
Antioxidant Capacities of Traditionally Formulated Thai Herbal Decoction and Its Effect on Cell Growth Using Saccharomyces cerevisiae Model
Ademola Ezekiel Adekoya*, Sasitorn Chusri, Eugene Ong Boon Beng, and Anthony Temitope IdownPublished Date : 2020-12-17
DOI : https://doi.org/10.12982/CMUJNS.2021.012
Journal Issues : Number 1, January-March 2021
Abstract Polyherbal tea is widely consumed for rejuvenating of health in Thailand. Among them are Ya-Tri-Phigut (YTP), Ya-Tri-Kasornmas (YTK), Ya-Plook-Fithat (YPF), and Ya-Benjakul (YB). In addition, they have been listed as essential medicines with several others in the National List of Essential Medicines, Thailand. To ascertain their nutraceutical potentials, aqueous extracts of these herbal formulations were screened for their antioxidant activities. Extracts obtained from YTP showed higher yield (8.70 %) than YPF (6.40 %), YTK (6.30 %) and YB (6.30 %) (P <0.05). YTP also showed better antioxidant capacities than other tested formulations (P <0.05). Thus, it was used to test for its ability to support yeast cell. The extract (YTP) supported the yeast cell growth than the standard used called cryptotanshinone. Thus, YTP formulated herbal tonic can serve as a rich nutraceutical agent to combat oxidative stress and health related diseases.
Keywords: Antioxidant, Healthcare and Extract, Herbal tea
Citation: Adekoya, A.E., Chusri, S., Beng, E.O.B., and Idown, A.T. 2021. Antioxidant capacities of traditionally formulated Thai herbal decoction and its effect on cell growth using Saccharomyces cerevisiae model. CMUJ. Nat. Sci. 20(1): e2021012.
INTRODUCTION
Traditional medicine such as herbal supplement and condiment has a pivotal function in the health care system of both industrialized and developing countries (Chanthasri et al., 2018). A recent survey estimated the percentage of herbal supplements consumption to be 20% in Europe (Garcia-Alvarez et al., 2014). In addition, countries, like Turkey (Soner et al., 2013), Nigeria (Onyeka et al., 2012), Thailand (Tangkiatkumjai et al., 2013) and Saudi Arabia (Mohammad et al., 2015) showed more than 40% of informants that have used herbal medicine. Herbal beverage consumption specifically have been embraced as part of daily menu in some countries (Petrovska, 2012). For instance, in India (Amir et al., 2011), Thailand (Booranasubkajorn et al., 2017), China (Ji et al., 2016), tonic beverages prepared from the addition of two or more medicinal plants (herbal formulations) either as decoctions or teas are usually consumed for their health benefits (Halberstein, 2005). Among the southeast Asian countries, Thailand is known for its folk medicines especially within the southern local community members where polyherbal decoctions are consumed as rejuvenating tea tonics (De Guzman and Siemonsma, 1999). Among such formulations are Ya-Tri-Phigut, Ya-Tri-Kasornmas, Ya-Plook-Fithat, and Ya-Benjakul which have been listed among other herbal formulations as essential medicines in the National List of Essential Medicines, Thailand (Chewchinda et al., 2018). However, these herbal formulations have not been fully explored scientifically to ascertain their antioxidant capacities.
Toxic substances and free radicals have been known to trigger Oxidative-stress related diseases such as cancer, cardiovascular diseases, neurodegenerative disorders, and aging (Migliore and Coppedè, 2009; Chanthasri et al., 2018). Meanwhile, aging is a multidimensional process that could have negative influence on the functions of different physiological systems (Amarya et al., 2015). It is generally associated with consistent accumulation of toxins at both cellular and molecular levels (Rizzo et al., 2014). Different studies have reported the use of murine, fruit flies (Drosophila melanogaster), nematodes (Caenorhabditis elegans) and the baker yeast (Saccharomyces cerevisiae) for aging models and cell growth studies (Neff et al., 2013; Qiao et al., 2019; Heydari et al., 2020; Olmedo et al., 2020). However, unicellular eukaryotes such as Saccharomyces cerevisiae is widely preferred because of the availability of their full sequenced genetic make-ups, many alternate knockout plasmids, insightful DNA replicating process, protein turn over, stress response with longevity and cell death mechanisms (Bitterman et al., 2003). Additionally, over 30% of the yeast gene have mammalian similarities (Yang et al., 2007). Genes responsible for human diseases have their yeast orthologues. Yeast cells are cheap, less time consuming and are applicable for non-ethical experimental models (Fruhmann et al., 2017).
Since herbal tea have been observed to be rich source of natural antioxidant that can slow-down or inhibit the oxidation of biomolecules that are responsible for several health damages (Lobo et al., 2010). Therefore, it is expedient to investigate Thai herbal formulations; Ya-Tri-Phigut, Ya-Tri-Kasornmas, Ya-Plook-Fithat, and Ya-Benjakul for their antioxidant capacities. Hence, this study aimed at examining the antioxidant activities of the above herbal formulations. In addition, herbal formulation with the best antioxidant potential was selected to study its ability to enhance cell growth using Saccharomyces cerevisiae model.
MATERIALS AND METHODS
Herbal materials and crude extraction
Four herbal formulations (Ya-Tri-Phigut, Ya-Tri-Kasornmas, Ya-Plook-Fithat, and Ya-Benjakul) listed in the National List of Essential Medicines, Thailand which are used as health rejuvenating tonics were selected. The plant components of the formulas were bought from an authorized herbal drug outlet, Triburi Orsot; located in the province of Songkhla, Thailand. Identification of the herbal materials was done against reference samples of the materia medica at the Traditional Thai Medicine Faculty, Prince of Songkla University, Thailand. The plant materials were cleaned with sterile distilled water, drying was done at 60°C in the electric oven (Mamaru MR-1214, Mamaru Co., Ltd., Bangkok, Thailand) for 72 hr and then grounded. The obtained powdered herbs were subjected to sieving (16-mesh sieve size) and weighed. Thereafter, they were stored in vacuum-sealed bags at 4 °C until further use. Equal proportions of varying types of plant parts were used to formulate the mixtures as described in Table 1.
