla pinnata with 50% covered area
C: Azolla pinnata with 75% covered area
Experimental procedure
Preparation of Experimental Tanks
The experimental tanks is a semi-outdoor tarpaulin pond with a size of 2.5 x 1.5 x 0.75 m3. The experimental containers, clean and rinse using fresh water and then dried, before used. Sand excavated water is put into each experimental tanks with a height of 50 cm. The initial Fe concentration was measured before the experiment was carried out, while the final Fe concentration was measured after the experiments had ended.
Preparation of Azolla pinnata
Before use, A. pinnata is cleaned using clean water to remove impurities, eggs of other organisms, and insect larvae that may be attached to plants. After cleaning, the A. pinnata was then put into the phytoremediation experimental tanks according to treatments.
Phytoremediation Process
The remediation process takes place until the Fe concentration is in accordance with the water quality standard, that is 0.03 mg/L (WHO). During the remediation process, no water was changed. Measurement of Fe in water is carried out every seven days.
Response Assessment
Fe concentration
The response of Azolla pinnata's ability to reduce iron (Fe) in water is measured in terms of: percentage of decrease in Fe in water (Sidek et al., 2018), translocation factor (TF) (Mellem et al., 2012), and bioconcentration factor (BCF) (Ghosh and Singh, 2005). As supporting data, dissolved oxygen (DO), temperature and pH of the water are also measured. Water samples were tested at the Integrated Laboratory and Technology Innovation Center of the Lampung University (referring to the EPA 200.7 Rev. 5 method).
The percentage reduction in Fe concentration was calculated by the equation of Sidek et al., (2018) as follows:

Bioconcentration factors (BCF) calculated by the equation, according to Ghosh and Singh (2005); Kamar et al. (2009).
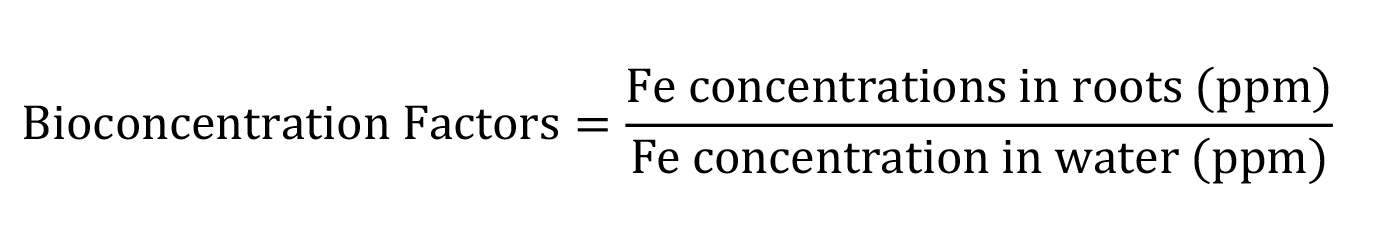
Water Quality Measurement
Water quality measurements include temperature, pH and dissolved oxygen DO. Measurements were taken at each unit of the experiment twice every day during the phytoremediation experiment
RESULTS
Water Quality
Measurement of the physical and chemical water quality was conducted in the morning and evening. Average dissolved oxygen (DO) reaches 5.1 - 6.6 mg/L in the morning and 6.3 - 7.1 mg/L in the afternoon (Table 1). DO is determined by the balance of oxygen production and consumption in the waters. Oxygen is produced by autotrophic organisms through photosynthesis during the day. A. pinnata releases oxygen in the roots so that oxygen in the water will increase (Haberl and Langergraber, 2002). Whereas at night, oxygen is consumed by all organisms through respiration and for the decomposition of organic matter and produce carbon dioxide (CO2). The concentration of CO2 in the water will affect the pH value, the higher the level of CO2, the lower the pH of the waters and vice versa.
Table 1.The concentration of dissolved oxygen (mg/L) based on the treatments.
|
Treatment |
1st day |
7th day |
14th day |
21st day |
||||
|
Morning |
Afternoon |
Morning |
Afternoon |
Morning |
Afternoon |
Morning |
Afternoon |
|
|
A |
6.0 – 6.8 |
6.5 – 7.7 |
4.6 - 8.7 |
5.5 - 8.6 |
6.4 - 8.4 |
6.6 - 8.8 |
6.3 - 7.2 |
6.7 - 8.0 |
|
B |
5.1 – 5.4 |
6.1 – 7.2 |
5.1 -7.6 |
6.1 - 8.6 |
5.7 - 7 |
6.4 - 8.5 |
6.9 - 6.8 |
6.5 - 8.1 |
|
C |
4.7 – 5.4 |
6.1 – 7.2 |
3.4 - 6.5 |
5.4 - 8.2 |
5.1 - 7 |
6.7 - 7.6 |
5.7 - 7 |
6 - 7.4 |
|
D |
4.7 – 5.6 |
6.4 – 8.2 |
4.2- 6.4 |
3.8 - 7 |
5.8 - 7 |
6.3 - 7.4 |
5.6 - 6.3 |
5.6 - 7 |
|
Averages |
5.2 |
6.3 |
6.1 |
7.1 |
6.6 |
7.3 |
6.5 |
6.9 |
Table 2. Range of pH based on the treatments.
