
Fabrication of Blended Gelatin-Polyvinyl Alcohol-Chitosan Scaffold for Wound Regeneration
Hongxiang Yin, Suruk Udomsom, and Fashai Kantawong*Published Date : 2020-09-01
DOI : https://doi.org/10.12982/CMUJNS.2020.0058
Journal Issues : Number 4, October-December 2020
ABSTRACT A single type polymer has very limited function and property which is not enough to be applied in the complex real situation. This study aimed to prepare the composited scaffolds by blending the gelatin, polyvinyl alcohol and chitosan together. The blended scaffolds were fabricated by making the final concentration of 7% gelatin, 0.5 %PVA and 0.1% chitosan and crosslinking by glutaraldehyde. The Young’s modulus was investigated by using atomic force microscopy (AFM). The pore size was investigated using the scanning electron microscope (SEM). The swelling rate of scaffolds were tested by water displacement method. The degradation rate of the scaffolds was studied using lysozyme digestion. The MTT assay was applied within this study in order to find out the relative cell viability upon culturing with the gelatin and the blended scaffolds compared to tissue culture plates. Collagen type IV expression was investigated in mouse fibroblasts cultured on both scaffolds for 10 days using real time PCR. The results showed that Young’s moduli of gelatin and blended scaffold were 53.30±26.80 kPa and 98.01±17.50 kPa, respectively. The average pore size of gelatin and blended scaffolds were 336.33±52.25 μm and 68.17±8.91 μm, respectively. The sample’s porosity of gelatin and blended scaffolds were 85.41±2.11% and 21.48±1.01%, respectively. The swelling rate and the degradation rate of gelatin scaffold were higher than blended scaffold. The MTT assay showed that the blended scaffold supported cell proliferation better than gelatin scaffold. Collagen type IV expression of mouse fibroblasts cultured on blended scaffolds was higher than gelatin scaffolds. In conclusion, these results illustrated that blended scaffolds were able to provide a better environment for fibroblast proliferation and collagen type IV expression.
Keywords: Gelatin, Chitosan, Polyvinyl alcohol, Wound regeneration
INTRODUCTION
There is increasing requirement for safer and more effective therapeutic methods for wound coverage and skin tissue repairing in a variety of clinical situations, such as acute skin wounds, burn wounds, and chronic skin ulcers. The split thickness skin autograft is the dominant desirable therapeutic method for coverage of excised burn wounds when the available donor sites for autografting are so limited, especially in instances where patients are faced with very large areas of burn wounds. These wound coverages require repeated harvesting from available donor sites, which leads to pain and scar at the donor sites that extend the time for skin recovery, and such patients have to stay longer in the hospital (Huang and Fu, 2011). Because of the limitations with regard to application of autografts and allografts, and the tremendous need for the same in clinical applications for wound regeneration in patients with various wound situations, bioengineered skin substitutes have been developed quickly so that new alternative methods are provided for clinicians to restore skin and solve a variety of skin defects (Rendon et al., 2010; Shishatskaya et al., 2016; Yang et al., 2016). The substitutes should have some fundamental properties to guarantee that they can create the proper environment for promoting wound healing, such as having appropriate physical and mechanical properties and having a controlled degradation rate. The desirable materials of skin substitutes should fulfill the following requirements: (1) be able to maintain local moist environment of the skin; (2) be able to protect the wound from side-infection; (3) should have the ability to absorb the wound fluids and exudates; (4) be able to minimize the wound surface necrosis; (5) be able to stimulate cell growth and differentiation; and (6) be elastic, non-toxic, non-antigenic, biocompatible, and biodegradable (MacNeil, 2007).
With regard to these characteristics, a variety of biomaterials, both of natural origin and of synthetic origin, have been used for medical applications, such as chitosan, alginate, collagen, gelatin, polyglycolic acid, polycaprolactone, and polylactic acid. All these substances are currently the most-used materials for tissue engineering applications since these materials have great capacity to reproduce the properties that match those of native organic skin tissues.
Gelatin is derived from hydrolysis of collagen. Gelatin is a very frequently used material for producing tissue engineering scaffolds because gelatin is water-soluble. Good biodegradability and being inexpensive enough for large scale production are characteristics that make gelatin a good choice as biomaterial in the production of tissue engineering scaffolds. Because gelatin is produced from collagen, it has the main peptide sequences or structures of collagen, which means that gelatin has some common features of collagen such as RGDs that could promote cell adhesion and migration. RGDs refers to Arg-Gly-Asp-Ser peptide sequence which is important as the binding motif of fibronectin to cell adhesion molecules. Furthermore, gelatin possesses excellent foaming ability; therefore, it always functions as a suitable colloid stabilizer and foaming agent (Santoro et al., 2014; Shevchenko et al., 2014). A previous study showed that photocurable hydrogel made from pure gelatin possesses ultrasoft property which induces neural differentiation in adipose derived stem cells (Kantawong et al., 2015). When hydroxyapatite was added to pure gelatin, it was found that the material could induce neural differentiation in hMSCs (Kantawong et al., 2016). If gelatin is applied on its own as the material of the scaffold, it would not be possible to obtain the kind of scaffold that can provide enough mechanical and physical properties for wound regeneration. Thus, a combination of gelatin and chitosan was developed and successfully applied in biomedical fields (Parvez et al., 2012).
Polyvinyl alcohol (PVA) is one of the most frequently used materials and also the oldest synthetic polymer hydrogel (Vashisth et al., 2016). PVA has good biocompatibility, and it has been proven to be of much use in many advanced biomedical applications, such as wound dressing (Ahmed et al., 2017; Saeed et al., 2017), drug delivery systems (Tavakoli and Tang, 2017), and contact lens production (Kita et al., 1990). However, the elasticity of the PVA hydrogel is not sufficient to match the elasticity of natural skin since PVA always acts as a stiff membrane and has limited hydrophilicity, which limits its functions when it is applied on its own as a wound dressing polymeric material (Kamoun et al., 2015; Kamoun et al., 2017). The Young's modulus (E) of the skin fluctuates between 0.42 MPa and 0.85 MPa for the torsion tests, between 4.6 MPa and 20 MPa (Pawlaczyk et al., 2013) but Elastic Modulus of 10% PVA is around 0.04 ± 0.01 MPa (Scholten et al., 2011).
The chemical name of chitosan is (1-4)-linked-2-amino-2-deoxy-b-glucan (Suh and Matthew, 2000; Croisier and Jerome, 2013). Chitosan is a by-product of N-deacetylation of chitin. Chitosan is an essential as is abundant constituent of crab and shrimp shells, and cuticles of insects (Bano et al., 2014; Figueiredo et al., 2015); it has excellent biocompatibility and very good wound healing capability and antimicrobial activity (Goy et al., 2016). Chitosan can form membranes after mixing with a proper amount of the cross-linker agent. These membranes present three-dimensional networks, which have good ability to absorb and retain a huge amount of water while being able to maintain their structures. In addition, chitosan membranes are widely used in the field of medicine (such as in tissue engineering, as material for dressing burns, and as a controller of drug release systems) and as packaging material for food (Baldino et al., 2014).
Since a single material or polymer is not able to create the proper environment for application in wound regeneration, to obtain materials of better potential for use as clinical skin substitutes, this study fabricated a composited scaffold by blending gelatin, polyvinyl alcohol, and chitosan together in the proper ratio. The blended scaffolds were tested to see if they could overcome the weakness of a single material, and if they possessed the necessary excellent properties for wound healing. The findings could provide significant information which would help further studies.
MATERIAL AND METHODS
Materials
The materials sourced included the following: gelatin powder from bovine skin (Type B; HiMedia, India), 50% glutaraldehyde solution (Merck, Germany), 0.25% trypsin-EDTA (Gibco, USA), Dulbecco’s modified Eagle Medium (DMEM) powder (Gibco, USA), fetal bovine serum (Gibco, USA), Penicillin-Streptomycin (Gibco, USA), polyvinyl alcohol powder (Loba Chemie, India), and squid chitosan polymers (TMECO, Thailand).
Preparation of the scaffold
A 10wt% gelatin solution was prepared by the following method: gelatin powder was soaked in sterilized water at 50°C in a water bath and it was kept stirring until the solution became homogeneous. Then, a 5wt% PVA solution was prepared: PVA powder was soaked in sterilized water at 80°C and it was kept stirring until the powder was totally dissolved. A 0.5wt% chitosan solution was prepared by soaking chitosan flakes in 1% acetic acid solution and it was kept at room temperature for 1 week. Three types of solutions were mixed together to make up the final concentration of 7% gelatin, 0.5% PVA, and 0.1% chitosan. The mixed solution was stirred in a water bath until the solution became homogeneous. After that, the homogeneous solution was transferred to a blender and blended for 15 sec. After that, 3 ml of the blended solution was mixed with 7.5 µl glutaraldehyde to initiate the cross-link before setting in each of the plates, and all the plates were kept at room temperature for 24 h. Thereafter, all the specimens were rinsed with sterilized water for 15 min, 3 times. All the cross-linked materials were left in the water at room temperature overnight, again. The water was discarded, and the plates were kept in the freezer at −20°C for pre-freezing. All the specimens were dried in a lyophilizer at 662780 MTORR, at a shelf temperature of 21.1°C and a condenser temperature of −84.4°C for 26 h. To analyze the Young’s moduli of the specimens, all the samples were cut into pieces of size 1 cm2. For gelatin scaffolds, 10wt% gelatin solution was blended for 15 sec then the same protocol used for the blended gelatin-PVA-chitosan scaffolds was applied. Then, Young’s moduli of the dry specimens were analyzed using Park XE7 atomic force microscopy (AFM).