Table 1: Herbal components, methods of preparation and extraction yield obtained from water decoction of four formulations.
|
Formulations method |
Administration |
Herbal components (parts used) |
Amount |
used% Extraction yield (w/w) |
|
YB |
Anaxagorea javanica (root) Bauhinia integrifolia (rhizome) Pennisetum pedicellatum (root) Plumbago rosea (rhizome) Zingiber officinale (rhizome) |
20 g each of each herbal components were mixed to make up the mixture. |
As powder, pill, pellet and bolus 800 mg - 1 g should be eaten 3 times a day after meals. |
6.30±0.40c |
|
YPF |
Acorus calamus (rhizome) Ardisia polycephala (fruit) Citrus hystrix (fruit peel) Cyperus rotundus (tuber) Foeniculum vulgare (fruit) Iresine herbstii (any part) Piper longum (fruit) Piper nigrum (fruits) Piper ribesoides (stem) Piper sarmentosum (root) Zingiber officinale (rhizome) |
The formula was made by mixing equal amount of the powdered herbal components as described in Ya-Benjakul. |
500 mg - 1.5 g daily 3 times before meals should be taken. |
6.40±0.15b |
|
YTK |
Aegle marmelos (unripe fruit) Jatropha multifida (back) Nelumbo nucifera (pollen) |
Equal proportion as described in Ya-Benjakul |
should be taken about four times each day before meal and before bed |
6.30±0.11c |
|
YTP |
Phyllanthus emblica (fruit) Plumbago indica (root) Piper nigrum (fruit) Piper pendulispicum (stem) Piper retrofractum (fruit) Piper sarmentosum (root) Terminalia bellirica (fruit) Terminalia chebula (fruit) Zingiber officinale (rhizome) |
Equal proportion of each herbal component in powdered form was mixed to prepare the formula. as described in Ya-Benjakul |
250 - 500 mg 3 times a day before meals |
8.70±0.09a |
Note: All information were extracted from the National List of Essential Medicine of Thailand except for the extraction yield which were calculated using the dry weight of extracts and the dry weight of the samples. Ya-Tri-Phigut (YTP), Ya-Tri-Kasornmas (YTK), Ya-Plook-Fithat (YPF), and Ya-Benjakul (YB). Different superscripts in the same column indicate significant differences (P < 0.05) (for extraction yield only).
Herbal mixtures and preparation
To prepare the extract, 10 g of powdered herbal mixture was placed in a tea bag. Thereafter, 180 ml of boiled water at 100 ± 0.00 °C was added and stirred continuously for 20 min. The obtained liquid extracts were filtered using a Whatman No. 1 filter paper. The obtained extract was allowed to cool at room temperature and then lyophilized with a freeze-dryer (CoolSafe 55, ScanLaf A/S, Lynge, Denmark). Extraction yield was calculated as detailed by Idowu et al. (2019) Thereafter, each herbal formulation were subjected to antioxidant activities.
Determination of antioxidant activities
DPPH radical scavenging activity
The ability of the polyherbal extracts to scavenge DPPH radical was performed as detailed by Wong et al. (2014) Two-fold serial dilution of each sample were prepared (2500-1.22 µg/ml). In a 96-well plate containing 20 µl of the extracts, 180 µl of (80 µM) DPPH solution prepared in 70% ethanol was added. The mixture was kept at a steady state at 27 °C in the dark for 30 mins. The color change in DPPH solution was measured by absorbance reading at 520 nm. Trolox was used as control with an appropriate blank measurement. The percentage scavenging capacity of extracts and standard solution against DPPH radical was calculated using the equation:
Scavenging activity (%) = (OD control-OD sample) x 100/OD control
ABTS radical scavenging activity
The ability of the aqueous extract of the various polyherbal formulations to scavenge ABTS radical was carried out as reported by Wong et al. (2014). Aqueous ABTS+ solution was prepared by the addition of 2 mM ABTS and 2.45 mM potassium persulfate in equal proportion. The mixture was incubated in dark for 16 hours then it was mixed with phosphate buffer saline (PBS) to give an absorbance between 0.68 and 0.72 at 734 nm. Two hundred microliters of the resultant ABTS+ solution was added to the two-fold serially diluted extracts (2500-1.22 µg/ml) in a 96-wells microplate. Shortly (6 mins) after the addition, the absorbance was read at 734 nm. The reading was taken with Trolox as control and an appropriate blank measurement. The percentage scavenging capacity of extracts and standard solution towards ABTS radical was calculated from the equation below:
Scavenging activity (%) = (OD control-OD sample) x 100/OD control
This was further expressed as inhibition concentration (IC50; mg/ml).
Ferric reducing antioxidant power (FRAP) assay
The ability of the extracts to reduce ferric ion present in ferric-tripyridyl triazine (TPTZ) complex to ferrous-TPTZ was estimated as described by Chanthasri et al. (2018). The FRAP reagent was prepared by mixing 10 ml of 300 mM acetate buffer, 1 ml of 10 mM TPTZ solution, and 10 ml of 20 mM ferric chloride solution together. The extracts were dissolved in dimethyl sulfoxide (DMSO) to make two-fold serial dilution concentrations (2.5-0.001 mg/ml). Prepared FRAP reagent 1.35 ml was added to 150 µl of the extract and incubated at 37 °C for half an hour in the absence of light. The absorbance of the solution was read at 596 nm. The ability of the extract to reduce the ferric ion was given as mM of ferrous mg of sample (µM Fe2SO4/mg extract).
Total phenolic content determination (TPC)
Folin-Ciocalten (FC) technique was applied with slight modification (Abbasi et al. 2015). In brief, 120 µl (2.5 mg/ml) of each extract was diluted 10-fold and added to 1000 μl FC reagent. The reaction was set for 5 mins. Sodium carbonate solution (1000 μl; 20 % w/v) was added to the reaction and kept in the dark at room temperature for 90 minutes. Absorbance was read at 725 nm. The experiment was performed in triplicate with results given as µg of Gallic acid per gram of the samples.
Total flavonoid content determination (TFC)
Aluminum chloride colorimetric procedure was applied in the quantification of TFC of each extract as illustrated by Abbasi et al. (2015) with slight modification. Each extract (0.05 ml, 2.5 mg/ml) was added to a combined mixture of 4 ml sterile distilled water, 0.3 ml of 5% (w/v) sodium nitrite and 0.3 ml, 10% (w/v) aluminum trichloride. The resultant mixture was made to stand for 6 mins at 27 °C, then 1M sodium hydroxide (2 ml) was added to stop the reaction. This was made up to 10 ml by the addition of sterile distilled water and the absorbance was read at 510 nm after 10 mins. The value of TFC was calculated from the standard graph of catechin solution and the results given in µg of catechin equivalent per gram of sample.
