
Hepatoprotective Activity of Bergenin against Xenobiotics-Induced Oxidative Stress in Human Hepatoma (HepG2) Cells
Yollada Sriset, Waranya Chatuphonprasert, and Kanokwan Jarukamjorn*Published Date : 2020-12-17
DOI : https://doi.org/10.12982/CMUJNS.2021.011
Journal Issues : Number 1, January-March 2021
Abstract Bergenin, a natural derivative of gallic acid, has been shown to exert anti-oxidant and anti-inflammatory activities. This study aimed to determine hepatoprotective activity of bergenin against ethanol and tert-butyl hydroperoxide (TBHP) induced oxidative stress in human hepatoma (HepG2) cells. HepG2 cells (5x105 cells/well) were co-treated with ethanol (100 mM) or TBHP (100 µM) and either bergenin (75, 150, and 300 µM) or gallic acid (60 µM, positive control) for 24 h. Cell viability, hematoxylin and eosin staining of cell morphology, cellular injury biomarkers: lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and malondialdehyde (MDA), and antioxidant biomarkers: superoxide dismutase (SOD), catalase (CAT), and total glutathione (GSH) content, were determined. Ethanol and TBHP decreased cell viability (86.67% and 84.49% of control, respectively), increased LDH toxicity (12.53% and 15.91% of control, respectively), and increased AST, ALT, and MDA levels, compared with the control. Based on cell morphology, both ethanol and TBHP injured HepG2 cells causing the loss of cell nuclei. Treatment of HepG2 cells with either ethanol or TBHP reduced SOD and CAT activities and depleted total GSH content, compared with the control. Bergenin and gallic acid improved the cell morphology, elevated cell viability (95.94-99.20% and 97.72-99.62% of control, respectively), lowered LDH toxicity (8.14-9.10% and 7.82-8.92% of control, respectively), restored AST, ALT, and MDA levels, promoted SOD and CAT activities, and enhanced the total GSH content of ethanol- and TBHP-treated HepG2 cells. Bergenin exhibited hepatoprotective activity via restoration of the oxidant-antioxidant system and is a potential candidate for hepatoprotective treatment.
Keywords: Antioxidant biomarkers, Cellular injury biomarkers, Ethanol, Glutathione, Tert-butyl hydroperoxide
Funding: This work was supported by Research Group for Pharmaceutical Activities of Natural Products using Pharmaceutical Biotechnology (PANPB2563), Faculty of Pharmaceutical Sciences, Khon Kaen University, Thailand.
Citation: Sriset, Y., Chatuphonprasert, W., and Jarukamjorn, K. 2021. Hepatoprotective activity of bergenin against xenobiotics-induced oxidative stress in human hepatoma (HepG2) cells. CMUJ. Nat. Sci. 20(1): e2021011.
INTRODUCTION
The liver is a major organ for metabolism, detoxification, and regulation of physiological homeostasis (Polson and Lee, 2005). The liver is easily damaged by xenobiotics because it is the primary organ for their biotransformation, which can subsequently produce excessive free radicals, especially reactive oxygen species (ROS) (Birben et al., 2012). Ethanol is a xenobiotic-related to lifestyle that is mostly found in beverages and diet, but is also present in household and industrial products (Zakhari, 2013). Tert-butyl hydroperoxide (TBHP) is a powerful oxidizing agent used as a bleach and an initiator of polymerization in industrial processes and it is typically related to environmental pro-oxidant pollutant (Liu et al., 2002). Ethanol and TBHP present a high potential risk to the liver due to the creation of ROS by-products from hepatic metabolism (Liu et al., 2002; Zakhari, 2013). This phenomenon can result in an imbalance of the oxidant-antioxidant system, leading to oxidative stress-induced liver injury, and subsequent damage to biological structures through liver malfunction (Cichoż-Lach and Michalak, 2014). Hence, oxidative stress is closely associated with the pathogenesis of liver diseases. Augmentation of hepatic antioxidant activity has been proposed as an important strategy to protect the liver against oxidative stress (Li et al., 2015).
Polyphenols are known to provide health-improving benefits due to their strong antioxidant effects (Li et al., 2015). Bergenin (C-glucoside of polyphenol 4-O-methylgallic acid, Figure 1A), a natural derivative of gallic acid, is an isocoumarin found in Mallotus japonicus, M. repandus, M. philippinensis, Caesalpinia digyna, Ardisia colorata, Bergenia ciliata, and B. ligulata (Bajracharya, 2015). Bergenin has been claimed to possess antioxidant (Nazir et al., 2011), hepatoprotective (Ambika and Saravanan, 2016), nephroprotective (Aggarwal et al., 2016), antidiabetic (Kumar et al., 2012a), anti-inflammatory (Gao et al., 2015), and gastroprotective properties (Zhang et al., 2003). Previously, extracts of M. philippinensis and M. repandus have been shown to exhibit hepatoprotective activity in ethanol- and D-galactosamine-induced hepatotoxicity in rats (Geetha et al., 2011; Mondal et al., 2020) and both are good sources of bergenin; M. repandus contained bergenin 19% dry weight (Sriset et al., 2018) and M. philippinensis reported 6% dry weight (Haribabu et al., 2012). However, there are few studies examining the hepatoprotective effects of bergenin, and there is lack of evidence establishing the hepatoprotective mechanism of bergenin in the human hepatic system. In the present study, the human hepatoma (HepG2) cell line was employed as an in vitro model to investigate the mechanism of bergenin activity against xenobiotics-induced hepatotoxicity. HepG2 cells are considered to be similar to the human hepatic system since they possess several specialized functions of normal human hepatocytes such as xenobiotic metabolism and glutathione (GSH) synthesis (Scharf et al., 2003). As gallic acid (Figure 1B) has shown potential against hydrogen peroxide (H2O2), TBHP, and carbon tetrachloride-induced oxidative stress in HepG2 cells, mice, and rats (Choi et al., 2015; Jadon et al., 2007; Kong et al., 2016), it was chosen as a positive control.
The present study, therefore, aimed to evaluate the hepatoprotective mechanism of bergenin by examining its effects on cell morphology, cellular injury, oxidative stress biomarkers, and the antioxidative status of HepG2 cells subjected to ethanol- and TBHP-induced oxidative stress.
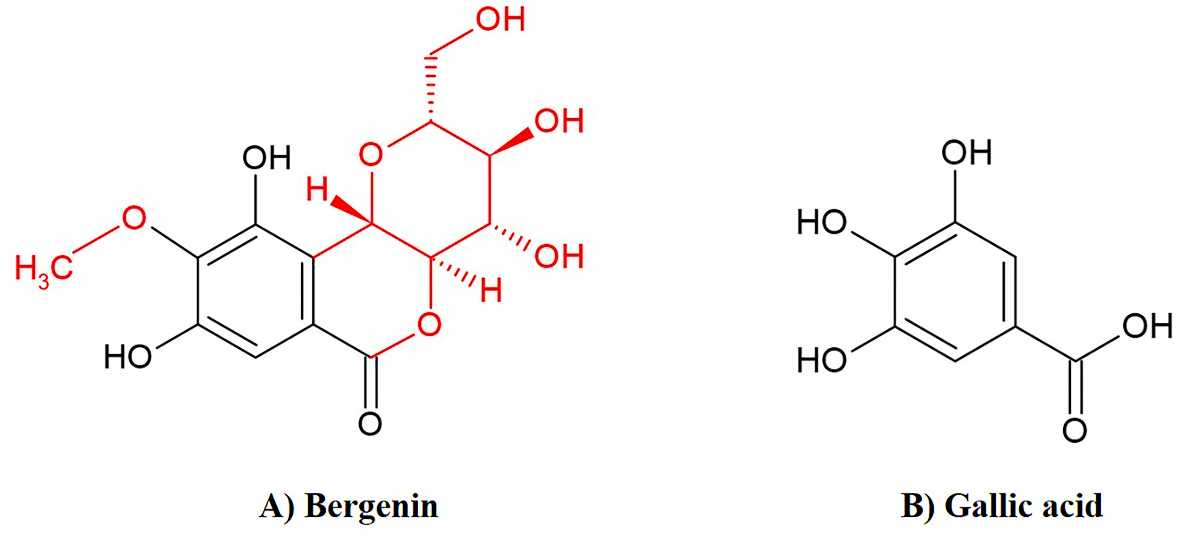
Figure 1. Chemical structure of A) bergenin and B) gallic acid.