AFM protocol
AFM measurements were performed by a XE 70 model (Park system, Korea) in contact mode with NCS36 cantilevers, with tip apex radius of curvature under 10 nm, the scan rate of 1Hz and scan area 5*5 μm2, to study surface morphology and was used to analysis Young’s modulus of scaffolds surface. Young’s modulus was calculated from Hertz and Sneddon model (Jan Domke 1998):
F = (2/π) [E/(1-ν2)]δ2 tan(α)
where F is a loading force, ν is a Poisson ratio of the sample, δ is an elastic indentation, α is a half opening angle of the indenting cone and E is an elastic or Young’s modulus.
Porosity
The volumes of the dry scaffolds were measured to get Vs (both blended scaffolds and gelatin scaffolds). Dry scaffolds were weighed and recorded as Wd (dry weight). The average weight of the scaffolds was calculated: the method of water displacement was applied in this study. All the scaffolds were soaked in sterilized distilled water and kept at room temperature overnight (to make sure that the scaffolds were completely equilibrated with water). The water was discarded, and the residual water was removed with tissue papers, again. The wet samples were weighed again to get Ww (wet weight). The volumes of the water inside the scaffolds were calculated using the following equation to get Vw:
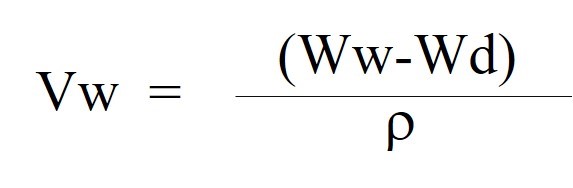
ρ = 1 X 103 kg/m3
The volumes of the dry scaffolds were measured using the following equation to get Vs:
Vs = πr2h
And then, the porosity of the scaffolds was calculated using the following equation:
Porosity = Vw/Vs
Triplicates of gelatin and blended scaffolds were studied for the statistical analysis.
Swelling ratio
Dry scaffolds were weighed and recorded as Wd. After that, the scaffolds were immersed in 70% ethanol for 30 min, and then washed with sterilized distilled water for 15 min, 3 times. PBS was added into the all plates and all the specimens were kept in an incubator at 37°C. All the specimens were weighed again after 3 h, 7 h, and 24 h incubation to get Ws. The swelling ratios of the scaffolds were calculated using the following equation:
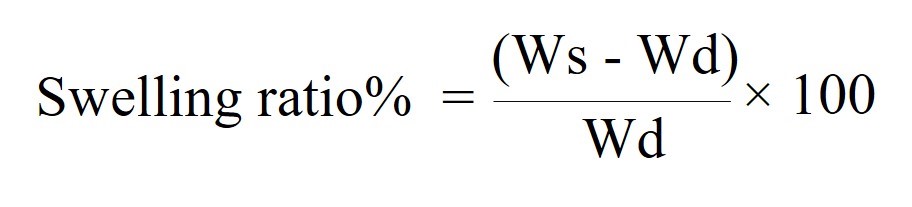
Degradation rate
Dry scaffolds were weighed and recorded as W0. After that, the scaffolds were immersed in 7% alcohol for 30 min, and then washed with sterilized distilled water for 30 min, 3 times. Sterile water was added into all the plates and all of the specimens were left at 4°C overnight in a cold room. The water was discarded, and the residual water was removed with tissue papers, again. A volume of 1 ml of lysozyme solution (1.6 µg/ml) was added to each scaffold, followed by addition of PBS with 1% Pen–Strep to cover the surface of the scaffolds. All the scaffolds were incubated at 37°C. PBS with 1% Pen–Strep was added every 2 days to make sure that all the samples were soaked in the lysozyme solution all the time. After 0, 3, 7, and 10 days of incubation, the scaffolds were washed with distilled water for 15 min, 3 times. All the water was removed from the scaffolds. All the scaffolds were frozen to −20°C before lyophilization. After the lyophilization process, the dry scaffolds were weighed again for calculating the degradation rate. The W0 means the original dry weight of scaffold before the degradation, and the Wt means the dry weight of scaffold after degradation.
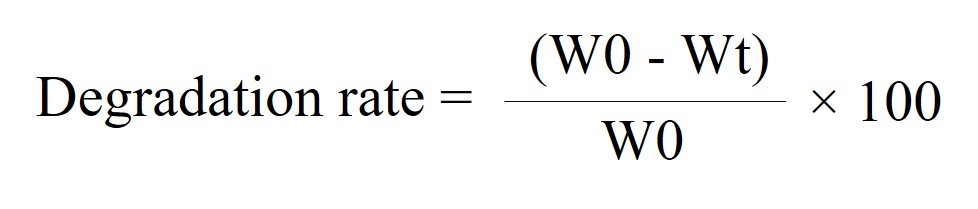
Fibroblast culture
The scaffolds were immersed in 70% alcohol for 30 min, and then washed with sterilized distilled water for 15 min, 3 times. Complete culture medium (10% FBS, 1% Pen/Strep-containing DMEM) was added into all the plates and all of the specimens were kept in the incubator at 37°C for 24 h. The culture medium was discarded and NIH/3T3 fibroblasts were seeded onto the scaffolds at 1.8 × 105 cells and cultured for 24 h. The medium was discarded, and the scaffolds were washed and rinsed with PBS. The NIH/3T3 fibroblasts (in the scaffolds) were fixed with 4% paraformaldehyde for 5–10 min and stained with methylene blue for 5 min. The NIH/3T3 fibroblasts (in scaffolds) were rinsed with tap water until the cells could be observed under an inverted microscope. The magnifying power of the eyepiece was 10x and the magnifying power of the objective lens was 20x (Nikon Eclipse TS100).
Characterization of morphology of scaffolds
The scaffolds were immersed in 70% alcohol for 30 min, and then washed with sterilized distilled water for 15 min, 3 times. The complete culture medium was added into all the plates and all of the specimens were kept in an incubator at 37°C for 24 h. The culture medium was discarded and the NIH/3T3 fibroblasts were seeded onto the scaffolds at 6.5 × 104 cells. The NIH/3T3 cells were cultured on the scaffolds for 4 days. The medium was discarded, and the samples were rinsed with phosphate buffer saline (PBS) once before fixing with 2.5% glutaraldehyde in PBS at 4°C for 1 week. The glutaraldehyde was discarded, and the scaffolds were washed with PBS for 10 min, 3 times. The scaffolds were post-fixed with 2% osmium in PBS for 2 h; then, the solution was discarded, and the scaffolds were washed with PBS for 5 min, 2 times. The samples were sequentially dehydrated in 50% alcohol for 5 min, 2 times; 70% alcohol for 15 min; 85% alcohol for 15 min; 95% alcohol for 15 min; and 100% alcohol for 15 min. The samples were dried using the critical point drying (CPD) procedure and the samples were coated with Au before observing under an SEM (JSM-6610LV Scanning Electron Microscope from JEOL, USA). The pore sizes were measured from the SEM images and the average pore size was calculated.
