
Body Size Distribution and Ovarian Histology of Pisodonophis boro (Hamilton, 1822) (Anguillifomes: Ophichthidae) from Pranburi River Estuary, Thailand
Phakorn Na Lampang, Amphornphan Palasai, Sinlapachai Senarat*, Wannee Jiraungkoorskul, Gen Kaneko, and Jes Kettratad*Published Date : 2020-12-17
DOI : https://doi.org/10.12982/CMUJNS.2021.001
Journal Issues : Number 1, January-March 2021
Abstract Reproductive characteristics on the snake eel Pisodonophis boro, a commercially important and high-value food source species in Thailand, have never been reported. We determined the body size distribution and gonadal structure of P. boro during the reproductive cycle. Healthy specimens were collected by local Pranburi River estuarine fishermen during March 2015 to March 2016. The total length of P. boro ranged from 24 cm to 97 cm with mean value of 66.28 ± 2.59 cm (N = 105, mean ± SD). Subsequent macroscopic observation demonstrated that the gonad of P. boro is a paired and elongated organ located parallel to the digestive tract. Surprisingly, the 105 specimens were all female, suggesting the protogynous sex reversal or spatial displacement of sexes in this species. Furthermore, only early and late perinucleolar stage oocytes were histologically identified throughout this study (synchronous developing type), which is often observed in semelparous fish species. These unique reproductive features of this eel in Thailand warrants further investigations on the male-female distribution and precise reproductive mode.
Keywords: Pranburi estuary, Reproductive organ, Snake eel, Thailand
Funding: This research was supported by grants funded by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund).
Citation: Na Lampang, P., Palasai, A., Senarat, S., Jiraungkoorskul, W., Kaneko, G., and Kettratad, J. 2021 Body size distribution and ovarian histology of Pisodonophis boro (Hamilton, 1822) (Anguillifomes: Ophichthidae) from Pranburi river Estuary, Thailand. CMUJ. Nat. Sci. 20(1): e2021001.
INTRODUCTION
The histological analysis on fish gonads provides key information for assessing the reproductive cycle, sex ratio and the cellular-level structure of gametes. Structural evidence for the gonadal development pattern particularly promotes better understanding of the reproductive biology of fish (Neidig et al., 2000). Such histological data acquired for economically important species such as Dicentrachus labrax and Centropomus undecimalis have been incorporated into policy making of fisheries management (Mayer et al., 1988; Neidig et al., 2000; Dietrich and Krieger, 2009).
The order Anguilliformes is a distinct group of teleosts comprising more than 700 species (Nelson, 2006). This order includes several groups of eels with unique life cycles such as catadromous eels of the Family Anguillidae (freshwater eel) and the anadromous eels of the Family Ophichthidae (snake eel) (Nelson, 2006; Froese and Pauly, 2016a). The catadromous eels spawn in the ocean and their larvae migrate to freshwater and estuarine habitats, while the anadromous eels do the opposite (Tsukamoto et al., 2002; Aoyama, 2009). Gonads of the family Anguillidae have been extensively studied in the context of reproductive histology (Colombo and Grandidr, 1996) because of their economic importance. Oocyte differentiation stages of Anguillids have been well classified and continuously used to understand the reproductive physiology in many species including Anguilla anguilla, A. rostrate, A. dieffenbachia, and A. australis (Sorensen and Pankhurst, 1988; Colombo and Grandidr, 1996; Lokman et al., 1998). It is also known that mature Anguillids can have the hermaphrodite reproductive mode, in which male gonads contain both spermatogonia and oogonia (called a Syrski organ) (Colombo and Grandidr, 1996; Kearney et al., 2011). On the other hand, females develop ovaries directly from the undifferentiated primordial gonad as reported in A. australis and A. dieffenbachii (Kearney et al., 2011).
