
Role of Amino Acid Cysteine in the Suppression of Xanthomonas citri pv. glycines Bacterial Pustule Disease of Soybean
Namfon Pluemjit, Supisara Sripo-ngam, Ekkachai Khwanbua, and Tiyakhon Chatnaparat*Published Date : 2020-09-01
DOI : https//:doi.org/10.12982/CMUJNS.2020.0052
Journal Issues : Number 4, October-December 2020
ABSTRACT Bacterial pustule is a major bacterial disease of soybean caused by Xanthomonas citri pv. glycines. The discovery of safety organic compounds that can control plant disease could provide an alternative strategy that may help reduce the pathogen resistance against antibiotics. Thus, the role of amino acids in suppressing the virulence of X. citri pv. glycines 12-2 was investigated. X. citri pv. glycines 12-2 were grown in M9 minimal medium (M9) and rich medium nutrient broth (NB) supplemented with each of 11 amino acids at 10 mM for tested the ability of amino acids to inhibit the growth of the pathogen. Cysteine, serine, and threonine were found to be the most effective amino acids to inhibit the growth of X. citri pv. glycines in M9 minimal medium but only cysteine could significantly inhibit the growth of X. citri pv. glycines in NB. Minimum inhibitory concentration (MIC) value of cysteine in inhibiting the growth of X. citri pv. glycines was 2.5 mM. We demonstrated that a short time exposure of 10 mM cysteine caused a cell death rather than just inhibiting the growth of X. citri pv. glycines 12-2 when determined by live/dead cells staining. Foliar application of cysteine mixed with X. citri pv. glycines on soybean were able to reduce the severity of bacterial pustule on soybean. In addition, glutamic acid and threonine have a protective effect on bacterial pustule disease. The results presented here indicated amino acids cysteine has the potential to be used for managing bacterial pustules of soybean.
Keywords: Soybean, Bacterial pustule, Plant disease control, Amino acids, Antibacterial compounds
INTRODUCTION
Bacterial pustule is one of the most serious diseases of soybean in several parts of the world including Thailand. In Thailand, the disease can occur at any time and is strongly favored by warm weather with frequent showers (Prathuangwong and Amnuaykit, 1987). The causal agent of bacterial pustule of soybean is a gram-negative bacteria Xanthomonas citri pv. glycines. This bacterium enters in plant tissue through stomata or wound and multiplies in the intercellular spaces (apoplast) of leaves. A typical symptom of the bacterial pustule disease is a pustule leaf spot, formed by hypertrophy and hyperplasia of parenchyma cells surrounded by chlorotic (yellow halos) on leaves (Groth and Braun, 1986). Severe disease results in premature defoliation and decreasing yield and seed quality (Morgan 1963; Weber et al., 1966).
The ability of X. citri pv. glycines to gain entry to and proliferate inside the plant depends on several virulence factors include a type III secretion system (T3SS) that delivers type III effectors (T3Es) into the plant cell; the exoenzymes cellulase, protease, endo-β-1,4-mannanase, and pectate lyases; quorum sensing system; the secreted plant hormones indole acetic acid and cytokinin (Kaewnum et al., 2006; Thowthampitak et al., 2008; Athinuwat et al., 2009). In addition, several nutrient utilization and virulence factors present in X. citri pv. glycines appear to be driven by the plant environment such as nutrients (Chatnaparat et al., 2016).
Like other bacterial pathogens, disease management strategies are mainly dependent on the usage of antibiotics and copper-based chemicals (McManus et al., 2002). However, several recent studies have shown that bacterial plant pathogens have developed high resistance against these chemicals (McManus et al., 2002; Surette and Wright, 2017; Sundin and Wang, 2018). For example, the streptomycin-resistance genes were found in Erwinia amylovora, Pseudomonas syringae, and Xanthomonas campestris, and these genes have been transferred from other bacteria under antibiotic selection (Sundin and Wang 2018). Recently, the use of antibiotics in agriculture is questioned because of the possibility of horizontal transfer of cross-resistance from plant-associated bacteria to closely related human and animal pathogens (Cooksey 1990; Hau 1990; Surette and Wright, 2017). Therefore, alternative antimicrobial compounds in agriculture for plant-disease control, with low toxicity and reduced negative environmental impact are required.
Amino acids and their metabolites play key roles in development, homeostasis, and growth of both plant and microorganism (Andel 1966; Galili et al. 2016). Amino acids in the environment were responsible for the various cellular process of bacterial pathogens such as cell wall biogenesis, biofilm formation, and growth inhibition (Aliashkevich et al., 2018). For example, the application of exogenous amino acid D-leucine was found to inhibit biofilm formation by Xanthomonas citri subsp. citri on different abiotic surfaces and host leaves (Li and Wang 2014). N-acetylcysteine was also found to decrease bacterial growth, motility and biofilm formation of X. citri subsp. citri (Picchi et al. 2016). Moreover, some amino acids could be suppressing the type III secretion effector genes of Pseudomonas syringae pv. tomato and Erwinia amylovora (Ancona et al., 2015; Chatnaparat et al., 2015).