MATERIALS AND METHODS
Materials
Bergenin (Cat. No. BP0258, purity > 98%) was a product of Biopurify Phytochemicals (Chengdu, China). Absolute ethanol (≥ 99.9% v/v) and gallic acid were obtained from Merck (Darmstadt, Germany). Catalase (CAT), 2,4-dinitrophenylhydrazine (DNPH), 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB), glutathione reductase, L-glutathione reduced, β-nicotinamide adenine dinucleotide 2′-phosphate (NADPH), resazurin sodium salt, superoxide dismutase (SOD), and tert-butyl hydroperoxide (TBHP, 70% w/v) were products of Sigma-Aldrich Chemical (St. Louis, Missouri, USA). α-Ketoglutarate, L-alanine, L-aspartate, malondialdehyde (MDA), and thiobarbituric acid (TBA) were purchased from Sigma-Aldrich Chemical. Aqueous solutions of 1% Eosin Y and Mayer’s hematoxylin were the products of Bio Optica (Milan, Italy). Dulbecco’s modified Eagle medium (DMEM, Cat. No. 11885-084), Dulbecco’s modified Eagle medium nutrient mixture F-12 (DMEM/F-12 without phenol red, Cat. No. 21041-025), and fetal bovine serum (FBS) were products of Gibco® (New York, USA). All other laboratory chemicals were of the highest purity and obtained from chemical suppliers.
Cell culture and treatment protocol
HepG2 cells (ATCC® HB-8065TM, Lot No. 61983117) were purchased from the American Type Culture Collection (Manassas, Virginia, USA). The cells were cultured in DMEM supplemented with 10% v/v FBS, 1× Glutamax®, and 1× penicillin-streptomycin-neomycin antibiotic mixture under 95% humidity in an atmosphere of 5% CO2 at 37°C.
The cells were seeded into 6-well plates (5×105 cells/well) in DMEM. After 48 h, the medium was replaced with DMEM/F-12 without phenol-red (supplemented with 10% FBS, 1×Glutamax®, and antibiotic mixture) containing ethanol (100 mM) or TBHP (100 µM) and either bergenin (75, 150, and 300 µM) or gallic acid (60 µM; positive control). Stock solutions of bergenin and gallic acid were prepared in 0.2% dimethyl sulfoxide (DMSO). Control was incubated with DMEM/F-12 without phenol-red containing 0.2% DMSO. After 24 h, %cell viability was determined by resazurin assay and the medium was kept to measure %lactate dehydrogenase (LDH) toxicity, and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. Cell morphology was examined using hematoxylin and eosin (H & E) staining. The cells were harvested in 1×phosphate buffered saline (PBS) by a cell scraper to determine SOD and CAT activities, total glutathione (total GSH), and MDA contents.
Assessment of cell viability
The percentage cell viability was fluorometrically measured by resazurin assay based on the ability of cellular enzymes to reduce resazurin (blue and non-fluorescent dye) to resorufin, a pink fluorescent compound (Miret et al., 2006). Briefly, 1 mM resazurin solution was added to culture medium (1:10) before incubation in 5%CO2 at 37°C for 1 h. The percentage cell viability was calculated from the amount of resorufin, which was measured at 530 nm excitation and 580 nm emission (Chatuphonprasert et al., 2020).
Assessment of LDH toxicity
The percentage LDH toxicity was assessed by determining LDH activity in culture medium using an LDH assay kit (Roche®, Mannheim, Germany). This assay measures activity of LDH released into culture medium upon a loss of cell membrane integrity. The percentage of HepG2 cell toxicity was calculated by comparison with a standard curve of Triton X-100 (6.25-50% toxicity) (Sriset et al., 2019).
Determination of AST and ALT levels
Culture medium was incubated with either AST substrate (10 mM L-aspartate and 1.7 mM α-ketoglutarate) or ALT substrate (300 mM L-alanine and 0.7 mM α-ketoglutarate) at 37°C for 30 or 20 min, respectively. Then DNPH was added to a final concentration of 0.5 mM and left for 20 min, followed by addition of sodium hydroxide to a final concentration of 1 M. Absorbance was measured at 505 nm. The levels of AST and ALT were determined as international units per liter (IU/L) by comparison with a standard curve of sodium pyruvate (100-500 µM) (Sriset et al., 2019).
Examination of HepG2 cell morphology by H & E staining
Culture medium was removed, and cells were washed with 0.01 M phosphate buffered saline (PBS) 3 times. The cells were immersed in 10% v/v neutral-buffered formalin for 30 min, followed by 60% v/v isopropanol for 1 min. Then the cells were stained with hematoxylin for 3 min and eosin for 30 sec. Morphological features of HepG2 hepatocytes were examined at 200-fold magnification using an inverted microscope (AE2000; Motic Incorporation, Ltd., Causeway Bay, Hong Kong). Images were recorded with the Moticam 5.0MP digital camera coupled with the Motic images plus 3.0 software (Motic Incorporation, Ltd.) (Qin et al., 2010).
Determination of protein content
HepG2 cells were lysed in 0.01 M phosphate buffered saline with a hand homogenizer in an ice-bath. Protein content of HepG2 cell lysate was quantified using the Bradford method with a few modifications (Chatuphonprasert et al., 2013). Briefly, 40 µL of the diluted HepG2 cell lysate was mixed with 160 µL of the Bradford reagent (Bio-Rad, Hercules, CA, USA). The measurement of protein-dye complexes at a wavelength of 595 nm was performed and protein content was calculated by comparison with a standard curve of bovine serum albumin (BSA; 12.5-150 µg/mL).
Assessment of SOD activity
SOD activity was evaluated based on the inhibition of formation of nitroblue tetrazolium (NBT) products (formazan), as described previously (Sriset et al., 2019). In brief, 50 µL of HepG2 cell lysate was mixed with chloroform and ethanol (1:1.67) and subsequently centrifuged at 14,000×g at 4°C for 30 min. A reaction mixture of 1.1 mM xanthine, 0.1 mM ethylenediamine tetraacetic acid, 0.6 mM NBT, 56 mM sodium carbonate, and 70 µg/mL BSA was mixed with the supernatant before incubation with 20 µUnits/µL xanthine oxidase at room temperature for 20 min, followed by addition of 0.1 mM copper (II) chloride to stop the reaction. Formazan product was measured at 550 nm and SOD activity was calculated by comparison with the SOD standard (40 mUnits/µL).
Assessment of CAT activity
CAT activity was assessed by the colorimetric method using H2O2 as the substrate. HepG2 cell lysate was incubated with 150 µM H2O2 at 37°C for 1 min. After incubation, ammonium molybdate was added to a final concentration of 26 mM, followed by measurement at 405 nm. CAT activity was calculated by comparison with the CAT standard (2.5 Units/µL) (Sriset et al., 2019).
Assessment of total glutathione (GSH) content
HepG2 cell lysate was extracted with 5% w/v sulfosalicylic acid (1:5) and centrifuged at 10,000×g at 4°C for 10 min to obtain supernatant. A freshly prepared reaction mixture of 7 mM potassium phosphate buffer (pH 7.0), 0.04 Units/mL glutathione reductase, and 30 µg/mL DTNB was mixed with the supernatant, followed by addition of 40 µg/mL NADPH to start the reaction. Absorbance was measured at 405 nm every 30 sec for 10 min. Total GSH content was calculated by comparison with a slope of GSH standard curve (6.25-50 µM) (Sriset et al., 2019).
Assessment of lipid peroxidation by thiobarbituric acid reactive substances (TBARS) assay
TBARS assay was performed as described previously (Sriset et al., 2019). In brief, HepG2 cell lysate was mixed with 10% w/v trichloroacetic acid (1:1) and subjected to centrifugation at 2,300×g at 4°C for 10 min. Supernatant was transferred to a new tube and mixed with an equal volume of 0.8% w/v TBA, before boiling at 100°C for 15 min, followed by immediate cooling in an ice-bath. TBARS formed by the reaction of TBA and MDA (a by-product of lipid peroxidation) were measured at 520 nm excitation and 590 nm emission and content was calculated by comparison with a standard curve of MDA (0.125-1 µM).
Statistical analysis
Results are expressed as mean ± standard deviation (SD) (n = 4). All data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using Statistical Package for Social Studies (SPSS) version 22.0 (Armonk, New York).
P < 0.05 was considered statistically significant.
RESULTS
Effect of bergenin on cell viability and cytotoxicity in ethanol- and TBHP-treated HepG2 cells
HepG2 cells treated with ethanol and TBHP showed significant decreases in %cell viability compared with the control (P < 0.05), but cell viability remained more than 80% (Table 1). Substances can be considered cytotoxic at % LDH over 10 (Sriset et al., 2019), and exposure to ethanol and TBHP was certainly toxic to HepG2 cells compared to the control (P < 0.05) (Table 1). All concentrations of bergenin (ranging from 75 to 300 µM) and gallic acid (60 µM) improved %cell viability and decreased %LDH toxicity in HepG2 cells treated with ethanol and TBHP to levels comparable to the control
(P < 0.05).
