
Comparison of Antioxidant and α-Glucosidase Inhibitory Activities in Different Cultivars of Five Mango (Mangifera Indica L.) Leaf Extracts
Savita Chewchinda, Orasa Suriyaphan, Pimpikar Kanchanadumkerng, Hitoshi Sato and Vilasinee Hirunpanich Sato*Published Date : 2020-12-17
DOI : https://doi.org/10.12982/CMUJNS.2021.014
Journal Issues : Number 1, January-March 2021
Abstract The objectives of study were to evaluate and compare the antioxidant, total phenolic, total flavonoid, mangiferin content and antidiabetic activities of five young mango cultivars leaf extract, namely, ‘Apple’, ‘Nam Dok Mai’, ‘Bao’, ‘Ok-Rong’ and ‘Kiew Savoey’. Antioxidant effect was investigated by DPPH, ABTS radical scavenging activity, and ferric reducing power (FRAP) assays. Inhibitory on α-glucosidase activity and type of enzyme inhibition were evaluated by using Lineweaver Burk plot analysis. Mangiferin, major active compound, was quantified by HPTLC method. Furthermore, the hypoglycemic effect was determined using streptozotocin (STZ) –nicotinamide (NA) -induced type 2 diabetic mice. Young mango cv. ‘Apple’ leaf extract demonstrated the strongest antioxidant activity in all assays. Moreover, it contains highest amounts of total phenolic and mangiferin to the values of 311 mg GAE/g extract and 197 mg/g extract, respectively. It possessed potent α-glucosidase inhibitory activity with IC50 value of 0.50 µg/mL. Lineweaver-Burk plot analysis demonstrated a non-competitive inhibition of α-glucosidase activity with the inhibition constant (Ki) of 2.98 µg/mL. Co-administration of young mango cv. ‘Apple’ leaf extract at dose of 1,000 mg/kg significantly reduced the total blood glucose level by 13.43% in STZ-NA-induced type 2 diabetic mice when compared with control diabetic mice in oral glucose tolerance test (OGTT) model. Inhibition of glucose absorption may be one of the possible mechanism of its hypoglycemic effect. In conclusion, young mango cv. ‘Apple’ leaf extract possesses the strongest antioxidant and antidiabetic activities which has a potential to develop as nutraceutical products.
Keywords: Antioxidant, α-Glucosidase, Diabetes, Mangifera indica, Mangiferin
Citation: Chewchinda, S., Suriyaphan, O., Kanchanadumkerng, P., Sato, H., and Sato, V.H. 2021. Comparison of antioxidant and α-glucosidase inhibitory activities in different cultivars of five mango (Mangifera Indica L.) leaf extracts. CMUJ. Nat. Sci. 20(1): e2021014
INTRODUCTION
Diabetes is one of the significant health problems which has been estimated by World Health Organization (WHO) as the third highest risk factor for premature death in adults after hypertension and smoking (World Health Organization, 2009). Pharmacological approaches for management of type 2 diabetes are biguanides, sulfonylureas, thiazolidinediones, DPP-4 inhibitors, SGLT2 inhibitors, α-glucosidase inhibitors, and insulins. However, unaccepted side effects are the major concerns (American Diabetes Association, 2017). Hyperglycemia is associated with the promotion of auto-oxidation of glucose to form free radicals beyond the scavenging abilities of endogenous antioxidant defenses, thus resulted in macro and microvascular dysfunction (Bajaj and Khan, 2012).Therefore, antioxidants have been focused to be a candidate to reduce the risk of diabetic complications by protection of free radicals derived from glycation reaction (Montonen et al., 2004).
Mangifera indica Linn., a perennial woody plant belonging to family Anacardiaceae, is commonly known as mango. The leaves are perennial, simple alternate and yellow green-purple in color when young while changes to leathery, glossy, and deep green color in mature leaves. It is an economic crop which is widely grown throughout Thailand. Several well-known cultivars of Thai mango are Nam Dok Mai, Kiew Savoey, Keaw, Chok Anan, Ok Rong (Chayamarit, 1994, Masud Parvez, 2016). Various pharmacological activities of mango leaves have been reported including antioxidant, analgesic, anti-inflammatory, and hypolipidemic properties (Masud Parvez, 2016, Ediriweera et al., 2017). The infusion of fresh mango leaves has been used for treatment of diabetes in several countries including India and Thai (Masud Parvez, 2016, Ediriweera et al., 2017). Phytochemical screening of M. indica showed the presence of highly effective bioactive compounds including flavonoids, tannins, alkaloids, terpenoids, anthraquinones, saponins, cardiac glycosides and steroids (Barreto et al., 2008, Aiyelaagbe and Osamudiamen, 2009, Berardini et al., 2009). Interestingly, quantification of phytochemical compositions revealed higher level of bioactive components found in young leaves more than in mature leaves especially polyphenolic and flavonoid compounds (Bhuvaneshwari et al., 2014). Among these compounds, mangiferin, a glucosyl xanthone (1, 3, 6, 7-tetrahydroxyxanthone-C2-β-D-glucoside) is a prominent polyphenolic constituent mostly found in mango which exhibits hypoglycemic effect (Sellamuthu et al., 2013, Anbalagan et al., 2019). However, the comparative study of the antioxidant and hypoglycemic effect of young leaves of several mango cultivars have not been determined yet.
Therefore, the aims of the present study were to evaluate and compare the antioxidant activities, total phenolic, total flavonoid, and mangiferin content of five popular cultivars of young mango leaf extracts. Inhibitory effect of α-glucosidase enzyme and the modes of enzyme inhibition and the correlation between the antioxidant and inhibitory effect on α-glucosidase were also examined. Moreover, the hypoglycemic effect of young mango cv. ‘Apple’ leaf extract which possess the strongest α-glucosidase inhibitory activity was also determined using streptozotocin (STZ) –nicotinamide (NA) -induced type 2 diabetic mice.
MATERIALS AND METHODS
Chemicals
Acarbose, α-glucosidase from Saccharomyces. cerevisiae, gallic acid, Folin-Ciocalteu reagent, quercetin, trolox, 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Aluminum chloride, ascorbic acid, disodium hydrogen phosphate, potassium persulfate, and sodium carbonate were obtained from Ajax Finechem, Australia. All other chemical reagents were of analytical grade and used without further purification.
Plant materials
Young leaves located at the first five leaves from the branch ends and characterized by soft with light green color of five cultivars of mango namely, ‘Apple’, ‘Nam Dok Mai’, ‘Kiew Savoey’, ‘Ok Rong’ and ‘Bao’ were collected in March, 2017. The plant samples were identified using the identification key provided in Flora of Thailand. The voucher specimens (SC2017001 - SC2017005) were deposited at Laboratory of Department of Food Chemistry, Faculty of Pharmacy, Mahidol University, Thailand.
Plant extraction
All leaves samples were cleaned and dried at temperature at 50°C. The dried leaves were pulverized into powder. Twenty grams of each mango leaf powder were separately macerated with 150 ml of 50% ethanol at room temperature for 24 h. The marc was re-extracted twice more. The pooled extract was filtered through Whatman filter paper No. 1, then concentrated by a rotary evaporator under low pressure at
40°C. The residue was stored in a sealed container at 4°C until used.