In vitro relative cell viability test
The scaffolds were immersed in 70% alcohol for 30 min, and then washed with sterilized distilled water for 15 min, 3 times. The complete culture medium was added into all the plates and all of the specimens were kept in an incubator at 37°C for 24 h. The culture medium was discarded and the NIH/3T3 fibroblasts were seeded onto the scaffolds at 6.5 × 104 cells/well and cultured for 4 days. Then, MTT assay was applied to test the relative cell viability. The culture medium was discarded and 2 ml of the culture medium (containing MTT dye 0.5 mg/ml) was added before incubation at 37°C for 2 h. The MTT solution was discarded and the samples were rinsed with PBS once. DMSO (7 ml) was added to each sample before shaking on the rotator for 15 min. The absorbance of DMSO was read at 570 nm and 630 nm using Shimadzu UV Mini 1240 Uv-vis spectrophotometer. The absorbance of DMSO at 630 nm was used as background correction (OD570–OD630 nm). Four pieces of gelatin and blended scaffolds were used for statistical analysis. The percent cell viability was calculated and compared to the control group cultured on 4 wells of tissue culture plates (polystyrene), using the following equation:
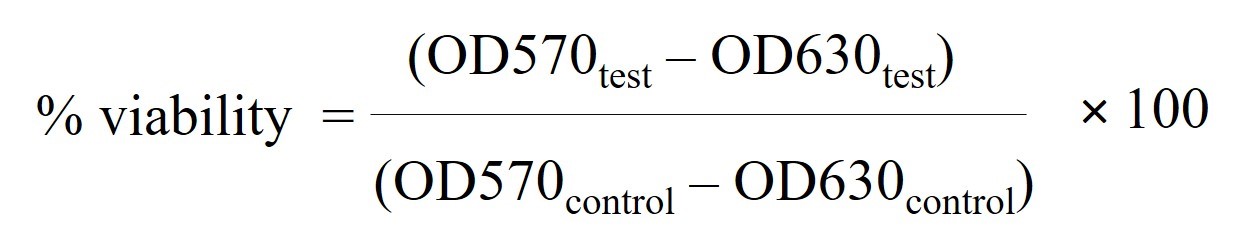
Gene expression
The scaffolds were immersed in 70% alcohol for 30 min, and then washed with sterilized distilled water for 15 min, 3 times. The complete culture medium was added into all the plates and all of the specimens were kept in an incubator at 37°C for 24 h. The culture medium was discarded and the NIH/3T3 fibroblasts were seeded onto the scaffolds at 3 × 104 cells/well and cultured for 10 days. The medium was discarded and rinsed with sterilized PBS. RNA isolation was performed using NucleoSpin RNA - MACHEREY-NAGEL. A volume of 350 µl of RA1 was mixed with 3.5 µl β-mercaptoethanol before adding into each sample to lyse the cells. The lysis solution was filtered for separation of cell lysates by spinning at 11,000 × g for 1 min. A volume of 350 µl of 70% ethanol was added to the filtrate before loading into the silica membrane and spinning at 11,000 × g for 1 min. A volume of 350 µl of MDB was added into the silica membrane and spun at 11,000 × g for 1 min to desalt the silica membrane. A volume of 95 μl of the DNase reaction mixture was added to the silica membrane and incubated at room temperature for 15 min to digest the DNA. The silica membrane was washed with 200 µl of RAW2 and spun at 11,000 × g for 30 s, followed by second washing by 600 µl of RA3 and spinning at 11,000 × g for 30 sec; 250 µl of RA3 was added for the final washing before spinning at 11,000 × g for 2 min. The total RNA was eluted with 60 µl of RNase-free H20 and spun at 11,000 × g for 1 min. The RNA samples were kept at−20°C for the next step. Then the RNA was converted to cDNA using ReverTra Ace -α-® (Toyobo) in an Eppendorf Mastercycler®. The cDNA was synthesized using the following program: priming at 25°C for 5 min; then, transcription at 42°C for 30 min; and, finally, inactivation at 85°C for 5 min. The NO-RT reaction was prepared as a negative control. SYBR Green Master Mix (SensiFAST SYBR® No-ROX Kit-Bioline) was applied for real-time PCR. The cDNA was diluted at a dilution of 1:2; the 10 µl reaction was composed of 5 µl of SYBR Green RT-PCR Master Mix, 4 µl of cDNA, and 1 µM of target-specific primer. LightCycler® 480 (Roche) was applied for RT-PCR. The polymerase chain reaction protocol consisted of 95°C pre-incubation for 2 min, followed by 40 cycles of 95°C for 5 sec, then 60°C for 10 sec, and, finally, 72°C for 20 sec. The melting peak analysis was performed at 95°C for 5 sec, 65°C for 1 min, and 97°C continuously. The cooling step was performed at 40°C for 30 sec. 18sRNA and GAPDH were used as reference genes. Relative quantification was performed with LightCycler® 480 software 1.5. The primers used in this study are shown in Table 1.
Table 1. List of the primer sequences used in this study.
|
Target gene |
Primer sequence (5'->3') |
|
1. 18s rRNA |
F: GGCCCTGTAATTGGAATGAGTC |
|
R: CCAAGATCCAACTACGAGCTT |
|
|
2. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) |
F: AAATCCCATCACCATCTTCCAGGAGC |
|
R: CATGGTTCACACCCATGACGAACA |
|
|
R: GATGCAGAACTTGGAACTAT |
|
|
3. COL4A1 (Type IV collagen) |
F: CGCTTACAGCTTTTGGCTCG |
|
R: GACGGCGTAGGCTTCTTGAA |
Statistical analysis
Triplicate of gelatin and blended scaffolds were studied for the statistical analysis. The results were presented as mean ± SD. The P-value was calculated using independent t-test. Statistically significant was determined when P< 0.05.
RESULTS
Characterization of scaffolds with AFM
Both gelatin scaffolds and blended scaffolds are shown in Figure 1. Young’s modulus of dried scaffolds was tested by AFM, and the results are as shown in Figure 2. Young’s moduli of the gelatin and the blended scaffolds were 53.30 ± 26.80 kPa and 98.01 ± 17.50 kPa, respectively. The P -value is 0.0001, this difference is considered to be extremely statistically significant. The blended scaffolds had higher Young’s moduli than the gelatin scaffolds, which implies that blended scaffolds possess stronger structure than gelatin scaffolds.
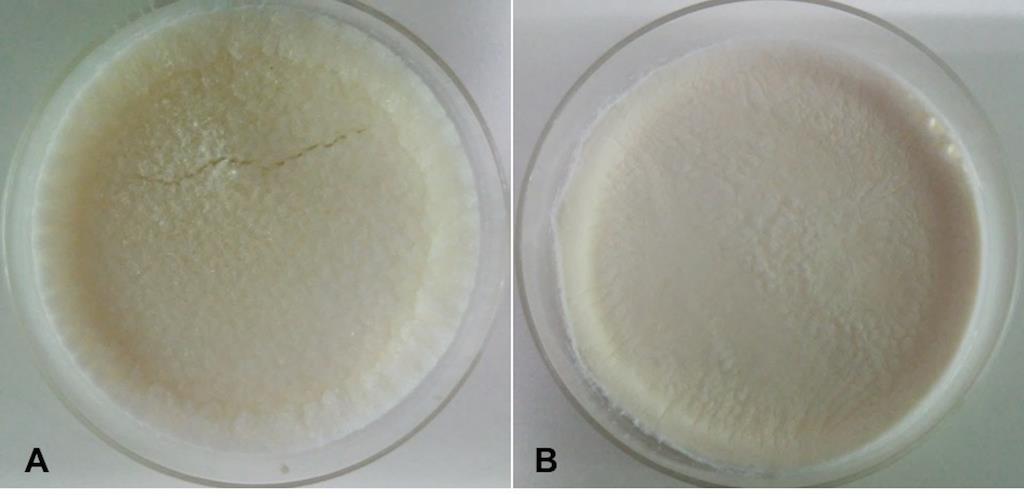
Figure 1. (A) Dried gelatin scaffold. (B) Dried blended scaffold.
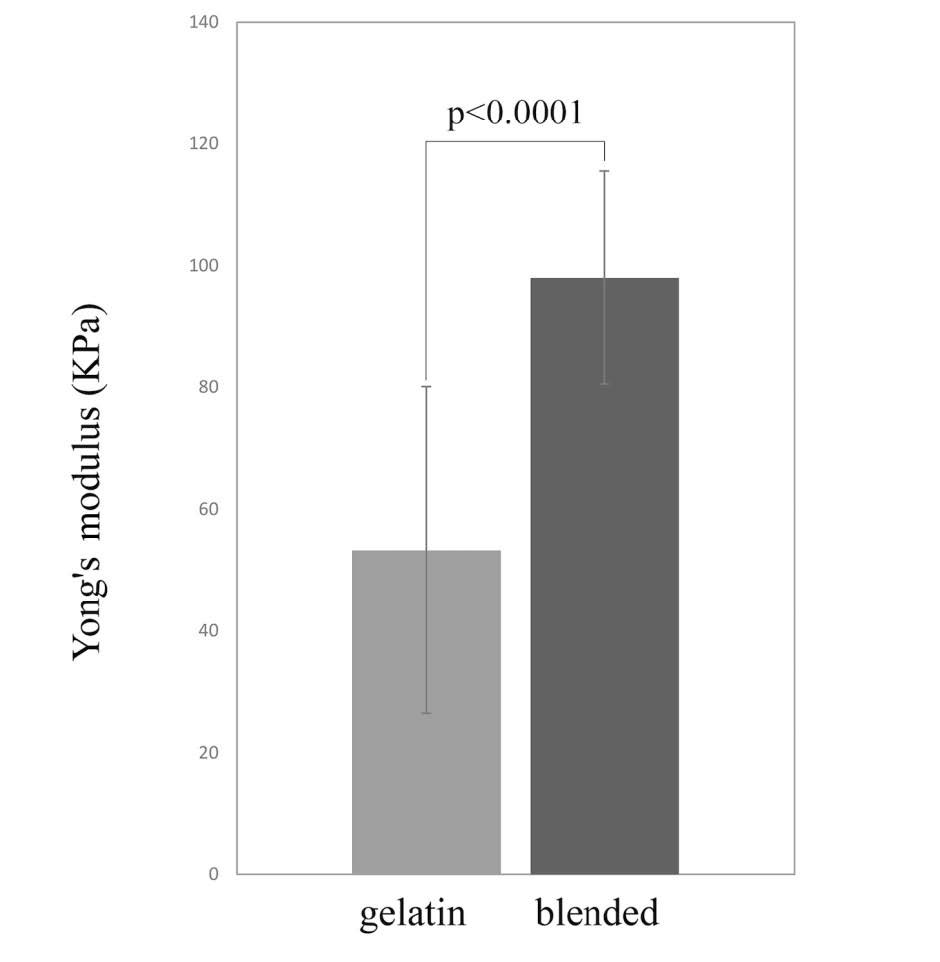
Figure 2. Young’s modulus of gelatin scaffolds and blended scaffolds. The blended scaffolds had higher Young’s modulus compared to gelatin scaffolds. The P-value is less than 0.0001.