Table 1. Effect of bergenin on cell viability and LDH toxicity in ethanol- and TBHP-treated HepG2 cells
|
Treatment |
Cell viability (%) |
LDHa toxicity (%) |
||
|
Normal |
Control |
100.00 ± 0.00 |
6.55 ± 0.34 |
|
|
Ethanol induction |
Ethanol |
86.67 ± 3.88* |
12.53 ± 0.84* |
|
|
(100 mM) |
Gallic acid |
|
|
|
|
|
60 µM |
99.62 ± 1.56# |
7.82 ± 1.49# |
|
|
|
Bergenin |
|
|
|
|
|
75 µM |
98.67 ± 1.41# |
8.53 ± 1.34# |
|
|
|
150 µM |
98.76 ± 0.67# |
8.41 ± 0.61# |
|
|
|
300 µM |
99.20 ± 0.21# |
8.66 ± 1.33# |
|
|
TBHP induction |
TBHP |
84.49 ± 1.30* |
15.91 ± 0.73* |
|
|
(100 µM) |
Gallic acid |
|
|
|
|
|
60 µM |
97.72 ± 0.94$ |
8.92 ± 1.46$ |
|
|
|
Bergenin |
|
|
|
|
|
75 µM |
95.94 ± 0.98$ |
9.10 ± 0.07$ |
|
|
|
150 µM |
96.11 ± 1.07$ |
8.59 ± 0.25$ |
|
|
|
300 µM |
96.66 ± 2.89$ |
8.14 ± 0.11$ |
|
Note: The data are presented as mean ± SD (n = 4). a, lactate dehydrogenase. A significant difference was determined by one-way analysis of variance followed by Tukey’s post hoc test. *P < 0.05 versus control; #P < 0.05 versus ethanol induction; $P < 0.05 versus TBHP induction.
Effect of bergenin on AST and ALT levels in ethanol- and TBHP- treated HepG2 cells
Ethanol and TBHP dramatically increased AST (Figure 2A) and ALT (Figure 2B) levels in HepG2 cells, compared with the control (P < 0.05). Bergenin concentration-dependently reduced AST and ALT levels in ethanol- and TBHP-treated HepG2 cells. Correspondingly, 60 µM gallic acid also significantly lowered levels of both enzymes in ethanol- and TBHP-treated HepG2 cells.
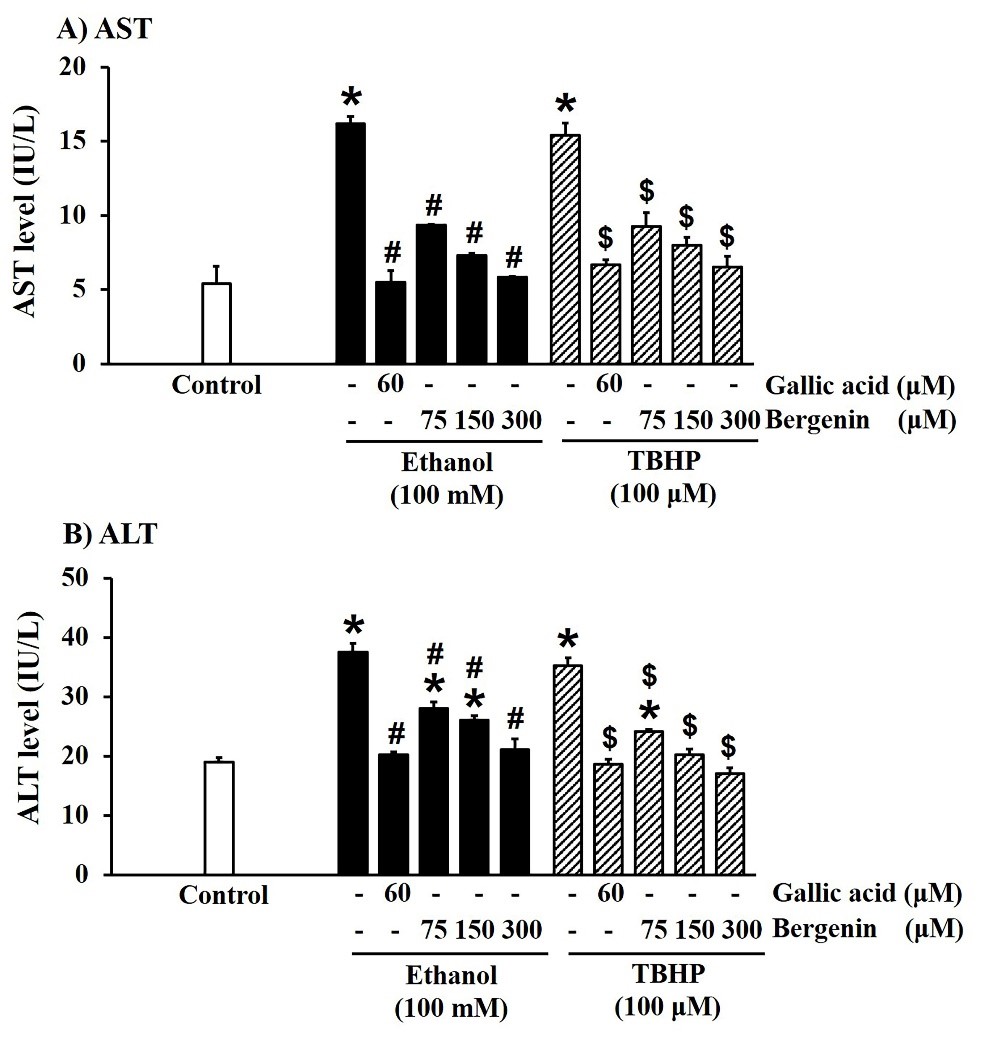
Figure 2. Effect of bergenin on cellular injury biomarkers in ethanol- and TBHP-treated HepG2 cells. A) AST and B) ALT levels. The data are presented as mean ± SD (n = 4). A significant difference was determined by one-way analysis of variance followed by Tukey’s post hoc test. *P < 0.05 versus control; #P < 0.05 versus ethanol induction; $P < 0.05 versus TBHP induction.
Effect of bergenin on cell morphology in ethanol- and TBHP-treated HepG2 cells
Normal HepG2 cell morphology is shown in Figures 3A and 4A (control). Ethanol and TBHP induced HepG2 cell morphological changes including modified round cell shape (blue arrows), and a loss of cell nuclei (orange triangles) (Figures 3B and 4B). Bergenin (Figures 3D-3F and 4D-4F) and gallic acid (Figure 3C and 4C) prevented ethanol- and TBHP-induced HepG2 cell damage.