Phytochemical analysis
Quantitative analysis of mangiferin by high-performance thin-layer chromatography (HPTLC). HPTLC analysis was determined according to the method of Sethiya et al. (2015). The chromatogram was performed on 20 x 10 cm aluminum plate coated with silica gel 60 F254. The samples and mangiferin standard were applied on the plates as 7.0 mm wide bands, positioned 10 mm from lower edge of the plate using Linomat V applicator (Camag, Switzerland) with a nitrogen flow providing delivery from the syringe. The mobile phase was consisted of ethyl acetate: acetic acid: formic acid: water in ratio of 10: 0.5: 0.5: 1.5 (v/v/v/v). Saturation time was 30 min at room temperature and developed the plates with a distance of 80 mm from lower edge. The developed plate was scanned at wavelength of 254 nm with TLC scanner 3 (Camag, Switzerland). Slit dimension was set at 6.00 x 0.45 mm. Mangiferin reference standard solution, corresponding to 0.25 - 1.25 µg/band, and each young mango leaf extract was applied to the HPTLC plate (n = 3). The results were evaluated by winCATS software (Camag, Switzerland).
Total phenolic content. Total phenolic content of young mango leaf extract was evaluated according to the previous method (Singleton et al., 1999). Each sample was mixed with 50 µL of Folin-Ciocalteu’s solution and allowed to stand at room temperature for 3 min. Then, 80 µL of 7.5% w/v sodium carbonate was added and incubated for 2 h in the dark at room temperature. The absorbance was analyzed at 765 nm using microplate reader (Tecan, Switzerland). Each experiment was performed in triplicate. Gallic acid was used for the standard curve. Total phenolic contents in extracts was expressed as mean ± SD of mg gallic acid equivalent (GAE)/g extract.
Total flavonoid content. Total flavonoid content was carried out following the previous study (Stankovic, 2011). Briefly, 100 µL of sample solution was mixed with 100 µL of 2% w/v aluminium chloride solution, then incubated for 10 min at room temperature. The absorbance was measured at 415 nm using microplate reader (Tecan, Switzerland). The samples were performed in triplicate and quercetin was used as a standard. The content of flavonoids in the extract were expressed as mean ± SD of mg quercetin equivalent (QE)/g extract.
In vitro antioxidant activities
Determination of DPPH-scavenging assay. DPPH scavenging assay was evaluated according to previous method of Sithisarn et al. (2015). In 96-well plate, 100 μL of sample solution was mixed with 100 μL of freshly prepared methanolic DPPH solution (152 μM). The mixture was incubated for 30 min in the dark at room temperature. The absorbance was recorded at 517 nm using microplate reader (Tecan, Switzerland). Ascorbic acid was used as a standard. The percent inhibition was then calculated as follow;
% inhibition = [(Ac-As)/Ac ] × 100
Where Ac and As were the absorbance of control solution and sample solution at 517 nm, respectively. IC50 value, the concentration of sample required for 50% inhibition of DPPH free radical scavenging activity, was calculated from linear equation of the plot between % inhibition and concentration. Each sample was performed in triplicate, and the average IC50 value was reported as mean ± SD.
Determination of ferric reducing power (FRAP) assay. Ferric reducing power assay was carried out according to the previous method with some modifications (Lim and Quah, 2007). Five hundred µL of each sample was mixed with 500 µL of 0.2 M potassium phosphate buffer (pH 6.6) and 500 µL of 1% w/v potassium ferricyanide solution in test tube. The mixture was incubated at 50°C for 20 min. Then, 2 mL of trichloroacetic acid was added to stop the reaction. In 96-well plate, 100 µL of supernatant of the mixture was mixed with 100 µL of deionized water and 20 µL of
0.1% w/v ferric chloride solution. The mixture was allowed to stand for 30 min before measuring the absorbance at 700 nm using microplate reader (Tecan, Switzerland). The procedure was carried out in triplicate. Ferrous sulfate was used for the standard curve. FRAP values were calculated as mean ± SD and expressed in mmol ferrous sulfate equivalents/g extract.
Determination of ABTS radical-scavenging assay. ABTS radical-scavenging assay was evaluated according to the previous method with some modifications (Thaipong et al., 2006). The ABTS+ stock solution was prepared by mixing 20 mg of ABTS and 3.5 mg of potassium persulfate in 10 mL of deionized water. The mixture was allowed to stand for 12-16 h in the dark at room temperature. The working solution was prepared by diluting 1 mL of ABTS+ stock solution with 24 mL of methanol in order to obtain an absorbance of 1.100 ± 0.020 units at 734 nm using a microplate reader (Tecan, Switzerland). Fresh ABTS+ solution was prepared for each assay. Each sample (20 µL) was mixed with 200 µL of ABTS radical solution. The absorbance was determined after 6 min of initial mixing at 734 nm. All determinations were carried out in triplicate. Trolox was used for the standard curve. Results were expressed in mg Trolox equivalents antioxidant capacity (TEAC)/g extract.
In vitro anti-diabetic effect
Determination of in vitro α-glucosidase inhibitory activity. The α-glucosidase inhibitory activity was determined according to spectrophotometric method described by Supasuteekul et al. (2016) with some modifications. In 96-well plate, 10 µL of sample was preincubated with 40 µL of 0.1 U/mL α-glucosidase at 37°C for 10 min. Then 50 µL of 2 mM pNPG was added to the mixture and further incubated at
37°C for 20 min. The reaction was terminated by adding 100 µL of 1 M sodium carbonate. The absorbance of p-nitrophenol was determined using microplate reader at 405 nm. Acarbose was used as positive control. The experiment was performed in triplicate. Results were expressed in term of percentage of α-glucosidase inhibitory and calculated as follow;
% α-glucosidase inhibitory activity = [(Ac-As)/Ac ] × 100
Where Ac and As were the absorbance of control and sample solution, respectively. IC50 value was determined.
Kinetics of α-glucosidase inhibition. In order to examine the type of inhibition of young mango leaf extract on α-glucosidase, pNPG in the various concentrations was used as substrate. Enzyme activity was determined in the absence or presence of young mango leaf extract at different concentrations. Optimal concentration of young mango leaf extract was determined based on the results from inhibitory activity assay as described above. The inhibition constant (Ki), the Michaelis-Menten constant (Km), and the maximum velocity (Vmax) for the α- glucosidase inhibition by young mango leaf extracts were determined by using the observed data to fit with the different types of Michaelis-Menten equations, using Solver Add-in equipped with Microsoft Excel 2010. Lineweaver-Burk was plotted to determine the enzyme kinetic parameters of young mango leaf extracts on α- glucosidase.
Correlation between total phenolic, total flavonoid, mangiferin content, antioxidant activities and α –glucosidase inhibitory effect
Pearson correlation (SPSS statistics version 18.0, IBM Corporation, USA) was applied to investigate the relationship between total phenolic, total flavonoid, mangiferin content, DPPH, ABTS radical scavenging activity, FRAP, and α-glucosidase inhibitory effect of young mango cv. ‘Apple’ leaf extract.
In vivo hypoglycemic effect of young mango cv. ‘Apple’ leaf extract
Animals. Male ICR mice (30-40 g) were purchased from the National Laboratory Animal Center, Mahidol University, Nakhonpathom and housed in the animal center at Faculty of Pharmacy, Mahidol University at a constant temperature (25°C) with a 12 h light-dark cycle and had free access to standard diet and water ad libitum. The mice were acclimatized for 7 days before starting the experiment. The protocol of in vivo determination was submitted to the animal ethics committee of Faculty of Pharmacy, Mahidol University prior to performing the experiment. The experimental protocol was approved by the animal ethics committee of Faculty of Pharmacy, Mahidol University (permission number: PYT 007/2560).