Although some amino acids have been used to control a variety of plant pathogens, to date, there are no studies about the application of exogenous amino acids to control X. citri pv. glycines in soybean. Therefore, the objective of this study was to investigate the efficacy of exogenous amino acids on disease severity of bacterial pustule in soybean. Our data demonstrated that cysteines could inhibit cell growth and rapidly kill X. citri pv. glycines. Furthermore, the application of exogenous amino acid cysteine, glutamic acid and threonine showed the protective effect to control bacterial pustule disease.
MATERIALS AND METHODS
Microbial strains and culture conditions
The bacterial strain Xanthomonas citri pv. glycines 12-2, a causal agent of bacterial pustule of soybean was used in the study. Bacterial cells were stored in nutrient broth (NB) medium supplemented with glycerol (25%) and maintained at −80 °C. X. citri pv. glycines 12-2 was cultured at 28 °C in nutrient broth (NB) or nutrient agar (NA) for routine work or in the minimal medium M9 (Sambrook et al., 1989). Escherichia coli DH5 strains were routinely grown in LB medium at 37 °C.
Eleven amino acids were tested in the study: aspartic acid, glutamic acid, asparagine, glutamine, lysine, threonine, arginine, serine, proline, leucine, histidine, alanine, methionine, isoleucine, cysteine, and valine, were purchased from HiMedia Laboratories LLC. Stock solutions of all amino acids were prepared at a concentration of 200 mM in sterilized de-ionized water and filter sterilized through a 0.22-μm-pore-size filter.
Antibacterial activity assay of amino acids
X. citri pv. glycines 12-2 were grown overnight in NB at 28 °C. The bacterial cells were then resuspended in the M9 minimal medium or in NB medium supplemented with 10 mM of each amino acid, starting at an OD600 of 0.05, and inoculated in 96 microtiter plates. Microplates were incubated in a rotator at 250 rpm and 28 °C for 24 h. Positive controls contained water instead of each amino acid, and negative controls contained each amino acid without bacterial suspension. The growth was measured using the SpectrostarNANO Microplate Reader (BMG Labtech). Two independent experiments were performed, and 8 replicates were used in each experiment.
Determination of the minimum inhibitory concentration (MIC)
Minimum inhibitory concentrations (MICs) of cysteine was tested by a two-fold serial dilution method (Clinical and Laboratory Standards Institute, 2015). X. citri pv. glycines 12-2 were grown overnight in NB at 28 °C. The cultures were adjusted to an OD600 of 0.05 in NB supplemented with different concentrations of cysteine. Then, 200 μl of bacterial cultures were aliquoted into wells of a 96-well plate. Plates were incubated in a rotator at 250 rpm and 28°C for 24 h. After 24 h incubation, the optical density (OD600) was measured using the SpectrostarNANO Microplate Reader (BMG Labtech). The MIC for X. citri pv. glycines 12-2 was defined as the lowest concentration of cysteine resulting in no growth after 24 h compared with the control samples.
Generation of X. citri pv. glycines expressing GFP
The plasmid pKT-trp consists of a green fluorescent protein marker gene (gfp) driven by the trp promoter from Salmonella enterica serovar Typhimurium (Hallmann et al., 2001),was isolated from Escherichia coli DH5 by using a FavorPrep™ Plasmid DNA Extraction Mini Kit (Favorgen, Ping-Tung , Taiwan). The plasmid pKT-trp was then transferred into X. citri pv. glycines 12-2 by electroporation according to standard procedures (Sambrook et al., 1989). X. citri pv. glycines containing pKT-trp plasmid were then selected on nutrient agar (NA) amended with 50 µg/ml of kanamycin and examined for fluorescence after 1 to 2 days under epifluorescence microscopy using an Axio microscope equipped with a ×40 objective (Zeiss Inc., Oberkochen, Germany).
Epifluorescence Microscopy
X. citri pv. glycines 12-2 (pKT-trp) exhibit constitutive expression of green fluorescens protein (GFP) was used to determine cell viability under epifluorescence microscopy. The effect of cysteine on cell viability of X. citri pv. glycines 12-2 GFP was determined after growth for 15 and 60 mins in NB medium with or without the addition of 10 mM cysteine. Cells were harvested and stained with propidium iodide (10 μg/ml) for 10 min in the dark at room temperature (Monier and Lindow, 2005). Ten microliters of the suspension were deposited on a slide, covered with a coverslip, and immediately observed under epifluorescence microscopy using an Axio microscope equipped with a ×40 objective (Zeiss Inc., Oberkochen, Germany). A filter set for fluorescein, FITC was used to visualize green and red fluorescent cells in the same field of view. On the resulting image, live cells appeared green, and dead cells appeared red, respectively.