The ovarian histology in the family Ophichthidae has been paid less attention and described only in limited species such as Pisodonophis cruentifer and Ophichthus rufus (Casadevall et al., 2001; Wenner, 2011). Such information is still lacking in Pisodonophis boro, one of the most important commercial teleosts in Thailand. This species widely distributes in Thailand, especially at the Pranburi River estuary (Paphavasit et al., 2014; Na Lampang et al., 2020), as well as the Indo-West Pacific to Sri Lanka (Froese and Pauly, 2016b). It is a high-value food source for the local consumer, but the wild population is dramatically declining partly because of the strong fishing effort and deterioration of their natural habitat (Moravec et al., 2007; Paphavasit et al., 2014). Cheevaporn and Menasveta (2003) reported that the Pranburi River estuary has been suffering from high concentration of toxic compounds such as lead and other heavy metals. If the population decline continues at the current rate, P. boro may extinct from the Pranburi River estuary in near future. To solve this problem, extensive efforts have been devoted to developing the aquaculture with various manipulative strategies (i.e. hormone priming and artificial fertilization) (Zohar and Mylonas, 2001). However, these strategies require the basic knowledge about reproductive characterizations to move to the application phase.
Therefore, in this study, we described the ovarian structure and development of P. boro in Pranburi River estuary, Thailand, during its reproductive season. Body size distribution of P. boro was also observed to associate the histological data with reproductive biology. This work shall provide new insights into the provision of scientific advice to aquaculture development and fisheries management strategies of P. boro.
MATERIALS AND METHODS
Fish collection
Monthly collection was done from March 2015 to March 2016. The 105 healthy specimens of Pisodonophis boro were captured by local fishermen from the middle to lower Pranburi River estuary, Prachuap Khiri Khan Province, Thailand (N 12°24'08.5" / E 099°59'00.2") (Figure 1). Species identification was conducted based on the taxonomic key of FAO (Rainboth, 1996). Then fish were euthanized by rapid cooling shock (Wilson et al., 2009). The experimental protocol was approved by the Animal Care and Use Committee of Faculty of Science in accordance with the guide for the care and use of laboratory animal prepared by Chulalongkorn University (Protocol Review No. 1523005).
