
In vitro Storage Techniques of Fritillaria imperialis Lubra Maxima, a Wild Rare and Critically Endangered Ornamental Species
Shima Seydi, Shahram Sedaghathoor*, and Behzad KavianiPublished Date : 2020-07-17
DOI : https://doi.org/10.12982/CMUJNS.2020.0040
Journal Issues : Number 3, July-September 2020
ABSTRACT Wild population of Fritillaria imperialis Lubra Maxima is at the risk of extinction, mainly because of many harvesting and pest and pathogens infestation. Therefore, this plant needs urgent protection. Here and for the first time, this study aimed to evaluate the efficiency of in vitro storage techniques (cold preservation and cryopreservation by encapsulation–dehydration and cryopreservation by encapsulation–vitrification) as the methods of medium– and long–term conservation of this valuable ornamental species. The bulb scale was used as explant. Explants were encapsulated in alginate beads. This study was carried out in three sections, namely, cold preservation with steps of encapsulation, preculture with dehydration with 0.75 M sucrose and air desiccation followed by storage at 4°C; cryopreservation with steps of encapsulation, preculture with dehydration with 0.75 M sucrose and air desiccation followed by direct immersion in liquid nitrogen (LN); and cryopreservation with steps of encapsulation, loading solution, vitrification with plant vitrification solution 3 (PVS3) solution followed by direct immersion in LN. After 6 months cold storage, maximum survival (67.0%) was observed for encapsulated explants dehydrated by air desiccation. After thawing, maximum survival of 74.3 and 81.6% was obtained for cryopreserved explants pretreated by encapsulation–dehydration and encapsulation–vitrification (for 20 min), respectively. The optimized in vitro conservation techniques can be utilized for germplasm preservation, breeding programs, and exchange of F. imperialis genetic resources.
Keywords: Cryoprotectants, Germplasm conservation, In vitro conservation, Liquid nitrogen, Slow–growth storage
Introduction
Fritillaria (Liliaceae) is a genus of about 100 species of bulbous perennials found in a range of habitats, from woodland to open meadows and high screes, distributed throughout the temperate regions of the N. hemisphere, particularly the Mediterranean, S.W. Asia and W. North America. Each bulb of Fritillaria has 2 or more scales. Fritillaria imperialis Lubra Maxima is a perennial plant with high medicinal and ornamental importance. Some species of F. imperialis are native to Iran (De Hertogh and Le Nard, 1993). Wild populations of F. imperialis are mostly found in high altitudes (>2,000 m) of western parts of Iran, particularly in three provinces, Chahar Mahal–va–Bakhtiari, Kohkyluyeh–va–Bouyrahmad and Ilam. Thanks to attractive red and yellow flowers, this plant reveals a great commercial potential (De Hertogh and Le Nard, 1993). The bulbs of F. imperialis contain alkaloids, non–alkaloid and high starch content. In Iran, wild populations of two important species, F. imperialis and F. persica, are considered critically endangered, because of many harvesting, lack of protecting rules, changing the pastures to dry farmlands, and pest and pathogens invasions. Therefore, there is an urgent need to conserve it before go extinct. In vitro conservation techniques must be utilized to preserve F. imperialis. Two most important techniques of in vitro conservation are slow growth storage and cryopreservation. The use of synthetic seed technology is ideal for these techniques (Divakaran et al., 2006). Cold preservation or slow–growth storage technique as a medium–term conservation can be established by maintenance of plant materials in an adverse chemical and/or physical conditions such as modifying the medium compositions particularly the use of growth retardants, maintaining at a low temperature (usually, 4–15°C) and low light intensity and covering the explants to reduce the oxygen available to the cells (Singh et al., 2015). This technique reduces the frequency of subculture, manual labor, costs and the risk of contamination. Successful slow–growth storage procedures have been reported for some important ornamental species like Vanilla (Divakaran et al., 2006), Hibiscus moscheutos (West et al., 2006), Dendrobium (Teixeira da Silva et al., 2014), Trichosanthes dioica Roxb. (Singh et al., 2015), Cynara cardunculus (Tavazza et al., 2015), Cypripedium lentiginosum (Jiang et al., 2017), and Nymphaea lotus (Blidar et al., 2017).
Cryopreservation as a long–term conservation is a superior, safe and efficient method for preservation of plant genetic resources. Cryopreservation techniques are based on storage of explants at ultra–low temperature of LN (−196°C). At this temperature, explants can be stored for unlimited duration (Bernard et al., 2002). Cryopreservation offer minimum storage requirements, space, contaminants and maintain genetic diversity and phenotype and genotype stability (Kulus and Zalewska, 2014). The number of cryopreserved species by combined techniques especially encapsulation–dehydration and encapsulation–vitrification has been increased (Kaviani and Negahdar, 2017). Since, cryopreservation induces a high stress to the plant tissues, reduction of the freezable water from cells physically by desiccation or chemically through the use of highly concentrated solutions particularly containing sucrose (0.30 – 0.75 M) is necessary.