|
Treatment |
1st day |
7th day |
14th day |
21st day |
||||
|
Morning |
Afternoon |
Morning |
Afternoon |
Morning |
Afternoon |
Morning |
Afternoon |
|
|
A |
5.3 – 5.9 |
5.4 – 6.0 |
5.5 - 6.4 |
5.3 - 6.5 |
5.4 - 6,6 |
6.0 – 6.1 |
6.2 - 6.1 |
5.6 - 6 |
|
B |
5.7 – 5.9 |
5.8 – 6.0 |
5.4 6.9 |
5.8 - 6.4 |
5.1 - 6.3 |
5.2 - 6.5 |
5.4 - 6.2 |
5.8 - 6.5 |
|
C |
5.6 – 5.8 |
5.6 – 5.9 |
5.5 - 6.2 |
5.6 - 6.3 |
4.8 - 6.3 |
4.8 - 6.5 |
5.4 - 6.2 |
5.6 - 6.4 |
|
D |
5.7 – 5.8 |
5.6 – 5.9 |
5 - 6.3 |
5.6 - 6.4 |
5.1 - 6.4 |
5.2 - 6.4 |
5.8 - 6.2 |
5.8 - 6.4 |
|
Averages |
5.7 |
5.8 |
5.9 |
6.0 |
6.0 |
6.2 |
6.2 |
6.3 |
The pH is a parameter associated with the concentration of CO2 in water. The balance between photosynthesis and respiration also determinate by CO2. Photosynthesis is a process that requires CO2 so that it can increase the pH of the waters, while respiration produces CO2 into the waters so that the pH of the waters decreases. The pH value in the study increases every week because A. pinnata is able to bind acids through the mechanism of decomposition of organic matter by associated microorganisms in the roots (Arimby, 2014). The process of decomposition of organic matter by microorganisms will produce OH- ions (Bwapwa et al., 2017). Increased pH can also occur by the photosynthesis (Gerloff-Elias et al., 2005). Intake of H+ ions in water for photosynthesis causes a decrease in H + ions in water so that the pH of the water increases. In this study, the pH values in all treatments ranged from 4.8 to 6.9 (Table 2). this value tends to be low, causing iron to tend to be in a dissolved form (Fe2+) which is more easily absorbed by the plants (Gonzales and Guo, 2018). This condition causes Fe to be absorbed well by A. pinnata. The optimal pH for plants to absorb metals or Fe is 4.6 -7.4 (Colombo et al., 2014; Ahmadpour et al., 2015).
A. pinnata has a high-temperature tolerance of 5 - 35˚C (Mentari et al., 2016). In this study, the temperature of each treatment was stable, ranging from 26.1 - 30.5˚C (Table 3), thus allowing A. pinnata to live optimally. According to Mentari et al., (2016), optimal temperature for Azolla's growth is in the range 18-28˚C. The increase of temperature can increase the diffusion of ions to the roots, so that it can accelerate the absorption of metal ions (Fritioff et al., 2005).
Table 3. Temperature range (˚C) of ex-sand mining water by remediation treatment by A. pinnata.
|
Treatment |
1st day |
7th day |
14th day |
21st day |
||||
|
|
morning |
Afternoon |
morning |
Afternoon |
morning |
Afternoon |
morning |
Afternoon |
|
A |
26.7 – 27.4 |
28.9 – 29.1 |
26.5 - 27.9 |
28 - 30.8 |
26.1 - 28.1 |
26.4 - 27.5 |
26.1 - 27.5 |
27.5 - 30 |
|
B |
27.4 - 27.8 |
28.6 – 29.6 |
27 - 27.4 |
28 - 29.6 |
26.3 - 27.2 |
28 - 29 |
26 - 27.6 |
28 - 29 |
|
C |
27.1 – 27.7 |
29 – 29.1 |
26.8 - 27.7 |
27.7 - 29.5 |
26.1 - 27.6 |
27.4 - 28.7 |
26.1 - 27.6 |
27.4 - 29.1 |
|
D |
27 – 27.9 |
28.7 – 30.7 |
26.7 - 27.9 |
27.8 30.7 |
26.4 - 27.4 |
27.5 - 28.9 |
26.4 - 27.7 |
26.4 - 28.9 |
|
Averages |
27.4 |
29.2 |
27.2 |
29.0 |
27.0 |
28.7 |
26.9 |
28.7 |
Concentration of Fe in the water
All of the phytoremediation treatments showed a significant decrease in Fe concentration. The highest reduction was shown by treatment D (98.104%). Furthermore, treatment C (93.474%), treatment B (93.319%) and lowest in treatment A (64.134%) (Table 4).