Body size distribution
All P. boro specimens were measured for total length (from the snout to the caudal fin to the nearest 1 cm). We determined the body size distribution following the criterion for ranges and size classes in a previous report (Kearney et al., 2011).
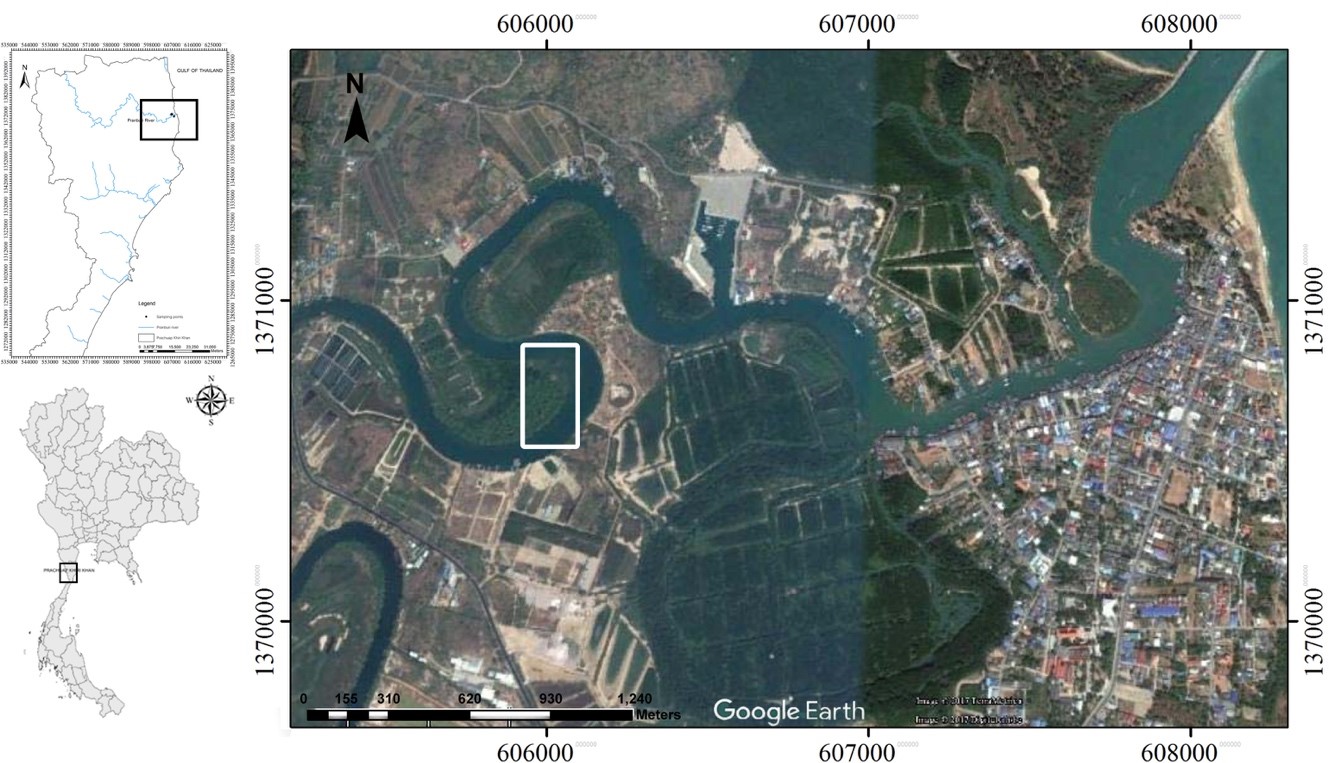
Figure 1. Study areas (boxed) of Pranburi River estuary, Prachuap Khiri Khan Province, Thailand, from which all fish specimens were collected.
Histological and histochemical examinations
The ovarian tissue from all fish were dissected out, classified into three regions (anterior, middle and posterior regions) and fixed in Davidson’s fixative at 4 ºC for about 48 hrs. They were then processed by standard histological techniques (Presnell and Schreibman, 1997; Suvarna et al., 2013). The paraffin blocks were serially sectioned at 4 µm thickness by a rotary microtome. The paraffin sections were deparaffinized with xylene, rehydrated through a series of ethyl alcohol and stained with Delafield’s heamatoxylin and eosin (H&E) to study the basic structure of gonadal tissue. Some histological sections were stained with Periodic acid schiff (PAS) and alcian blue pH 2.5 (AB) to observe the chemical components (Presnell and Schreibman, 1997; Suvarna et al., 2013). Sex organs and gonadal structures were identified following the standard criteria from Dietrich and Krieger (2009) and Wenner (2011). Photographs were taken at light microscopic levels using a Leica DM750-U microscope. Additionally, the scientific diagram of the gonadal structure was drawn using illustration software (Adobe Illustrator CS6).
RESULTS
Body size distribution
The body size of Pisodonophis boro ranged from 24 cm to 97 cm with the mean total length of 66.28 ± 2.59 cm (mean ± SD, n = 105) (Figure 2). The total length of majority of the specimens was in the range from 50.1 cm to 80 cm (n = 91). The difference in the size may be due to the difference of natural habitat and collecting method; however, this observation requires continuous monitoring.
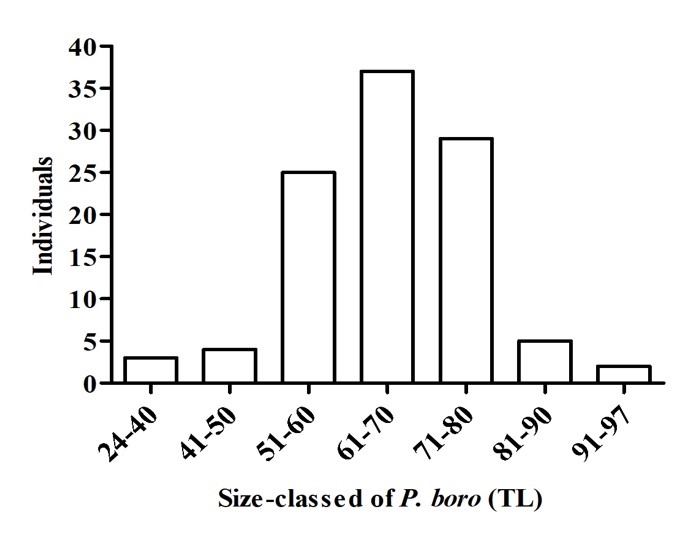
Figure 2. Size class distribution of Pisodonophis boro.