Some ornamental species are on the brink of extinction. Cryopreservation of plant materials by encapsulation–dehydration has been successfully used for many ornamental plants such as Chrysanthemum × grandiforum (Sakai et al., 2000), Ceratopetalum gummiferum (Shatnawi and Johnson, 2004), Dendrobium (Lurswijidjarus and Thammasiri, 2004), Chrysanthemum morifolium (Halmagyi et al., 2004), Saintpaulia ionantha (Moges et al., 2004), Rhododendron simsii (Verleysen et al., 2005), Dianthus caryophyllus (Halmagyi and Deliu, 2007), Iris (Jevremović et al., 2009), and Buxus hyrcana (Kaviani and Negahdar, 2017). Encapsulation–dehydration technique is the most popular among ornamental plants especially important with slow–growing species. Ice crystal formation inside the plant cells can be prevented through the use of plant vitrification solutions PVS2 (dimethyl sulfoxide + ethylene glycol + glycerol) and PVS3 (sucrose + glycerol) (Sakai, 2000). Encapsulation–vitrification technique has been applied to a number of species, but their routine use is still limited (Engelmann, 2004). The vitrification procedure was successfully used for cryopreservation of Chrysanthemum × grandiflorum (Sakai et al., 2000; Halmagyi et al., 2004; Martin and Gonzalez–Benito, 2005), Iris sp. (Jevremović et al., 2009), Gentiana sp. (Sakai and Engelmann, 2007), Photinia serrulata (Yan, 2006), and Saintpaulia ionantha (Moges et al., 2004). Vitrification is a popular method of cryopreservation for orchids (Vendrame et al., 2007). Cryopreservation of Lilium ledebourii (Baker) Bioss. germplasms by encapsulation–vitrification, and encapsulation–dehydration techniques as well using sucrose and dehydration as pretreatments was performed (Kaviani et al., 2008; Kaviani et al., 2009; Kaviani, 2010; Kaviani et al., 2010). There are no reports regarding the application of in vitro conservation by cold storage and cryopreservation by using encapsulation–dehydration and encapsulation–vitrification for F. imperialis Lubra Maxima, and this highlights the relevance of this research. Therefore, the present study focused on the development of medium– and long–term in vitro conservation protocols using cold preservation and cryopreservation by encapsulation–dehydration and encapsulation–vitrification on bulb scales of F. imperialis Lubra Maxima, a wild rare and critically endangered ornamental plant.
Materials and methods
Plant materials and sterilization
Fritillaria imperialis Lubra Maxima is growing in mountain regions and Zagros altitudes, Ilam province, Iran (Figure 1A and B). Two–years–old natural–grown plants of F. imperialis were used as mother plants. The bulbs of F. imperialis (Figure 1C and D) were harvested and used as the starting material for the experiments. Bulbs were transferred to the plant tissue culture and biotechnology laboratory, Amol, Mazandaran Province, Iran. All the experiments were conducted at this laboratory and its experimental greenhouse since 2017 to 2019. In laboratory, bulbs (5–8 cm long) were washed under running tap water for half an hour to remove mud and dirt. The bulbs were put into a vessel filled with water and a few drops of dish–washing liquid for 10 min. Then, these were washed in running tap water for half an hour, again. Cleaned bulbs (Figure 1E) were decontaminated with a fungicide (0.10 mg l–1 benomile + carbendazim, rural T.S.) for 20 min followed by once washing for 10 min. in distilled water. After thorough rinse in distilled water, the bulbs were transported to aseptic condition in laminar air hood. The bulbs were disinfected by immersing in 0.01 g l–1 mercuric chloride (HgCl2) for 15 min with continuous stirring using magnetic stirrer, then by 20% sodium hypochlorite (NaOCl) solution (commercial bleach) for 15 min followed by three times rinsing (each for 10 min.) in sterile distilled water and finally the bulbs were dipped in 70% ethanol for 60 sec. The scales were rinsed in sterile distilled water for three times. Dried bulbs (by placing on paper for 5 min.) were vertically cut into 10 × 10 × 2 mm under aseptic conditions to obtain twin scales as explants (Figure 1F). Outer scales directly in contact with disinfectant during sterilization were removed before obtaining double scale explants.

Figure 1. Fritillaria imperialis L. growing at its natural habitat (mountain regions and Zagros altitudes, Ilam province, Iran) in flowering period (A and B); plant eradicated from the soil (C); young plants transferred to the laboratory (D); bulbs ready for sterilization (E); to cut cleaned bulbs as slices (scales) used for explants in all experiments (F) (scale bar = 10 mm).
Encapsulation-dehydration of bulb scales
MS basal medium was used in all experiments. For encapsulation, 3% sodium alginate (w/v) and 100 mM calcium chloride (CaCl2·2H2O) prepared in MS basal medium containing 0.75 M sucrose was applied. Encapsulation was accomplished by mixing the bulb scales explants into the Na-alginate solution for 2 h and then dropping them (1 bulb scale/drop) into 100 mM CaCl2·2H2O solution, so as to form round beads. The explants were allowed to remain in the solution for 2 h with gentle shaking for proper bead formation. Encapsulated bulb scales were used for cold preservation and cryopreservation by encapsulation–dehydration. For physical dehydration, encapsulated explants were transferred to uncovered sterile Petri dishes and maintained for 2 h under aseptic condition in laminar air hood with velocity of 50 cm per second, temperature of 24 ± 2ºC and relative humidity of 40-50% for air desiccation.
Steps for cold preservation and cryopreservation by encapsulation-dehydration
Steps consisted of preculture by 0.75 M sucrose for 2 h in MS liquid medium, physically dehydration by putting the explants under clean air flow of laminar flow cabinet for 2 h, and encapsulation in medium containing 3% Na–alginate for 60 min followed by plunging in medium containing 100 mM CaCl2 for 60 min.