Porosity measurement
Porosity was tested by using the water displacement method based on the difference in weights between the dry and the wet scaffolds. Figure 3 shows that gelatin scaffolds presented higher porosity than blended scaffolds. The porosity of the blended scaffolds was 21.48 ± 1.01%, while the porosity of gelatin scaffolds was 85.41 ± 2.11%. Student t test were used to find P-value and statistical significance: P value is less than 0.0001.
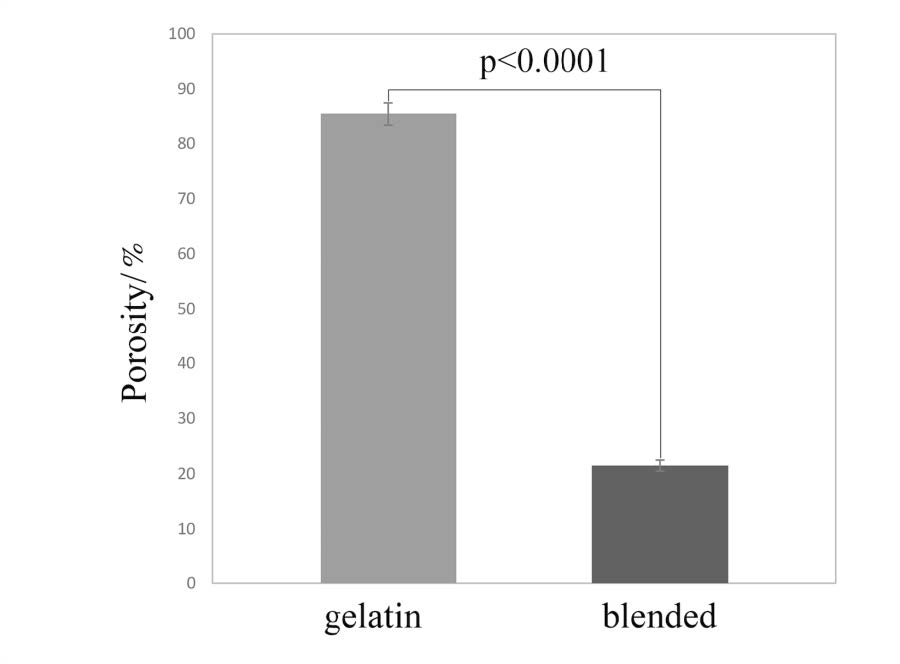
Figure 3. Porosity of gelatin scaffolds and blended scaffolds. Gelatin scaffolds possessed higher porosity compared to blended scaffolds.
Swelling ratio measurement
Table 2 presents the swelling ratios of both the gelatin and the blended scaffolds at 3 h, 7 h, and 24 h, respectively. The result showed that both the blended scaffolds and the gelatin scaffolds had very good swelling capacity. However, the swelling capacity of the gelatin scaffolds was higher than that of the blended scaffolds. In both types of scaffolds, the swelling capacity was observed to increase with time, the swelling capacity at 24 h was more than that at 3 h.
Table 2. Swelling Capacity of gelatin scaffolds and blended scaffolds investigated at different time intervals: 3 h, 7 h, and 24 h.
|
Swelling ratio |
3 h |
7 h |
24 h |
||
|
gelatin scaffold |
1189.56 ± 147.23 |
1442.28 ± 143.65 |
1904.81 ± 166.06 |
||
|
blended scaffold |
863.84 ± 97.32 |
1126.65 ± 58.52 |
1474.59 ± 65.50 |
||
Note: Results show as mean and standard error of both types of scaffolds’ swelling capacity.
In vitro degradation rate test
The degradation rate was tested at 0, 3, 7, and 10 days, and the results are as illustrated in Figure 4. The degradation rate of the gelatin scaffolds was higher than that of the blended scaffolds.
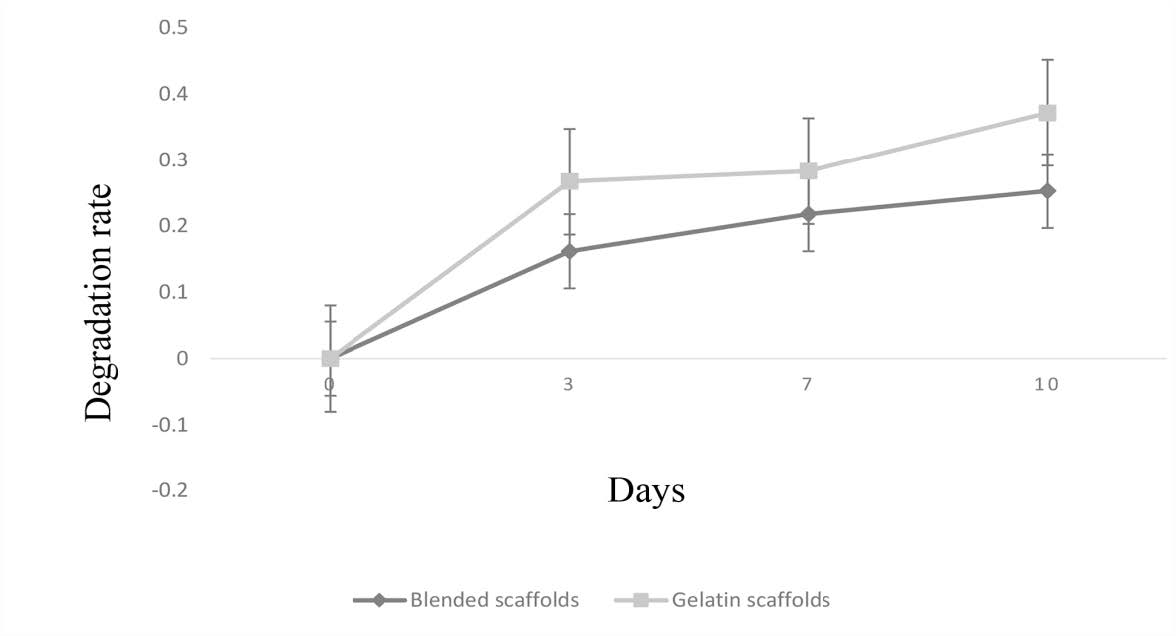
Figure 4. Degradation rate of gelatin scaffolds and blended scaffolds from 0 day to 10 days. Gelatin scaffolds showed higher degradation rate compared to blended scaffolds.
Cell culture
NIH/3T3 cells cultured on gelatin and blended scaffolds for 24 h are shown in Figure 5. The cells were aggregated on the gelatin scaffolds, while they were spread on the blended scaffolds. At 4 days of culture, the NIH/3T3 cells cultured on the gelatin and the blended scaffolds were observed with SEM, and the results are as shown in Figure 6. The result demonstrates that the average pore size of the gelatin scaffolds was 336.3 3 ± 52.25 µm, while the average pore size of the blended scaffolds was 68.17 ± 8.91 µm. NIH/3T3 proliferated and covered the surfaces of both the scaffolds at 4 days of culture.