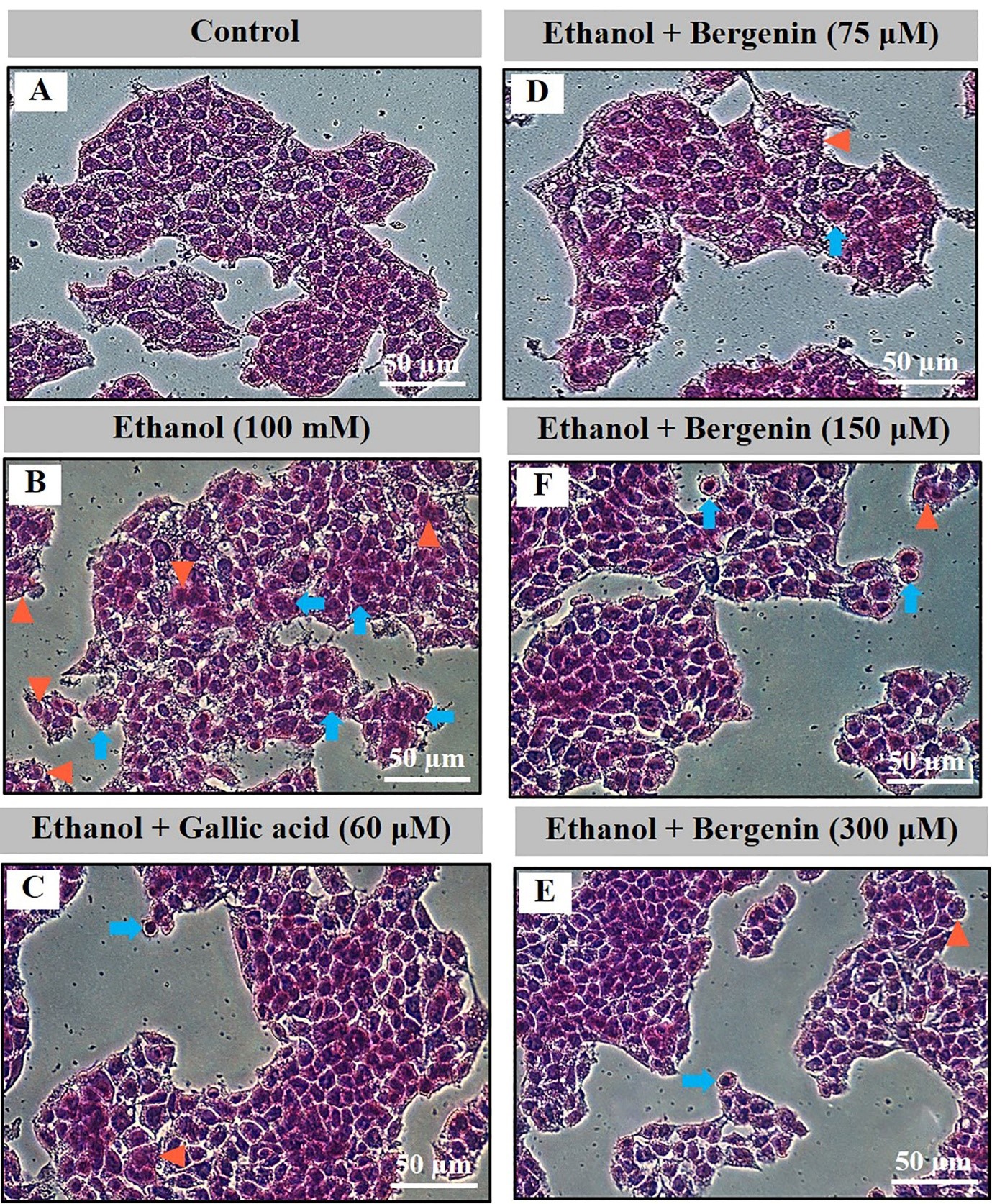
Figure 3. Effect of bergenin on cell morphology by H & E staining in ethanol-treated HepG2 cells. A) control, B) ethanol (100 mM), C) co-treatment of ethanol and gallic acid (60 µM), and D-F) co-treatment of ethanol and bergenin (75, 150, and 300 µM) for 24 h. Images are 200-fold magnification. Blue arrows, round-shaped cells; Orange triangles, loss of cell nuclei.
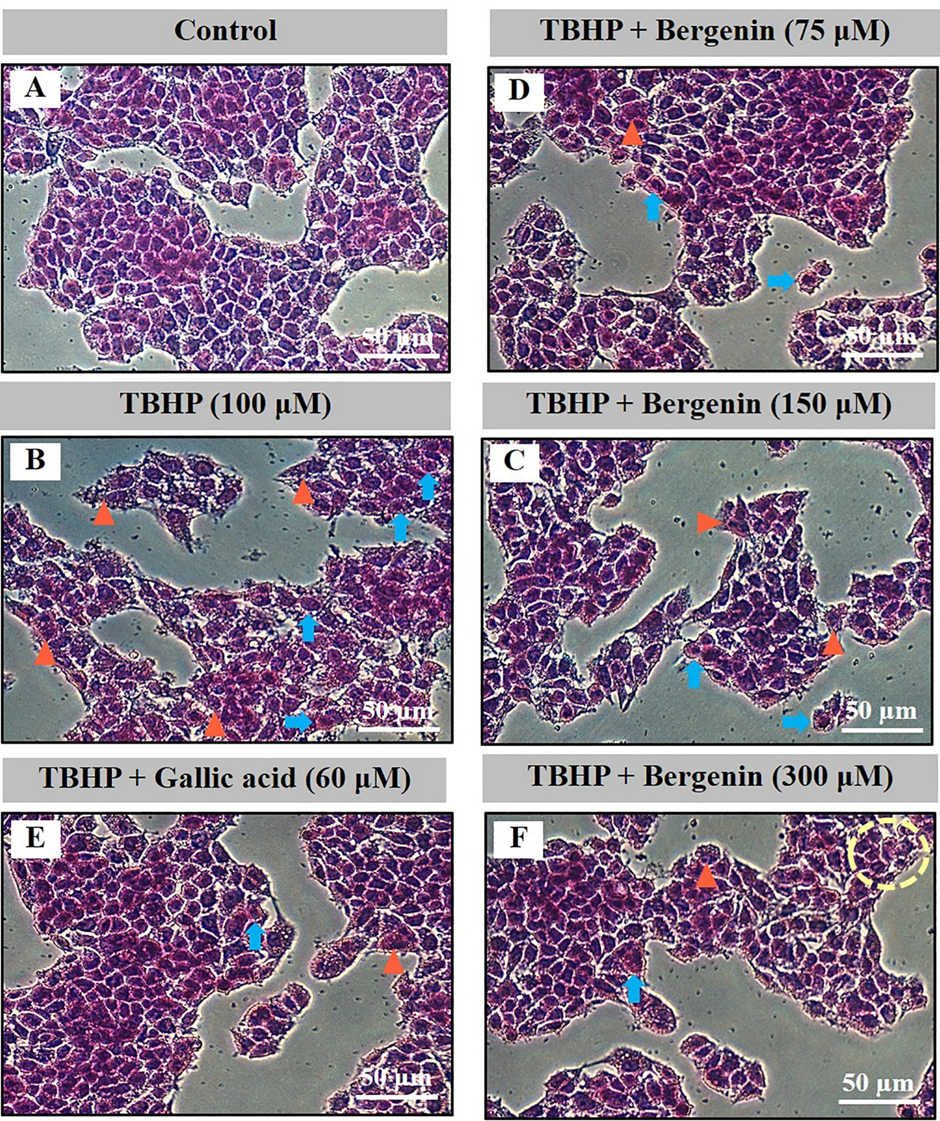
Figure 4. Effect of bergenin on cell morphology by H & E staining in TBHP-treated HepG2 cells. A) control, B) TBHP (100 µM), C) co-treatment of TBHP and gallic acid (60 µM), and D-F) co-treatment of TBHP and bergenin (75, 150, and 300 µM) for 24 h. Images are 200-fold magnification. Blue arrows, round-shaped cells; Orange triangles, loss of cell nuclei.
Effect of bergenin on cellular antioxidant defenses in ethanol- and TBHP-treated HepG2 cells
Cellular antioxidant defenses, including SOD and CAT activities and total GSH content, were examined in ethanol- and TBHP- induced HepG2 cells. Ethanol and TBHP significantly decreased SOD and CAT activities (Figure 5A and 5B) and total GSH content (Figure 6A), compared with the control (P < 0.05). Gallic acid (60 µM) and bergenin treatment significantly increased SOD and CAT activities (Figure 5A and 5B) and total GSH content (Figure 6A) in HepG2 cells treated with ethanol and TBHP (P < 0.05). The effects of bergenin on the cellular antioxidant defenses were concentration-dependent.