Table 4. Concentration (mg /L) and percentage of Fe decrease in water after treatment by A. pinnata
|
|
Treatment |
|||||||
|
Day To |
A (0%) |
B (25%) |
C (50%) |
D (75%) |
||||
|
Fe |
% |
Fe |
% |
Fe |
% |
Fe |
% |
|
|
0 |
0.987 |
|
0.943 |
|
0.702 |
|
1.002 |
|
|
7 |
0.689 |
30.193 |
0.354 |
62.460 |
0.508 |
27.635 |
0.427 |
57.385 |
|
14 |
0.413 |
58.156 |
0.145 |
84.624 |
0.212 |
69.801 |
0.120 |
88.024 |
|
21 |
0.354 |
64.134 |
0.063 |
93.319 |
0.046 |
93.447 |
0.019 |
98.104 |
The results of this study indicate that the coverage area of A. pinnata has a significant effect (P <0.05) on the decrease in Fe concentration in the sand mining water. Least Significance Different (LSD) tests showed that treatment A was significantly different from treatment B, C, and D. While the treatments for B, C, and D were not significantly different (Figure 1).
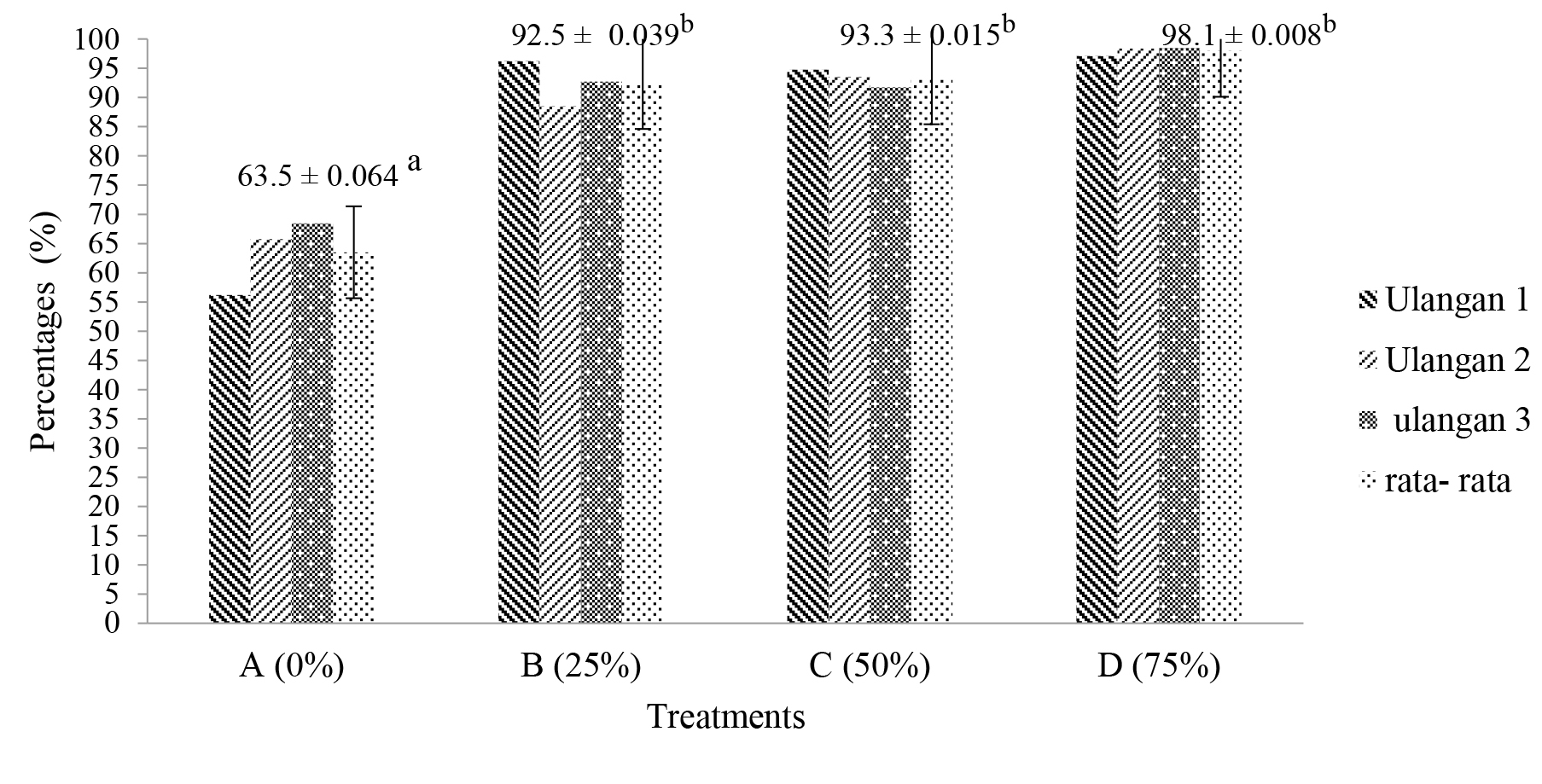
Figure 1. Graph of percentage of Fe concentration reduction by A. pinnata
Note: * The same letter notation shows results that are not significantly different from the 95 % confidence interval
* Different letter notation shows significantly different results with confidence interval 95 %
Concentration of Fe in Azolla pinnata plants
Fe in the water is absorbed by the epidermal cells of the root. Partly of Fe stored in the root and then put into the xylem through the simpas and apoplas to be transported from the root to the canopy. After xylem transport, heavy metals are transplanted and stored in leaf cells and redistributed again through phloems transport (Irawati, et al., 2017; Khatri et al., 2017). The lowest Fe and root concentrations of Fe occurred in treatment C because Fe concentrations in water were also low at 0.702 ppm (Table 4). Concentrations of Fe in the roots and leaves of each tretment are presented in Table 5, Figure 2 and Figure 3.