Plant test in greenhouse.
Soybean cultivar SJ5 was used in this study. X. citri pv. glycines 12-2 were cultured in NB medium overnight at 28 °C and resuspended in sterile distilled water. Cell suspensions at an OD600 of 0.2 were mixed with 10 mM of each selected amino acid. Then, bacterial cells of X. citri pv. glycines 12-2 were inoculated on 1-month-old soybean plants by spraying method (Chatnaparat et al., 2012). Inoculated plants were maintained in the greenhouse during the experiments. At 14 days after inoculation, disease severity was assessed by counting the number of lesions on each leaf. Three trifoliate leaves (one each collected from the top, middle, and basal portion of three plants) from each of five replicate pots were evaluated for each strain.
RESULTS
Growth of X. citri pv. glycines 12-2 was inhibited by cysteine.
Eleven amino acids were tested for their ability to inhibit the growth of X. citri pv. glycines 12-2 in M9 minimal medium. The addition of 10 mM of each cysteine, serine, and threonine significantly reduced the growth of X. citri pv. glycines 12-2 in M9 minimal medium. Conversely, growth increase was achieved by the addition of glutamic acid, leucine, aspartic acid, glutamine, valine, proline, and alanine (Figure 1A). Then, the amino acids cysteine, serine, and threonine were selected to test their ability in suppressing the growth of X. citri pv. glycines 12-2 in NB, which is the rich medium that rapidly promotes the growth of X. citri pv. glycines 12-2. In the NB medium, the addition of 10 mM cysteines was found to suppress the growth of X. citri pv. glycines 12-2, whereas the addition of other amino acids did not affect the growth of this strain (Figure 1B). The results showed that 10 mM cysteine was able to inhibit the growth of X. citri pv. glycines 12-2 in complex medium, suggesting that 10 mM cysteines have toxicity effect on X. citri pv. glycines 12-2.
To further evaluate the toxicity of cysteine, the MIC was determined in NB medium. The inhibition of X. citri pv. glycines 12-2 growth was significantly observed with 0.625 mM cysteine and almost complete inhibition was obtained with 2.5 mM cysteine (Figure 2) suggesting that low concentration of cysteine was toxic to the cells of X. citri pv. glycines 12-2 and cysteine had an antimicrobial effect on this bacterium in a dose-dependent manner.
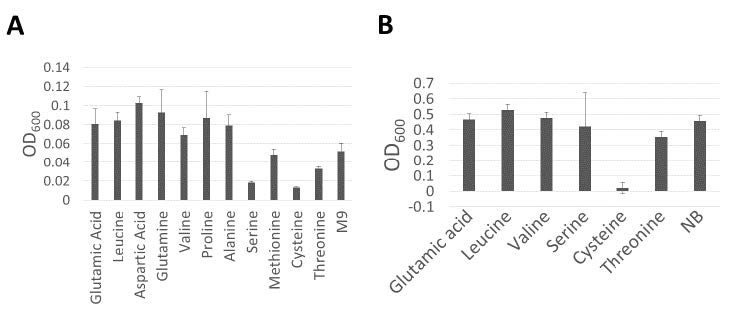
Figure 1. Effect of different amino acids on the growth of Xanthomonas citri pv. glycines 12-2 in minimal medium (A) and nutrient broth (NB) medium (B). The experiments were repeated three times with similar results with eight replicates (eight wells), and the error bars indicated standard deviations.
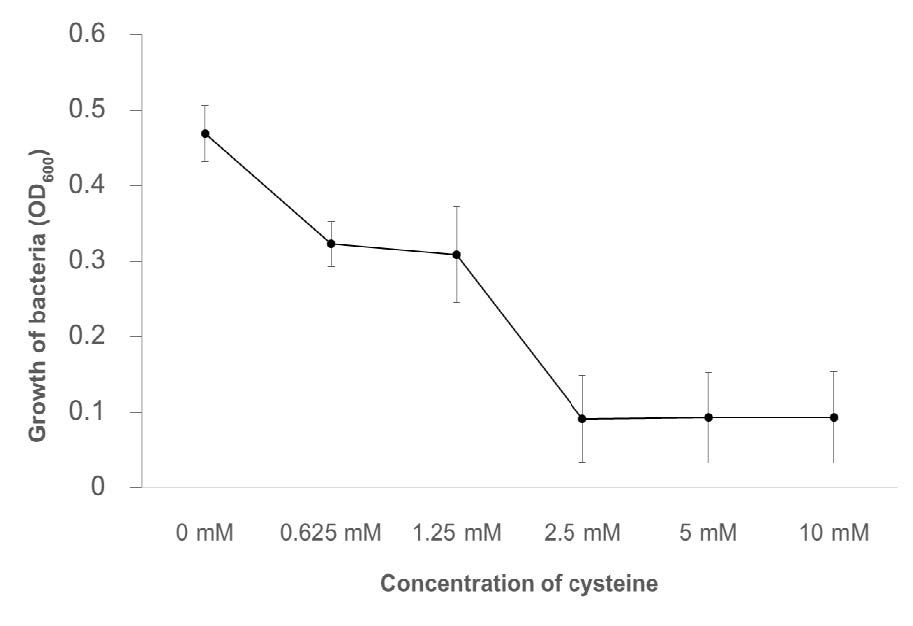
Figure 2. Effect of different concentrations of cysteine on the growth of Xanthomonas citri pv. glycines 12-2 in nutrient broth (NB) medium. The experiments were repeated three times with similar results with eight replicates (eight wells), and the error bars indicated standard deviations.