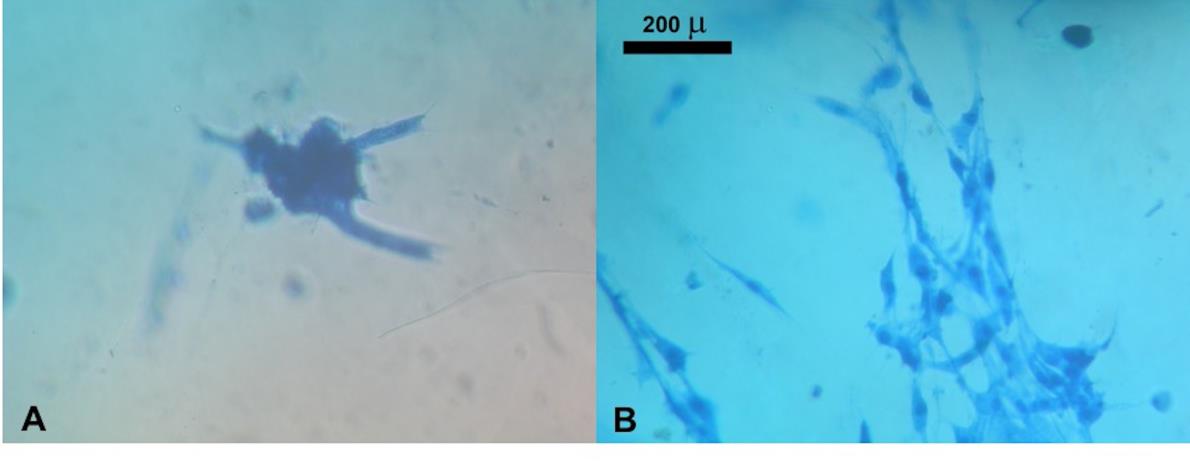
Figure 5. Morphology of NIH/3T3 fibroblasts cultured on gelatin scaffolds and blended scaffolds for 24 h. Cells were spread with good morphology on blended scaffolds, but they were aggregated on gelatin scaffolds.
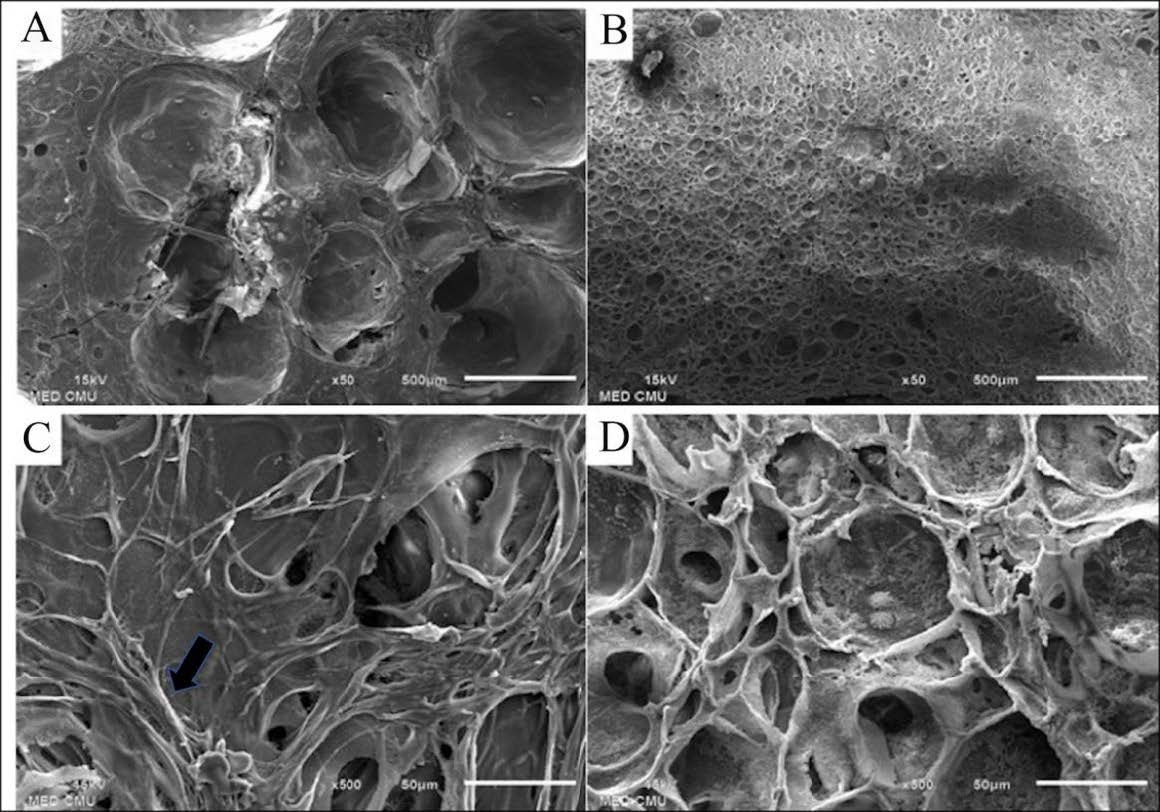
Figure 6. SEM imaging of gelatin scaffolds and blended scaffolds seeded with NIH/3T3 fibroblasts. (A&B) SEM imaging of gelatin scaffolds and blended scaffolds at 50 x magnification showed that pore size of gelatin scaffolds was bigger than blended scaffolds. (C&D) At 4 days cultivation NIH/3T3 fibroblasts could proliferate and cover the surfaces of both gelatin scaffolds and blended scaffolds (500 x magnification). The black arrow indicated the layer of fibroblasts on the scaffold.
In vitro relative cell viability
The result of the MTT assay is as shown in Figure 7. The %viability of the gelatin and the blended scaffolds were compared to that of the control cells on tissue culture plates. The relative cell v iability of the gelatin and the blended scaffolds were 12.82%, 30.16%, and p-value equals 0.0003 respectively. The color of the formazan product produced on the control group, the gelatin scaffolds, and the blended scaffolds was correlated with the result of the %viability, as shown in Figure 8.
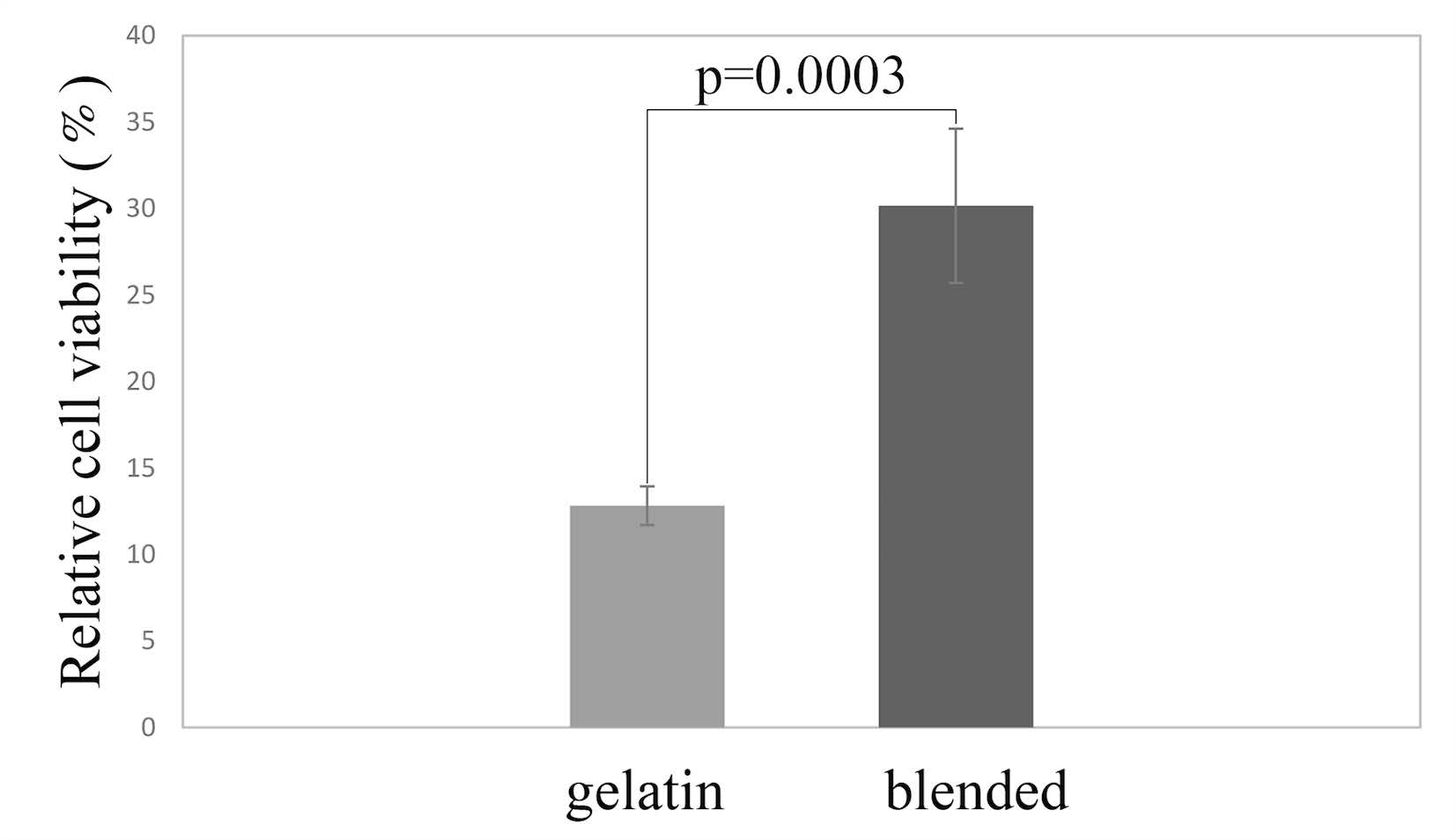
Figure 7. Relative cell viability of NIH/3T3 fibroblasts on gelatin scaffolds was lower than that of blended scaffolds.