Table 5. Concentrations of Fe in roots and leaves of A. pinnata.
|
Treatments |
roots (mg/kg) |
leaves (mg/kg) |
|||
|
|
Day 0 |
Day 21 |
Day 0 |
Day 21 |
|
|
B |
70.946 |
1210.10 |
76.089 |
1237.13 |
|
|
C |
67.504 |
774.84 |
72.260 |
816.21 |
|
|
D |
65.481 |
1060.16 |
70.125 |
1078.30 |
|
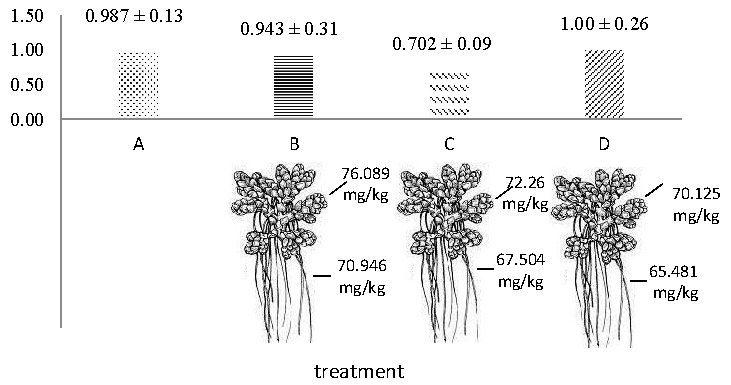
Figure 2. The concentration of Fe in water and plants before the experiment.
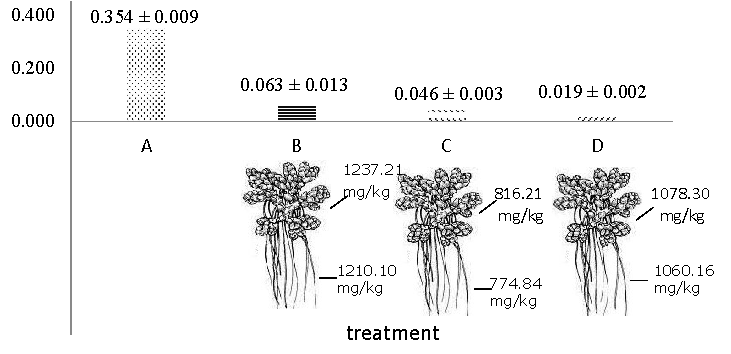
Figure 3. The concentration of Fe in water and plants after the experiment.
Bioconcentration factor (BCF) and translocation factor (TF) function to evaluate the feasibility of A. pinnata as a metal phytoremediation agent. In this study, the BCF value showed a high value reaching 1283.83 and the lowest being 1058.57 (Table 6). According to Testi et al., (2019) if the BCF value > 1000 then the plant has a high ability to absorb metals. Thus A. pinnata belongs to the accumulator plant against Fe. Accumulator plants are the ability of plants to absorb pollutants into their tissues (Sood et al., 2012; Thayaparan et al., 2013).