Induction of type 2 diabetes. Type 2 diabetes in mice was induced by a single intraperitoneal (i.p.) injection of 120 mg/kg streptozotocin (STZ) dissolved in freshly prepared 0.1 M citrate buffer (pH 4.5), 15 minutes after a single-dose i.p. administration of 100 mg/kg nicotinamide (NA) dissolved in normal saline. Mice were fed with glucose solution (10%) for 12 h to avoid an initial mortality due to drug-induced hypoglycemia. After 7 days, the progression of diabetes was assessed by measuring blood glucose after overnight fasting using a commercial glucometer (Accu-Chek®, Roche, Thailand) with glucose test strips. Only mice with blood glucose exceeding 200 mg/dL were considered to be diabetic and employed in the present study. These mice were referred to as STZ-NA-induced type 2 diabetic mice.
Oral glucose tolerance test (OGTT) in normal and diabetic mice. In order to examine whether young mango cv. ‘Apple’ leaf extract produce the hypoglycemic in normal mice or not, the extract at dose of 500 and 1,000 mg/kg were orally administered in the normoglycemic mice before administration of glucose solution (2 g/kg). Then, fasting blood glucose level was determined by using a glucometer with glucose test strips (Accu-Chek®, Roche, Thailand) after glucose administration at 0, 30, 60, 90 and 120 min.
After 7 days of induction of diabetes, controlled mice and STZ-NA-induced type 2 diabetic mice employed in this study were divided into 5 groups according to the treatments as in the follows;
Group 1: Normoglycemic mice; normal mice were orally administered with 0.5 % carboxymethyl cellulose (CMC), served as normal control.
Group 2: STZ-NA-induced diabetic mice; mice were orally administered with 0.5 % carboxymethyl cellulose and served as diabetic control.
Group 3: STZ-NA-induced diabetic mice orally administered with 5 mg/kg of glibenclamide dissolved in distilled water and served as positive control.
Group 4: STZ-NA-induced diabetic mice orally treated with 500 mg/kg of young mango cv. ‘Apple’ leaf extract dissolved in 0.5% CMC and served as experimental group 1.
Group 5: STZ-NA-induced diabetic mice orally treated with 1,000 mg/kg of young mango cv. ‘Apple’ leaf extract dissolved in 0.5% CMC and served as experimental group 2.
All mice were fasted overnight for 12 h before the day of treatment. Each treatment was administered at 30 min before loading glucose. After the mice were orally fed with 2 g/kg of glucose solution, blood was then withdrawn from the tail vein at 0, 30, 60, 90 and 120 min after glucose administration. The glucose blood level was measured using a glucometer with glucose test strips (Accu-Chek®, Roche, Thailand).
In the separated experiment, mice were simultaneous orally fed with assigned test compounds and 2 g/kg of glucose solution. Blood was then withdrawn from the tail vein at 0, 30, 60, 90 and 120 min after glucose administration. The glucose blood level was measured using a glucometer (Accu-Chek®, Roche, Thailand) with glucose test strips.
At the end of the study, mice were sacrificed using CO2 inhalation. Oral glucose tolerance curves for normal and diabetic mice were determined by plotting fasting blood glucose concentrations (mg/dL) against time after glucose administration (0-120 min). Area under the curve for glucose (AUCglucose at 0-120 min) over time was calculated by a linear trapezoidal rule methods shown in Equation 1,
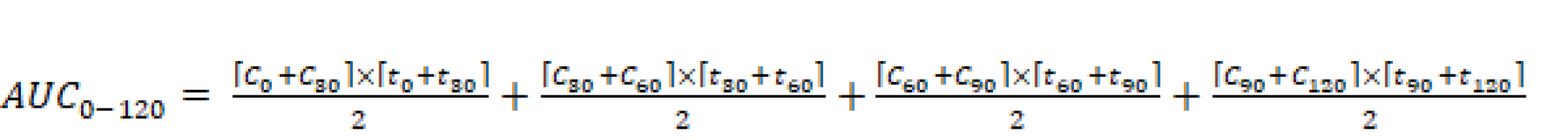 Eq (1)
Eq (1)
where C0, C30, C60, C90, and C120 are fasting blood glucose concentration (mg/dL) at 0, 30, 60, 90 and 120 min after glucose administration, respectively, and t0, t30, t60, t90, and t120 are time (min) after glucose administration, respectively.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) from triplicated experiments. The data were statistically analyzed using one-way analysis of variance (ANOVA) followed by Scheffe’s test. The statistical probability (P-value) less than 0.05 indicated a statistical significant difference between groups. SPSS statistics version 18.0 was used for all statistical procedures.
RESULTS
Quantitative analysis of total phenolic, total flavonoid, and mangiferin content in five cultivars of young mango leaf extract.
All young mango leaf extracts had brownish-green in color, sour and astringent taste with characteristic odor. The percentage yield of extraction were in the range of 25-47% (Table 1). HPTLC chromatogram of mangiferin in young mango leaf extracts showed peak with Rf value of 0.32 which corresponded with the peak of mangiferin standard (Figure 1). From the results, young mango cv. ‘Apple’ leaf extract contained the highest mangiferin contents while the lowest amount appeared in cv. Ok Rong (Table 1).
Table 1. Extraction yield, total phenolic, total flavonoid and mangiferin content of fiveCultivar of young mango leaf extracts.
|
cultivars of young mango leaf extracts. |
% Yield extraction |
Mangiferin content (mg/ g extract) |
Total phenolic content (mg GAE/g extract) |
Total flavonoid (mg QE/g extract) |
|
cv. ‘Apple’ |
47.44 |
197.32 ± 23.07 |
310.63 ± 3.85 |
26.69 ± 0.43 |
|
cv.‘Nam Dok Mai’ |
41.87 |
186.48 ± 7.67 |
266.65 ± 4.58 |
37.40 ± 0.46 |
|
cv. ‘Bao’ |
30.33 |
151.94 ± 2.28 |
225.63 ± 3.29 |
28.12 ± 0.64 |
|
cv. ‘Ok Rong’ |
25.28 |
105.18 ± 3.667 |
213.24 ± 2.36 |
33.90 ± 1.46 |
|
cv. ‘Kiew Savoey’ |
34.27 |
120.43 ± 7.26 |
174.49 ± 1.29 |
27.10 ± 0.60 |
Note: Data were expressed as mean ± SD (n = 3), GAE = gallic acid equivalent, QE = quercetin equivalent.
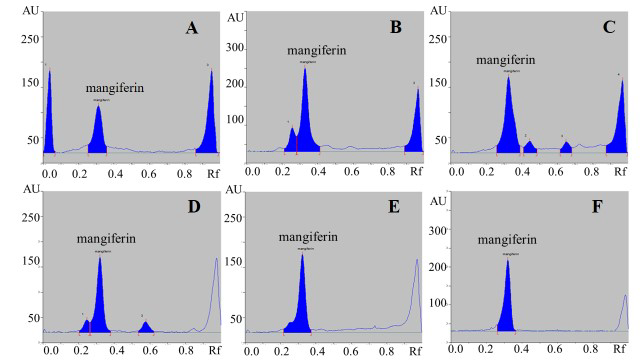
Figure 1. HPTLC chromatograms of five cultivars of young mango leaf extracts: ‘Apple’ (A), ‘Nam Dok Mai’ (B), ‘Bao’ (C), ‘Ok Rong’ (D), ‘Kiew Savoey’ (E), and mangiferin reference standard (F).