Antioxidant analysis of individual components of selected herbal formulation
The two most potent herbal formulations with higher antioxidant capacities were selected for further analysis. The components of those herbal formulation were investigated for their individual antioxidant capacities. They were subjected to metal chelating activities (MCA) as detailed by Wong et al. (2014). In addition, DPPH radical scavenging activity, ABTS, FRAP, TPC and TFC were studied as described in section 2.
General growth condition of S. cerevisiae cells
Cells stored at -80 °C were revived and re-cultured at the start of each experiment carried out in this experiment. Yeast cells were cultured using yeast extract peptone dextrose (YPD) medium and YPD agar (YPDA). All media were autoclaved at 100 kPa, 121 °C for 15 minutes before use. The incubating temperature was 30 °C with shaking speed of 200 rpm all through the study. The yeast strain used was grown in 7 ml-sized Bijou bottles with a final culture volume of 1 ml or 96-well flat bottom microplate with lid without shaking. The above condition of yeast was applied throughout the study as stated by Gietz and Schiestl (2007).
Growth effects of medicinal samples on yeast cells
Herbal extract with the highest antioxidant capacities was selected for cytotoxicity study in Saccharomyces cerevisiae cell. The effects of the herbal extracts on the growth of yeast were examined according to the method of Powers et al. (2006) with some modifications. Briefly, from a frozen stock of the cells, a loop was taken and streaked on a YEPD (1% yeast extract, 2% bacto-peptone, 2% agar, 2% glucose) agar plate and was incubated at 30 °C for 48 hours for the formation of distinct colonies. After this period, a single colony was picked from the plate and inoculated into YPD medium. After 24 hours of incubation at 30 °C, the cells were standardized appropriately and aliquot measure of the standardized cells were put into bottles containing the various extract samples. The cell culture in synthetic complete (SC) media (Yeast nitrogen base and synthetic defined medium (-TRP), 100X L-tryptophan, and 20% glucose) with YTP extracts at a final concentration of 0.1μg/ml, cryptotanshinone (CPT) at varying concentration (5, 2.5 and 1.25 μg/ml) and were put into 96 well plate and the optical density taken at 600 nm for 24 hours; with readings taken every 30 mins.
Statistical analysis
Experiments were run in triplicate. Analysis of variance (ANOVA) was carried out. Means were compared using the Duncan’s multiple range test. The Statistical Package for Social Science (SPSS 11.0 for Windows, SPSS Inc, Chicago, IL, USA) was used for statistical analysis.
RESULTS
Extraction yield of herbal extracts
The extraction yield of the four herbal extracts are shown in Table 1. YTP had the highest extraction yield of 8.7 % (w/w) than others (P <0.05). Yield of YPF was 6.4% (w/w) while YTK and YB showed a yield of 6.3% (w/w), respectively under the same condition.
Antioxidant activities of herbal formulations
DPPH radical scavenging activity
DPPH activities of herbal formulations as well as standard ranged between 0.08 ± 0.00 -2.02 ± 0.16 (IC50, mg/ml). Different herbal formulation of YTP, YTK, YPF and YB showed varying DPPH radical scavenging activity (Table 2).
ABTS radical scavenging activity
ABTS activity of all herbal formulations are presented in Table 2. YTK had activities of 0.17 ± 0.00, YTP (0.20 ± 0.00), YB (2.73 ± 0.05), YPF (2.38 ± 0.03) and Trolox (0.07 ± 0.00). YTK and YTP showed similar ability to terminate ABTS radicals (P >0.05).
Ferric reducing antioxidant power (FRAP) assay
In this study, YTP showed highest activity 61.78 ± 0.39, YTK (39.53 ± 0.19), YPF (13.32 ± 0.41) and YB(11.99 ± 0.30) (P <0.05) (Table 2).
Total phenolic content (TPC)
In this study, YTP showed the highest TPC value of 26.2 ± 0.04 µg of gallic acid equivalent/g extract, closely followed by that of YTK having 24.3 ± 1.37 gallic acid equivalent/g extract (P <0.05) (Table 2).
Total flavonoid content (TFC)
In this study, result obtained indicated that YTK had the highest TFC of all the tested herbal formulations (P <0.05). This was followed by YTP, YPF and YB with the values of 5.71 ± 0.02, 1.88 ± 0.03 and 1.64 ± 0.14 catechin equivalent/ g extract respectively as shown in Table 2.
Table 2. Antioxidant activities and bioactive contents of herbal formulations.
|
Extracts |
Free radical scavenging assays |
FRAP FeSO4 equivalent (uM FeSO4/ mg sample |
TPC (µg of Gallic acid equivalent/g extract)
|
TFC (µg of Catechin equivalent/ g extract) |
|
|
|
DPPH |
ABTS |
|||
|
|
IC50 (mg/ml) |
IC50 (mg/ml) |
|||
|
YTK |
1.24 ± 0.02c |
0.17 ± 0.00b |
39.53 ± 0.19b |
24.3 ± 1.37b |
6.60 ± 0.10a |
|
YTP |
0.25 ± 0.00b |
0.20 ± 0.00b |
61.78 ± 0.39a |
26.2 ± 0.04a |
5.71 ± 0.02b |
|
YB |
2.02 ± 0.16e |
2.73 ± 0.05d |
11.99 ± 0.30d |
2.06 ± 0.10d |
1.88 ± 0.03c |
|
YPF |
1.73 ± 0.18d |
2.38 ± 0.03c |
13.32 ± 0.41c |
2.27 ± 0.00c |
1.64 ± 0.14d |
|
Trolox |
0.08 ± 0.00a |
0.07 ± 0.00a |
N/A |
N/A |
N/A |
Note: Ya-Tri-Phigut (YTP), Ya-Tri-Kasornmas (YTK), Ya-Plook-Fithat (YPF), and Ya-Benjakul (YB). Values in the same column with different superscripts are significantly different (P <0.05); Data given as mean ± standard deviation (S.D). N/A: not applicable.
In vitro antioxidant activities of individual components of selected herbal extracts
In vitro antioxidant activities of individual herbal components in YTP
Metal chelating activity (MCA)
In the present study, MCA ranged between 0.15 ± 0.00 - 0.32 ± 0.00 (Inhibitory activity, IC50; mg/ml) in all tested plant component of YTP (Table 3).
DPPH radical scavenging activity
For DPPH activity of YTP constituents, activity ranged between 0.05 ± 0.00 - 7.30 ± 1.01, IC50; mg/ml (Table 3).
ABTS radical scavenging activity
Activity ranged from 0.03 ± 0.00 - 1.54 ± 0.01 (IC50; mg/ml) in Table 3.