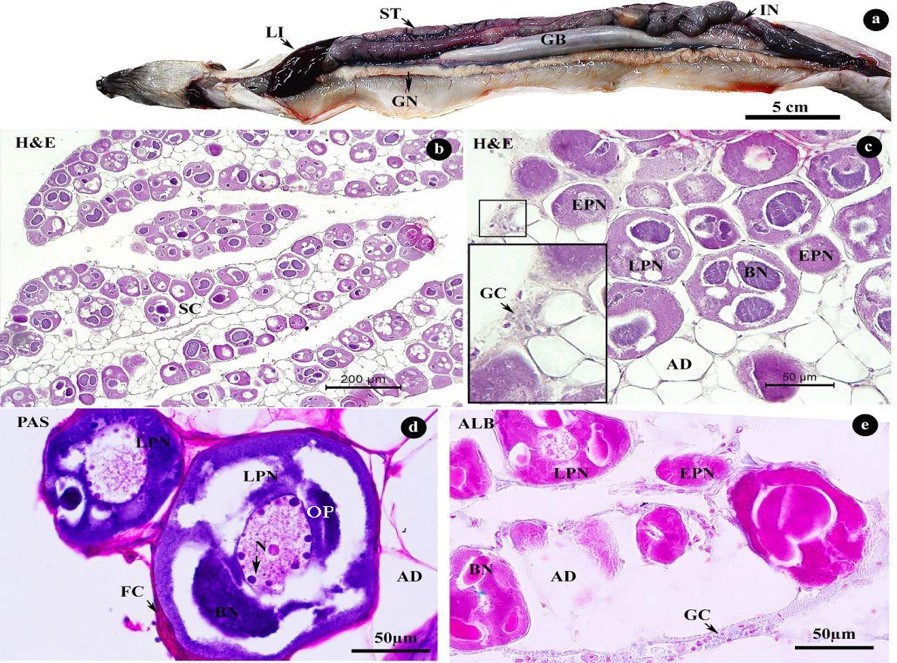
Figure 3. Morphology (a) and histology (b–e) of Pisodonophis boro ovary consisting of early perinucleolar (EPN) and late perinucleolar (LPN) stages. Arrows in c and d indicate GC and N, respectively. Abbreviations: AD = adipose tissue, BN = Balbiani bodies, FC = follicle cell, GC = germinal cell, GB = gallbladder, GN = gonad, IN = intestine, LI = liver, N = nucleus, SC = stromal compartment, ST = stomach
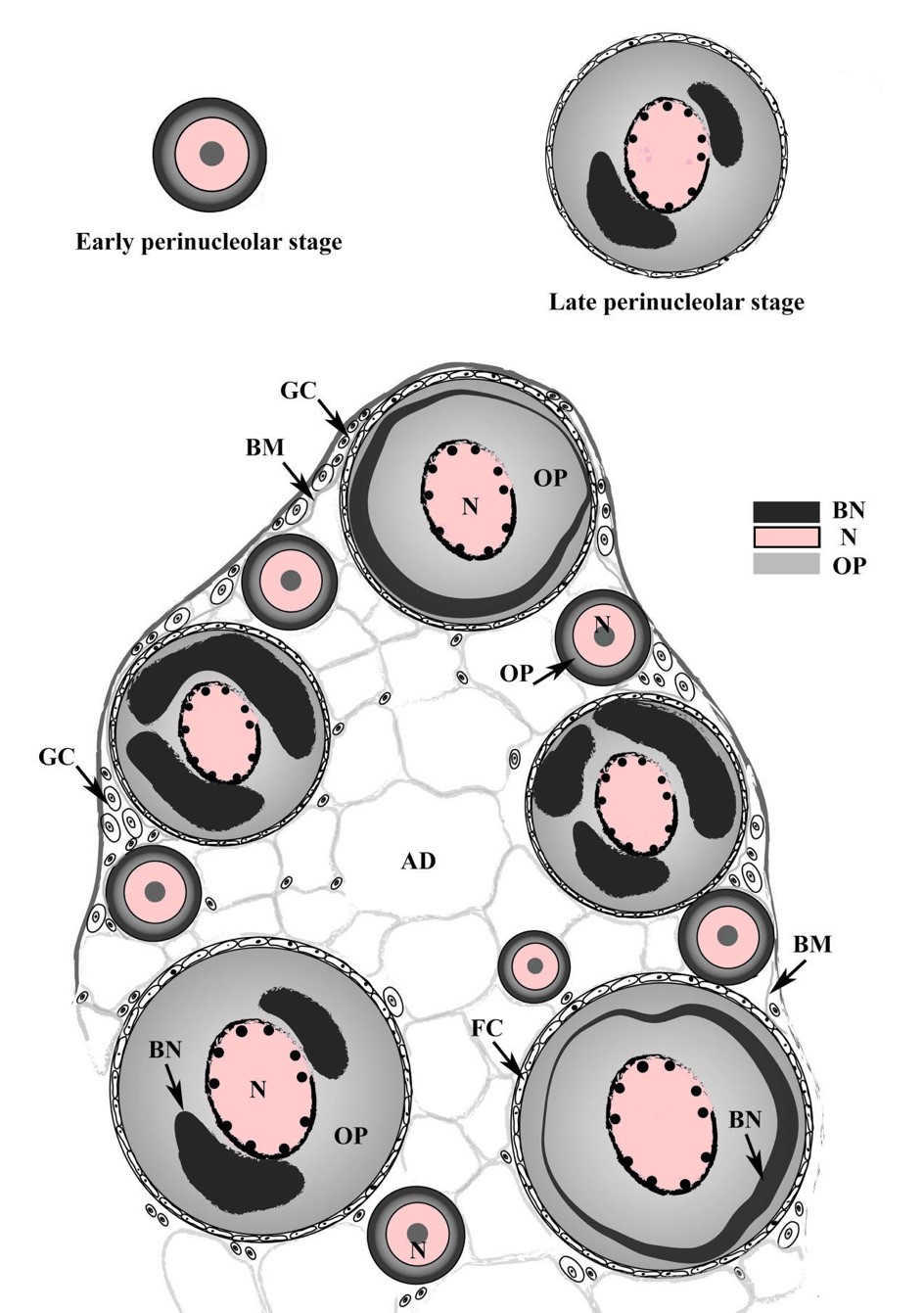
Figure 4. Schematic diagram of Pisodonophis boro ovary and oocytes having two stages including early perinucleolar and late perinucleolar stages. Abbreviations: AD = adipose tissue, BM = basement membrane, BN = Balbiani bodies, FC = follicle cell, GC = germinal cell, N = nucleus, OP = ooplasm.
DISCUSSION
Observation of the gonad of P. boro was firstly showed as a gymnovarian type fish, which directly releases oocytes to the body cavity rather than through reproductive duct prior to spawning (Simon and Tomelleri, 2011). This is similar to previous observations of P. cruentifer (Wenner, 2011) and Ophichthus rufus (Casadevall et al., 2001). Neither of them has a reproductive duct.