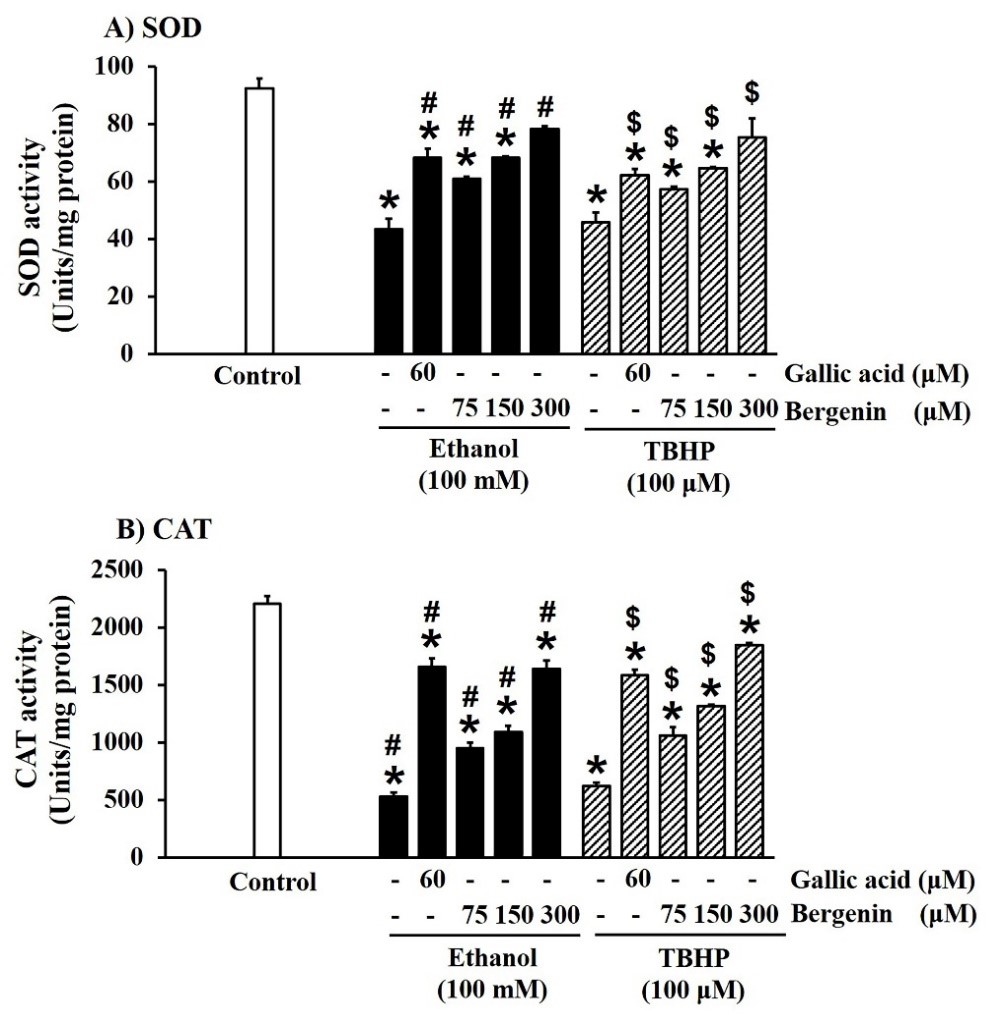
Figure 5. Effect of bergenin on SOD and CAT activities in ethanol- and TBHP-treated HepG2 cells. The data are presented as mean ± SD (n = 4). A significant difference was determined by one-way analysis of variance followed by Tukey’s post hoc test. *P < 0.05 versus control; #P < 0.05 versus ethanol induction; $P < 0.05 versus TBHP induction.
Effect of bergenin on lipid peroxidation in ethanol- and TBHP-treated HepG2 cells
Lipid peroxidation was assessed by quantification of MDA, a final product of this reaction (Sriset et al., 2019). Ethanol and TBHP significantly increased MDA levels in HepG2 cells, compared with the control (P < 0.05) (Figure 6B). Gallic acid and bergenin, in a concentration-dependent manner, greatly reduced MDA levels in ethanol- and TBHP-treated HepG2 cells (P < 0.05) (Figure 6B).
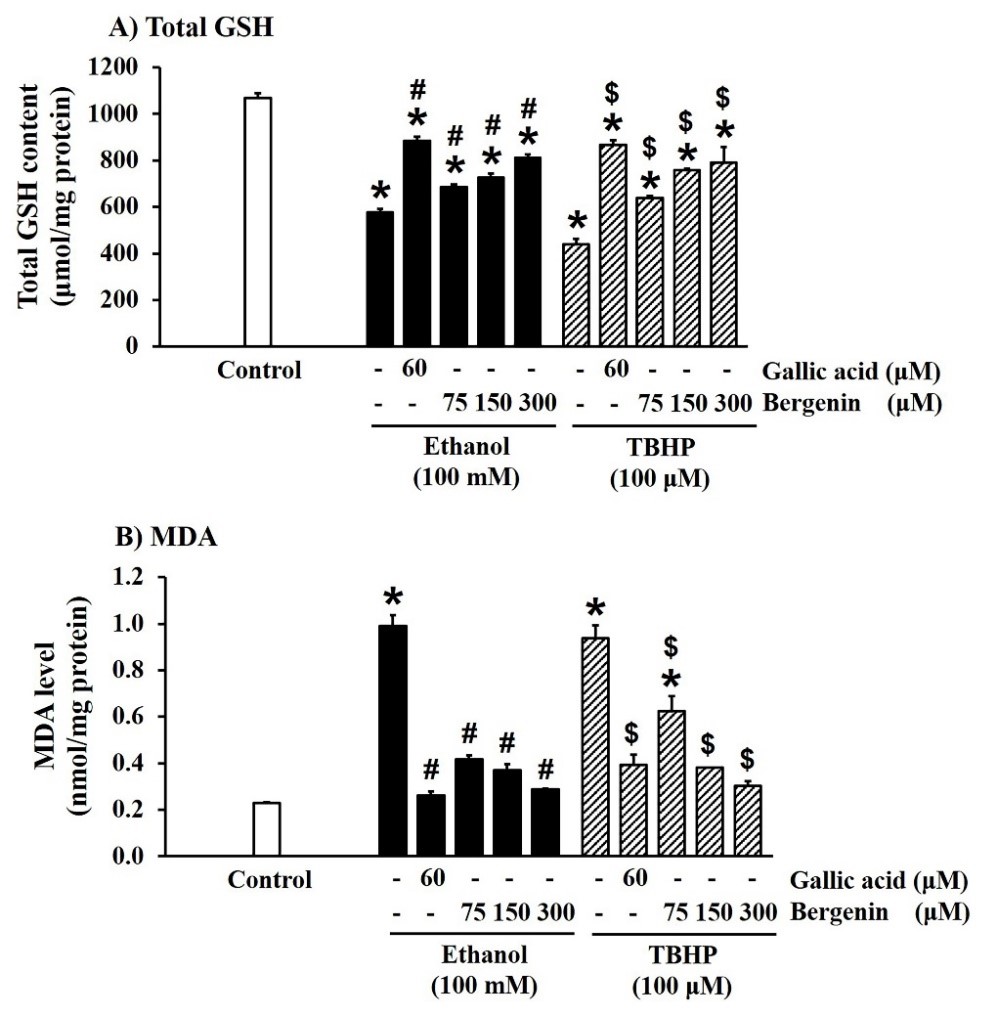
Figure 6. Effect of bergenin on total GSH content and MDA level in ethanol- and TBHP-treated HepG2 cells. The data are presented as mean ± SD (n = 4). A significant difference was determined by one-way analysis of variance followed by Tukey’s post hoc test. *P < 0.05 versus control; #P < 0.05 versus ethanol induction; $P < 0.05 versus TBHP induction.
DISCUSSION
Over-production of ROS contributes to hepatocyte damage (Li et al., 2015). Among the pathophysiological mechanisms of liver injury, xenobiotics-generated ROS are one of the main factors causing oxidant-antioxidant imbalance, leading to oxidative stress which is considered a key step in the progression of liver diseases (Cichoż-Lach and Michalak, 2014). Ethanol and TBHP are well-known xenobiotics encountered in daily life and in industrial pollution, respectively (Liu et al., 2002; Zakhari, 2013). Due to their highly reactive and toxic properties, both ethanol and TBHP are considered hazardous chemicals to humans (Cichoż-Lach and Michalak, 2014). Moreover, hepatic metabolism of ethanol and TBHP can be involved in excessive ROS production, cellular antioxidant depletion, and hepatoxicity (Liu et al., 2002; Zakhari, 2013). Therefore, ethanol and TBHP can be employed as inducers to evoke hepatocyte damage initiated by oxidative stress and to disturb the cellular antioxidant status in HepG2 cells. Furthermore, the HepG2 cell model is often used to study the cellular antioxidant system, including both the enzymatic and non-enzymatic mechanisms, as the main biological defense in the liver (Alía et al., 2005; Kozics et al., 2013; Pareek et al., 2013).
Ethanol is metabolized in the liver through three pathways consisting of alcohol dehydrogenase (ADH), the microsomal ethanol oxidizing system, and CAT (Zakhari, 2013). The major pathway of ethanol metabolism is ADH, which produces a toxic metabolite acetaldehyde. Furthermore, each pathway generates ROS by-products, especially superoxide anion (Ό2-) and hydroxyl radicals (Lee et al., 2008; Zakhari, 2013). Ethanol is known to strongly induce cytochrome P450 2E1 as the major component of the microsomal ethanol oxidizing system (Liu et al., 2010). This results in the generation of excess ROS that rapidly react with lipid membranes, leading to lipid peroxidation and oxidative stress (Farshori et al., 2015). In the present study, remarkably elevated levels of MDA, the product of lipid peroxidation, were observed in HepG2 cells treated with ethanol. These findings suggest that the microsomal ethanol oxidizing system is a key pathway of ethanol metabolism to induce oxidative stress in HepG2 cells.