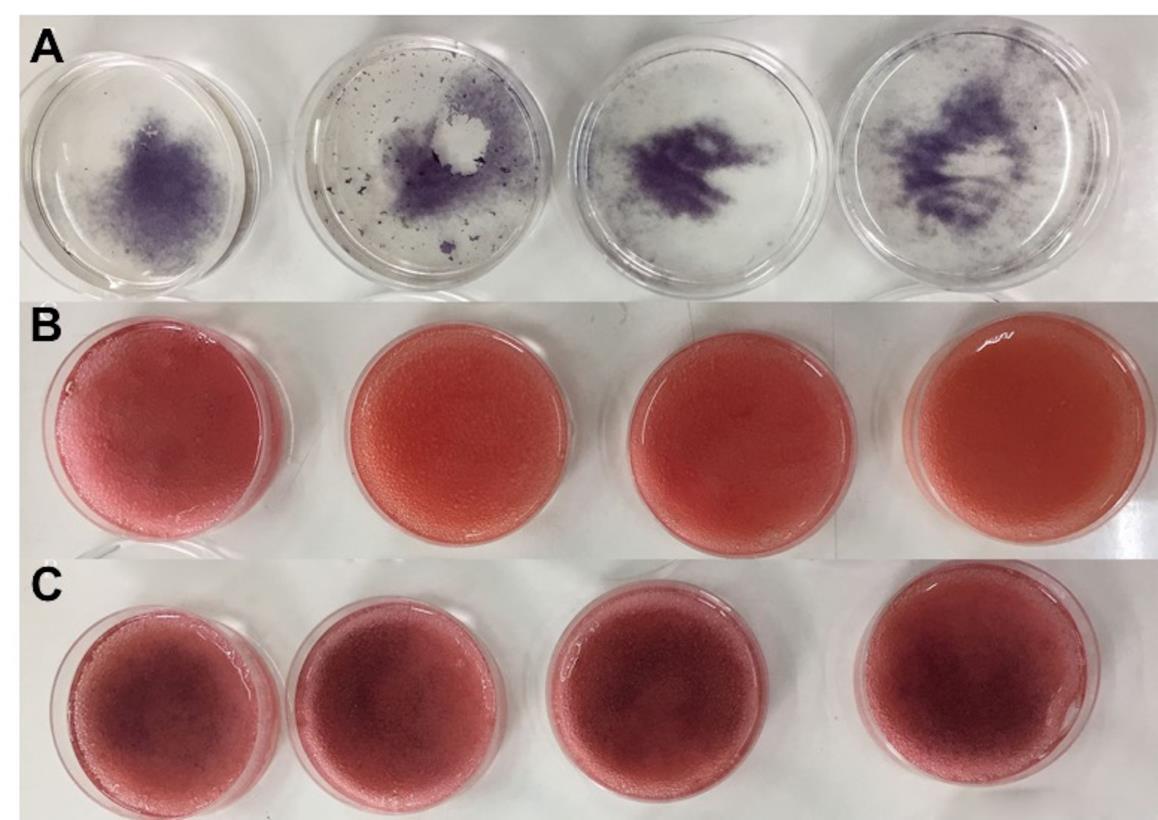
Figure 8. The purple color of formazan product on (A) tissue culture plates, (B) gelatin scaffolds and (C) blended scaffolds. The color of formazan product was correlated to the %cell viability.
Gene expression
Figure 9 presents the relative expression of collagen type IV in NIH/3T3 cells. At 10 days of culture, the blended scaffolds showed higher expression of collagen type IV than the gelatin scaffolds. Cells on both the types of scaffolds showed higher collagen type IV expression when compared to cells on the tissue culture plates. P-value of gelatin group and blended group is less than 0.0011, which is considered to be very statistically significant.
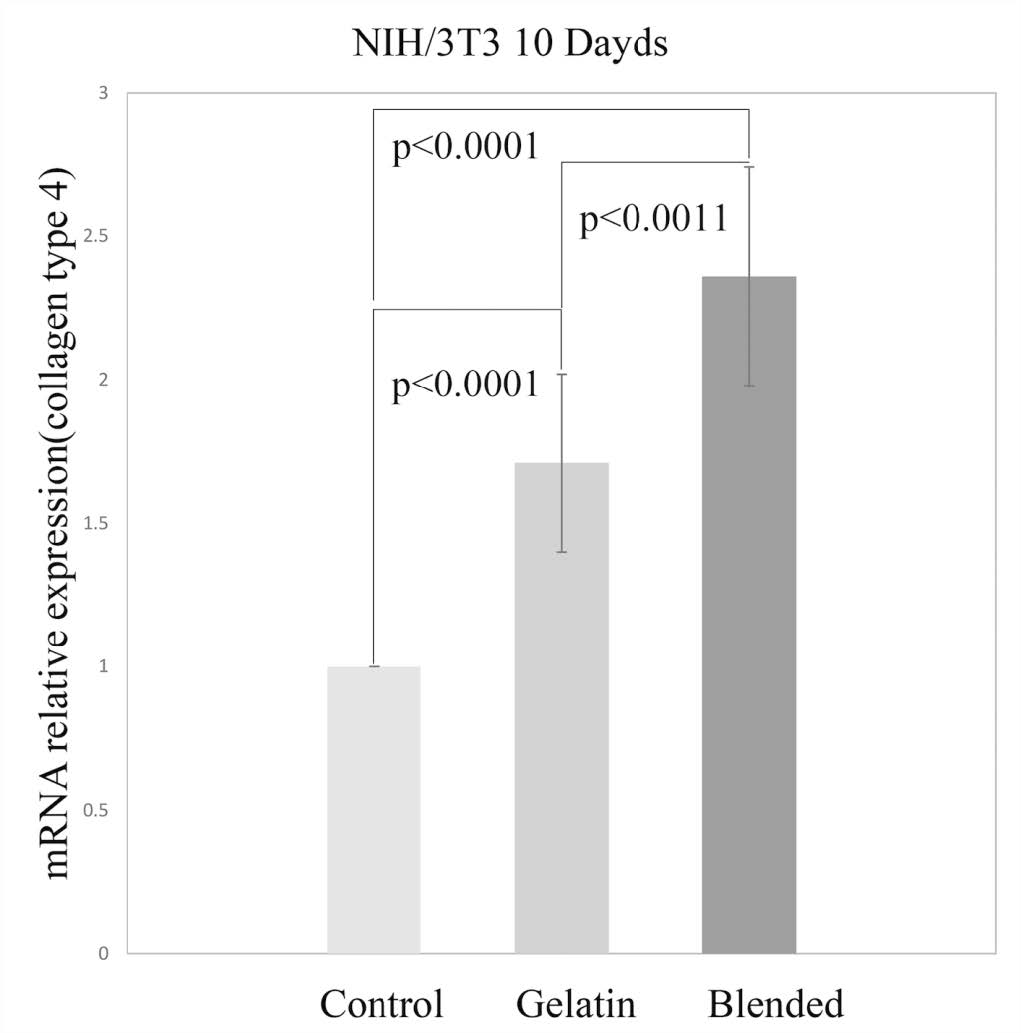
Figure 9. The relative expression of collagen type 4 at 10 days culture. NIH/3T3 fibroblasts on blended scaffold showed higher collagen type4 expression than that of gelatin scaffolds. The P-value1 was gotten from control group and gelatin group; P-value2 from control group and blended group; P-value3 from gelatin group and blended group.