Other examples of synchronous developing type fish including anadromous salmon (Oncorhynchus spp.) and catadromous eels (Anuguilla spp.) (Casadevall et al., 2001; McMillan, 2007) were documented, which spawn once in their lifetime and die after spawning. A northwest Mediterranean eel Ophichthus rufus (Anguilliformes) also has the synchronous developing type oocytes (Casadevall et al., 2001). Although there is no detailed report about the reproductive cycle of Op. rufus, it is possible that this species has a single and short breeding season (Dietrich & Krieger, 2009). Likewise, our histological observation that P. boro has a synchronous ovary suggests that this species may spawn only once in their lifetime.
Another striking finding in this study is that our histological analysis did not find any males in the 105 specimens tested. Four possible explanations could account for this extremely skewed sex ratio: a) males mature at smaller size than females and were not included in our sample population of total length from 24 cm to 97 cm (Kartas and Quignard, 1985), b) presence of hermaphrodite individuals and/or parthenogenesis (Colombo and Grandidr, 1996), c) a protogynous sex reversal, and d) spatial displacement of sexes (where the female and male living different habitats) (Cau and Manconi, 1983). The first possibility has explained a similar observation in Op. rufus, where males were not observed in samples of a large size (>39 cm in the body length) (Casadevall et al., 2001). Although the sex-dependent difference in maturation size is commonly found in teleosts, this may not be the case in P. boro because the smallest individual (24 cm in total length) was female as determined by histological technique. Continuous field monitoring will further substantiate this idea.
It is known that many Anguilla species show the hermaphroditic mode of reproduction, in which their gonads contain both spermatogonia and oogonia and are termed as the ‘Syrski organ,’ as reported in A. australis, A. anguilla, A. rostrata and A. japonica (Colombo and Grandidr, 1996; Kearney et al., 2011; Geffroy et al., 2013). However, neither P. boro (present study) nor Op. rufus (Casadevall et al., 2001) was confirmed to have the Syrski organ. Therefore, hermaphroditism might not be a common mode of reproduction in non-Anguilla eels. On the other hand, parthenogenetic reproduction has been reported from more than 70 taxa of vertebrates (Neaves and Baumann, 2011; Wootton and Smith, 2014). Parthenogenetic reproduction used to be regarded as minor in teleosts because only 8 taxa (about 0.03% of all teleost species) are reported to have this reproductive mode (Pandian, 2011; Wootton and Smith, 2014). Also, the parthenogenetic mode is found in specific families, mostly in the Poeciliids and rarely in Atheriniformes and Cypriniformes (Pandian, 2011). However, recently, the parthenogenetic reproduction has been found in the captive female white spotted bambooshark for the first time (Straube et al., 2016). Straube and colleagues (2016) had claimed that this event was the first genetically confirmed evidence for second-generation facultative parthenogenesis in vertebrates and significantly support the evolutionary of parthenogenetic reproductive mode as an alternative to sexual reproduction. Hence, we cannot rule out the possibility that many other teleost species, including P. boro, may display the parthenogenetic mode of reproduction under certain conditions. Nonetheless, since the parthenogenesis has not been reported in any Anguilliforms species, further studies should be carefully conducted to test this hypothesis. Influence of male-to-female sex changes of this eel population in the Pranburi River estuary should also be investigated with seasonal sampling.
Lastly, the spatial displacement of sexes represents a more probable assumption to explain the skewed sex ratio of P. bolo. There is a report of spatial displacement of sexes in an Anguillid Conger conger (Cau and Manconi, 1983). Cau and Manconi (1983) demonstrated that C. conger males were absent at the depth below 400 m. Between 400 m to 800 m, the sex ratio of C. conger was close to 0.5 and this is where the reproductive activity takes place. If this is the case in P. boro, the Pranburi River estuary system is the feeding ground but not the spawning ground. To test the possibility, P. boro specimens should be collected from various other places.
- boro is one of the most important commercial teleosts in Thailand. An important strategy for establishing sustainable fisheries is to avoid the capture of juvenile fish. The present study suggests that P. boro may spawn once in their lifetime, and individuals in the Pranburi River estuary are juveniles are premature adults. Along with the effort to establish aquaculture of this species, fisheries management strategy such as creating fisheryexclusion zones should be implemented.