In vitro antioxidant activity
As present in Table 2, the results of antioxidant activities determined by all different methods consistently showed that young mango cv. ‘Apple’ leaf extract possessed the strongest antioxidant activity as compared with other cultivars. Moreover, its scavenging activity on DPPH radical and ferric reducing power was equivalent to those of mangiferin.
Table 2. DPPH, ABTS radical scavenging capacity and ferric reducing power of five cultivars of young mango leaf extracts.
|
Cultivar of young mango leaf extracts.
|
DPPH radical scavenging capacity IC50 value (µg/ml) |
ABTS radical scavenging capacity (mg TEAC/g extract) |
Ferric reducing power (mmol FeSO4/g extract) |
|
cv. ‘Apple’ |
5.42 ± 0.20 |
1800.50 ± 5.39 |
7.18 ± 0.09 |
|
cv. ‘Nam Dok Mai’ |
5.73 ± 0.16 |
1486.09 ± 78.11 |
5.95 ± 0.16 |
|
cv. ‘Bao’ |
7.27 ± 0.34 |
1404.25 ± 3.00 |
4.83 ± 0.41 |
|
cv. ‘Ok Rong’ |
8.14 ± 0.17 |
1256.28 ± 26.72 |
4.80 ± 0.21 |
|
cv. ‘Kiew Savoey’ |
11.13 ± 0.27 |
1110.34 ± 85.13 |
3.71 ± 0.15 |
|
Mangiferin |
4.13 ± 0.32 |
3160.83 ± 95.18 |
7.90 ± 0.19 |
Note: Data were expressed as mean ± SD (n = 3), IC50 = median inhibition concentration, TEAC = trolox equivalent antioxidant capacity.
Effect on α-glucosidase inhibitory activity
The inhibitory effect of all extracts were shown as IC50 value in Table 3. All of the extracts showed an inhibition on α-glucosidase activity in a dose-dependent manner. The young leaf extract of mango cv. ‘Apple’ revealed the highest inhibitory activity with an IC50 value of 0.50 ± 0.03 µg/ml, followed by cv. ‘Nam Dok Mai’, ‘Bao’, ‘Kiew Savoey’ and ‘Ok Rong’ young leaf extract, respectively. Its α-glucosidase inhibitory activity was markly stronger than acarbose and mangiferin.
Figure 2 shows the Lineweaver–Burk plots analysis of each cultivar of young mango leaf extract. The results suggested that mango cv. ‘Apple’, ‘Nam Dok Mai’, ‘Bao’, ‘Ok Rong’ and ‘Kiew Savoey’ exhibit the non-competitive inhibition type with Km of 2.25, 12.74, 6.81, 8.80, 5.76 mM, respectively and Ki of 2.98, 8.15, 8.96, 32.02 and 39.83 µg/mL, respectively. The results indicated that mango cv. ‘Apple’ leaf extract exhibits the strongest inhibitory effect (lowest Ki) on α-glucosidiase enzyme.
Table 3. The IC50 values of α-glucosidase inhibitory activity from 5 cultivars of young mango leaf extracts.
|
Cultivar of young mango leaf extracts |
IC50 value (µg/ml) |
|
cv. ‘Apple’ |
0.50 ± 0.03 |
|
cv. ‘Nam Dok Mai’ |
1.02 ± 0.08 |
|
cv. ‘Bao’ |
1.22 ± 0.04 |
|
cv. ‘Ok Rong’ |
1.82 ± 0.02 |
|
cv. ‘Kiew Savoey’ |
1.80 ± 0.10 |
|
Mangiferin |
170.44 ± 7.06 |
|
Acarbose |
577.39 ± 16.60 |
Note: Data were expressed as mean ± SD (n = 3).
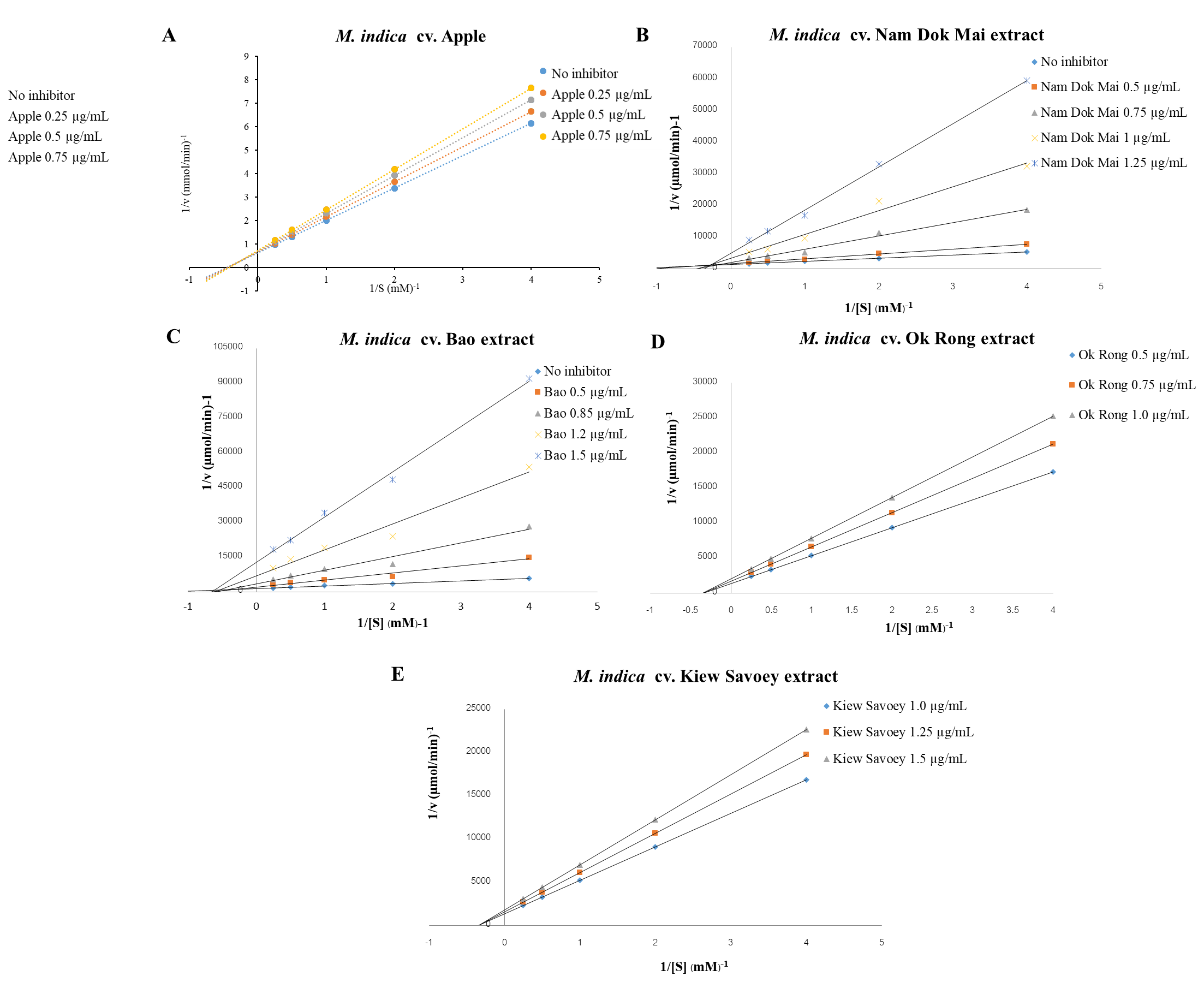
Figure 2. Lineweaver-Burk plots of α-glucosidase inhibition of young mango leaf extract of cultivar ‘Apple’ (A) ‘Nam Dok Mai’ (B), ‘Bao’ (C), ‘Ok Rong’ (D) and ‘Kiew Savoey’ (E).