Steps for cryopreservation by encapsulation-vitrification
Bulb scales were immersed in MS medium containing 0.75 M sucrose and 3% Na–alginate for 60 min followed by plunging in medium containing 0.75 M sucrose and 100 mM CaCl2 for 60 min. Encapsulated explants were placed in 5 ml cryovial and treated with 4 ml mixture of 1 M glycerol and 0.4 M sucrose (loading solution) for 20 min at room temperature. For vitrification, loading solution was then removed with a Pasteur pipette and replaced with 4 ml sterilized PVS3 (50% glycerol + 50% sucrose, both w/v) for 10, 20 and 30 min at room temperature.
Cold preservation and cryopreservation conditions
For cold preservation, pretreated and control bulb scales were transferred to Petri dishes, sealed with parafilm and stored at 4°C under dark condition for 6 months. There were five explants in each Petri dish. For cryopreservation, following encapsulation–dehydration and encapsulation–vitrification, pretreated and control beads containing bulb scales were placed in 2 ml cryovials and frozen in LN by direct immersion (stored at least for 1 h).
Post-storage processes and regeneration medium
After at least 1 h storage of bulb scales in LN for cryopreservation, cryovials holding explants were removed from LN and thawed rapidly by immersing in warm water (40°C) for 2 min. After rewarming, the PVS3 was discarded form the cryovials and replaced with basal culture medium containing 1.2 M sucrose (unloading solution) for 20 min.
Cold-preserved and cryopreserved explants were cultured in Murashige and Skoog’s (MS) medium (1962) fortified with 3% sucrose, gelled with 0.7% agar and enriched with 0.50 mg l–1 KIN together with 1.00 mg l–1 NAA, obtained earlier for multiplication (data not shown). The pH of the medium was adjusted to 5.7 ± 1.0 before autoclaving at 121°C for 20 min. The same regeneration medium and environmental conditions were utilized in cold preservation and cryopreservation experiments. All the cultures were kept at 23 ± 2°C under a16 h photoperiod of 50 μmol m−2 s−1 photosynthetic photon flux provided by cool–white fluorescent lamps (standard culture conditions).
Post–cold and post–cryo regrowth
The survival percentage was recorded 60 days after culture initiation. The regrowth capacity in term of percentage of germinated beads was evaluated 30 days after culturing. Germination was considered as emergence of the leaves or roots from the beads. In this paper, the survival percentage was calculated as the percentage of the number of beads germinated among the total countered number of beads.
Experimental design and data analysis
The experiments were performed in a completely randomized design. Each treatment consisted of 4 replicates and each replicate included 5 specimens. Replicates contained four Petri dishes, and each Petri dish contains 5 cold preserved and cryopreserved bulb scales. All the statistical analyses were done by using Microsoft Excel 2013 and SAS v 9.2. The analysis of variance (ANOVA) procedure was used to test for significant effect of treatments, followed by LSD test for comparisons of different means of different treatments.
Results
Cold preservation by encapsulation–dehydration
For inducing minimal growth in F. imperialis Lubra Maxima, pretreated and non–pretreated explants (bulb scales) were storage at 4°C. Survival data was recorded after 6 months. There were statistically significant differences in survival percentage between pretreated and non–pretreated explants (Table 1). All pretreated explants had better survival (more than 40.0%) than non–pretreated explants (with about 25.0%) after culture in regeneration medium (Figure 2). Pretreated explants started to growth when they were transferred to standard culture conditions, with normal growth in 35–40 days (Figure 3). Maximum survival (67.0%) was obtained in encapsulated bulb scales treated with dehydration. The untreated explants showed reduced growth rate (25.0%). No morphological changes were observed in cold–conserved and non–conserved plantlets. Sixty seven percentage of the encapsulated–dehydrated bulb scales retrieved from the cold storage survived after in vitro establishment.
Table 1. Analysis of variance of the effect of different pretreatments on survival percentage of Fritillaria imperialis Lubra Maxima grown in regeneration medium.
|
Source of variations |
df |
Cold preservation |
Cryopreservation by encapsulation–dehydration |
df |
Cryopreservation by encapsulation–vitrification |
|
Treatments |
7 |
499.00** |
715.00** |
3 |
148.00** |
|
Error |
14 |
49.03 |
47.10 |
6 |
13.60 |
|
CV (%) |
- |
14.07 |
14.04 |
- |
7.32 |
Note: **: Significant at the 0.01 probability level.