CONCLUSION
This study demonstrated that the gonad of P. boro is a paired and elongated organ parallel to the digestive tract. Importantly, the ovary was composed of only perinucleolar stage oocytes, suggesting that this species is the synchronous development type, which typically spawn once in their lifetime. Moreover, our samples contained only female specimen, which warrants further investigations of reproductive mode and male-female distribution of this species. Our results provide a cue for understanding of reproductive mode of P. boro, an important food resource in the Pranburi River estuary.
REFERENCES
Aoyama, J. 2009. Life history and evolution of migration in Catadromous eels (Genus Anguilla). Aqua-BioScience Monographs. 2: 1–42.
Casadevall, M., Munoz, M., Carrasson, M., and Matallanas, J. 2001. The reproductive cycle of Ophichthus rufus (Anguilliformes) in the northwest Mediterranean. Cybium. 25: 53-65.
Cau, A., and Manconi, P. 1983. Sex-ratio and spatial displacement in Conger conger (L.). Rapports et Procѐs-Verbaux des Rѐunions. CIESM. 28: 93–96.
Cheevaporn, V., and Menasveta, M. 2003. Water pollution and habitat degradation in the Gulf of Thailand. Marine Pollution Bulletin. 47: 43–51.
Colombo, G., and Grandidr, G. 1996. Histological study of the development and sex differentiation of the gonad in the European eel. Journal of
Fish Biology. 48: 493–512.
Dietrich, D., and Krieger, H.O. 2009. Histological analysis of endocrine disruptive effects in small laboratory fish. New Jersey: John Wiley & Sons.
Froese, R., and Pauly, D. 2016a. FishBase. World Wide Web electronic publication. Anguilliformes. Retrieved from www.fishbase.org
Froese, R., and Pauly, D. 2016b. FishBase. World Wide Web electronic publication. Pisodonphis boro. Retrieved from www.fishbase.org
Geffroy, B., Guiguen, Y., Fostier, A., and Bardonnet, A. 2013. New insights regarding gonad development in european eel: evidence for a direct ovarian differentiation. Fish Physiology and Biochemistry. 39: 1129– 1140.
Kartas, F., and Quignard, J.P. 1985. La fécondité des poissons téléostéens. Paris: Masson.
Kearney, M., Jeffs, A., and Lee, P. 2011. Development and early differentiation of male gonads in farmed new zealand shortfin eel. New Zealand Natural Sciences. 36: 33–44.
Lokman, P., Vermeulen, G., Lambert, J., and Young, G. 1998. Gonad histology and plasma steroid profiles in wild New Zealand freshwater eels (Anguilla dieffenbachii and A.australis) before and at the onset of the natural spawning migration. I. Females*. Fish Physiology and Biochemistry. 19: 325–338.
Mayer, I., Shackley, S.E. and Rylan, J.S. 1988. Aspects of the reproductive biology of the bass, Dicentrarchus labrax L. an histological and histochemical study of oocyte development. Journal of Fish Biology. 33: 609–622.
McMillan, D.B. 2007. Female genital systems of fish: female reproductive systems. Netherlands: Springer.
Moravec, F., Taraschewski, H., Anantaphruti, M.T., Maipanich, W., and Laoprasert, T. 2007. Heliconema longissimum (Ortlepp, 1923) (Nematoda: Physalopteridae) from Pisodonophis boro (Teleostei: Ophichthidae) in Thailand, with remarks on the taxonomy of the Proleptinae Schulz, 1927. Systematic Parasitology. 66: 73–80.
Na Lampang, P., Palasai, A., Senarat, S., Kettratad, J., Jiraungkoorskul, W., and Boonyoung, P. 2020. The existence of argyrophilic endocrine cells in the digestive system of snake eels (Pisodonophis boro, Hamilton, 1822). Veterinary Integrative Sciences. 18: 75-83.