Analysis of the correlation between total phenolic, total flavonoid, mangiferin content, DPPH, ABTS radical scavenging, FRAP, and α-glucosidase inhibitory activity.
According to the highest antioxidant and α-glucosidase inhibitory activities of young mango cv. ‘Apple’leaf extract, correlation analysis between chemical constituents and its antioxidant and α-glucosidase inhibitory effects was performed. As shown in Table 4, there were high correlation between the results of DPPH scavenging activity and total phenolic and total flavonoid content (r = 0.943 and 0.809). Several studies were reported that antioxidant activities were correlate to the content of total phenolic and total flavonoid compounds (Maisuthisakul et al., 2007, Ling et al., 2009). These high correlations support that total phenolic and total flavonoid compounds may responsible for the antioxidant activities of young mango leaf extract. Moreover, mangiferin content was also highly correlate with ABTS radical scavenging activity (r = 0.991). The result was in accordance with previous study of HPLC coupled with an online ABTS assay that mangiferin pentoside possessed the highest antioxidant capacity in young leaves of mango cv. ‘Talapnak’, ‘Chok Anan’ and ‘Nam Dok Mai’ (Prommajak et al., 2014).
Table 4. Pearson’s correlation between biological activities and chemical constituents in young mango cv. ‘Apple’ leaf extract.
|
|
TPC |
TFC |
DPPH |
ABTS |
FRAP |
Mangiferin Content |
a-glucosidase |
|
TPC |
1 |
|
|
|
|
|
|
|
TFC |
.959 |
1 |
|
|
|
|
|
|
DPPH |
.943 |
.809 |
1 |
|
|
|
|
|
ABTS |
-0.430 |
-0.156 |
-0.707 |
1 |
|
|
|
|
FRAP |
-.983 |
-.995 |
-.866 |
.258 |
1 |
|
|
|
Mangiferin |
-.309 |
-.026 |
-.609 |
.991 |
-.969 |
1 |
|
|
a-glucosidase |
-.199 |
-.470 |
.139 |
-.799 |
.375 |
-.870 |
1 |
|
a-glucosidase |
-.199 |
-.470 |
.139 |
-.799 |
.375 |
-.870 |
1 |
* Correlation is significant at the 0.05 level (2-tailed).
In vivo antidiabetic activity
From the results, young mango cv. ‘Apple’ leaf extract exhibited the strongest antioxidant activity among five cultivars. It also revealed the greatest α-glucosidase inhibitory activity and high level of total phenolic and mangiferin contents. Therefore, young mango cv. ‘Apple’ leaf extract was chosen for further in vivo evaluation of hypoglycemic effect in STZ-NA-induced type 2 diabetic mice.
Administration of young mango cv. ‘Apple’ leaf extract at a dose of 500 and 1,000 mg/kg did not altered blood glucose level and area under the curve (AUC) within 120 minutes in normoglycemic mice as shown in Figure 3a and 3b.
Administration of the young mango cv. ‘Apple’ leaf extract at a dose of 500 and 1,000 mg/kg at 30 min before glucose loading showed no hypoglycemic effect in diabetic mice determined by OGTT model (Figure 4a and Figure 5). While, glibenclamide significantly reduced blood glucose level at 30 min and 120 min and the overall glucose during 120 min (AUCglucose (0-120)) as compared with the untreated-diabetic group.
Figure 4b shows that the co-administration of the extract at a dose of 1,000 mg/kg with glucose loading significantly attenuated blood glucose level at 30, 90 and 120 min. Moreover, simultaneously administration of the young mango cv. ‘Apple’ leaf extract 1,000 mg/kg with glucose reduced AUC0-120 min significantly by about 13.43% as compared with untreated-DM mice. Its hypoglycemic activity was higher than that of glibenclamide (Figure 5).
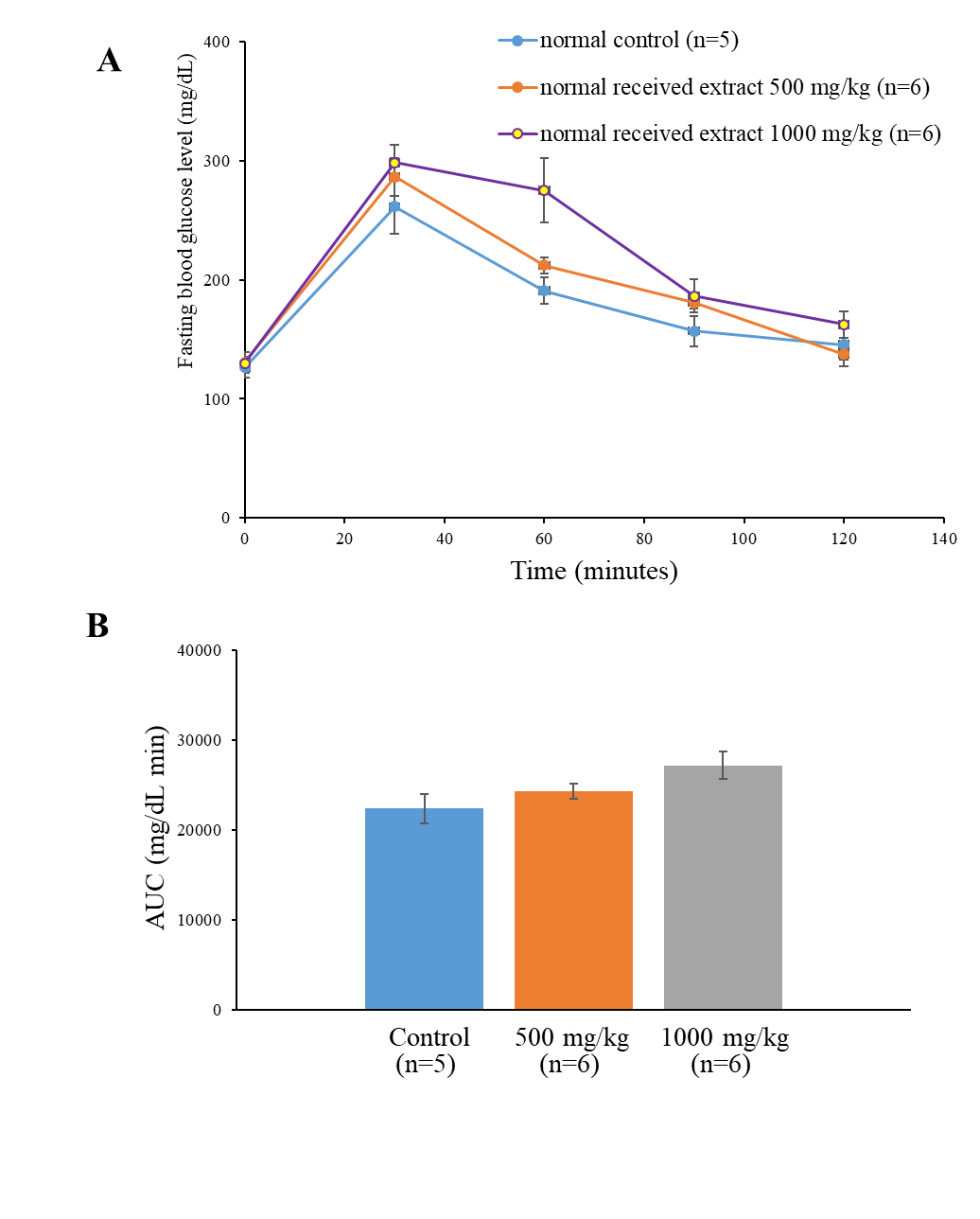
Figure 3. Fasting blood glucose and Area under the curve (AUC) of glucose after single administration of young mango cv. ‘Apple’ leaf extract for 30 min before loading with 2 g/kg of glucose solution in normoglycemic mice determined by Oral Glucose Tolerance Test (OGTT). Data are mean ±SEM (n = 5-6).