Table 6. Bioconcentration Factor and Fe Translocation Factors in A. pinnata roots.
|
Treatments |
Bioconcentration |
Translocation |
|
B |
1283.85 |
1.02 |
|
C |
1104.46 |
1.05 |
|
D |
1058.57 |
1.02 |
The value of Fe translocation from roots to leaves averaged 1.02. This value shows that Azolla pinnata has the ability of Metal Fe phytoextraction mechanism because TF> 1 (Testi et al., 2019). Phyto-extraction is the process of absorbing heavy metals by plant roots which are then translated into the stems and leaves. Fe is absorbed by root epidermal cells. Some Fe is stored in the roots, then put into the xylem through a simplas and apoplasts to be transported from the roots to the canopy. After xylem transport, heavy metals are transplanted and stored in leaf cells and redistributed again through phloem transport (Irawati et al., 2017; Khatri et al., 2017)
DISCUSSION
The phytoremediation treatments of the water from ex-sand mining land showed a significant decrease in Fe concentration. This condition is thought to be because A. pinnata has rapid growth thereby increasing Fe absorption. Increased A. pinnata growth causes the amount of Azolla that can absorb Fe also increases properly. Although under specific conditions, differences in the number of plants can show the opposite response (Santoso et al., 2010; Mutmainah et al., 2015). This condition occurs because of the influence of bacteria and microalgae that can affect the availability of nutrient in the waters (Purwaningsih, 2009), which causes changes in the form of Fe in the waters (Syahputra, 2005).
The ability of A. pinnata to reduce metals in water has been conveyed by Shafi et al., (2015), that after ten days A. pinnata has accumulated Cu (0.90ppm), Pb (0.42ppm), Cr (0.27ppm), Cd (0.042ppm) and Zn (2.1ppm). Other studies have shown that A. pinnata can reduce the concentration of Pb and Cd in water by 70-94% (Soodet al., 2012). A. pinnata was also able to reduce Pb metal concentration by 83% during the four days of treatment (Thayaparan et al., 2013). The study by Noorjahan and Jamuna (2015) showed that A. microphylla after 96 hours of treatment was able to reduce n heavy metals such as Cr (Biountreated 84.74% and Biotreated 100%), Cu (Biountreated 87.21% and Biotreated 89.43%), Zn (Biountreated 99.74% and Biotreated 100%). 07% and Biotreated 100%). Therefore, it can be concluded that A. pinnata is a potential candidate to remove Fe in the ex sand mining water.
Fe in the waters have several forms, including Fe2+ and Fe3 + (Khatri et al., 2017; Runtti et al., 2018). Bacteria are microorganisms that help reduce Fe3+ to Fe2+ that can be absorbed by plants (Gonzalez and Lin Guo, 2018). This process occurs because organic materials that contain groups such as carboxylic acids, nitrogen, hydroxides, sulfates, phosphates that can form complex compounds with iron. Organic material at the beginning of its decomposition will increase the pH if it produces ammonia. However, ammonia which is converted to nitrate, will reduce pH. This decrease in pH will increase iron content because Fe3+ will be reduced to Fe2+ (Kathri et al., 2017; Gonzalez and Lin Guo, 2018).
The highest concentration of Fe in the roots and leaves was found in treatment B because A. pinnata cover area was lower than treatment C and D. It was possible for individuals of A. pinnata to absorb Fe more than treatments C and D. Concentrations of Fe accumulated in roots and leaves increased at the end of the study because the process of absorption of Fe by A. pinnata continued. High concentrations of Fe in plants cause a negative impact, which is a change in leaf colour A. pinnata turns yellowish. According to Deval et al., (2012), Fe concentrations can affect root length, leaf and biomass production. This condition can occur due to the inhibition of food hydrolysis and translocation, so that root growth becomes inhibited. In some cases, it can cause a decrease in the content of carbohydrates (Valavanidis et al., 2005). However, chlorophyll can be increased through an osmotic adjustment mechanism developed by experimental species. Similar to the results of this study, Sood et al., (2012) state that A. pinnata can reduce the concentration of heavy metals (Hg and Cd) with concentrations ranging between 310 and 740 mg/kg dry mass in Azolla’s tissue. Thayaparan et al., (2013) state that the efficiency of lead (Pb) removal by plants depends on the duration of exposure. The maximum absorption of lead is 1383 mg/kg dry weight of A. pinnata.
CONCLUSION
The difference in the coverage area of A. Pinnata affects the percentage of Fe reduction in water taken from ex-sand mining land. The results of measurement of Fe content in roots and stems, as well as the bioconcentration factor values, indicate that the A. pinnata plant has a high ability to absorb Fe, so that A. pinnata can be classified as a strong accumulator plant for Fe. This study provides evidence that A. pinnata is a strong candidate as a phytoremediation agent for the reduction of Fe in water. The success of Fe reduction provides opportunities for the management and use of ex-sand mining sites. Furthermore, the land can be used for aquaculture. Aquaculture activities can provide benefits as a source of income and welfare for the community around the ex-sand mining sites in Pasir Sakti Regency, Lampung. Indonesia.
REFERENCES
Abdi, C., Khair, R.M., and Saputra, M.W. 2016. Utilization of Kepok banana (Musa acuminate L.) skin waste as activated carbon for Banjarbaru city water treatment: Fe and Mn. Journal of Environmental Engineering. 1: 8-15. [In Indonesian].