Neaves, W.B., and Baumann, P. 2011. Unisexual reproduction among vertebrates. Trends Genet. 27: 81–88.
Nelson, J.S. 2006. Fishes of the world. 4th ed. New Jersey: Wiley & Sons.
Neidig, C.L., Skapura, D.P., Geier, H.J., and Dennis, C.W. 2000. Techniques for spawning common snook: broodstock handling, oocyte staging, and egg quality. North American Journal of Aquaculture. 62: 103–113.
Pandian, T.J. 2011. Sexuality in fishes. Enfield, New Hampshire: Science Publishers, Inc.
Paphavasit, N., Siriboon, S., Jaiperm, J., and Mookui, P. 2014. Sirinath RajiniMangrove Ecosystem Learing Center. . . . From mangrove plantation to mangrove forest enhaching human development. 1st ed. Bangkok: PTT co., Ltd. and Department of Science. Chulalongkorn University.
Presnell, J.K., and Schreibman, M.P. 1997. Humason's animal tissue techniques. Baltimore: Johns Hopkins University Press.
Rainboth, W.J. 1996. FAO Species identification field guide for fishesry purposes. Fishes of the Cambodian Mekong. Rome: FAO.
Simon, T.P., and Tomelleri, J.R. 2011. Fishes of Indiana: a field guide. Bloomington, IN: Indiana University Press.
Sorensen, P.W., and Pankhurst, N.W. 1988. Histological changes in the gonad, skin, intestine ando olfactory epithelium of artificially‐matured male American eels, Anguilla rostrata (LeSueur). Journal of Fish Biology. 32: 297–307.
Straube, N., Lampert, K.P., Geiger, M.F., Weiss, J.D., and Kirchhauser, J.X. 2016. First record of second-generation facultative parthenogenesis in a vertebrate species, the whitespotted bambooshark Chiloscyllium plagiosum. Journal of Fish Biology. 88: 668–675.
Suvarna, K.S., Layton, C., and Bancroft, J.D. 2013. Bancroft’s theory and practice of histological techniques. Canada: Elsevier.
Tsukamoto, K., Aoyama, J., and Miller, M.J. 2002. Migration, speciation, and the evolution of diadromy in anguillid eels. Canadian Journal of Fisheries and Aquatic Sciences. 59: 1989–1998.
Wenner, C.A. 2011. Aspects of the biology and morphology of the snake eel, Pisodonophis cruentifer (Pisces, Ophichthidae). Journal of the Fisheries Board of Canada. 33: 656–665.
Wilson, J.M., Bunte, R.M., and Carty, A.J. 2009. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science. 48: 785–789.
Wootton, R.J., and Smith, C. 2014. Reproductive biology of teleost fishes. London: John Wiley & Sons.
Zohar, Y., and Mylonas, C.C. 2001. Endocrine manipulations of spawning in cultured fish: From hormones to genes. Aquaculture. 197: 99–136.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Phakorn Na Lampang1, Amphornphan Palasai2, Sinlapachai Senarat3*, Wannee Jiraungkoorskul4, Gen Kaneko5, and Jes Kettratad1, 6*
1 Department of Marine Sciences, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
2 Faculty of Agriculture, Princess of Naradhiwas University, Narathiwat 96000, Thailand
3 Department of Marine Science and Environment, Faculty of Science and Fisheries Technology, Rajamangala University of Technology Srivijaya, Trang 92150, Thailand
4 Department of Pathobiology, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
5 School of Arts and Sciences, University of Houston-Victoria, Texas 77901, USA
6 Marine Ecology and Marine Resources Utilization Research Unit, Aquatic Resources Research Institute, Chulalongkorn University, Bangkok 10330, Thailand
Corresponding author: 1. Sinlapachai Senarat, E-mail: sinlapachai.s@rmutsv.ac.th 2. Jes Kettratad, E-mail: Jes.K@chula.ac.th
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: March 22, 2020;
Revised: May 20, 2020;
Accepted: May 22, 2020