Ferrous reducing antioxidant power (FRAP)
FRAP activity of YTP measured are shown in Table 3. Activities ranged between 0.09 ± 0.00 – 62.10 ± 0.00 (µM FeSO4/g sample).
Total phenolic content (TPC)
TPC in YTP constituents ranged from 0.06 ± 0.00 - 82.58 ± 0.00 in mg Gallic acid/g sample as highlighted in Table 3.
Table 3. Antioxidant activities, total phenolic and flavonoid contents of YTP herbal components.
|
Sample |
Inhibitory activity (IC50; mg/ml) |
FRAP |
TPC (mg Gallic acid/g sample) |
TFC (mg Catechin/g sampl |
||
|
MCA |
DPPH |
ABTS |
||||
|
P. emblica |
0.32 ± 0.01 |
0.15 ± 0.00 |
0.03 ± 0.00 |
7.23 ± 0.00 |
8.40 ± 0.05 |
1.05 ± 0.03 |
|
P. nigrum |
0.29 ± 0.02 |
7.30 ± 1.01 |
0.92 ± 0.01 |
0.26 ± 0.00 |
0.45 ± 0.00 |
0.12 ± 0.00 |
|
P. pendulispicum |
0.18 ± 0.00 |
3.33 ± 0.06 |
0.74 ± 0.01 |
0.09 ± 0.00 |
0.06 ± 0.00 |
0.03 ± 0.00 |
|
P. retrofractum |
0.18 ± 0.01 |
6.46 ± 0.81 |
0.95 ± 0.02 |
0.14 ± 0.00 |
0.20 ± 0.02 |
0.05 ± 0.00 |
|
P. sarmentosum |
0.26 ± 0.00 |
1.94 ± 0.19 |
1.54 ± 0.01 |
0.72 ± 0.03 |
1.04 ± 0.03 |
0.23 ± 0.01 |
|
P. indica |
0.24 ± 0.01 |
2.92 ± 0.02 |
0.27 ± 0.00 |
0.52 ± 0.00 |
1.16 ± 0.01 |
0.23 ± 0.01 |
|
T. bellirica |
0.15 ± 0.00 |
0.05 ± 0.00 |
0.04 ± 0.00 |
62.10 ± 0.00 |
82.58 ± 0.00 |
10.04 ± 0.33 |
|
T. chebula |
0.32 ± 0.00 |
0.13 ± 0.00 |
0.11 ± 0.00 |
41.91 ± 0.00 |
54.15 ± 0.28 |
5.81 ± 0.16 |
|
Z. officinale |
0.17 ± 0.00 |
3.13 ± 0.02 |
0.53 ± 0.01 |
3.91 ± 0.00 |
2.19 ± 0.12 |
0.82 ± 0.17 |
Note: Data given as mean ± standard deviation (S.D). Values of individual components are compared along the column.
Total flavonoid content (TFC)
TFC of YTP constituents are between 0.03 ± 0.00 - 10.04 ± 0.33 mg Catechin/g sample.
In vitro antioxidant activities of individual herbal components in YTK
Metal chelating activity
The MCA of those plant components ranged between 0.19 ± 0.00 - 1.43 ± 0.00 (IC50; mg/ml) (Table 4).
DPPH radical scavenging activity
DPPH activity of YTK constituents measured are shown in Table 4. Activities are 0.51 ± 0.00, 0.67 ± 0.00 and 2.11 ± 0.02 (IC50; mg/ml) for A. marmelos, J. multifidi and N. nucifera, respectively.
ABTS radical scavenging activity
ABTS activities of YTK constituents are highlighted in Table 4. A. marmelos had 0.04± 0.00, J. multifidi (0.03 ± 0.00), and N. nucifera (0.19 ± 0.00) (IC50; mg/ml).
Ferrous reducing antioxidant power (FRAP) assay
FRAP activity ranged from 1.88 - 6.20 µM FeSO4/g sample as highlighted in Table 4.
Total phenolic content (TPC)
Values obtained for TPC of YKT constituent are between 1.49 ± 0.00 - 11.67 ± 0.04 (mg Gallic acid/g sample).
Total flavonoid content (TFC)
TFC observed in the constituent of YTK ranged between 0.57 ± 0.05 - 5.09 ± 0.05 mg Catechin/g sample (Table 4).
Table 4: Antioxidant activities, total phenolic and flavonoid contents of YTK herbal components.
|
Sample |
Inhibitory activity (IC50; mg/ml) |
FRAP |
TPC (mg Gallic acid/g sample) |
TFC (mg Catechin/g sampl |
||
|
MCA |
DPPH |
ABTS |
||||
|
A. marmelos |
0.19 ± 0.00 |
0.51 ± 0.00 |
0.04 ± 0.00 |
6.20 ± 0.0 |
11.67 ± 0.00 |
0.04 ± 0.00 |
|
J. multifidi |
0.22 ± 0.00 |
0.67 ± 0.00 |
0.03 ± 0.00 |
1.88 ± 0.00 |
3.39 ± 0.00 |
1.00 ± 0.00 |
|
N. nucifera |
1.43 ± 0.00 |
2.11 ± 0.02 |
0.19 ± 0.00 |
1.95 ± 0.00 |
1.49 ± 0.00 |
0.57 ± 0.05 |
Note: Caption: See Table 3
Growth effect of YTP extracts and standard samples on yeast cells
The growth curve of yeast treated with YTP extracts and standard samples (cryptotanshinone) are shown in Figure 1. Cryptotanshinone (CPT) was used as a standard and supplemented to the medium at different concentrations of 5, 2.5 and 1.25 μg/ml and compared with the extract of YTP (0.1 μg/ml) (Figure 1).
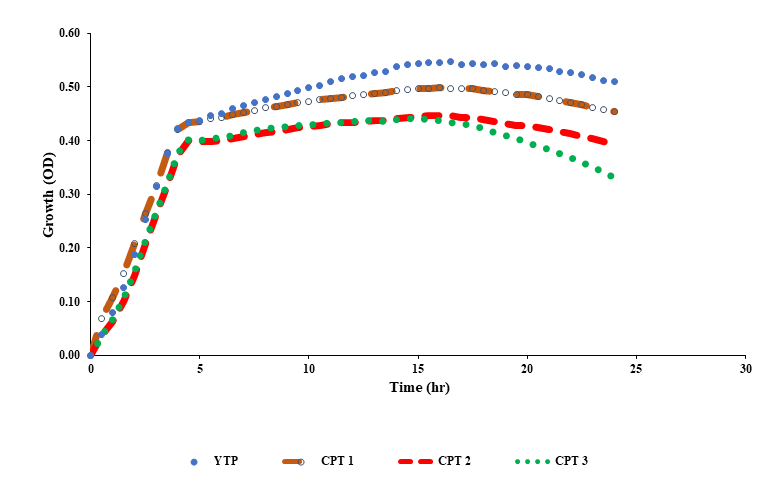
Figure 1. Growth curves of yeast treated with YTP extract and standard samples YTP: Ya-Tri-Phigut (0.1μg/ml), CPT: cryptotanshinone; CPT 1: (5μg/ml), CPT 2: (2.5μg/ml), CPT 3: (1.25μg/ml).