Cell viability of X. citri pv. glycines 12-2 was rapidly inhibited by cysteine.
To obtain insight into the mechanisms by which cysteine inhibits the growth of X. citri pv. glycines 12-2, we evaluated the effect of cysteine on cell viability, X. citri pv. glycines 12-2 harboring constitutive expression of green fluorescens protein plasmid (pTrp-GFP) was treated with 10 mM cysteine, the viable cells expressing GFP were measured using epifluorescence microscopy. Propidium iodide was used to distinguish membrane-compromised cells or dead cells (stained in red) from viable cells (retained in green). At the starting time, the majority of the X. citri pv. glycines 12-2 cells showed a green fluorescence due to the viable or live cells (retained in green), whereas only a small percentage of the cells showed red fluorescence denoting dead cells with non-permeable cell wall or membrane structure (Figures 3A and D). At 15 mins, in the absence of 10 mM cysteine, most X. citri pv. glycines 12-2 live cells were observed (Figure 3B), whereas the number of dead cells (retained in red) were increased in NB medium supplemented with cysteine (Figure 3E). At 60 mins, most of X. citri pv. glycines 12-2 cells in NB were detected as viable cells showed green fluorescence (Figure 3C), whereas the cells (almost 99%) treated with 10 mM cysteine exhibited red fluorescence indicating dead cells. (Figure 3F). These results indicate that the treatment of X. citri pv. glycines 12-2 with the cysteine leads to cell death. In addition, cysteine showed effective for rapidly killing X. citri pv. glycines 12-2.
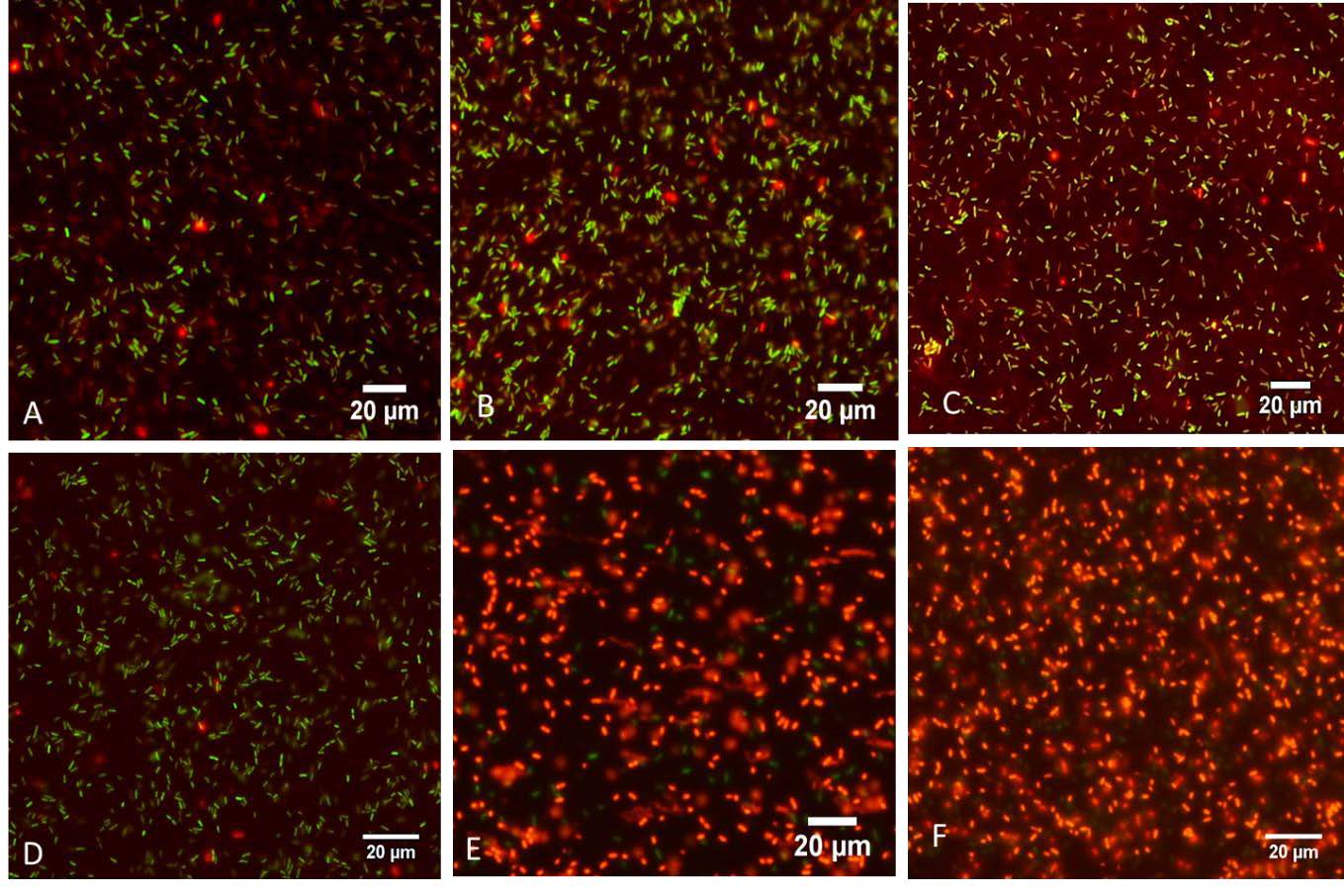
Figure 3. Microscopy images of Xanthomonas citri pv. glycines 12-2 constitutively expressing green fluorescent protein (GFP) stained with propidium iodide observed by epifluorescence microscope after grown in NB medium at 0, 15 and 60 minutes (A, B and C) and NB medium supplemented with 10 mM cysteine at 0, 15 and 60 minutes respectively (D, E, and F). GFP-labeled bacterial cells were green, dead bacterial cells stained with propidium iodide were red. Pictures were taken at 400 X magnification with a scale bar of 20 μm.
Amino acids reduce bacterial pustule symptom production in planta.