TBHP is biotransformed into toxic tert-butyl alcohol and ROS, mainly peroxyl and alkoxyl anions, via activation of cytochrome P450 2E1 and the glutathione peroxidase-glutathione reductase system (Cederbaum et al., 2001; Liu et al., 2002; Park et al., 2009). These ROS and toxic metabolites can induce breakdown of the hepatocyte membrane, releasing liver injury-related enzymes (LDH, AST, and ALT) from the cytoplasm into extracellular fluids (Cichoż-Lach and Michalak, 2014). Furthermore, polyunsaturated fatty acids in the hepatocyte membrane are attacked by ROS, resulting in lipid peroxidation that generates MDA and eventually injures the hepatocyte structure (Niki et al., 2005). Together, these events demonstrate that lipid peroxidation, triggered by ROS generation from xenobiotics such as ethanol, TBHP, and carbon tetrachloride, leads to oxidative damage in HepG2 cells (Gómez-Quiroz et al., 2003; Krithika et al., 2009; Sohn et al., 2005). Therefore, in the present study, the ROS produced by the metabolism of ethanol and TBHP evoked HepG2 cell injury, as seen in the destruction of cell morphology and loss of cell nuclei, the decrease in cell viability, the increase in leakage of LDH, AST, and ALT from the cytoplasm and a surge in MDA levels from increased lipid peroxidation.
SOD is the main antioxidant defense against the Ό2- free radical, which is converted into H2O2 and further catalyzed to water and oxygen by CAT (Limón-Pacheco and Gonsebatt, 2009). GSH, a non-enzymatic antioxidant, is a reducing agent that directly scavenges and neutralizes free radicals and detoxifies toxic metabolites (Forman et al., 2009). Hence, both enzymatic and non-enzymatic antioxidants are responsible for preventing oxidative stress. Nevertheless, excessive levels of ROS result in an imbalance of this cellular antioxidant system and a deterioration in antioxidant capability (Cichoż-Lach and Michalak, 2014). In this study, ethanol decreased SOD and CAT activities and total GSH content in HepG2 cells. These results correspond with a previous study that showed treatment of human primary hepatocytes with 100 mM ethanol for 24 h increased the amount of AST and LDH and depleted GSH stores, which was followed by an increase in MDA level (Yao et al., 2007). Likewise, HepG2 cells exposed to 100 mM ethanol for 24 h demonstrated increases in AST, ALT, MDA, and ROS levels with a reduction of GSH content (Kumar et al., 2012b). Furthermore, primary rat hepatocytes treated with 100 mM ethanol for 8 h showed increases in LDH and AST activities, followed by increased lipid peroxidation, reduction in SOD and CAT activities, and a decrease in GSH content (Liu et al., 2010). Corresponding to the study of Alía et al., 2005, our study found a decrease in cellular antioxidant status (reduced SOD and CAT activities and total GSH content) due to TBHP in HepG2 cells. Alía et al. found that TBHP at concentrations over 50 µM for 3 h elevated MDA level and reduced SOD and CAT activities in HepG2 cells (Alía et al., 2005). Moreover, TBHP at 200 µM for 3 h decreased GSH content and reduced SOD and CAT activities, followed by increases in MDA, LDH, and ROS in HepG2 cells (Alía et al., 2006; Cuello et al., 2007). TBHP at 250 µM retarded HepG2 cell viability by 47.33%, indicating suppression of cell growth, which was related to increases in ROS and MDA production along with GSH depletion (Sohn et al., 2005). The current study suggests that HepG2 cell damage and toxicity due to exposure to ethanol and TBHP can be mostly attributed to oxidative stress, which accords with our previous study (Sriset et al., 2019). In our previous study, we demonstrated that concentration- and time-dependent oxidative stress could be observed after cells were treated with either 10 to 500 mM ethanol for 24 to 48 h or 50 to 200 µM TBHP for 3 to 24 h, as verified by induction of LDH, AST, and ALT release, increased production of MDA, reduction in SOD and CAT activities, and depletion of total GSH stores (Sriset et al., 2019). Therefore, maintaining the oxidant-antioxidant balance by promoting antioxidant activity is an approach to minimize oxidative stress and liver damage (Li et al., 2015)
Gallic acid at a concentration of 60 µM has previously been shown to be a potent antioxidant and anti-necroptosis agent in human hepatoma HepG2 cells and human hepatocyte LO2 cells (Kong et al., 2016; Zhou et al., 2019). Moreover, our previous study demonstrated that gallic acid at 60 µM restored the antioxidant status (i.e. SOD and CAT activities, and total GSH content) in ethanol-, sodium selenite-, and TBHP-induced oxidative stress in HepG2 cells (Sriset et al., 2019). Therefore, the concentration of 60 µM was selected for gallic acid in the present study. The concentrations of bergenin that were selected for the present study were based on studies examining the inhibitory effects of bergenin against free radicals (Srinivasan et al., 2007). Bergenin showed in vitro antioxidant activity against hydrogen peroxide (Srinivasan et al., 2007) and in vitro anti-inflammatory activity against cyclooxygenase-1 (Nunomura et al., 2009) at concentrations of 75 and 100 µM. In addition, bergenin at 300 µM was reported to have hepatoprotective and antioxidant activities in primary rat hepatocytes (Kim et al., 2000; Lim et al., 2000). Hence, the selected concentrations of bergenin in the present study were 75, 150, and 300 µM.
Gallic acid (30 to 60 µM) reduced ROS and MDA formation and restored SOD and CAT activities in H2O2-induced HepG2 cells (Kong et al., 2016), while gallic acid at 100 µM prevented cumene hydroperoxide-induced ROS generation and lipid peroxidation, and augmented total GSH content in primary rat hepatocytes (Pourahmad et al., 2010). Our previous study demonstrated that 60 µM gallic acid restored the antioxidant status (both SOD and CAT, and total GSH content) in ethanol-, sodium selenite-, and TBHP-induced oxidative stress in HepG2 cells (Sriset et al., 2019). Hence, gallic acid was employed as a positive standard in the present study at a dose of 60 µM, representing the peak antioxidant and hepatoprotective activities. In the present study, LDH, as a stable cytoplasmic enzyme, was employed as a sensitive indicator of cell membrane damage. An increase in extracellular LDH level indicates leakage and can be used as an index of cytotoxicity, which is inverse to cell viability (Nagaraj et al., 2012). Bergenin protected HepG2 cells against ethanol- and TBHP-induced cytotoxicity, elevating cell viability and reducing LDH toxicity. However, these effects were not dose-dependent, with bergenin exerting its maximum effect on the mechanism underlying cell viability and LDH release at 75 µM. Similarly, AST and ALT are specific markers of liver function and can be used to assess liver integrity. The liver can be damaged directly by ROS-induced increases in cell membrane permeability, leading to leakage of AST and ALT (McGill, 2016). Thus, an increase in release of AST and ALT indicates damage to hepatocytes (Giannini et al., 2005). In contrast to LDH, bergenin concentration-dependently lowered ethanol- and TBHP-induced AST and ALT release from HepG2 cells, in our model. Moreover, the hepatoprotective action of bergenin against ethanol- and TBHP-induced liver damage was revealed through the restoration of normal HepG2 cell morphological features following enhancement of SOD and CAT activities, increase in total GSH content, and reduction in MDA level. Correspondingly, the damage to carbon tetrachloride-injured primary rat hepatocytes was attenuated by bergenin at concentrations ranging from 1 to 300 µM via maintenance of glutathione homeostasis (Kim et al., 2000). In addition, the hepatoprotective activity of bergenin (100 µM) could be noted by decreases in the levels of biochemical makers of liver injury including glutamic pyruvic transaminase and sorbitol dehydrogenase in D-galactosamine-intoxicated primary rat hepatocytes (Lim et al., 2000). The hepatoprotective activity of bergenin in a hepatic ischemia reperfusion model in human hepatocyte LO2 cells and male Balb/c mice was recently reported (Xiang et al., 2020). Bergenin improved cell viability by up to 70% by eliminating ROS and up-regulating peroxisome proliferator-activated receptor gamma expression in LO2 cells, which correlates with our findings (Xiang et al., 2020). In the present study, gallic acid attenuated HepG2 cell injury to restore cell viability, LDH, AST, ALT, and MDA to normal levels. The restoration of the antioxidant system (SOD, CAT, and total GSH) by gallic acid was also observed in ethanol- and TBHP-induced oxidative stress in HepG2 cells. The antioxidative potentials of gallic acid and bergenin were not different in the present study. Gallic acid is the active metabolite of 4-O-methylgallic acid and a major part of bergenin’s structure (Rastogi and Rawat, 2008; Song et al., 2013). Thus, the hydroxyl rich structures of bergenin and gallic acid (Figure 1) act in the same way to decrease oxidative stress by directly binding with free radicals through an electron donating mechanism (Lü et al., 2010; Chen et al., 2020).