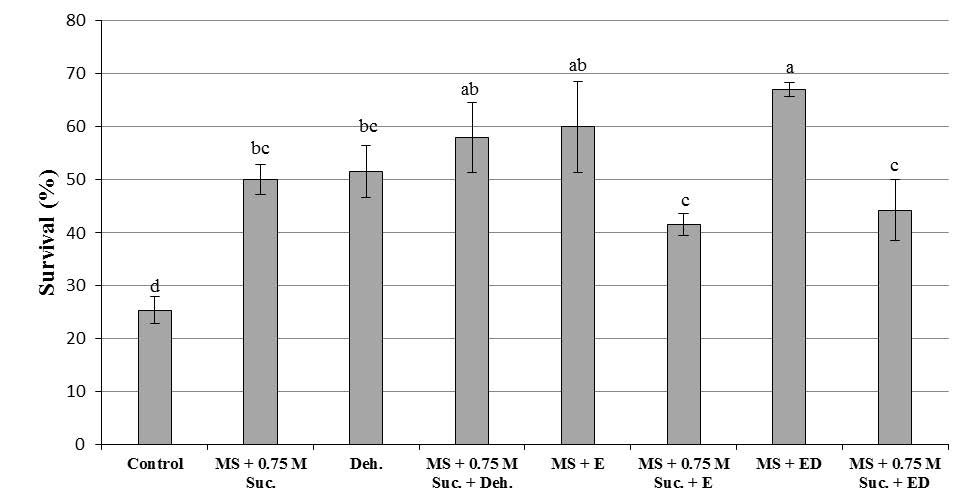
Figure 2. Effect of different treatments on survival percentage of cold preserved bulb scales of F. imperialis L. in MS regeneration medium after 6 months storage. For each treatment, different letters indicate percentages significantly different at P≤0.05. Control: no encapsulation (E), no preculture with 0.75 M sucrose (P), no dehydration (D); Cold MS + 0.75 M suc.: no E, P with 0.75 M sucrose for 2 h, no D; Cold Deh: no E, no P, D for 2 h; Cold MS + 0.75 M suc. + Deh: no E, P with 0.75 M sucrose for 2 h, D for 2 h; Cold MS + E: E (3% Na-alginate for 60 min and 100 mM CaCl2 for 60 min), no P, no D; Cold MS + 0.75 M suc. + E: E, P with 0.75 M sucrose for 2 h, no D; Cold MS + ED: with E and D; Cold MS + 0.75 M suc. + ED: E and D.

Figure 3. Cold preservation of bulb scales of F. imperialis L. pre-treated with encapsulation–dehydration. A) Encapsulated bulb scales; B) in vitro culture of bulb scales after 6 months of conservation; C) plant recovery of cold preserved bulb scales on MS medium supplemented with 0.50 mg l–1 KIN together with 1.00 mg l–1 NAA after 60 days (scale bar = 10 mm).
Cryopreservation by encapsulation–dehydration
Present study demonstrated that encapsulation–dehydration technique enhanced the tolerance to cryopreservation of F. imperialis bulb scales (Figures 4 and 5). Maximum viability (74.3%) was achieved for encapsulated–dehydrated bulb scales prior to LN exposure (just before LN exposure). All non–encapsulated–dehydrated shoot tips (control) died after exposure to LN. There was a significant difference between control and the treatments (Table 1). Figure 4 exhibits that all explants pretreated with 0.75 M sucrose and air desiccation showed higher survival than the non–pretreated explants. Concerning the dehydration type, pretreatment of bulb scales in air desiccation produced better results compared with sucrose enriched media (Figure 5). The present study exhibited the positive effect of encapsulation–dehydration treatment which was more favorable in cryopreserved bulb scales compared to 0.75 M sucrose, 0.75 M sucrose together with air desiccation and 0.75 M sucrose together with encapsulation–dehydration treatments.
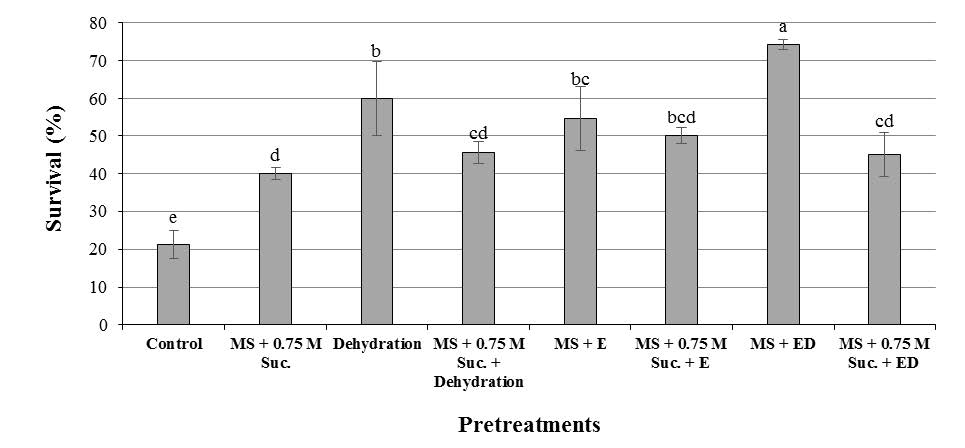
Figure 4. Effect of different treatments on survival percentage of cryopreserved bulb scales of F. imperialis L. in MS regeneration medium after plunge in LN. For each treatment, different letters indicate percentages significantly different at P≤0.05. Control: no encapsulation (E), no preculture with 0.75 M sucrose (P), no dehydration (D); LN MS + 0.75 M suc.: no E, P with 0.75 M sucrose for 2 h, no D; LN Deh: no E, no P, D for 2 h; LN MS + 0.75 M suc. + Deh: no E, P with 0.75 M sucrose for 2 h, D for 2 h; LN MS + E: E (3% Na-alginate for 60 min and 100 mM CaCl2 with 60 min), no P, no D; LN MS + 0.75 M suc. + E: E, P with 0.75 M sucrose for 2 h, no D; LN MS + ED: with E and D; LN MS + 0.75 M suc. + ED: E and D.