Plant inoculation by spray X. citri pv. glycines 12-2 along with cysteine decreased lesion numbers by about 87 % as compared with the water control (Table 1). Interestingly, the lesion numbers on soybean inoculated with X. citri pv. glycines 12-2 supplemented with either glutamic acid and threonine were also significantly reduced with disease reduction about 84.31 and 72.79 %, respectively. These results suggesting that cysteine, threonine and glutamic acid were able to reduce the virulence of X. citri pv. glycines 12-2 on soybean. Obviously, cysteine was able to suppress disease severity due to directly inhibit the growth of this pathogen. However, our data in vitro shown that glutamic acid and threonine were not directly inhibiting the growth of X. citri pv. glycines 12-2 but the application of glutamic acid and threonine to soybean reduced disease severity, suggesting that glutamic acid and threonine might be suppressing X. citri pv. glycines 12-2 by indirect mechanisms.
Table 1. Disease severity of Xanthomonas axonopodis pv. glycines on soybean treated with different amino acids.
|
Treatments |
Number of lesions1 |
Disease severity (%) |
Disease reduction (%) |
|
Xag12-2 |
136.00 a |
100 |
0 |
|
Cysteine/Xag12-2 |
17.67 b |
12.27 |
87.0 |
|
Serine/Xag12-2 |
161.33 a |
118.62 |
-11.8 |
|
Threonine/Xag12-2 |
37.33 b |
27.44 |
72.79 |
|
Glutamic acid/Xag12-2 |
21.33 b |
15.68 |
84.31 |
|
Leucine/Xag12-2 |
212.00 a |
155.88 |
-55.88 |
Note: 1Means within a column under each factor, means followed by a same letter are not significantly difference at the 5% level by DMRT.
DISCUSSION
The control of phytopathogenic bacteria using conventional bactericides, such as antibiotics, raises serious concerns about drug resistance and food safety. Therefore, alternative plant disease management strategies, which reduce the use of bactericides, are required. In particular, nutrients such as sugar and amino acids could affect the interaction between plants and pathogens. However, there is little information on the effect of amino acids on bacterial disease control and many factors that influence this response are not well understood. In this study, we found that amino acids cysteine, glutamic acid, and threonine had a protective effect on Xanthomonas citri pv. glycines, a causal agent of bacterial pustule disease of soybean.