Therefore, it can be concluded that the hepatoprotective potential of bergenin is comparable to gallic acid. Thus, the main finding of the present study is that traditional uses of medicinal plants containing bergenin are related to the hepatoprotective and antioxidant activities of bergenin. For Thai traditional medicine, M. repandus has been used to relieve muscle pain and to maintain homeostasis (Rivière et al., 2010; Hasan et al., 2018), while in Bangladesh M. repandus has been applied for the treatment of inflammation and liver toxicity (Rivière et al., 2010). In addition, M. japonicus and M. philippinensis have been traditionally used to treat gastrointestinal diseases in Korea and India, respectively (Rivière et al., 2010; Gangwar et al., 2014).
Bergenin exerted its hepatoprotective effect against ethanol- and TBHP-induced oxidative stress in HepG2 cells by maintenance of the enzymatic and non-enzymatic antioxidant systems. This brought down hepatic oxidative stress and protected the hepatocytes, which inhibited the leakage of LDH, AST and ALT and the formation of MDA. These findings suggest that the hepatoprotective activity of bergenin in HepG2 cells is related to its ability to restore oxidant-antioxidant homeostasis.
CONCLUSION
Ethanol and TBHP induced oxidative stress, suppressed cell viability, accelerated LDH, AST, and ALT leakage, increased production of MDA, decreased SOD and CAT activities and disturbed the glutathione system, resulting in damage to HepG2 cells. Bergenin ameliorated the impact of oxidative stress caused by ethanol and TBHP in HepG2 cells. The highest tested concentration of bergenin at 300 µM modulated the oxidant-antioxidant imbalance by restoring antioxidant enzyme activities and GSH homeostasis. Therefore, bergenin protected hepatocytes from ethanol- and TBHP-induced damage based on its antioxidant properties. This study provides primary evidence supporting the use of bergenin for hepatoprotection.
ACNKOWLEDGEMENTS
The authors thank Dr. Glenn Borlace, Khon Kaen University, for English language assistance.
REFERENCES
Aggarwal, D., Gautam, D., Sharma, M., and Singla, S.K. 2016. Bergenin attenuates renal injury by reversing mitochondrial dysfunction in ethylene glycol induced hyperoxaluric rat model. European Journal of Pharmacology. 791: 611-621.
Alía, M., Ramos, S., Mateos, R., Bravo, L., and Goya L. 2005. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). Journal of Biochemical and Molecular Toxicology. 19: 119-128.
Alía, M., Ramos, S., Mateos, R., Granado-Serrano, A.B., Bravo, L., and Goya, L. 2006. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicology and Applied Pharmacology. 212: 110-118.
Ambika, S., and Saravanan, R. 2016. Effect of bergenin on hepatic glucose metabolism and insulin signaling in C57BL/6J mice with high fat-diet induced type 2 diabetes. Journal of Applied Biomedicine. 14: 221-227.
Bajracharya, G.B. 2015. Diversity, pharmacology, and synthesis of bergenin and its derivatives: potential materials for therapeutic usages. Fitoterapia. 101: 133-152.
Birben, E., Sahiner, U.M., Sackesen, C., Erzurum, S., and Kalayci, O. 2012. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 5: 9-19.
Cederbaum, A.I., Wu, D., Mari, M., and Bai, J. 2001. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radical Biology and Medicine. 31: 1539-1543.
Chatuphonprasert, W., Nawaratt., and Jarukamjorn, K. 2020. Reused palm oil from frying pork or potato induced expression of cytochrome P450s and the SLCO1B1 transporter in HepG2 cells. Journal of Food Biochemistry. 44: 1-11.
Chatuphonprasert, W., Udomsuk, L., Monthakantirat, O., Churikhit, Y., Putalun, W., and Jarukamjorn, K. 2013. Effects of Pueraria mirifica and miroestrol on the antioxidation‐related enzymes in ovariectomized mice. Journal of Pharmacy and Pharmacology. 65: 447-456.
Chen, J., Yang, J., Ma, L., Li, J., Shahzad, N., and Kim, C.K. 2020. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Scientific Reports. 10: 2611.
Choi, M.K., Kim, H.G., Han, J.M., Lee, J.S., Lee, J.S., Chung, S.H., and Son, C.G. 2015. Hepatoprotective effect of Terminalia chebula against t-BHP-induced acute liver injury in C57/BL6 mice. Evidence-Based Complementary and Alternative Medicine. 1-11.
Cichoż-Lach, H., and Michalak, A. 2014. Oxidative stress as a crucial factor in liver diseases. World Journal of Gastroenterology. 20: 8082-8091.
Cuello, S., Ramos, S., Mateos, R., Martín, M.A., Madrid, Y., Cámara, C., Bravo, L., and Goya, L. 2007. Selenium methylselenocysteine protects human hepatoma HepG2 cells against oxidative stress induced by tert-butyl hydroperoxide. Analytical and Bioanalytical Chemistry. 389: 2167-2178.
Farshori, N.N., Al-Sheddi, E.S., Al-Oqail, M.M., Hassan, W.H.B., Al-Khedhairy, A.A., Musarrat, J., and Siddiqui, M.A. 2015. Hepatoprotective potential of Lavandula coronopifolia extracts against ethanol induced oxidative stress-mediated cytotoxicity in HepG2 cells. Toxicology and Industrial Health. 31: 727-737.
Forman, H.J., Zhang, H., and Rinna, A. 2009. Glutathione: overview of its protective roles, measurement, and biosynthesis. Molecular Aspects of Medicine. 30: 1-12.
Gangwar, M., Goel, R.K., and Nath, G. 2014. Mallotus philippinensis Muell. Arg (Euphorbiaceae): ethnopharmacology and phytochemistry review. BioMed Research International. 1-13.
Gao, X.J., Guo, M.Y., Zhang, Z.C., Wang, T.C., Cao, Y.G., and Zhang, N.S. 2015. Bergenin
plays an anti-inflammatory role via the modulation of MAPK and NF-kB signaling pathways in a mouse model of LPS-induced mastitis. Inflammation. 38: 1142-1150.
Geetha, K.M., Ramakrishna, S., Sridhar, C., and Murugan, V. 2011. Hepatoprotective activity of methanolic extract of Mallotus philippensis (Lam.) muell.-arg in rats. Asian Journal of Chemistry. 23: 1577-1580.
Giannini, E.G., Testa, R., and Savarino, V. 2005. Liver enzyme alteration: a guide for clinicians. Canadian Medical Association Journal. 172: 367-379.
Gómez-Quiroz, L., Bucio, L., Souza, V., Escobar, C., Farfán, B., Hernández, E., Konigsberg, M., Vargas-Vorackova, F., Kershenobich, D., and Gutiérrez-Ruiza, M.C. 2003. Interleukin 8 response and oxidative stress in HepG2 cells treated with ethanol, acetaldehyde or lipopolysaccharide. Hepatology Research. 26: 134-141.
Haribabu, K., Ajitha, M., Ramesh, B., Babu, K.S., and Rao, J.M. 2012. Quantification of bergenin from Mallotus philippinensis by HPLC-MS and study on different extraction methods. Journal of Planar Chromatography. 25: 445-449.
Hasan, R., Uddin, N., Sana, T., Hossain, M., Mondal, M., Kanta, I.J., and
Choudhuri, M.S.K. 2018. Analgesic and anti‑inflammatory activities of methanolic extract of Mallotus repandus stem in animal models. Oriental Pharmacy and Experimental Medicine. 18: 139-147.
Jadon, A., Bhadauria, M., and Shukla, S. 2007. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. Journal of Ethnopharmacology. 109: 214-218.
Kim, H.S., Lim, H.K., Chung, M.W., and Kim, Y.C. 2000. Antihepatotoxic activity of bergenin, the major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated hepatocytes. Journal of Ethnopharmacology. 69(1): 79-83.
Kong, K.W., Mat-Junit, S., Aminudin, N., Hassan, F.A., Ismail, A., and Aziz, A.A. 2016. Protective effects of the extracts of Barringtonia racemosa shoots against oxidative damage in HepG2 cells. Journal of Life and Environmental Sciences. 1-20.
Kozics, K., Klusová, V., Srančíková, A., Mučaji, P., Slameňová, D., Hunáková, L., Kusznierewicz, B., and Horváthová, E. 2013. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chemistry. 141: 2198-2206.
Krithika, R., Mohankumar, R., Verma, R.J., Shrivastav, P.S., Mohamad, I.L., Gunasekaran, P., and Narasimhan, S. 2009. Isolation, characterization and antioxidative effect of phyllanthin against CCl4-induced toxicity in HepG2 cell line. Chemico-Biological Interactions. 181: 351-358.
Kumar, R., Patel, D.K., Prasad, S.K., Laloo, D., Krishnamurthy, S., and Hemalatha, S. 2012a. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler. Fitoterapia. 83: 395-401.