DISCUSSION
Various factors such as input and duration of the extraction process have been reported to influence extraction yield from medicinal plants as highlighted in Table 1. They include the type of solvent used, methods of extraction, temperature and time involved in the extraction processes (Tambunan et al., 2017; Terblanche et al., 2017). Higher yield observed in YTP might be due to its greater solubility of its bioactive compounds in aqueous solvent. Molecular constituents could also have mass kinetic transfer rate which is higher than those of other formulations. Dhanani et al. (2017) reported that strong solute-matrix interaction between molecules and those of solvent could enhance extraction yield. Thus, variation in extraction yield of herbal extracts were observed.
DPPH assay is usually used to investigate antioxidative properties of compounds as a hydrogen donor or free radical scavengers (Idowu et al., 2019). Different activities were obtained in all samples and standard (P <0.05) (Table 2). However, YTP showed the highest activity than other herbal formulations (P <0.05). Thus, indicating its ability to scavenged more free radicals than others. It should be noted that the lower the IC50 value, the stronger the antioxidant potential. High phenolic contents have been reported to influence antioxidant activities of herbal extracts (Chanthasri et al., 2018). Therefore, the phenolic contents of the individual extracts might have contributed synergistically to the overall activity of the polyherbal tea. Thus, higher activity of YTP than other herbal formulations was obtained.
YB showed the least ABTS activity of all the tested herbal formulations (P <0.05) (Table 2). Thus, less free radical were stabilized to retard the chain reaction. Low amount of phenolics in YB extracts could have resulted in less ABTS activity. Generally, ABTS assay is used to evaluate the capacity of antioxidants to donate a hydrogen atom or an electron to free radicals, thus a nonradical species is formed (Idowu et al., 2019). ABTS assay is widely used for both hydrophilic and lipophilic compounds, while DPPH assay is effectual for lipophilic compounds (Re et al., 1999).
FRAP indicates the ability of tested compound to reduce TPTZ-Fe (III) complex to TPTZ-Fe (II) complex by providing an electron to the free radicals (Idowu et al., 2019). The FRAP value of YTP (61.78 ± 0.39 µM FeSO4/mg of sample) was almost doubled that of YTK (39.53 ± 0.19 µM FeSO4/mg of sample) higher than those of YPF and YB in order of activities (P <0.05) (Table 2).
Phenolics compounds have been reported to be crucial for major portion of the antioxidant capacity in many plants (Yogesh et al., 2014). In the current study, YTP demonstrated the highest TPC activity of 26.2 ± 0.04 gallic acid equivalent/g extract while YB had the least activity of 2.06 ± 0.00 gallic acid equivalent/g extract (P <0.05) (Table 2).
Flavonoids are common secondary metabolites such as flavonols, condensed tannins and flavones (Ghasemi et al., 2014). In vitro studies have shown flavonoids from plant studies showed free radical scavenging activity and can protect against oxidative stress (Ghasemi et al., 2014). Differences in TFC of herbal extract could be attributed to complementary or antagonist activity of the herbal formulation constituents (Table 2). Based on the high antioxidant activity of YTP and YTK as reported in this study in Table 2, both herbal formulations were selected to investigate the antioxidant activity and bioactive contents of their individual plant component.
MCA activities of individual components of YTP herbal formulation studied are highlighted in Table 3. YTP is made up of P. emblica, P. nigrum, P. pendulispicum, P. retrofractum, P. sarmentosum, P. indica, T. bellirica, T. chebula and Z. officinale. Generally, MCA is used to study the ability of antioxidant compound to chelate prooxidative metals . Transition metals like iron and copper ions are necessary in the Fenton-like reaction which could generate hydroxyl radicals that may react with essential biomolecules (such as proteins, lipids and nucleic acids) resulting in the damage of such biomolecules (Apak et al., 2016). T. bellirica showed the highest MCA inhibitory activity of 0.15 ± 0.00 (IC50; mg/ml). Similar MCA of 0.18 ± 0.00 (Inhibitory activity, IC50; mg/ml) each were observed in P. pendulispicum and P. retrofractum, activity. In P. emblica and T. chebula also showed similar activity of 0.32 ± 0.01 (IC50; mg/ml).
The highest DPPH inhibitory activity was observed in T. bellirica (0.05 ± 0.00, IC50; mg/ml), followed by T. chebula (0.13 ± 0.00, IC50; mg/ml) and P. emblica (0.15 ± 0.00, IC50; mg/ml) respectively, while P. nigrum showed the least inhibitory activity of 7.30 ± 1.01 (IC50; mg/ml) (Table 3). This indicated that T. bellirica showed higher potency to donate hydrogen during the reaction with DPPH, in order to scavenged the radicals in a lipophilic system.
ABTS activity of YTP constituents are reported in Table 3. P. emblica showed the highest inhibitory activity of 0.03 ± 0.00 (IC50; mg/ml), while P. sarmentosum showed the least inhibitory activity of 1.54 ± 0.01 (IC50; mg/ml). It can be deduced that P. emblica is a powerful antioxidant that can scavenge ABTS radical.
The FRAP result indicated that T. bellirica demonstrated the highest activity of 62.10 ± 0.00 µM FeSO4/g sample followed by T. chebula with activity of 41.91 ± 0.00 µM FeSO4/g sample. FRAP activity of T. bellirica reported was more than 8 times that of P. emblica (7.23 ± 0.00 µM FeSO4/g sample) (Table 3).
Highest TPC was observed in T. bellirica (82.58 ± 0.00 mg Gallic acid/g sample), while P. pendulispicum had the lowest TPC of 0.06 ± 0.00 mg Gallic acid/g sample (Table 3). High TPC of plant constituent have been reported to influenced their antioxidant activity (Škerget et al., 2005). T. bellirica have been reported for its notable therapeutic potential such as antioxidant activities (Deb et al., 2016). Thus, high phenolic profile of T. bellirica correlated well with its high antioxidant activity as reported in MCA, DPPH, ABTS and FRAP assays in Table 3.