Our results showed that cysteine, serine, and threonine were able to inhibit the growth of X. citri pv. glycines in M9 minimal medium, suggesting that X. citri pv. glycines could not utilize these amino acids as carbon or nitrogen sources. Serine and threonine were found to be particularly important amino acids in the plant and X. citri subsp. citri interaction. These amino acids quickly accumulated in the plant apoplast after pathogen inoculation and these required to effectively inhibit bacterial growth (Goto and Yamanaka, 1981). Thus, the role of serine and threonine in X. citri pv. glycines and soybean pathosystem should be further investigated. In a rich medium, however, the bacterium was able to grow on all tested amino acids except cysteine. It has been reported that cysteine strongly inhibits phytopathogenic bacteria such as X. citri subsp. citri and Pseudomonas syringae (Goto and Yamanaka, 1981). Several clinical bacterial pathogens such as Escherichia coli, Pseudomonas fluorescens, and Salmonella enterica were also inhibited by cysteine (Harris, 1981; Tuite et al., 2005; Demetrescu et al., 2018).
We also found that the concentration of cysteine over 2.5 mM could inhibit the growth of X. citri pv. glycines 12-2. Our result was similar to other research work that, cysteine concentrations greater than 3.0 mM inhibited the growth of Pseudomonas fluorescens NC3 (Himelbloom and Hassan, 1986). Cysteine is a source of sulfur for other cellular macromolecules and it generates hydrogen sulfide (H2S) as a by-product of cysteine degradation (Carlsson et al., 1979). H2S can also kill microorganisms by inhibiting antioxidant enzymes during induced oxidative stress (Fu et al., 2014). In addition, we demonstrated that a short time exposure of 10 mM cysteine caused a cell death rather than just inhibiting the growth of X. citri pv. glycines 12-2 when determined by live/dead cells staining. It has been shown that an excess level of intracellular L-cysteine promotes the Fenton reaction which is toxic to the cell by hydroxyl radicals damage nucleic acids, carbonylate proteins, and lipids (Zhao and Drlica, 2014; Park and Imlay, 2003). In E. coli, the cysteine treatment for a short time caused a cell death was due to an unusually rapid rate of DNA damage (Park and Imlay, 2003).
In addition, N‐acetylcysteine (NAC), which is a cysteine analogue, has been reported as a biofilm formation inhibitor and has antivirulence activities against bacterial plant pathogens, Xylella fastidiosa and X. citri subsp. citri (Muranaka et al., 2013; Picchi et al., 2016).Whether the mechanism of cysteine toxicity and anti-virulence proporties of cysteine on X. citri pv. glycines 12-2 remains to be characterized.
Interestingly, our results demonstrated that the application of exogenous amino acid cysteine, glutamic acid and threonine showed the protective effect to control bacterial pustule disease. In our experiment, cysteine was mixed with the bacterial suspension and immidately apply to soybean plant, data show that cysteine was able to reduce the bacterial pustule disease. However, concerning the effect of cysteine to control plant disease in the field, the pre or post treatment of plants with cysteine will be further investigated. Although, our results demonstrated that cysteine was able to directly inhibit the bacterium but cysteine, glutamic acid, and threonine might also suppress this bacterium by act as signaling amino acids in soybean plants to induced resistance to the pathogen. A recent study indicates that the application of the amino acid glutamic acid and cysteine in seed treatment and foliar application increased the activity of the antioxidant enzymes in soybean (Teixeira et al., 2017). In addition, exogenous application of glutamic acid to rice roots induced systemic disease resistance against rice blast in leaves (Kadotani et al., 2016). Several genes related to defense responses of rice are rapidly induced by glutamic acid (Kan et al., 2017). Further, it has been indicated that pretreatment of plants with threonine effectively suppresses the downy mildew pathogen Hyaloperonospora arabidopsidis in planta growth (Stuttmann et al., 2011). Thus, the application of cysteine, glutamic acid, and threonine to soybean might play a role in plant defense response against this bacterium; however, this needs to be further investigated.
ACKNOWLEDGMENTS
We are very grateful to S. Prathuangwong for helpful discussions and suggestions for this work. We thank S.E. Lindow at University of California, Berkeley for providing the pKT-trp plasmid. This work was supported by Grants from Kasetsart University Research and Development Institute, and Center for Advanced Studies for Agriculture and Food, CASAF, Kasetsart University, Thailand.
REFERENCES
Aliashkevich, A., Alvarez, L., and Cava, F. 2018. New insights into the mechanisms and biological roles of D-amino acids in complex eco-systems. Front. Microbiol. 9: 683. https://doi.org/10.3389/fmicb.2018.00683
Ancona, V., Lee, J.H., Chatnaparat, T., Oh, J., Hong, J.-I., and Zhao, Y. 2015. The bacterial alarmone (p)ppGpp activates the type III secretion system in Erwinia amylovora. J. Bacteriol. 197: 1433–1443. https://doi.org/ 10.1128/JB.02551-14
Andel, O.M.V. 1966. Amino acids and plant diseases. Annu. Rev. Phytopathol. 4: 349–368. https://doi.org/10.1146/annurev.py.04.090166.002025
Athinuwat, D., Prathuangwong, S., Cursino, L., and Burr, T. 2009. Xanthomonas axonopodis pv. glycines soybean cultivar virulence specificity is determined by avrBs3 homolog avrXg1. Phytopathology. 99: 996–1004. https://doi.org/10.1094/PHYTO-99-8-0996
Carlsson, J., Granberg, G.P., Nyberg, G.K., and Edlund, M.B. 1979. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl. Environ. Microbiol. 37: 383–390.