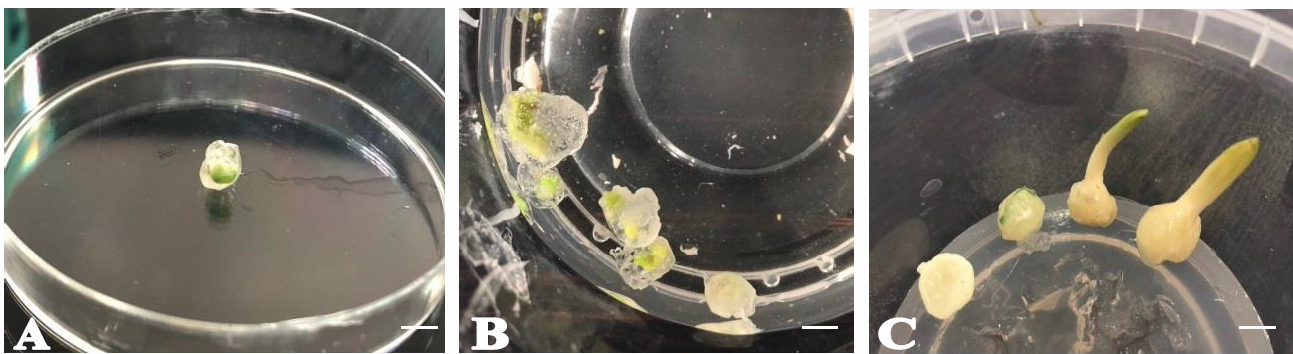
Figure 5. Cryopreservation of bulb scales of F. imperialis L. pre-treated with encapsulation–dehydration. A) Encapsulated bulb scales; B) in vitro culture of bulb scales after conservation in LN; C) regrowth of cryopreserved bulb scales that were exposed to encapsulation–dehydration prior to plunge in LN after 60 days of culture (scale bar = 10 mm).
Cryopreservation by encapsulation–vitrification
Bulb scales without treatment with PVS3 solution displayed lowest viability value. Figure 6 shows that the greatest viability was obtained with exposure to PVS3 treatment for 20 min. Maximum survival was obtained after this treatment with a percentage of 81.6% (Figures 6 and 7). A treatment with PVS3 for 10 and 30 min reduced the viability of bulb scales till 62.0 and 40.0%, respectively (Figure 6). An important factor in production of high post–cryopreservation survival rates is the optimization of exposure time to plant vitrification solution prior to cryopreservation.
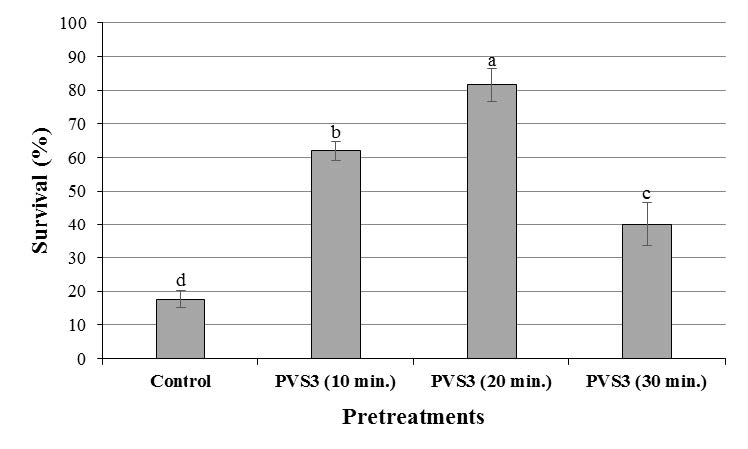
Figure 6. Effect of different treatments (exposure to various times of PVS3) on survival percentage of cryopreserved bulb scales of F. imperialis L. in MS regeneration medium after plunge in LN. For each treatment, different letters indicate percentages significantly different at P≤0.05.

Figure 7. Cryopreservation of bulb scales of F. imperialis L. pre-treated with encapsulation–vitrification. Regrowth of cryopreserved bulb scales that were exposed to encapsulation–vitrification for 10 min (A), 20 min (B) and 30 min (C) prior to plunge in LN after 60 days of culture on MS medium supplemented with 0.50 mg l–1 KIN together with 1.00 mg l–1 NAA (scale bar = 10 mm).
DISCUSSION
Measuring explants survival parameter at the end of storage highlighted the effectiveness of slow–growth pretreatments. Contrary to our result, Divakaran et al. (2006) reported that low temperatures (5°C) were not suitable for synthetic seed storage of Vanilla planifolia, as they lost their viability within 30 days. Germination and viability of non–encapsulated Cypripedium lentiginosum seeds stored at 5°C decreased after cold storage (Jiang et al., 2017). Similar results were reported in encapsulated Hibiscus moscheutos nodes (West et al., 2006), and Helianthus annuus shoot tips (Katouzi et al., 2011). Encapsulation of Begonia microshoots improved survival rate and germination percentage after cold storage irrespective of the medium type or storage environment compared with non–encapsulated explants in both in vitro and
ex vitro environments (Sakhanokho et al., 2013). These researchers reported 88, 53, 28, and 11% survival rates after 2, 4, 6, and 8 weeks cold storage at 4°C.