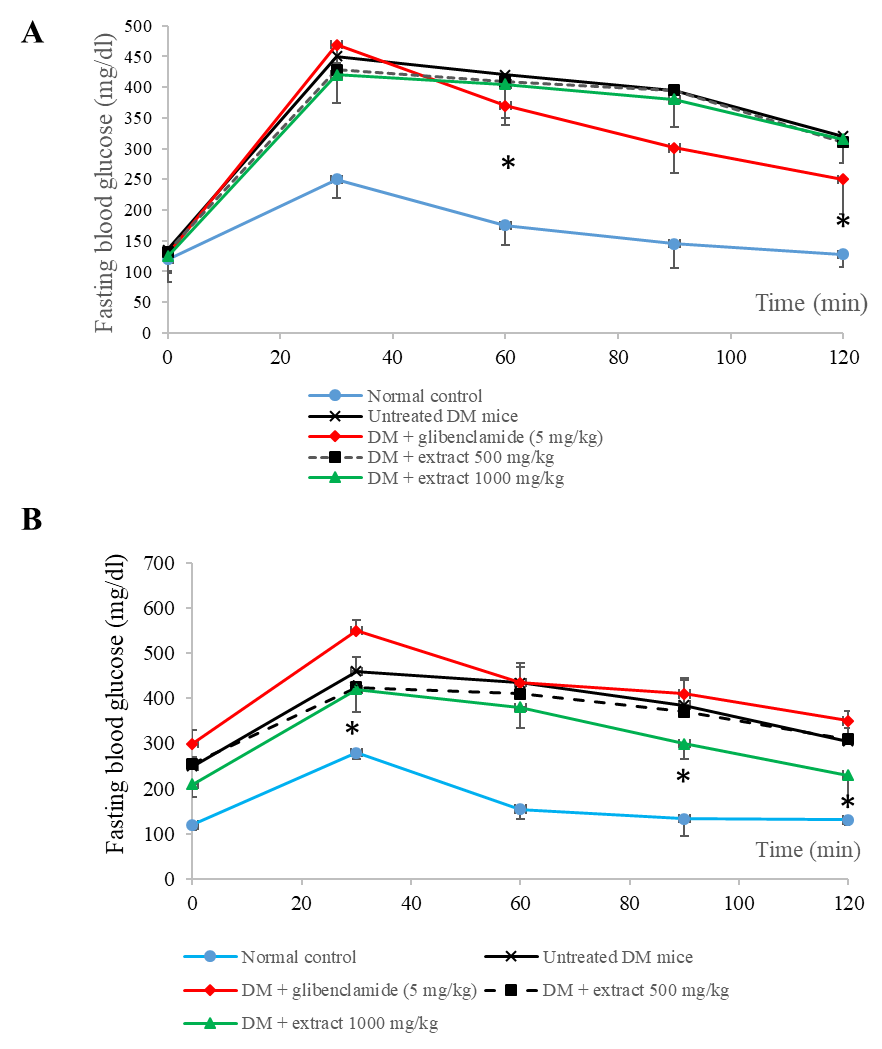
Figure 4. Effect of single administration of young mango cv. ‘Apple’ leaf extract with 2 g/kg of glucose solution on blood glucose level in Oral Glucose Tolerance Test (OGTT) in STZ-NA-induced type 2 diabetic mice: administered the extracts 30 minutes before 2 g/kg of glucose solution (A), administered the extracts simultaneously with 2 g/kg of glucose solution (B). Data are mean ±SEM (n = 5-6). * P <0.05 as compared with untreated-STZ-NA induced DM mice group examined using one way ANOVA followed by Scheffe’s test.
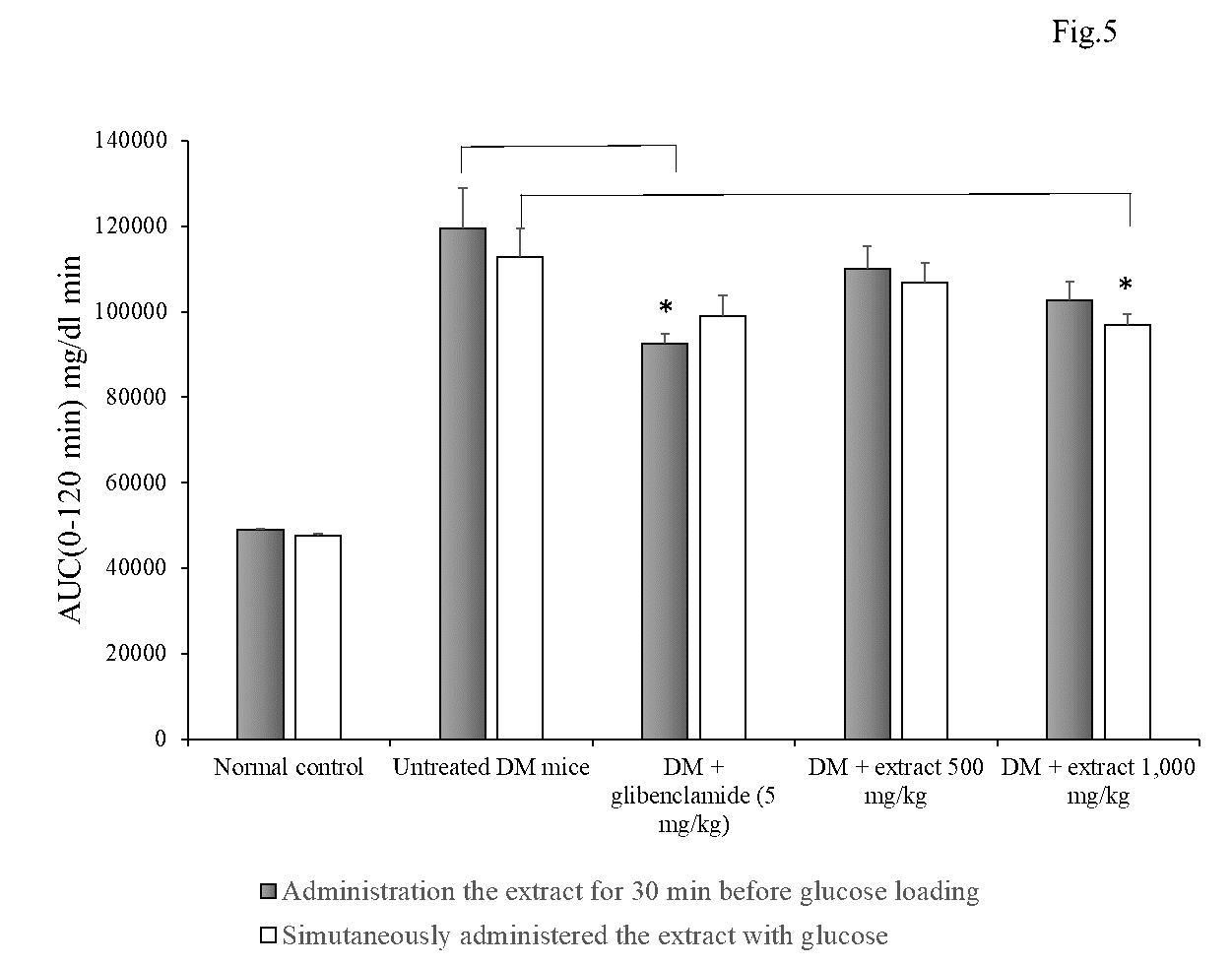
Figure 5. Area under the curve (AUC) of blood glucose level on Oral Glucose Tolerance Test (OGTT) in STZ-NA-induced diabetic mice during 0-120 min after glucose administration. Data are mean ± SEM (n = 5-6). * P <0.05 as compared with untreated-STZ-NA induced DM mice group examined using one way ANOVA followed by Scheffe’s test.