DISCUSSION
After gelatin was polymerized, it was rinsed with sterilized water for 15 min, 3 times. After that, gelatin was left in sterile distilled water at room temperature overnight. In the next day, water was discarded, and gelatin was kept in the freezer at −20°C before freeze drying. Gelatin was kept as dried scaffolds. In this step, most of glutaraldehyde was washed out because glutaraldehyde could interfere with freeze drying process resulting in cracking of dried scaffold. Moreover, before use, the dried scaffolds were immersed in 70% alcohol for 30 min, and then washed with sterilized distilled water for 15 min, 3 times before equilibration in complete culture medium in the incubator at 37°C for 24 h. There were many steps of washing so glutaraldehyde should be washed out. In this study, the result demonstrated that porosity of gelatin scaffolds is higher than that of blended scaffolds; then, obviously, the swelling ratio of gelatin scaffolds is higher too. Besides, the higher porosity and the larger pore size also allowed more water to enter the scaffold (Liu et al., 2014; Song et al., 2015). The higher porosity and the larger pore size contributed toward lowering the Young’s modulus of the gelatin scaffolds compared to the blended scaffolds (Yu et al., 2008). The swelling capacity of gelatin scaffolds is higher than that of blended scaffolds. A previous study mentioned that the molecular chain movement in blended scaffold was more limited, so lesser amount of water could be absorbed when the degree of cross-linking was higher (Song et al., 2015). One of the most important functions of a scaffold is maintaining of an optimal degradation rate so that the scaffold would be able to facilitate the growth of cells and tissues (Ye et al., 2014). This study used lysozyme because lysozyme is an enzyme present in certain human body fluids; so, lysozyme played a very important role in the biodegradation of the scaffolds (Baniasadi et al., 2015). The concentration of lysozyme was adjusted to be in accordance with the human serum concentration 0.95-2.45 mg/mL (~1.7 µg/mL) (Brouwer et al., 1984; Sydow et al., 2019). We could not find the concentration of skin lysozyme but we assumed that it should be lower than serum lysozyme concentration so we used the concentration of 1.6 µg/mL. The degradation rate of the gelatin scaffolds was higher than that of the blended scaffolds because the bigger pore size allowed more solution to pass into the gelatin scaffolds and promoted the degradation. More importantly, the higher content of the hydrophilic glycolic acid in gelatin facilitated the absorption and diffusion of water and promoted the hydrolysis (Wu and Ding, 2004; Liu et al., 2014). The blended scaffolds were able to provide a more stable environment for cell growth since cells prefer harder structures to proliferate. Herein this study, the final concentration of 7% gelatin, 0.5% PVA and 0.1% chitosan was chosen as the optimal ratios. If the higher chitosan and PVA concentration were used, the cross-linking of gelatin would be insufficient (Song et al., 2015). Previous research mentioned that the swelling ratio of the hybrid hydrogel scaffold is not only related to the degree of cross-linking but also proportional to the total concentration of the hydrogel scaffold. Movement of the molecular chains is more limited in blended scaffolds, so lesser amount of water is absorbed when the degree of cross-linking is higher (Song et al., 2015). The blended scaffolds possessed higher degree of cross-linking, which contributed to the lower swelling ratio compared to the gelatin scaffolds.
A previous study reported that there are specific integrin–ligand interactions between the cell and the surrounding ECM, which can influence cell attachment and migration. Therefore, it is very important for scaffolds to possess relatively high surface area for optimal cell attachment. Numerous researchers have found that the specific surface area decreases with increasing pore size. As a result, it was hypothesized that cell attachment would decrease linearly with increasing pore size (O’Brien FJ, 2005; Murphy et al., 2010). Because of these reasons, the surface area of the gelatin scaffolds decreased with increasing pore size, which led to a lower degree of cell survival than that in the blended scaffolds. The collagen type IV expression was used as a biomarker of skin formation because Type IV collagen is a type of collagen found primarily in the skin within the basement membrane zone or the dermal–epidermal junction, where it is mostly found in the lamina densa (Abreu-Velez and Howard, 2012; Matsuura-Hachiya et al., 2017). A previous study showed that a reasonably designed scaffold should be able to regulate cell morphology which, in turn, would regulate cellular functionality, such as proliferation and differentiation (Kumar et al., 2011).
The blended scaffolds had more surface area and more porosity which facilitated the transportation of nutrients and metabolites and supported cell adhesion, while gelatin scaffold led to the lesser surface area which contributed to the limitation in cell adhesion, and the cells simply migrated through the scaffold. Then the level of cell-to-cell contact was low, which resulted in the low proliferation rate (Murphy et al., 2010; Tsai et al., 2014), and this could be confirmed by MTT assay. The relative cell viability of the blended scaffolds was much higher than that of the gelatin scaffolds. For these reasons, the blended gelatin–PVA–chitosan scaffold got better results with regard to collagen type IV expression than the gelatin scaffold at 10 days. Thus, the blended gelatin–PVA–chitosan scaffold has better condition for cell survival than the gelatin scaffold. This research study demonstrated that blended scaffolds have better properties than pure gelatin scaffolds, which implies that blended scaffolds can provide a better environment for cell proliferation and differentiation
In conclusion, it can be stated that this research study demonstrated that blended gelatin–PVA–chitosan scaffolds have the potential to be used for skin regeneration. The blended scaffold has a more controllable degradation rate, which plays a very important role in terms of its usability as wound dressing material. When compared to pure gelatin scaffolds, the average pore size of blended scaffolds is more suitable for promoting cell growth because blended scaffolds provide more surface area. The fibroblasts were able to survive and proliferate on both gelatin scaffolds and blended scaffolds; however, the blended scaffolds produced better results. The change in the gene expression upon using real-time PCR reflected that the blended scaffolds were able to provide a better environment for fibroblast proliferation and gene expression. The blended scaffolds provided better biocompatibility, which is very promising for advanced biomedical applications.
CONCLUSIONS
In conclusion, this research showed that the blended gelatin-PVA-chitosan scaffolds had the potential to be used for skin regeneration. The blended scaffold has the more controllable degradation rate, which played a very important role in terms of wound dressing material. Compared with the pure gelatin scaffolds, the average pore size of blended scaffolds is more suitable for promoting cell growth because blended scaffolds provided more surface area. The fibroblasts were able to survive and proliferate on both gelatin scaffolds and blended scaffolds, however, the blended scaffolds got a better result. Change of gene expression using real time PCR reflected that the blended scaffolds were able to provide a better environment for fibroblast proliferation and gene expression. Blended scaffolds provided a better biocompatibility which was very promising for advanced biomedical applications.
ACKNOWLEDGMENT
The authors are grateful to Prof. Kidoaki, Institute for Materials Chemistry and Engineering, Kyushu University, for providing NIH/3T3 fibroblasts. The authors would like to thank Dr. Nutjeera Intasai for the MTT dye and the Dental Research Center, Faculty of Dentistry, Chiang Mai University, for supporting the study by providing LightCycler® 480. The authors would also like to acknowledge the support received from the National Research Council of Thailand (NRCT) [grant number 170327].
REFERENCES
Abreu-Velez, A.M., and Howard, M.S. 2012. Collagen IV in normal skin and in pathological processes. North American Journal Medical Sciences. 4(1): 1-8. https://doi.org/10.4103/1947-2714.92892
Ahmed, A.S., Mandal, U.K., Taher, M., Susanti, D., and Jaffri, J.M. 2017. PVA-PEG physically cross-linked hydrogel film as a wound dressing: experimental design and optimization. Pharmaceutical Development and Technology. 23(8): 1-25. https://doi.org/10.1080/10837450.2017.1295067
Baldino, L., Cardea, S., De Marco, I., and Reverchon, E. 2014. Chitosan scaffolds formation by a supercritical freeze extraction process. The Journal of Supercritical Fluids. 90: 27-34. https://doi.org/10.1016/j.supflu.2014.03.002
Baniasadi, H., Ramazani, S.A.A., and Mashayekhan, S. 2015. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. International Journal Biological Macromolcules. 74: 360-366. https://doi.org/10.1016/j.ijbiomac.2014.12.014
Bano, I., Ghauri, M.A., Yasin, T., Huang, Q., and Palaparthi A.D. 2014. Characterization and potential applications of gamma irradiated chitosan and its blends with poly(vinyl alcohol). International Journal of Biological Macromolcules. 65: 81-88.
Brouwer, J., van Leeuwen-Herberts, T., and Otting-van de Ruit, M. 1984. Determination of lysozyme in serum, urine, cerebrospinal fluid and feces by enzyme immunoassay. Clinica Chimica Acta. 142(1): 21-30. https://doi.org/10.1016/0009-8981(84)90097-4
Croisier, F., and Jerome, C. 2013. Chitosan-based biomaterials for tissue engineering. European Polymer Journal. 49(4): 780-792. https://doi.org/10.1016/j.eurpolymj.2012.12.009
Figueiredo, L., Moura, C., Pinto, L., Ferreira, F., and Rodrigues, A. 2015. Processing and characterization of 3D dense chitosan pieces, for orthopedic applications, by adding plasticizers. Procedia Engineering.110: 175-182. https://doi.org/10.1016/j.proeng.2015.06.182
Goy, R., Morais, S., and Assis O. 2016. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E-coli and S. aureus growth. Revista Brasileira De Farmacognosia-Brazilian Journal of Pharmacognosy.26(1): 122-127. https://doi.org/10.1016/j.bjp.2015.09.010
Huang, S., and Fu, X. 2011. Tissue-engineered skin: bottleneck or breakthrough. International Journal Burns Trauma. 1(1): 1-10.