Our finding showed that encapsulation–dehydration technique increased the tolerance to cryopreservation of F. imperialis bulb scales. Similar finding was reported by some researchers on other endangered ornamental plants particularly the members of Orchidacae, Liliaceae, Bromeliaceae and Cactaceae families (Kaviani et al., 2008; Thammasiri, 2008; Kaviani, 2010, 2011; Khoddamzadeh et al., 2011; Subramaniam et al., 2011). Today, cryopreservation is the most important and the most popular long–term storage method for preservation of endangered and rare ornamental plants (Kulus and Zalewska, 2014; Teixeira da Silva et al., 2014; Kaviani and Negahdar, 2017). Some studies showed that encapsulation–dehydration may provide better protection than PVS or sucrose pretreatment alone (Kaviani et al., 2008, 2010). Encapsulation–dehydration technique is widely applied with orchids (seeds and PLBs) such as Dendrobium (Subramaniam et al., 2011) and Phalaenopsis (Khoddamzadeh et al., 2011). Kaviani and Negahdar (2017) showed that encapsulation–dehydration increased the tolerance to freezing of Buxus hyrcana Pojark shoot tips. These researchers demonstrated that the maximum regrowth after freezing in LN was registered around 50% for encapsulated–dehydrated shoot tips. All non–encapsulated–dehydrated shoot tips died after exposure to LN. Contrary to our finding, Sakai et al. (2000) showed the lowest result (20% regeneration) using encapsulation–dehydration technique with chrysanthemum shoot tips.
Pre–treatment with a high concentration of sucrose or air desiccation greatly increases the tolerance of cryopreserved plant explants. Physical dehydration under laminar air flow and chemical dehydration by sucrose are the most widely used pre–treatments (Sakai et al., 2000). Seeds of Lilium ledebourii pretreated with 0.75 M sucrose and dehydration for 1 h under laminar air flow showed 75% survival after cryopreservation (Kaviani et al., 2008; Kaviani et al., 2009). No survival was observed in untreated explants. The presence of sucrose in the beads dehydrates the explants and stimulates faster recovery on the regeneration media. Halmagyi et al. (2004) reported proper results with Chrysanthemum shoot tips, after 4–48 h incubation in 0.75 M sucrose solution. In the present study, pretreatment of bulb scales in air desiccation produced better results compared with sucrose enriched media. Desiccation in a sterile air–flow cabinet allows for a faster reduction of moisture content (Moges et al., 2004). Some researchers have used a combination of encapsulation, high sucrose pretreatment and air desiccation before exposure of plant materials to LN in endangered ornamental species (Kaviani et al., 2008, 2009; Kaviani, 2010, 2011; Khoddamzadeh et al., 2011). The present study exhibited the positive effect of encapsulation–dehydration treatment which was more favorable in cryopreserved bulb scales compared to sucrose, sucrose along with air desiccation and sucrose along with encapsulation–dehydration treatments. The possible reason for this observation could be the effective dehydration from the cells.
An important factor in production of high post–cryopreservation survival rates in explants is the optimization of exposure time to PVS prior to cryopreservation (Sakai et al., 2008). The encapsulation–vitrification method is a valuable technique for cryopreservation of many species including endangered ornamentals (Halmagyi and Deliu, 2007; Sakai and Engelmann, 2007; Kaviani et al., 2010). Maximum viability in Dendrobium nobile Lindl. (75.9%) was observed with 150 min of PVS2 incubation (Mohanty et al. 2012). Some researchers demonstrated that the best result varies between 5 and 25 min (Halmagyi and Deliu, 2007). Kaviani et al. (2010) applied an encapsulation–vitrification technique for cryopreservation of Lilium ledebourii germplasms (seeds, embryonic axes, lateral buds and bulblets). In this study, a 16–h incubation was used which resulted in the death of most explants. Results revealed that around 10% of cryopreserved seeds and embryonic axes pretreated with PVS2, 0.75 M sucrose and encapsulation were able to sprouting, while there was no survival after LN storage of seeds and embryonic axes pretreated with PVS2 and sucrose without encapsulation (Kaviani et al., 2010). PVS3 solution is less toxic than PVS2; therefore, we utilized PVS3 as a treatment for cryopreservation.
Many researches have revealed that the combined techniques especially encapsulation–dehydration and encapsulation–vitrification provide enough protection against lethal cryo–damage than non–combined techniques (Kaviani, 2011; Kulus and Zalewska, 2014). The encapsulation–vitrification technique was utilized for Saintpaulia ionantha Wendle. (Moges et al., 2004), and Dendrobium spp. (Mohanty et al., 2012; Teixeira da Silva et al., 2014). The maximum survival rate (over 80%) of Saintpaulia ionantha, Iris pumila L. and Chrysanthemum × grandiflorum shoot tips was observed using vitrification (Halmagyi et al., 2004; Moges et al., 2004; Jevremović et al., 2009). However, vitrification with PVS2 was lethal for some endangered ornamental plants such as Lilium ledebourii (Kaviani et al., 2010). Investigation of Teixeira da Silva
et al. (2014) on cryopreservation of Dendrobium germplasms revealed that encapsulation–vitrification and encapsulation–dehydration are the most used protocols resulted in a high survival frequency after cryogenic storage of explants. Nevertheless, long time exposure of explants to the high concentration of PVS is potentially injurious. Our findings confirm recently reported results.
Conclusion
In conclusion, the endangered and rare plant species are in urgent need of protection against the extinction of generations. Nowadays, in order to protect rare and endangered ornamental species, modern biotechnological tools need to be utilized. The results of this study provide reliable protocols for protection of F. imperialis Lubra Maxima against extinction by cold preservation and cryopreservation techniques with encapsulation–dehydration and encapsulation–vitrification pretreatments. These combined methods allowed high viability rates (between 67.0 and 81.6%) after cryopreservation. Therefore, the use of encapsulation–vitrification technique appeared to be a promising procedure for the long-term conservation of F. imperialis. However, routine application of encapsulation–vitrification for long-term storage in gene banks is currently limited to ornamental plants. Future perspectives may include consideration of genetic stability of the in vitro conserved material, optimization of existing cryopreservation protocols, standardizing and simplifying the methods as well as innovative approaches to in vitro conservation of Fritillaria species.