Ahmadpour, P., Ahmadpour, P., Sadeghi, S.M., Tayefeh, F.H., Soleimani, M., and BinAbdu, A. 2015. Evaluation of four plant species for phytoremediation of copper-contaminated soil. Soil Remediation and Plants, Prospects and Challenges: 147-205.
Ajibade, F.O., Adeniran, K.A., and Egbuna, C.K. 2013. Phytoremediation efficiencies of water hyacinth in removing heavy metals in domestic sewage (A case study of University of Ilorin, Nigeria). The International Journal of Engineering and Science. 2: 16-27.
Akinbile, C.O., Ogunrinde, T.A., Che bt Man, H., and Aziz, H.A. 2016. Phytoremediation of domestic wastewaters in free water surface constructed wetlands using Azolla pinnata. International Journal of Phytoremediation. 18: 54-61.
Anjuli, P., Radha, P., and Singh, P.K. 2004. Biological significance of Azolla and its utilization in agriculture. Proceedings of the Indian National Science Academy. Part B, Biological Sciences. 70: 299-333.
Arimby, C., Lestari, W., and Azis, Y. 2014. Utilization of Azolla pinnata R. Br in Zn absorption from rubber plant liquid waste as phytoremediator. Online Journal of Mathematics and Natural Sciences Students of Riau University. 1: 1-8.
[in Indonesian]
Asabonga, M., Cecilia, B., Mpundu, M.C., and Vincent, N.M. 2017. The physical and environmental impacts of sand mining. Transactions of the Royal Society of South Africa. 1–5.
Aziz, T., Rizky, A., and Devah, V. 2016. Removal of heavy metals from contaminated soil using chelating agent (EDTA). Journal of Chemical Engineering. 21: 41-49. [in Indonesian].
Baby, J., Raj, J.S., Biby, E.T., Sankarganesh, P., Jeevitha, M.V., Ajisha, S.U., and Rajan, S.S. 2010. Toxic effect of heavy metals on aquatic environment. Internation Journal of Biology and Chemistry Sciences. 4: 939-952.
Bharti, S. and Banerjee, T.K. 2012. Phytoremediation of the coal mine effluent. Ecotoxicology and Environmental Safety. 8: 36-42.
Burke, A., Hasirci, N., Yilmas, E., and Yilmas, O. 2002. Iron (III) ion removal from solution through adsorption on chitosan. Journal of Applied Polymer Science 84: 1185 – 1192.
Bwapwa J.K., Jaiyeola, A.T., and Chetty, R. 2017. Bioremediation of acid mine drainage using algae strains: A review. South African Journal of Chemical Engineering. 62-70.
Colombo, M., Raposo, G., and Théry, C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology. 30: 255-289.
Commonwealth of Australia, 2016. Preventing acid and metalliferous drainage: leading practices in the sustainable development program for the mining industry. The Australian Ministry of Resources. Australia.
Darmayanti, N.C.E., Manaf, A., and Briyatmoko, B. 2000. Identification of the content of chemical compounds in mineral sands. Proceedings of the National Seminar on Magnet Materials I. Serpong Indonesia. October 11, 200: 40-43. [in Indonesian].
Deval, C.G., Mane, A.V., Joshi, N.P., and Saratale, G.D. 2012. Phytoremediation potential of aquatic macrophyte Azolla caroliniana with references to zinc plating effluent. Emirates Journal of Food and Agriculture (EJFA). 24: 208-223.
Fritioff, A., Kautsky, L., and Greger, M. 2005. Influence of temperature and salinity on heavy metal uptake by submerged plants. Environmental Pollution Elsevier Journal. 265-274.
Gavriletea, M.D. 2017. Review: environmental impacts of sand exploitation. analysis of sand market. Journal Sustainability. 1-26.
Gerloff-Elias, A., Spijkerman, E., and Proschold, T. 2005. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acidic lake
(pH 2.6). Plant, Cell and Environment. 1218–1229.
Giwa, A., Dufour, V., Al Marzooqi, F., Al Kaabi, M., and Hasan, S.W. 2017. Brine management methods: Recent innovations and current status. Desalination. 1-23.
Gonzalez, N.A., and Guo, L. 2018. The potensial of Lemna minor to uptake iron in water. Journal of Environmental Science and Engineering. 268-273.
Ghosh, M., and Singh, S. P. 2005. A comparative study of Cadmium phytoextraction by accumulator and weed species. Environmental Pollution. 133: 365-371.
Haberl, R., and Langergraber, H. 2002. Constructed Wetland: A chance to solve wastewater problem in developing countries. Water Science Technology. 11-17.