Chatnaparat, T., Li, Z., Korban, S.S., and Zhao, Y. 2015. The stringent response mediated by (p)ppGpp is required for virulence of Pseudomonas syringae pv. tomato and its survival on tomato. Mol. Plant. Microbe. Interact. 28: 776–789. https://doi.org/10.1094/MPMI-11-14-0378-R
Chatnaparat, T., Prathuangwong, S., Ionescu, M., and Lindow, S.E. 2012. XagR, a LuxR homolog, contributes to the virulence of Xanthomonas axonopodis pv. glycines to soybean. Mol. Plant. Microbe. Interact. 25: 1104–1117. https://doi.org/10.1094/MPMI-01-12-0008-R
Chatnaparat, T., Prathuangwong, and Lindow, S. E. 2016. Global pattern of gene expression of Xanthomonas axonopodis pv. glycines within soybean leaves. Mol. Plant. Microbe. Interact. 29: 508–522. https://doi.org/10.1094/MPMI-01-16-0007-R
Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard - tenth edition. CLSI document M07-A10, Clinical and Laboratory Standards Institute, Wayne, PA
Cooksey, D.A. 1990. Genetics of bactericide resistance in plant pathogenic bacteria. Annu. Rev. Phytopathol. 28: 201–219. https://doi.org/10.1146/annurev.py.20.090190.001221
Demetrescu, I., Dumitriu, C., Totea, G., Nica, C., Dinischiotu, A., and Ionita, D. 2018. Zwitterionic cysteine drug coating influence in functionalization of implantable Ti50Zr alloy for antibacterial, biocompatibility and stability properties. Pharmaceutics. 10(4): 220. https://doi.org/ 10.3390/pharmaceutics10040220
Fu, L.H., Hu, K.D., Hu, L.Y., Li, Y.H., Hu, L.B., Yan, H., Liu, Y.S., and Zhang, H. 2014. An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum J. Calvert, ed. PLoS One. 9:e104206. https://doi.org/10.1371/journal.pone.0104206
Galili, G., Amir, R., and Fernie, A.R. 2016. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 67: 153–178. https://doi.org/10.1146/annurev-arplant-043015-112213
Goto, M., and Yamanaka, K. 1981. Growth inhibition of Xanthomonas campestris pv. citri and its reversal by amino acids found in the intercellular fluids of citrus leaves. Ann, Phytopath, Soc. Japan 47: 618-626.
Groth, D.E., and Braun, E.J. 1986. Growth kinetics and histopathology of Xanthomonas campestris pv. glycines in leaves of resistant and susceptible soybeans. Phytopathology 76: 959-965. https://doi.org/10.1094/Phyto-76-959
Hallmann, J., Quadt-Hallmann, A., Miller, W.G., Sikora, R.A., and Lindow, S.E. 2001. Endophytic colonization of plants by the biocontrol agent Rhizobium etli G12 in relation to Meloidogyne incognita infection. Phytopathology. 91: 415–422. https://doi.org/10.1094/PHYTO.2001.91.4.415
Harris, C.L. 1981. Cysteine and growth inhibition of Escherichia coli: threonine deaminase as the target enzyme. J. Bacteriol. 145(2): 1031–1035
Hau, B. 1990. Analytic models of plant disease in a changing environment. Annu. Rev. Phytopathol. 28: 221–245. https://doi.org/10.1146/annurev.py.28.090190.001253
Himelbloom, B.H., and Hassan, H.M. 1986. Effects of cysteine on growth, protease production, and catalase activity of Pseudomonas fluorescens. Appl. Environ. Microbiol. 51(2): 418–421
Kadotani, N., Akagi, A., Takatsuji, H., Miwa, T., and Igarashi, D. 2016. Exogenous proteinogenic amino acids induce systemic resistance in rice. BMC Plant Biol. 16: 60. https://doi.org/10.1186/s12870-016-0748-x
Kan, C.-C., Chung, T.-Y., Wu, H.-Y., Juo, Y.-A., and Hsieh, M.-H. 2017. Exogenous glutamate rapidly induces the expression of genes involved in metabolism and defense responses in rice roots. BMC Genomics. 18: 186. https://doi.org/10.1186/s12864-017-3588-7
Kaewnum, S., Prathuangwong, S., and Burr, T.J. 2006. A pectate lyase homolog, xagP, in Xanthomonas axonopodis pv. glycines is associated with hypersensitive response induction on tobacco. Phytopathology. 96(11): 1230–1236. https://doi.org/10.1094/PHYTO-96-1230
Li, J., and Wang, N. 2014. Foliar application of biofilm formation-inhibiting compounds enhances control of citrus canker caused by Xanthomonas citri subsp. citri. Phytopathology. 104(2): 134–142. https://doi.org/10.1094/PHYTO-04-13-0100-R
McManus, P.S., Stockwell, V.O., Sundin, G.W., and Jones, A.L. 2002. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 40: 443–465. https://doi.org/10.1146/annurev.phyto.40.120301.093927
Monier, J.-M., and Lindow, S.E. 2005. Spatial organization of dual-species bacterial aggregates on leaf surfaces. Appl. Environ. Microbiol. 71: 5484–5493. https://doi.org/10.1128/AEM.71.9.5484-5493.2005
Morgan, F. L. 1963. Bacterial pustule of soybean. Soybean Dig. 23:8-9.