DISCUSSION
The results revealed the highest total phenolic, mangiferin content and strongest antioxidant activities were obtained from young mango cv. ‘Apple’ leaf extract. Not only total phenolic and total flavonoid compounds are correlated with antioxidant effect, the active compound, mangiferin is also play an important role in antioxidant effect of young mango leaves. The highest content of mangiferin was found in the young leaves of mango cv. ‘Apple’ (197.32 mg/g extract), while the lowest quantity appeared in cv. ‘Ok Rong’ (105.18 mg/g extract). Interestingly, the content of mangiferin in this study were higher than those found in the previous study of others cultivar of mangos in Thailand such as ‘Talapnak’ (37.92 mg/g dry weight), ‘Chok Anan’ (29.11 ± 11.42 mg/g dry weight) and ‘Nam Dok Mai’ (33.27 ± 2.65 mg/g dry weight), respectively (Prommajak et al., 2014). These results suggested that mangiferin may be responsible for the antioxidant activity of the extract. Mangiferin acts as an antioxidant principally by quenching the free radicals and also chelating the iron-complex ions (Fe2+/Fe3+), these result in the protection of membrane from lipid peroxidation. When mangiferin reacts with the free radicals, catechol moiety is oxidized and then liberates the quinone and semiquinone radicals. The number of hydroxyl groups and catechol moiety in the structure found to be related to the degree of antioxidant activity (Imran et al., 2016).
Our findings suggested that among five cultivars of young mango leaf extracts, mango cv. ‘Apple’ exhibits the considerable potential to possibly delay the absorption of glucose via the inhibition of α-glucosidase enzyme. All mango cultivars exhibited inhibition type of non-completive inhibition which is accordance with that of the major active compound, mangiferin (Shi et al., 2017).
In vivo study found that young mango cv. ‘Apple’ leaf extract at a dose of 500 and 1,000 mg/kg have no hypoglycemic effect on fasting blood glucose level in normoglycemic mice which is corresponded with previous study (Aderibigbe et al., 1999). Evaluation of mango leaf extract on antidiabetic activity provided an effective response in several studies (Aderibigbe et al., 1999, Aderibigbe et al., 2001). On the contrary, the present study found no hypoglycemic effect after oral administration of extract before loading glucose in OGTT model. However, hypoglycemic effect was detected when the extract and glucose were simultaneously co-administered in DM mice which is in accordance with the results found in previous studies (Bhowmik et al., 2009, Hossain et al., 2010). The overall blood glucose level, represented as AUCglucose
(0-120 min), in extract-treated mice at a dose of 1,000 mg/kg was significantly reduced by about 13.43% when compared with diabetic control mice. These findings suggested that the administration of young mango cv. ‘Apple’ leaf extract simultaneous with glucose tend to decrease blood glucose level in type 2 diabetic mice. The possible hypoglycemic mechanism may be due to the inhibition of glucose absorption in the gastrointestinal tract by interfering the intestinal glucose absorption. Moreover, the co-administration of the extract with glucose may promote the solubility and the absorption of mangiferin which is normally poor absorption with very low oral bioavailability (about 1.2% in rats) (Han et al., 2010, Wang et al., 2013), resulting in the improvement of the hypoglycemic effect of mangiferin. However, further evaluation of mechanisms responsible for hypoglycemic effect, for instance, stimulation of insulin secretion from β-cells, activation of insulin receptors and glucose uptake in peripheral tissues, modulation of hepatic glucose output or elevation of GLUT-4 expression should be confirmed in the further study.
Mango leaves consisted of several bioactive components as reported in many studies, for example, gallic acid, catechin and quercetin (Ediriweera et al., 2011). The hypoglycemic effect of the extract might be attributed to these containing compounds. Flavonoids including myricetin, quercetin and isoquercitrin had been appraised for their noncompetitively inhibition of glucose and fructose transport on GLUT-2 by acting as a potent luminal inhibitor, owing to their poor absorption attribute (Kwon et al., 2007). Gallic acid can increase GLUT-4 translocation concomitant with enhance glucose uptake activity (Prasad et al., 2010). Mangiferin and its metabolite, norathyriol, have been determined for anti-diabetic effects by various mechanisms such as lowering blood glucose level, improvement the glucose utilization and insulin sensitivity by up-regulation of the phosphorylation of AMPK (Aderibigbe et al., 1999, Imran et al., 2017, Shi et al., 2017,).
In this study, young mango cv. ‘Apple’ leaf extract exhibited not only an outstanding antioxidant capacity with the great contents of bioactive compounds, but also a remarkable inhibitory effect on the in vitro α-glucosidase activity with a fairly in vivo hypoglycemic effect in type 2 diabetic mice. The highest experimental dose of the extract at 1,000 mg/kg given the trend in gradually decreasing blood glucose level in mice, thus increasing dose of the extract might contribute to the greater reduction in blood glucose level. With regard to the toxicological aspects of mango leaf extracts, previous studies of both aqueous and methanolic extracts showed no acute toxicity with maximum dose of 18.4 g/kg (Zhang et al., 2014). Thus, mango leaves possessed a low level of toxicity and had been considered to be used as supplements or adjunctive therapy in type 2 diabetes management.
CONCLUSION
The present study is the first time of determination the hypoglycemic effect of young mango cv. ‘Apple’ leaf extract in STZ-NA- induced type 2 diabetic mice. Single oral administration of the extract at a dose of 1,000 mg/kg simultaneously with glucose provided the practicable trend in normalizing blood glucose level, possibly mechanism of α-glucosidase inhibitory activity in the non-competitive inhibition manner. Thus, it would be suggest that among the young mango leaves extract, young mango cv. ‘Apple’ leaf extract could be further develop as a potential source of nutraceutical products.
REFERENCES
Aderibigbe, A.O., Emudianughe, T.S., and Lawal, B.A.S. 1999. Antihyperglycaemic effect of Mangifera indica in rat. Phytotherapy Research. 13: 504-507.
Aderibigbe, A.O., Emudianughe, T.S., and Lawal, B.A.S. 2001. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytotherapy Research. 15: 456-458.
Aiyelaagbe, O.O. and Osamudiamen, P.M. 2009. Phytochemical screening for active compounds in Mangifera indica leaves from Ibadan, Oyo State. Plant Science and Research. 2: 11-13.
American Diabetes Association, 2017. Pharmacologic approaches to glycemic treatment. Diabetes care. 40(Suppl 1): S64- S74.