T. bellirica showed the highest TFC of 10.04 ± 0.33 mg Catechin/g sample while P. pendulispicum had the least TFC of 0.03 mg Catechin/g sample. Overall, the choice of solvent used as extraction medium could have affected the antioxidant activities of all constituents studied. Of all YTP constituents, T. bellirica demonstrated the highest antioxidant activity. It can be inferred that T. bellirica possibly influenced the high antioxidant activity of YTP over other herbal formulation.
Antioxidant activities of individual YTK herbal formulation are shown in Table 4. YTK is made up plant components such as A. marmelos, J. multifidi and N. nucifera. The highest MCA was observed in A. marmelos (0.19 ± 0.00, inhibitory activity, IC50; mg/ml) while N. nucifera had the lowest MCA (1.43 ± 0.00 Inhibitory activity, IC50; mg/ml).
For DPPH of YTK constituent, the highest activity was observed in A. marmelos (0.51 ± 0.00, Inhibitory activity, IC50; mg/ml), while N. nucifera (2.11 ± 0.02, Inhibitory activity, IC50; mg/ml) showed the least activity.
For ABTS radical scavenging activity of YTK constituent, J. multifidi exhibited the highest activity of 0.03 ± 0.00 (Inhibitory activity, IC50; mg/ml), closely followed by A. marmelos (0.04 ± 0.00, Inhibitory activity, IC50; mg/ml), while N. nucifera had the least inhibitory activity of 0.19 ± 0.00 (IC50; mg/ml) (Table 4).
For FRAP, A. marmelos showed the highest activity (6.20 ± 0.00, µM FeSO4/g sample), while J. multifidi showed the least activity of 1.88 ± 0.00 µM FeSO4/g sample.
TPC of YTK constituent, A. marmelos had the highest TPC of 11.67 ± 0.04 mg Gallic acid/g sample. The high phenolic contents of A. marmelos corresponded well with its increased MCA, DPPH, ABTS and FRAP reported (Table 4). Low TPC of J. multifidi observed could have influenced its low antioxidant activity reported.
In correlation to the result obtained in TPC, A. marmelos demonstrated the highest TFC of 5.09 mg Catechin/g sample (Table 4). This further strengthen their potency to function as a good antioxidant. Rahman and Parvin (2014) reported the functionality of A. marmelos aqueous extract as a good therapeutic agent. In general, A. marmelos exhibited the highest antioxidant activity of all YTK constituent. In addition, between both extracts studied, YTP showed better antioxidant characteristics than YTK as reported in table 3 and 4. Thus, it was selected for cytotoxicity study in a yeast (Saccharomyces cerevisiae) model.
Cryptotanshinone is a major active component extracted from the dried root of Salvia miltiorrhiz otherwise known as Danshen, a Chinese traditional herbal medicine notable for the treatment of metabolic disease syndromes (Pan et al., 2008). It has been reported that a lot of phytochemicals which are beneficial at low concentrations could be toxic at increased levels (Wiegant et al., 2009). This resulted to the selection of low concentration of 0.1 μg/ml for YTP extracts coupled with the initial preliminary studies. No toxicity was observed as indicated by the gradual growth in cell with supplementation of CPT and YTP (Figure 1). However, CPT 1 showed a slightly higher cell growth than YTP extracts from initiation time of 0-3 hr, but maintained similar progression afterwards to 5 hr. It could be that some metabolized nutrients such as protein in culture medium are slowly released. Protein concentration in culture medium may be used as a marker of yeast cell viability (Bayliak and Lushchak, 2011). Also, YTP extracts could have acted as a mild stressor which resulted to the effect observed. After 5 hr, slow progression in cell growth was observed for all standard samples up until 15 hr, while for YTP extract cell growth observed was slightly higher indicating higher yeast viability in cultures supplemented with YTP extracts. No growth was observed from 15- 20 hr for standard samples while YTP extract was constant. Antioxidant enzymes such as catalase and superoxide dismutase (SOD) are key antioxidant enzymes in yeast stationary phase and its isoenzymes are required for long-term yeast survival (Bayliak and Lushchak, 2011). SOD, in particular play a sensitive role in protection of Saccharomyces cerevisiae cell against oxidative damage from superoxide anion and other reactive oxygen species (ROS) (Longo et al., 1996; Fabrizio et al., 2003a). The production of ROS has been reported to increase during prolonged incubation of stationary cultures of S. cerevisiae (Jakubowski et al., 2000). Reactive oxygen species (ROS) generated in respiratory metabolism have been proposed to trigger oxidative damage of cellular components coupled with decline of antioxidant defense are responsible for yeast cell aging, decline and death (Jakubowski et al., 2000; Costa and Moradas-Ferreira, 2001). Thus, after 20 hr, a decline in cell growth was observed in CPT (standard samples) and in YTP extract. Loss in yeast cell viability or reproduction have also been linked to SOD attack from ROS produced during respiratory metabolism of yeast cell (Costa and Moradas-Ferreira, 2001; Bayliak and Lushchak, 2011). In our study, the decrease in yeast cell viability could be as a result of decrease in SOD activity during long-term incubation which promoted oxidative damage. Alternatively decrease in protein turnover or protein synthesis in the stationary cultures could also be responsible for decline in cell growth as specified by Gray et al. (2004). Inactivation of mitochondrial aconitase which is the primary target of superoxide have also been confirmed to be responsible for aging, decline in reproducibility and viability of yeast cell (Fabrizio and Longo, 2003b). Apart from the influence of the extracts, cell defense mechanisms, such as the intensity of the heat shock proteins which occur likely to worms, or inhibited the depletion of glutathione, a low-molecular mass antioxidant, that perform a key function in yeast survival at stationary phase (Stephen and Jamieson, 1996) Also, at the stationary phase, increase in activity of antioxidant enzymes in both YTP and CPT extracts treated yeast might not be necessary. Rather, the weakness of defense mechanisms and agglomeration of damaged molecules at late stages of cultivation, as detailed above, could have led to decrease in yeast cell growth. Overall, this study confirmed that YTP extracts at low concentration supported cell growth than the known CPT extracts at higher concentration. It could be inferred that YTP extract showed a favourable effect on yeast cell growth than cryptotanshinone. Thus, YTP extracts at low concentration could support cell growth than cryptotanshinone.