References
Bernard, F., Shaker-Bazarnov, H., and Kaviani, B. 2002. Effect of salicylic acid on cold preservation and cryopreservation of encapsulated embryonic axes of persian lilac (Melia azedarach L.). Euphytica. 123(1): 85–88. https://doi.org/10.1023/A:1014416817303
Blidar, C.F., Tripon, I.M., and Ilea, C. 2017. In vitro conservation of genetic resources of Nymphaea lotus var. thermalis (DC.) Tuzs., an endangered plant species. Romanian Biotechnological Letters. 24(3): 448–457. https://doi.org/10.25083/rbl/24.3/448.457
De Hertogh, A.A., and Le Nard, M. 1993. General chapter on summer flowering bulbs. In: De Hertogh, A.A., Le Nard, M., editors. The physiology of flowering bulbs. Amsterdam, London, New York, Tokyo: Elsevier. p. 741–774.
Divakaran, M., Nirmal Babu, K., and Peter, K.V. 2006. Conservation of Vanilla species, in vitro. Scientia Horticulturae. 10(2): 175–180. https://doi.org/10. 1016/j.scienta.2006.07.003
Engelmann, F. 2004. Plant cryopreservation: progress and prospects. In Vitro Cellular and Developmental Biology – Plant. 40(5): 427–433. https://doi.org/10.1079/IVP2004541
Halmagyi, A., and Deliu, C. 2007. Cryopreservation of carnation (Dianthus caryophyllus L.) shoot tips by encapsulation-vitrification. Scientia Horticulturae. 113(3): 300–306. https://doi.org/10.1016/j.scienta.2007.04.002
Halmagyi, A., Fischer-Kluver, G., Mix-Wagner, G., and Schumacher, H.M. 2004. Cryopreservation of Chrysanthemum morifolium (Dendranthema grandiflora Ramat) using different approaches. Plant Cell Reproduction 22. (6): 371–375. https://doi.org/10.1007/s00299-003-0703-9
Jevremović, S., Benelli, C., De Carlo, A., Subotić, A., and Lambardi, M. 2009. Development of cryopreservation procedures for dwarf irises (Iris spp.). In: 1st International Symposium: Cryopreservation in Horticultural Species, Book of Abstract. Leuven: ISHS. p. 45.
Jiang, H., Chen, M.C., and ILee, Y. 2017. In vitro germination and low-temperature seed storage of Cypripedium lentiginosum P.J.Cribb and S.C.Chen, a rare and endangered lady's slipper orchid. Scientia Horticulturae. 225(18): 471–479. https://doi.org/10.1016/j.scienta.2017.07.040
Katouzi, S.S.S., Majd, A., Fallahian, F., and Bernard, F. 2011. Encapsulation of shoot tips in alginate beads containing salicylic acid for cold preservation and plant regeneration in sunflower (Helianthus annuus L.). Australian Journal of Crop Science. 5: 1469–1474.
Kaviani, B. 2010. Cryopreservation by encapsulation-dehydration for long-term storage of some important germplasm: seed of lily [Lilium ledebourii (Baker) Bioss.], embryonic axe of Persian lilac (Melia azedarach L.), and tea (Camellia sinensis L.). Plant Omics Journal. 3(6): 177–182.
Kaviani, B. 2011. Conservation of plant genetic resources by cryopreservation. Australian Journal of Crop Science. 5(6): 778–800.
Kaviani, B., Abadi, D.H., Torkashvand, A.M., and Hoor, S.S. 2009. Cryopreservation of seeds of lily (Lilium ledebourii Baker Bioss): use of sucrose and dehydration. African. Journal of Biotechnology. 8(16): 3809–3810.
Kaviani, B., Dahkaei, M., Hashemabadi, D., and Darabi, A. 2010. Cryopreservation of Lilium ledebourii (Baker) Bioss. by encapsulation-vitrification and in vivo media for planting of germplasm. American-Eurasian Journal of Agricultural and Environmental Sciences. 8(5): 556–560.
Kaviani, B., and Negahdar N. 2017. Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark., an endangered ornamental shrub. South African Journal of Botany. 111: 326–335.
Kaviani, B., Safari-Motlagh, M.R., Padasht-Dehkaei, M.N., Darabi, A.H., and Rafizadeh, A. 2008. Cryopreservation of lily [Lilium ledebourii (Baker) Bioss.] germplasm by encapsulation-dehydration. International Journal of Botany. 4(4): 491–493. http://doi.org/10.3923/ijb.2008.491.493
Khoddamzadeh, A.A., Sinniah, U.R., Lynch, P., Kadir, M.A., Kadzimin, S.B., and Mahmood, M. 2011. Cryopreservation of protocorm-like bodies (PLBs) of Phalaenopsis bellina (Rchb. f.) Christenson by encapsulation-dehydration. Plant Cell, Tissue and Organ Culture. 107(3): 471–481. http://doi.org/10.1007/s11240-011-9997-4
Kulus, D., and Zalewska, M. 2014. Cryopreservation as a tool used in long term storage of ornamental species—a review. Scientia Horticulturae. 168: 88–107. https://doi.org/10.1016/j.scienta.2014.01.014
Lurswijidjarus, W., and Thammasiri, K. 2004. Cryopreservation of shoot tips of Dendrobium Walter Oumae by encapsulation/dehydration. Science Asia. 30: 293–299. http://doi.org/10.2306/scienceasia1513-1874.2004.30.293
Martin, C., and Gonzalez-Benito, E. 2005. Survival and genetic stability of Dendranthema grandiflora Tzvelev shoot apices after cryopreservation by vitrification and encapsulation-dehydration. Cryobiology. 51(3): 281–289. https://doi.org/10.1016/j.cryobiol.2005.08.001
Moges, A.D., Shibli, R.A., and Karam, N.S. 2004. Cryopreservation of African violet (Saintpaulia ionantha Wendl.) shoot tips. In Vitro Cellular and Developmental Biology – Plant. 40(4): 389–395. https://doi.org/10.1079/IVP2004536
Mohanty, P., Das, M.C., Kumaria, S., and Tandon, P. 2012. High-efficency cryopreservation of the medicinal orchid Dendrobium nobile Lindl. In Vitro Cellular and Developmental Biology – Plant. 109(2): 297–305. https://doi.org/10.100/s11240-011-0095-4
Murashige, T., and Skoog, F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 15(3): 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Sakai, A. 2000. Development of cryopreservation techniques. In: Engelmann, F., Takagi, H., editors. Cryopreservation of Tropical Plant Germplasm. International of Plant Genetic Resources Institute, Rome, p. 1-7.