Anbalagan, K., Kumar, M.M., Ilango, K., Mohankumar, R., and Priya, R.L. 2019. Prelusive scale extraction of mangiferin from Mangifera indica leaves: Assessing solvent competency, process optimization, kinetic study and diffusion modelling. Industrial Crops and Products. 140: 111703.
Bajaj, S., and Khan, A. 2012. Antioxidants and diabetes. Indian Journal of Endocrinology and Metabolism. 16: S267-S71.
Barreto, J.C., Trevisan, M.T., Hull, W.E., Erben, G., de Brito, E.S., Pfundstein, B., and Owen, R.W. 2008. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.) Journal of Agricultural and Food Chemistry. 56: 5599-5610.
Berardini, N., Fezer, R., Conrad, J., Beifuss, U., Carle, R., and Schieber, A. 2005. Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol- o and xanthone c-glycosides, anthocyanins, and pectin. Journal of Agricultural and Food Chemistry. 53: 1563-1570.
Bhowmik, A., Khan, L.A., Akhter, M., and Rokeya, B. 2009. Studies on the antidiabetic effects of Mangifera indica stem-barks and leaves on nondiabetic, type 1 and 2 diabetic model rats. Bangladesh Journal of Pharmacology. 4: 110-114.
Bhuvaneshwari, J., Khanam, S., and Devi, K. 2014. In-vitro enzyme inhibition studies for antidiabetic activity of mature and tender leaves of Mangifera indica var. Totapuri. Journal of Microbiology and Biotechnology. 3: 36-41.
Chayamarit, K. 1994. Preliminary checklist of the family Anacardiaceae in Thailand. Thai Forest Bulletin (Botany). 22: 1-25.
Ediriweera, M.K., Tennekoon, K.H., and Samarakoon, S.R. 2017.A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (Mango). Evidence-Based Complementary and Alternative Medicine. 2017.
Han, D., Chen, C., Zhang, C., Zhang, Y., and Tang, X. 2010. Determination of mangiferin in rat plasma by liquid-liquid extraction with UPLC-MS/MS. Journal of Pharmaceutical and Biomedical Analysis. 51: 260-263.
Hossain, M.S., Khan, M.R.I., Anisuzzaman, A.S.M., Ahmed, M., Amran, M.S., and Islam, A. 2010. Antidiabetic and glycogenesis effects of different fractions of ethanolic extract of leaves of Mangifera indica Linn. in normal and aloxan induced diabetic rats. Journal of Medical Sciences. 10: 80-86.
Imran, M., Arshad, M.S., Butt, M.S., Kwon, J.H., Arshad, M.U., and Sultan, M.T. 2017. Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids in Health and Disease. 16: 84.
Kwon, O., Eck, P., Chen, S., Corpe, C.P., Lee, J.H., Kruhlak, M., Levine, M. 2007. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. The FASEB Journal. 21: 366-377.
Lim, Y.Y. and Quah, E.P.L. 2007. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chemistry 103: 734-740.
Ling, L.T., Yap, S.A., Radhakrishnan, A.K., Subramaniam, T., Cheng, H.M., and Palanisamy, U.D., 2009. Standardised Mangifera indica extract is an ideal antioxidant. Food Chemistry 113: 1154-1159.
Maisuthisakul, P., Suttajit, M., Pongsawatmanit, R., 2007. Assessment of phenolic content and free radical scavenging capacity of some Thai indigenous plants. Food Chemistry 100: 1409-1418.
Masud Parvez, G.M. 2016. Pharmacological activities of mango (Mangifera indica): A review. Journal of Pharmacognos and Phytochemistry. 5: 1-7.
Montonen, J., Knekt, P., Jarvinen, R., and Reunanen, A. 2004. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 27: 362-366.
Prasad, C.N.V., Anjana, T., Banerji, A., and Gopalakrishnapillai, A. 2010. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Letter. 584: 531-536.
Prommajak, T., Kim, S.M., Pan, C.H., Kim, S.M., Surawang, S., and Rattanapanone, N. 2014. Identification of antioxidants in young mango leaves by LC-ABTS and LC-MS. Chiang Mai University Journal of Natural Sciences. 13: 317-330.
Sellamuthu, P.S., Arulselvan, P., Kamalraj, S., Fakurazi, S., and Kandasamy, M. 2013. Protective nature of mangiferin on oxidative stress and antioxidant status in tissues of streptozotocin-induced diabetic rats. ISRN pharmacology. 2013: 750109.
Sethiya, N.K., Trivedi, A., and Mishra, S.H. 2015. Rapid validated high performance thin layer chromatography method for simultaneous estimation of mangiferin and scopoletin in Canscora decussata (south Indian Shankhpushpi) extract. Revista Brasileira de Farmacognosia. 25: 193-198.
Shi, Z.L., Liu, Y.D., Yuan, Y.Y., Song, D., Qi, M.F., Yang, X.J., Wang, P., Li, X.Y., Shang, J.H., and Yang, Z.X. 2017. In vitro and in vivo effects of norathyriol and mangiferin on α-glucosidase. Biochemistry Research International. 2017: 1206015.
Singleton, V.L., Orthofer, R., and Lamuela-Raventós, R.M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. 299: 152-158.
Sithisarn, P., Rojsanga, P., Sithisarn, P., and Kongkiatpaiboon, S. 2015. Antioxidant activity and antibacterial effects on clinical isolated Streptococcus suis and Staphylococcus intermedius of extracts from several parts of Cladogynos orientalis and their phytochemical screenings. Evidence-Based Complementary and Alternative Medicine. 2015: 908242.
Stankovic, M.S. 2011. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac Journal of Science. 33: 63-72.
Supasuteekul, C., Nonthitipong, W., Tadtong, S., Likhitwitayawuid, K., Tengamnuay, P., and Sritularak, B. 2016. Antioxidant, DNA damage protective, neuroprotective, and α-glucosidase inhibitory activities of a flavonoid glycoside from leaves of Garcinia gracilis. Revista Brasileira de Farmacognosia. 26: 312-320.
Thaipong, K., Boonprakob, U., Crosby, K., Cisneros-Zevallos, L., and Byrne, D.H. 2006. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis. 19(6): 669-675.
Wang, X., Gu, Y., Ren, T., Tian, B., Zhang, Y., Meng L, and Tang X. 2013. Increased absorption of mangiferin in the gastrointestinal tract and its mechanism of action by absorption enhancers in rats. Drug development and industrial pharmacy. 39(9): 1408-1413.
World Health Organization, 2009. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva, Switzerland: World Health Organization.
Zhang, Y., Li, J., Wu, Z., Liu, E., Shi, P., Han L, Guo, L., Gao, X., and Wang Tet. Acute and long-term toxicity of mango leaves extract in mice and rats. Evidence-Based Complementary and Alternative Medicine. 2014: 691574.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Savita Chewchinda1, Orasa Suriyaphan1, Pimpikar Kanchanadumkerng1, Hitoshi Sato2 and Vilasinee Hirunpanich Sato3,*
1 Department of Food Chemistry, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand
2 Department of Pharmacology, Toxicology and Therapeutics, Division of Pharmacokinetics and Pharmacodynamics,
School of Pharmacy, Showa University, Tokyo 142-8555, Japan
3 Department of Pharmacology, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand
Corresponding author: Vilasinee Hirunpanich Sato, E-mail: vilasinee.sat@mahidol.ac.th
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 11, 2020;
Revised: September 2, 2020;
Accepted: September 8, 2020