Kumar, S., Liao, J.W., Xiao, J.H., Vani, M.G., and Wang, S.Y. 2012b. Hepatoprotective effect of lucidone against alcohol-induced oxidative stress in human hepatic HepG2 cells through the up-regulation of HO-1/Nrf-2 antioxidant genes. Toxicology in Vitro. 26: 700-708.
Lee, S. I., Kim, H. J., and Boo, Y. C. 2008. Effect of green tea and (-)-epigallocatechin gallate on ethanol-induced toxicity in HepG2 cells. Phytotherapy Research. 22: 669-674.
Li, S., Tan, H.Y., Wang, N., Zhang, Z.J., Lao, L., Wong, C.W., and Feng, Y. 2015. The role of oxidative stress and antioxidants in liver diseases. International Journal of Molecular Sciences. 16: 26087-26124.
Lim, H.K., Kim, H.S., Chung, M.W., and Kim, Y.C. 2000. Protective effects of bergenin, the major constituent of Mallotus japonicus, on D-galactosamine-intoxicated rat hepatocytes. Journal of Ethnopharmacology. 70: 69-72.
Limón-Pacheco, J., and Gonsebatt, M.E. 2009. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutation Research. 674: 137-147.
Liu, S., Hou, W., Yao, P., Zhang, B., Sun, S., Nüssler, A.K., and Liu, L. 2010. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicology in Vitro. 24: 516-522.
Liu, C.L., Wang, J.M., Chu, C.Y., Cheng, M.T., and Tseng, T.H. 2002. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food and Chemical Toxicology. 40: 635-641.
Lü, J.M., Lin, P.H., Yao, Q., and Chen, C. 2010. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. Journal of Cellular and Molecular Medicine. 14: 840-860.
McGill, M.R. 2016. The past and present of serum aminotransferases and the future of liver injury biomarkers. Experimental and Clinical Sciences. 15: 817-828.
Miret, S., De Groene, E.M., and Klaffke, W. 2006. Comparison of in vitro assays of cellular toxicity in the human hepatic cell line HepG2. Journal of Biomolecular Screening. 11: 184-193.
Mondal, M., Hossain, M., Hasan, R., Tarun, T.I., Islam, A.F., Choudhuri, M.S.K., Islam, M.T., and Mubarak, M. S. 2020. Hepatoprotective and antioxidant capacity of Mallotus repandus ethyl acetate stem extract against D-galactosamine-induced hepatotoxicity in rats. ACS Omega. 5: 6523-6531.
Nagaraj, S., Rajarama, M.G., Arulmurugana, P., Baskaraboopathya, A., Karuppasamy, K., Jayappriyana, K.R., Sundararaj, R., and Rengasamy R. 2012. Antiproliferative potential of astaxanthin-rich alga Haematococcus pluvialis Flotow on human hepatic cancer (HepG2) cell line. Biomedicine and Preventive Nutrition. 2: 149-153.
Nazir, N., Koul, S., Qurishi, M.A., Najar, M.H., and Zargar, M.I. 2011. Evaluation of antioxidant and antimicrobial activities of bergenin and its derivatives obtained by chemoenzymatic synthesis. European Journal of Medicinal Chemistry. 46: 2415-2420.
Niki, E., Yoshida, Y., Saito, Y., and Noguchi, N. 2005. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochemical and Biophysical Research Communications. 338: 668-676.
Nunomura, R.C.S., Oliveira, V.G., Da Silva, S.L., and Nunomura, S.M. 2009. Characterization of bergenin in Endopleura uchi bark and its anti-inflammatory activity. Journal of the Brazilian Chemical Society. 20: 1060-1064.
Pareek, A., Godavarthi, A., Issarani, R., and Nagori, B.P. 2013. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. Journal of Ethnopharmacology. 150: 973-981.
Park, S., Kim, A.J., and Lee, M. 2009. Synergic effects of α-tocopherol and β-carotene on tert-butyl hydroperoxide-induced HepG2 cell injury. Toxicology and Industrial Health. 25: 311-320.
Polson, J., and Lee, W.M. 2005. The management of acute liver failure. Hepatology. 41: 1179-1197.
Pourahmad, J., Eskandari, M.R., Shakibaei, R., and Kamalinejad, M. 2010. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food and Chemical Toxicology. 48(3): 854-858.
Qin, X.G., Hua, Z., Shuang, W., Wang, Y.H., and Cui, Y.D. 2010. Effects of matrine on HepG2 cell proliferation and expression of tumor relevant proteins in vitro. Pharmaceutical Biology. 48: 275-281.
Rastogi, S., and Rawat, A.K.S. 2008. A comprehensive review on bergenin, a potential hepatoprotective and antioxidative phytoconstituent. Herba Polonica. 54: 66-79.
Rivière, C., Nguyen Thi Hong, V., Tran Hong, Q., Chataigne, G., Nguyen Hoai, N., Dejaegher, B., Tistaert, T., Nguyen Thi Kim, T., Vander Heyden, Y., Chau Van, M., and Quetin-Leclercq, J. 2010. Mallotus species from Vietnamese mountainous areas: phytochemistry and pharmacological activities. Phytochemistry Reviews. 9: 217-253.
Scharf, G., Prustomersky, S., Knasmuller, S., Schulte-Hermann, R., and Huber, W. W. 2003. Enhancement of glutathione and γ-glutamylcysteine synthetase, the rate limiting enzyme of glutathione synthesis, by chemoprotective plant derived food and beverage components in the human hepatoma cell line HepG2. Nutrition and Cancer. 45: 74-83.
Sohn, J.H., Han, K.L., Lee, S.H., and Hwang, J.K. 2005. Protective effects of panduratin A against oxidative damage of tert-butylhydroperoxide in human HepG2 cells. Biological and Pharmaceutical Bulletin. 28: 1083-1086.
Song, H., Wang, J., Zhang, R., Liu, X., Yuan, G., Wei, C., Zhao, W., Li, R., Wang, B., and Guo, R. 2013. In vivo metabolism study of bergenin in rats by HPLC-QTOF mass spectrometry. Biomedical Chromatography. 27: 1398-405.
Srinivasan, R., Chandrasekar, M.J.N., Nanjan, M.J., and Suresh, B. 2007. Antioxidant activity of Caesalpinia digyna root. Journal of Ethnopharmacology. 113: 284-291.
Sriset, Y., Chatuphonprasert, W., and Jarukamjorn, K. 2018. Quantitative determination of bergenin in Mallotus repandus (Willd.) Muell. Arg. stem extract by reverse phase-high performance liquid chromatography. Isan Journal of Pharmaceutical Sciences. 14: 67-74.
Sriset, Y., Chatuphonprasert, W., and Jarukamjorn, K. 2019. Optimized models of xenobiotic-induced oxidative stress in HepG2 cells. Tropical Journal of Pharmaceutical Research. 18: 1001-1007.
Xiang, S., Chen, K., Xu, L., Wang, T., and Guo, C. 2020. Bergenin exerts hepatoprotective effects by inhibiting the release of inflammatory factors, apoptosis, and autophagy via the PPAR-γ pathway. Drug Design, Development and Therapy. 14: 129-143.
Yao, P., Nussler, A., Liu, L., Hao, L., Song, F., Schirmeier, A., and Nussler, N. 2007. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. Journal of Hepatology. 47: 253-261.
Zakhari, S. 2013. Alcohol metabolism and epigenetics changes. Alcohol Research. 35: 6-17.
Zhang, Y.H., Fang, L.H., Lee, M.K., and Ku, B.S. 2003. In vitro inhibitory effects of bergenin and norbergenin on bovine adrenal tyrosine hydroxylase. Phytotherapy Research. 17: 967-969.
Zhou, Y., Jin, H., Wu, Y., Chen, L., Bao, X., and Lu, C. 2019. Gallic acid protects against ethanol-induced hepatocyte necroptosis via an NRF2-dependent mechanism. Toxicology in Vitro. 57: 226-232.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Yollada Sriset1 , Waranya Chatuphonprasert2 , and Kanokwan Jarukamjorn1,*
1 Research Group for Pharmaceutical Activities of Natural Products using Pharmaceutical Biotechnology (PANPB), Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen 40002, Thailand
2 Faculty of Medicine, Mahasarakham University, Maha Sarakham 44000, Thailand
Corresponding author: Kanokwan Jarukamjorn, E-mail: kanok_ja@kku.ac.th
Total Article Views
Editor: Wasu Pathom-aree, Chiang
Mai University, Thailand
Article history:
Received: June 6, 2020;
Revised: August 1, 2020;
Accepted: August 31, 2020