Hidayati, N. 2013. Physiological mechanisms of heavy metal hyperaccumulator plants. Journal of Environmental Technology. 14: 75-82. [in Indonesian]
Irawati, W., Riak, S., Sopiah, N. and Sulistia, S. 2017. Heavy metal tolerance in indigenous bacteria isolated from the industrial sewage in Kemisan River, Tangerang, Banten, Indonesia. Biodiversitas. 18: 1481-1486.
Khatri, N., Tyagi, S., and Rawtani, D. 2017. Recent strategies for the removal of iron from water: a review. Journal of Water Process Engineering. 291-304.
Kumar, V., Kumar, P., Singh, J., and Kumar, P. 2019. Potential of water fern (Azolla pinnata R.Br.) in phytoremediation of integrated industrial effluent of SIIDCUL, Haridwar, India: removal of physicochemical and heavy metal pollutants. International Journal of Phytoremediation. 1-12.
Lapo, B., Demey, H., Carchi, T., and Sastre, A.M. 2019. Antimony Removal from Water by a Chitosan-Iron(III)[ChiFer(III)] Biocomposite. Journal Polymers. 11: 1-14.
Lee, J.H. 2013. An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnology and Bioprocess Engineering. 18: 193-210.
Leung, W.C., Chua, H., Wong, M.F., and Jung Lo, W. 2010. Removal and recovery of heavy metals by bacteria isolated from activated sludge treating industrial effluents and municipal wastewater. Water Science & Technology. 41: 266-277.
Malik, A. 2017. Impact of natural resource exploitation on community welfare in the view of islamic business ethics (case study of the galian c mine in pasir sakti district, east lampung). Nizham Journal of Islamic Studies. 5: 58-76. [in Indonesian].
Mellem, J.J., Baijnath, H., and Odhav, B. 2012. Bioaccumulation of Cr, Hg, As, Pb, Cu and Ni with The Ability for Hyperaccumulation by Amaranthus dubius. African Journal of Agricultural Research. 7: 591-596.
Mentari, A., Probosunu, N., and Adharini, R. I. 2016. Utilization of azolla sp. to decreass cod (chemical oxygen demand) content in laundry waste water. Journal of Fisheries, Gadjah Mada University. 18: 67-72. [in Indonesian]
Mutmainnah, F., Arinafril, and Suheryanto. 2015. Phytoremediation of lead metal (pb) using hydrilla verticillata and najas indica. Journal of Environmental Engineering. 12: 90-103. [in Indonesian].
Ndimele, P.E., and Jimoh, A.A. 2011. Water hyacinth (Eichhornia crassipes (Mart.) Solms.) in phytoremediation of heavy metal polluted water of Ologe Lagoon, Lagos, Nigeria. Research Journal of Environmental Sciences. 5: 424-433.
Noorjahan, C.M., and Jamuna, S. 2015. Biodegradation of sewage waste water using azolla microphylla and its reuse for aquaculture of fish tilapia mossambica. Journal of Environmental Science, Toxicology and Food Technology. 9: 75-80.
Nur'Aini, P.E.R., and Wilopo, W. 2017. Utilization of the consortium of natural sulfate and zeolite reducing bacteria in precipitating mn (reducing sulphate and natural zeolite in mn metal sedimentation using bacteria consortium). Saintek Research Journal. 22: 37-48. [in Indonesian].
Purwaningsih, I.S. 2009. Effect of nutrition addition on the effectiveness of phytoremediation using water hyacinth plants (Eichhornia crassipes) against orto-chlorophenol waste. Journal of Process Engineering. 3: 5-9. [in Indonesian].
Rahmadian, F., and Dharmawan, A.H. 2014. The ideology of actors and community perceptions of the impact of sand mining in the Galunggung mountain village. Journal of Rural Sociology. 2: 83-95. [in Indonesian].
Rajeshkumar, S., and Xiaoyu Li. 2018. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicology Reports 5: 288–295.
Rizqan, A., Mahyudin, I., Rahman, M., and Hadie, J. 2016. Status of river water quality around the sand mining area in batang alai river wawai village, south kalimantan. EnviroScienteae. 12: 1-6. [in Indonesian].
Robertson, A., Kawashima, N., Smart, R., and Schumann, R. 2015. ‘Management of pyrrhotite tailings at Savannah Nickel Mine: a decade of experience and learning’, in Proceedings of the 10th International Conference on Acid Rock Drainage and International Mining and Water Association Annual Conference, Santiago, Chile. 21–24.
Runtti, H., Tynjälä, P., Tuomikoski, S., Kangas, T., Hu, T., Rämö, J., and Lassi, U. 2017. Utilisation of barium-modifiedanalcime in sulfate removal: isotherms, kinetics and thermodynamics studies. Journal Water Process. Eng. 319-328.
Rusianto, T., Wildan, M.W., and Abraha, K. 2012.The potential of iron sand from the coast south of Bantul Yogyakarta as raw ceramic magnet materials. Journal Teknologi. 5: 62-69.