Muranaka L.S., Giorgiano, T.E., Takita, M.A., Forim, M.R., Silva, L.F.C., Coletta‐Filho, H.D., Machado, M.A. and de Souza, A.A. 2013. N‐acetylcysteine in agriculture, a novel use for an old molecule: focus on controlling the plant pathogen Xylella fastidiosa . PLoS One, 8, e72937. https://doi.org/10.1371/journal.pone.0072937
Park, S., and Imlay, J.A. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 185(6): 1942–1950. https://doi.org/10.1128/JB.185.6.1942-1950.2003
Picchi, S.C., Takita, M.A., Coletta-Filho, H.D., Machado, M.A., and de Souza, A.A. 2016. N -acetylcysteine interferes with the biofilm formation, motility and epiphytic behaviour of Xanthomonas citri subsp. citri. Plant Pathol. 65: 561–569. https://doi.org/10.1111/ppa.12430
Prathuangwong, S., and Amnuaykit, K. 1987. Studies on tolerance and rate reducing bacterial pustule of soybean cultivars/lines. Kasetsart J. (Nat. Sci.) 21: 408-420
Sambrook, J., Fritsch, E.F., and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, U.S.A.
Stuttmann, J., Hubberten, H.-M., Rietz, S., Kaur, J., Muskett, P., Guerois, R., Bednarek, P., Hoefgen, R., and Parker, J.E. 2011. Perturbation of Arabidopsis amino acid metabolism causes incompatibility with the adapted biotrophic pathogen Hyaloperonospora arabidopsidis. Plant Cell. 23: 2788–2803. https://doi.org/10.1105/tpc.111.087684
Sundin, G.W., and Wang, N. 2018. Antibiotic resistance in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 56: annurev–phyto–080417–045946. https://doi.org/10.1146/annurev-phyto-080417-045946
Surette, M.D., and Wright, G.D. 2017. Lessons from the environmental antibiotic resistome. Annu. Rev. Microbiol. 71: 309–329. https://doi.org/10.1146/annurev-micro-090816-093420
Teixeira, W.F., Fagan, E.B., Soares, L.H., Umburanas, R.C., Reichardt, K., and Neto, D.D. 2017. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 8: 327. https://doi.org/10.3389/fpls.2017.00327
Thowthampitak, J., Shaffer, B.T., Prathuangwong, S., and Loper, J.E. 2008. Role of rpfF in virulence and exoenzyme production of Xanthomonas axonopodis pv. glycines, the causal agent of bacterial pustule of soybean. Phytopathology. 98(12): 1252–1260. https://doi.org/10.1094/PHYTO-98-12-1252
Tuite, N.L., Fraser, K.R., and O’Byrne, C.P. 2005. Homocysteine toxicity in Escherichia coli is caused by a perturbation of branched-chain amino acid biosynthesis. J. Bacteriol. 187(13): 4362–4371. https://doi.org/10.1128/JB.187.13.4362-4371.2005
Weber, C.R., Dunleavy, J.M., and Fehr, W.R. 1966. Effect of bacterial pustule on closely related soybean lines. Agron. J. 58: 544-545.
Zhao, X., and Drlica, K. 2014. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 21: 1–6. https://doi.org/10.1016/j.mib.2014.06.008
Namfon Pluemjit, Supisara Sripo-ngam, Ekkachai Khwanbua, and Tiyakhon Chatnaparat*
Department of Plant Pathology, Faculty of Agriculture, Kasetsart University, Bangkok 10900, Thailand
*Corresponding author. E-mail: fagrtkc@ku.ac.th
Total Article Views
Article history:
Received: September 27, 2019;
Revised: December 16, 2019;
Accepted: January 2, 2020