Sakai, A., and Engelmann, F. 2007. Vitrification, encapsulation-vitrification and droplet-vitrification: a review. Cryo Letter. 28(3): 151–172.
Sakai, A., Hiraii, D., and Niino, T. 2008. Development of PVS-based vitrification and encapsulation–vitrification protocols. In: Reed, B. editors. Plant cryopreservation—a practical guide. New York: Springer Science Business Media, LLC. P. 33–57. https://doi.org/10.1007/978-0-387-72276-4 3
Sakai, A., Matsumoto, T., Hirai, D., and Niino, T. 2000. Newly development encapsulation-dehydration protocol for plant cryopreservation. Cryo Letter. 21(1): 53–62.
Sakhanokho, H.F., Cecil, T., Pounders, L., and Blythe, E.K. 2013. Alginate encapsulation of Begonia microshoots for short-term storage and distribution. Hindawi Publishing Corporation. The Scientific World Journal. 1-7. https:// doi.org/10.1155/2013/341568
Shatnawi, M.A., and Johnson, K.A. 2004. Cryopreservation by encapsulation-dehydration of ‘Christams bush’ (Ceratopetalum gummiferum) shoot tips In Vitro Cellular and Developmental Biology – Plant. 40: 239–244.
Singh, H., Kumar, S., and Singh, B.D. 2015. In vitro conservation of pointed gourd (Trichosanthes dioica) germplasm through slow-growth shoot cultures: Effect of flurprimidol and triiodobenzoic acid. Scientia Horticulturae. 182: 41–46. https://doi.org/10.1016/j.scienta.2014.11.009
Subramaniam, S., Sinniah, U.R., Khoddamzadeh, A.L., Periasamy, S., and James, J.J. 2011. Fundamental concept of cryopreservation using Dendrobium Sonia-17 protocorm-like bodies by encapsulation-dehydration technique. African Journal of Biotechnology. 10(19): 3902–3907
Tavazzaa, R., Nestor Alonso Reyb, N.A., Papacchiolia, V., and Pagnotta, M.A. 2015. A validated slow-growth in vitro conservation protocol for globeartichoke germplasm: a cost-effective tool to preserve from wild to elite genotypes. Scientia Horticulturae. 197: 135–143. https://doi.org/10.1016/j.scienta.2015.09.024
Teixeira da Silva, J.A., Zeng, S., Galdiano Jr., R.F., Dobránszki, J., Cardoso, J.C., and Vendrame, W.A. 2014. In vitro conservation of Dendrobium germplasm. Plant Cell Reproduction. 33(9): 1413–1423. https://doi.org/10.1007/s00299-014-1631-6
Thammasiri, K. 2008. Cryopreservation of some Thai orchid species. Acta Horticulturae. 788: 53–62. https://doi.org/10.17660/ActaHortic.2008.788.5
Vendrame, W.A., Carvalho, V.S., and Dias, J.M.M. 2007. In vitro germination and seedling development of cryopreserved Dendrobium hybrid mature seeds. Scientia Horticulturae. 114(3): 188–193. https://doi.org/10.1016/j.scienta.2007.06.006
Verleysen, H., Van Bockstaele, E., and Debergh, P. 2005. An encapsulation–dehydration protocol for cryopreservation of the azalea cultivar ‘Nordlicht’ (Rhododendron simsii Planch.). Scientia Horticulturae. 106(3): 402–414. https://doi.org/10.1016/j.scienta.2005.04.004
West, T.P., Ravindra, M.B., and Preece, J.E. 2006. Encapsulation, cold storage, and growth of Hibiscus moscheutos nodal segments. Plant Cell, Tissue and Organ Culture. 87(3): 223–231. https://doi.org/10.1007/s11240-006-9155-6
Yan, W.Y.L. 2006. Cryopreservation of Photinia serrulata shoot-tips by vitrification. Scientia Silvae Sinica. 12: 23.
Shima Seydi, Shahram Sedaghathoor*, and Behzad Kaviani
Department of Horticultural Science, Rasht Branch, Islamic Azad University, Rasht, Iran
*Corresponding author. E-mail: sedaghathoor@yahoo.com
Total Article Views
Article history:
Received: April 10, 2020;
Revised: May 23, 2020;
Accepted: May 27, 2020