CONCLUSION
The herbal formulations investigated such as Ya-Benjakul (YB), Ya-Plook-Fithat (YPF), Ya-Tri-Kasornmas (YTK) and Ya-Tri-Phigut (YTP) showed antioxidant activities. However, Ya-Tri-Phigut was preferred for its good antioxidant activities. In addition, Ya-Tri-Phigut improved cell growth in Saccharomyces cerevisiae model better than standard rejuvenating compound cryptotanshinone. A main active component in Danshen of Chinese herbal remedy made from the dried root of Salvia miltiorrhiz. Since oxidative stress is known as a promoter of aging and age-related diseases, YTP with good antioxidant properties and ability to promote cell growth can serve as good nutraceutical agent to combat oxidative stress as well as aging and age-related diseases.
CONFLICT OF INTEREST
The authors at this moment declared no competing interest.
REFERENCES
Abbasi, A.M., Shah, M.H., Li, T., Fu, X., Guo, X., and Liu, R.H. 2015. Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. Journal of Ethnopharmacology. 162: 333-345.
Amarya, S., Singh, K., and Sabharwal, M., 2015. Changes during aging and their association with malnutrition. Journal of Clinical Gerontology Geriatrics. 6: 78-84.
Amir, M., Khan, A., Mujeeb, M., Ahmad, M.A., and Siddiqui, N.A., 2011. Phytochemical screening and in vitro antioxidant activity of Jawarish Amla-A poly herbal formulation. Pharmacognosy Journal. 3: 54-60.
Apak, R., Özyürek, M., Güçlü, K., and Çapanoğlu, E., 2016. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. Journal of Agriculture and Food Chemistry. 64: 997-1027.
Bayliak, M.M., and Lushchak, V.I., 2011. The golden root, Rhodiola rosea, prolongs lifespan but decreases oxidative stress resistance in yeast Saccharomyces cerevisiae. Phytomedicine. 18: 1262-1268.
Bitterman, K.J., Medvedik, O., and Sinclair, D.A., 2003. Longevity regulation in Saccharomyces cerevisiae: linking metabolism, genome stability, and heterochromatin. Microbiology and Molecular Biology Revision. 67: 376-399.
Booranasubkajorn, S., Huabprasert, S., Wattanarangsan, J., Chotitham, P., Jutasompakorn, P., Laohapand, T., Akarasereenont, P., and Tripatara, P., 2017. Vasculoprotective and vasodilatation effects of herbal formula (Sahatsatara) and piperine in spontaneously hypertensive rats. Phytomedicine. 24: 148-156.
Chanthasri, W., Puangkeaw, N., Kunworarath, N., Jaisamut, P., Limsuwan, S., Maneenoon, K., Choochana, P., and Chusri, S., 2018. Antioxidant capacities and total phenolic contents of 20 polyherbal remedies used as tonics by folk healers in Phatthalung and Songkhla provinces, Thailand. BMC Complementary and Alternative Medicine. 18: 73.
Chewchinda, S., Lomarat, P., and Sithisarn, P., 2018. Validated thin-layer chromatography-densitometric method for simultaneous determination of piperine and plumbagin in “Benjakul” Thai Polyherbal formulation and its antioxidant activities. Thai Journal of. Pharmacetical Science (TJPS). 42:23-29
Costa, V., and Moradas-Ferreira, P., 2001. Oxidative stress and signal transduction in Saccharomyces cerevisiae: insights into ageing, apoptosis and diseases. Molecular Aspects of Medicine. 22: 217-246.
De-Guzman, C.C., and Siemonsma, J., 1999. Plant resources of South-East Asia no 13: spices, Backhuys Publishers.
Deb, A., Barua, S., and Das, B., 2016. Pharmacological activities of Baheda (Terminalia bellerica): a review. Journal of Pharmacognosy and Phytochemistry. 5: 194-199.
Dhanani, T., Shah, S., Gajbhiye, N., and Kumar, S. 2017. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arabian Journal of Chemistry. 10: S1193-S1199.
Fabrizio, P., Liou, L.-L., Moy, V.N., Diaspro, A., Valentine, J.S., Gralla, E.B., and Longo, V.D., 2003a. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics.163: 35-46.
Fabrizio, P., and Longo, V.D. 2003b. The chronological life span of Saccharomyces cerevisiae. Aging cell. 2: 73-81.
Fruhmann, G., Seynnaeve, D., Zheng, J., Ven, K., Molenberghs, S., Wilms, T., Liu, B., Winderickx, J., and Franssens, V., 2017. Yeast buddies helping to unravel the complexity of neurodegenerative disorders. Mechanical Ageing and Development. 161: 288-305.
Garcia-Alvarez, A., Egan, B., De Klein, S., Dima, L., Maggi, F.M., Isoniemi, M., Ribas-Barba, L., Raats, M.M., Meissner, E.M., and Badea, M. 2014. Usage of plant food supplements across six European countries: findings from the PlantLIBRA consumer survey. PloS one. 9.
Ghasemi, P.A., Siahpoosh, A., Setayesh, M., and Craker, L., 2014. Antioxidant activity,
total phenolic and flavonoid contents of some medicinal and aromatic plants used as herbal teas and condiments in Iranian. Journal of Medicinal Food. 17: 1151-1157.
Gietz, R.D., and Schiestl, R.H., 2007. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Natural Products. 2, 31-34.
Gray, J.V., Petsko, G.A., Johnston, G.C., Ringe, D., Singer, R.A., and Werner-Washburne, M. 2004. ‘Sleeping beauty’: quiescence in Saccharomyces cerevisiae. Microbiology and Molecular Biology Revision. 68: 187-206.
Halberstein, R.A. 2005. Medicinal plants: historical and cross-cultural usage patterns. Annals of Epidemiology. 15: 686-699.
Heydari, S., Siavoshi, F., Ebrahimi, H., Sarrafnejad, A., and Sharifi, A.H., 2020. Excision of endosymbiotic bacteria from yeast under aging and starvation stresses. Infection and Genetics Evolution. 78: 104141.
Idowu, A.T., Benjakul, S., Sinthusamran, S., Sookchoo, P., and Kishimura, H., 2019. Protein hydrolysate from salmon frames: Production, characteristics and antioxidative activity. Journal of Food Biochemistry. 43: e12734.
Jakubowski, W., Biliński, T., and Bartosz, G., 2000. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radical Biology Medicine. 28: 659-664.