Jan, D., and Manfred, R. 1998. Measuring the elastic properties of thin polymer filmswith the atomic force microscope. 14: 3320-3325. https://doi.org/10.1021/la9713006
Kamoun, E., Chen, X., Eldin, M., and Kenawy, E. 2015. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: a review of remarkably blended polymers. Arabian Journal of Chemistry. 8(1): 1-14. https://doi.org/10.1016/j.arabjc.2014.07.005
Kamoun, E.A., Kenawy, E.S, and Chen, X. 2017. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. Journal of Advanced Research. 8(3): 217-233. https://doi.org/10.1016/j.jare.2017.01.005
Kantawong, F., Kuboki, T., and Kidoaki, S. 2015. Redox gene expression of adipose-derived stem cells in response to soft hydrogel. Turkish Journal of Biology. 39 (5): 682-691. https://doi.org/10.3906/biy-1412-29
Kantawong, F., Tanum, J., Wattanutchariya, W., and Sooksaen, P. 2016. Variation of hydroxyapatite content in soft gelatin affects mesenchymal stem cell differentiation. Brazilian Archives Of Biology And Technology. 59. https://doi.org/10.1590/1678-4324-2016150650
Kita, M., Ogura, Y., Honda, Y., Hyon, S.H., Cha, W., and Ikada, Y. 1990. Evaluation of polyvinyl alcohol hydrogel as a soft contact lens material. Graefe's Archive for Clinical and Experimental Ophthalmology. 228(6): 533-537. https://doi.org/10.1007/bf00918486
Kumar, G., Tison, C.K., Chatterjee, K., Pine, P.S., McDaniel, J.H., Salit, M.L., Young, M.F., and Simon, C.G.Jr. 2011. The determination of stem cell fate by 3D scaffold structures through the control of cell shape. Biomaterials. 32(35): 9188-9196. https://doi.org/10.1016/j.biomaterials.2011.08.054
Liu, Y.S., Huang, Q.L., Kienzle, A., Muller, W.E., and Feng, Q.L. 2014. In vitro degradation of porous PLLA/pearl powder composite scaffolds. Materials Science & Engineering. C, Materials for Biological Applications. 38: 227-234. https://doi.org/10.1016/j.msec.2014.02.007
MacNeil, S. 2007. Progress and opportunities for tissue-engineered skin. Nature. 445(7130): 874-880. https://doi.org/10.1038/nature05664
Matsuura-Hachiya, Y., Arai, K.Y., Muraguchi, T., Sasaki, T., and Nishiyama, T. 2017. Type IV collagen aggregates promote keratinocyte proliferation and formation of epidermal layer in human skin equivalents. Experimental Dermatology. 27(5): 443-448. https://doi.org/10.1111/exd.13328
Murphy, C.M., Haugh, M.G., and O'Brien, F.J. 2010. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 31(3):461-466. https://doi.org/10.1016/j.biomaterials.2009.09.063
O'Brien, F.J., Harley, B.A., Yannas, I.V., and Gibson, L.J. 2005. The effect of pore size on cell adhesion in collagen-GAG scaffold. Biomaterials. 26: 433-441. https://doi.org/10.1016/j.biomaterials.2004.02.052
Parvez, S., Rahman, M., Khan, M., Khan, M., Islam, J., Ahmed, M., Rahman, M., and Ahmed, B. 2012. Preparation and characterization of artificial skin using chitosan and gelatin composites for potential biomedical application. Polymer Bulletin. 69(6): 715-731. https://doi.org/10.1007/s00289-012-0761-7
Pawlaczyk, M., Lelonkiewicz, M., and Wieczorowski, M. 2013. Age-dependent biomechanical properties of the skin. Postepy Dermatol Alergol. 30 (5): 302-306. https://doi.org/10.5114/pdia.2013.38359
Rendon, M.I., Berson, D.S., Cohen, J.L., Roberts, W.E., Starker, I., and Wang, B. 2010. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. Journal of Clinical and Aesthetic Dermatology. 3(7):32-43.
Saeed, S.M., Mirzadeh, H., Zandi, M., and Barzin, J. 2017. Designing and fabrication of curcumin loaded PCL/PVA multi-layer nanofibrous electrospun structures as active wound dressing. Progress in Biomaterials. 6(1-2): 39-48. https://doi.org/10.1007/s40204-017-0062-1
Santoro, M., Tatara, A.M., and Mikos, A.G. 2014. Gelatin carriers for drug and cell delivery in tissue engineering. Journal of Controlled Release. 190: 210-218. https://doi.org/10.1016/j.jconrel.2014.04.014
Scholten, P.M., Ng, K.W., Joh, K., Serino, L.P., Warren, R.F., Torzilli, P.A., and Maher, S.A. 2011. A semi-degradable composite scaffold for articular cartilage defects. Journal of Biomedical Materials Research, Part A. 97 (1): 8-15. https://doi.org/10.1002/jbm.a.33005
Shevchenko, R.V., Eeman, M., Rowshanravan, B., Allan, I.U., Savina, I.N., Illsley, M., Salmon, M., James, S.L., Mikhalovsky, S.V., and James, S.E. 2014. The in vitro characterization of a gelatin scaffold, prepared by cryogelation and assessed in vivo as a dermal replacement in wound repair. Acta Biomater. 10(7): 3156-3166.https://doi.org/10.1016/j.actbio.2014.03.027
Shishatskaya, E.I., Nikolaeva, E.D., Vinogradova, O.N., and Volova, T.G. 2016. Experimental wound dressings of degradable PHA for skin defect repair. Journal of Materials Sciences, Materials in Medicine. 27(11): 165. https://doi.org/10.1007/s10856-016-5776-4
Song, K., Li, L., Li, W., Zhu, Y., Jiao, Z., Lim, M., Fang, M., Shi, F., Wang, L., and Liu, T. 2015. Three-dimensional dynamic fabrication of engineered cartilage based on chitosan/gelatin hybrid hydrogel scaffold in a spinner flask with a special designed steel frame. Materials Science & Engineering. C, Materials for Biological Application. 55: 384-392. https://doi.org/10.1016/j.msec.2015.05.062
Suh, J.K., and Matthew, H.W. 2000. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 21 (24): 2589-2598. https://doi.org/10.1016/s0142-9612(00)00126-5
Sydow, S., Aniol, A., Hadler, C., and Menzel H. 2019. Chitosan-azide nanoparticle coating as a degradation barrier in multilayered polyelectrolyte drug delivery systems. Biomolecules. 9(10). https://doi.org/10.3390/biom9100573
Tavakoli, J., and Tang., Y. 2017. Honey/PVA hybrid wound dressings with controlled release of antibiotics: Structural, physico-mechanical and in-vitro biomedical studies. Materials Science and Engineering. C, Materials for Biological Application. 77(1): 318-325. https://doi.org/10.1016/j.msec.2017.03.272
Tsai, R.Y., Hung, S.C., Lai, J.Y., Wang, D.M., and Hsieh, H.J. 2014. Electrospun chitosan–gelatin–polyvinyl alcohol hybrid nanofibrous mats: Production and characterization. Journal of the Taiwan Institute of Chemical Engineers. 45(4): 1975-1981. https://doi.org/10.1016/j.jtice.2013.11.003
Vashisth, P., Nikhil, K., Roy, P., Pruthi, P.A., Singh, R.P., and Pruthi, V. 2016. A novel gellan-PVA nanofibrous scaffold for skin tissue regeneration: Fabrication and characterization. Carbohydrate Polymers. 136: 851-859. https://doi.org/10.1016/j.carbpol.2015.09.113
Wu, L., and Ding, J. 2004. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 25(27): 5821-5830. https://doi.org/10.1016/j.biomaterials.2004.01.038
Yang, J.D., Cho, I.G., Kwon, J.H., Lee, J.W., Choi, K.Y., Chung, H.Y., and Cho, B.C. 2016. Feasibility of the Use of RapiGraft and Skin Grafting in Reconstructive Surgery. Archives of Plastic Surgery. 43(5): 418-423. https://doi.org/10.5999/aps.2016.43.5.418
Ye, M., Mohanty, P., and Ghosh, G. 2014. Morphology and properties of poly vinyl alcohol (PVA) scaffolds: impact of process variables. Materials Science & engineering. C, Materials for Biological Applications. 42: 289-294. https://doi.org/10.1016/j.msec.2014.05.029
Yu, H., Matthew, H.W., Wooley, P.H., and Yang, S.Y. 2008. Effect of porosity and pore size on microstructures and mechanical properties of poly-epsilon-caprolactone- hydroxyapatite composites. Journal of Biomedical Materials Research. Part B, Applied Biomaterials. 86(2): 541-547. https://doi.org/10.1002/jbm.b.31054
Hongxiang Yin1, Suruk Udomsom1, and Fashai Kantawong2*
1Biomedical Engineering Institute, Chiang Mai University, Chiang Mai 50200, Thailand
2Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. Email: fahsai.k@cmu.ac.th
Total Article Views
Article history:
Received: December 3, 2019;
Revised: March 18, 2020;
Accepted: March 20, 2020