Santoso, R.S. 2010. Water hyacinth efficiency in allowance for organic materials in tugel gunung tugel purwokerto leachate. Purification Journal. 11: 163-170. [in Indonesian].
Saviour, M.N., and Stalin, P. 2016. Soil and sand mining: causes, consequences and management. IOSR Journal of Pharmacy (IOSRPHR). 2: 01-06.
Schumann, R., Robertson, A.M., Gerson, A.R., Fan, R., Kawashima, N., Li, J. and Smart, S.C. 2015. Iron sulfides ain’t iron sulfides: a comparison of acidity generated during oxidation of pyrite and pyrrhotite in waste rock and tailing materials. In Proceedings of the 10th International Conference on Acid Rock Drainage and International Mining and Water Association Annual Conference, 21–24 April 2015. Chile: Santiago.
Shafi, N., Pandit, A.K., Kamili, A.N., and Mushtaq, B. 2015. Heavy metal accumulation by Azolla pinnata of Dal Lake Ecosystem, India. Journal of Environment Protection and Sustainable Development. 1: 8-12. http://www.publicscience
framework.org/journal/jepsd.
Sidek, N.M., Abdullah,S.R.S., Ahmad, N,U., Draman, S.F.S., Rosli, M.M.M., and Sanusi, M.F. 2018. Phytoremediation of abandoned mining lake by water hyacinth and water lettuces in constructed Wetlands. Jurnal Teknologi (Sciences & Engineering). 80: 87-93.
Singh, D., Tiwari, A., and Gupta, R. 2012. Phytoremediation of lead from wastewater using aquatic plants. Journal of Agricultural Technology. 8: 1-11.
Siswanto, B., Krisnayani, B.D., Utomo, W.H., and Anderson, C.W.N. 2012. Rehabilitation of artisanal gold mining land in West Lombok, Indonesia: Characterization of overburden and the surrounding soils. Journal of Geology and Mining Research. 4: 1-7.
Sood, A., Uniyal, P.L., Prasanna, R., Ahluwalia, A.S. 2012. Phytoremediation potential of Aquatic Macrophyte, Azolla. Review Paper. Journal Ambio. 122–137.
Syahputra, R. 2005. Phytoremediation of Cu and Zn Metals with water hyacinth plants (Eichhornia crassipes. Mart Solms). Logic Journal. 2. 57-62. [in Indonesian]
Syauqiah, I., Amalia, M., and Kartini, H.A. 2011. Analysis of time and speed mixing variants in the adsorption process of heavy metal waste with active charcoal. Journal of Technical Info. 12: 11-20. [in Indonesian].
Thayaparan M., Iqbal, S.S., Chathuranga, P.K.D., and Iqbal, M.C.M. 2013. Rhizofiltration of Pb by Azolla pinnata. International Journal of Environmental Science. 3: 1811-1821.
Testi, E. H., Soenardjo, N., and Pramesti, R. 2019. Pb metal in Avicennia marina Forssk, 1844 (Angiosperms: Acanthaceae) in the Water, Sedimentary Environment, on the East Coast of Semarang. Journal of Marine Research. 8: 211-217. [in Indonesian].
Valavanidis, A., Fiotakis, K., Bakeas, E., and Vlahogianni, T. 2005. Electron paramagnetic resonance study of the generation of reactive oxygen species catalysed by transition metals and quinoid redox cycling by inhalable ambient particulate matter. Redox Report. 10: 37-51.
Vaseem, H., and Banerjee, T. K. 2012. Phytoremediation of the toxic effluent generated during recovery of precious metals from polymetallic sea nodules. International Journal of Phytoremediation. 14: 457-466.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Qadar Hasani1,2,*, Niken T.M. Pratiwi1, Hefni Effendi3,4, Yusli Wardiatno3,4, Jupendi A. Raja Guk Guk2, Henni Wijayanti Maharani2, and Miftahur Rahman5
1 Postgraduate School of Aquatic Resources Management, Faculty of Fisheries and Marine Sciences, IPB University, Dramaga, Bogor. West Java. Indonesia 16880
2 Department of Aquatic Resources, Faculty of Agriculture, Lampung University, Lampung, Indonesia 35145
3 Center of Coastal and Marine Resources Studies, IPB University, Dramaga Bogor West Java, Indonesia 16680
4 Center for Environmental Research, Institute for Research and Community Development,
IPB University, Dramaga Bogor West Java, Indonesia 16680
5 Integrated Laboratory and Center for Technological Innovation, Lampung University, Lampung, Indonesia 35145
Corresponding author: Qadar Hasani, E-mail: masqod@fp.unila.ac.id
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: July 11, 2020;
Revised: October 4, 2020;
Accepted: October 22, 2020