Ji, H.-J., Wang, D.-M., Wu, Y.-P., Niu, Y.-Y., Jia, L.-L., Liu, B.-W., Feng, Q.-J., and Feng, M.-L. 2016. Wuzi Yanzong pill, a Chinese polyherbal formula, alleviates testicular damage in mice induced by ionizing radiation. BMC Complementary and Alternative Medicine. 16: 509.
Lobo, V., Patil, A., Phatak, A., and Chandra, N. 2010. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Revision. 4: 118.
Longo, V.D., Gralla, E.B., and Valentine, J.S., 1996. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae Mitochondrial production of toxic oxygen species in vivo. Journal of Biology and Chemistry. 271: 12275-12280.
Migliore, L., Coppedè, F., 2009. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Muta. Research /Genetic Toxicology and Environmental Mutage. 674: 73-84.
Mohammad, Y., Al-Ahmari, A., Al-Dashash, F., Al-Hussain, F., Al-Masnour, F., Masoud, F., and Jradi, H., 2015. Pattern of traditional medicine use by adult Saudi patients with neurological disorders. BMC Complementary and Alternative Medicine. 15: 102.
Neff, F., Flores-Dominguez, D., Ryan, D.P., Horsch, M., Schröder, S., Adler, T., Afonso, L.C., Aguilar-Pimentel, J.A., Becker, L., Garrett, L., 2013. Rapamycin extends murine lifespan but has limited effects on aging. The Journal of Clinical Investigation. 123: 3272-3291.
Olmedo, M., Mata‐Cabana, A., Jesús, M., Rodríguez‐Palero, S., García‐Sánchez, A., Fernández‐Yañez, M., and Artal‐Sanz, M., 2020. Prolonged quiescence delays somatic stem cell‐like divisions in Caenorhabditis elegans and is controlled by insulin signaling. Aging cell. 19: e13085.
Onyeka, T.C., Ezike, H.A., Nwoke, O.M., Onyia, E.A., Onuorah, E.C., Anya, S.U., and Nnacheta, T.E., 2012. Herbal medicine: a survey of use in Nigerian presurgical patients booked for ambulatory anaesthesia. BMC Complementary and Alternative Medicine. 12: 130.
Pan, Y., Bi, H.-C., Zhong, G.-P., Chen, X., Zuo, Z., Zhao, L.-Z., Gu, L.-Q., Liu, P.-Q., Huang, Z.-Y., Zhou, and S.-F., 2008. Pharmacokinetic characterization of hydroxylpropyl-β-cyclodextrin-included complex of cryptotanshinone, an investigational cardiovascular drug purified from Danshen (Salvia miltiorrhiza). Xenobiotics. 38: 382-398.
Petrovska, B.B., 2012. Historical review of medicinal plants’ usage. Pharmacognosy Reviews. 6: 1.
Powers, R.W., Kaeberlein, M., Caldwell, S.D., Kennedy, B.K. and Fields, S., 2006. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Development. 20: 174-184.
Qiao, Y., Zhang, F., Wu, C., Qin, X., and Zhang, J., 2019. Advances in three important signaling pathways related to aging in Drosophila melanogaster and screening of anti-aging traditional Chinese medicine." Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China Journal of Chinese Materia Medica. 44: 4412.
Rahman, S., Parvin, R., 2014. Therapeutic potential of Aegle marmelos (L.)-An overview. Asian Pacific Journal of Tropical Diseases. 4: 71-77.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C., 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology Medicine. 26: 1231-1237.
Rizzo, C., Caruso, C., and Vasto, S., 2014. Possible role of ABO system in age-related diseases and longevity: a narrative review. Immunology and Ageing. 11: 16.
Škerget, M., Kotnik, P., Hadolin, M., Hraš, A., Simonič, M., Knez, Z., 2005. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chemistry. 89: 191-198.
Soner, B.C., Sahin, A.S., and Sahin, T.K., 2013. A survey of Turkish hospital patients’ use of herbal medicine. European Journal of Integrated Medicine. 5: 547-552.
Stephen, D.W., and Jamieson, D.J., 1996. Glutathione is an important antioxidant molecule in the yeast Saccharomyces cerevisiae. FEMS Microbiology Letters. 141: 207-212.
Tambunan, A.P., Bahtiar, A., and Tjandrawinata, R.R., 2017. Influence of extraction parameters on the yield, phytochemical, TLC-densitometric quantification of quercetin, and LC-MS profile, and how to standardize different batches for long term from Ageratum conyoides L. leaves. Pharmacognosy Journal. 9.
Tangkiatkumjai, M., Boardman, H., Praditpornsilpa, K., and Walker, D.M., 2013. "revalence of herbal and dietary supplement usage in Thai outpatients with chronic kidney disease: a cross-sectional survey. BMC Complementary and Alternative Medicine. 13: 153.
Terblanche, U., Semakalu, C., Mtunzi, F., and Pillay, M., 2017. Screening of variables influencing extraction yield of Cotyledon orbiculata: 23 full factorial design. Internat. Journ. Pharmacognosy and Phytochemical Research. 9: 303-312.
Wiegant, F., Surinova, S., Ytsma, E., Langelaar-Makkinje, Wikman, E., Post, J., 2009. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 10: 27-42.
Wong, F.-C., Yong, A.-L., Ting, E.P.-S., Khoo, S.-C., Ong, H.-C., Chai, T.-T., 2014. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iranian Journal of Pharmaceutical Research (IJPR). 13(4): 1409.
Yang, C., Bolotin, E., Jiang, T., Sladek, F.M. and Martinez, E., 2007. Prevalence of the initiator over the TATA box in human and yeast genes and identification of DNA motifs enriched in human TATA-less core promoters. Genetics. 389: 52-65.
Yogesh, K., Jha, S., Ahmad, T., 2014. Antioxidant potential of aqueous extract of some food grain powder in meat model system. Journal of Food Science and Technology. 51: 3446-3451.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Ademola Ezekiel Adekoya1,*, Sasitorn Chusri1, Eugene Ong Boon Beng2, and Anthony Temitope Idown3
1 Department of Thai Traditional Medicine, Prince of Songkla University, Hat Yai, Songkhla 90112, Thailand
2 Institute for Research in Molecular Medicine, Universiti Sains Malaysia, Malaysia
3 Department of Food Science and Technology, Faculty of Agro-Industry, Prince of Songkla University, Hat Yai, Songkhla 90112, Thailand
Corresponding author: Ademola Ezekiel Adekoya, E-mail: ademolaezekieladekoya@yahoo.com
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: July 4, 2020;
Revised: August 31, 2020;
Accepted: September 1, 2020

