
Antiplasmodial Compounds from Indonesian Marine Sponge, Xestospongia sp, against Plasmodium falciparum 3D7
Murtihapsari, Dikdik Kurnia, Tati Herlina, Dewa gede Katja, Kadarusman, Khalijah Awang, Yoshihito Shiono, and Unang Supratman*Published Date : 2020-07-17
DOI : https://doi.org/10.12982/CMUJNS.2020.0032
Journal Issues : Number 3, July-September 2020
ABSTRACT A part of our continuing search for antiplasmodial compounds from Indonesian marine sponge, two steroidal alkaloids, epoxysarcovagenine-D (1) and epoxyepapakistamine-A (2), have been isolated from the ethyl acetate extract of the Indonesian marine ponge, Xestospongia sp. Their structures were identified on the basis of spectroscopic data analysis and by comparison with published spectroscopic and physicochemical properties data. Compounds 1 and 2 were isolated first time from marine sponge, Xestospongia sp., and showed strongest plasmodial activity against Plasmodium falciparum 3D7 strain with IC50 values of 0.013 and 0.158 µM, respectively.
Keywords: Steroidal alkaloid, Xestospongia sp, Petrosiidae, Antiplasmodial activity
INTRODUCTION
The genus Xestospongia is the marine sponge belong to Petrosiidae family, comprises approximately 40 species that are mainly distributed in north-western Australia, Papua New Guinea, Solomon Island, Thailand and Indo-Malaysia Peninsula (Fromont, 1991; Calcul, 2003; Laurent et al., 2006; Aguinaga et al., 2010). Xestospongia is known to settle and grow on a variety of substrates, such as sand, rock beds, dead coral rubble and coral heads (Kerr and Borges, 1994; Williams et al., 1998; Krisanapuntu et al., 2001; Bell and Smith, 2004; Armstrong et al., 2006). Chemical investigations on the species of this genus have led to the isolation of a large array of structurally diverse secondary metabolites with significant biological activities including antimalarial alkaloids (Girard et al., 2004; Darumas et al., 2007; Ashok et al., 2014), antifungal alkaloids (Moon et al., 2012), cytotoxic and inhibition of the aspartic protease of the quinones and hydroquinones (Aguinaga et al., 2010; Dai et al., 2010), antimalarial quinones (Laurent et al., 2006), antiplasmodial benzaldehyde (Murtihapsari et al., 2019) and antiplasmodial sterol (Renga et al., 2012).
During the course of our continuing search for antiplasmodial compounds from Indonesian marine sponge, the methanolic extract of Xestospongia sp exhibited a significant antiplasmodial activity against Plasmodium falciparum 3D7 strain. Xestospongia sp is distributed in the eastern part of Indonesia, and previous investigation have led to the isolation of several steroid with antiplasmodial activity (Muptihapsari et al., 2019). Owing to our interest in antiplasmodial compounds from this species, we investigated the ethyl acetate extract of the Xestospongia sp and obtained two antiplasmodial steroidal alkaloid. Here, we describe the structural identification of the isolates and their antiplasmodial activity.
MATERIALS AND METHODS
General
Melting points were obtained on an electrothermal melting point apparatus. Optical rotations were measured on an ATAGO AP-300 automatic polarimeter. IR spectra were obtained with a Perkin Elmer spectrum-100 spectrophotometer using KBr pellets. Mass spectra were recorded on Water Qtof HR-MS XEVotm mass spectrometers. 1D and 2D-NMR spectra were run on a JEOL ECZ A-600 spectrometer with tetramethyl silane as an internal standard. Chemical shifts (δ) were expressed in ppm with reference to the solvent signals. Column chromatography was performed on silica gel (70-230 and 200-400 mesh, Merck, Germany) and RP-18 gel (20−45 μm, Fuji Silysia Chemical Ltd., Japan). Fractions and spots were monitored by TLC (GF254, Merck, Germany), and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH followed by heating.
Sponge materials
Material of porifera Xestospongia sp, was obtained by scuba in about 10 m depth in the south west of Kaimana, West Papua, Indonesia (GPS: 4°20.341’ S-133°30.265’E). It has been identified taxonomically as Xestospongia sp. (Darumas et al., 2007), the species belongs to the Xestospongia. Identification taxonomic and nomenclature were provided by the Laboratory Biology and Conservation, Jakarta Fisheries University, Ministry of Marine Affairs and Fisheries (LIN BIOVASI, No: 041/STP-V/2016, Catalogue: MS041.1-5).
Parasite cultivation
Plasmodium falciparum strain 3D7, which is resistant to chloroquine (Singh and Rosenthal, 2001), was cultured in sealed flask at 37 oC, in a 3% O2, 5% CO2 and 91% N2 atmosphere in RPMI 1640, 25 µM HEPES, pH 7.4, supplemented with heat inactivated 10% human serum and human erythrocytes to achieve a 2% haematocrit. Parasites were synchronized in the ring stage by serial treatment with 5% sorbitol and studied at 1% parasitemia (Lambros et al., 1979).
In vitro antiplasmodial
Extracts and isolated compounds were prepared as 20 µg/mL stock solutions in DMSO (dimethyl sulfoxide), diluted as needed for individual experiment and tested in triplicate. The stock solutions were diluted in supplemented RPMI (Rosewell Parla Memorial Institute medium product) 1640 medium so as to have, at most, 0.2% DMSO in the final reaction medium. An equal volume of 1% parasitemia, 4% haematocrit culture was there after added and gently mixed thoroughly. Negative controls contained equal concentration of DMSO. Positive control contained an artemisinin. Cultures were incubated at 37 oC for 48 hours (one parasite erythrocytic life cycle). Parasites at ring stage were thereafter fixed by replacing the serum medium by an equal volume of 1% formaldehyde in PBS. (Phosphate Buffered Saline) aliquots (50 µL) of each culture were then added to 5 mL round-bottom polystyrenes tubes containing 0.5 mL 0.1% Triton X-100 and 1 nM YOYO nuclear dye (barand YOYO®-1) in PBS. Parasitemias of treated and control cultures were compared using a Becton-Dickinson FAC (brand BD-FAC™) Sort flow cytometer to count nucleated (parasitized) erythrocytes. An antiplasmodial examination was used 96 wells, each well filled by parasitemia culture 1%. About 50 µL of the compounds filled into the well with the following concentration 10-9 to 10-2 µg/mL. These data were normalized to percent control activity and 50% inhibitory concentration (IC50) calculated using Table probit.
Different concentration of the extract and isolated compounds were incubated at 37 oC with cultured 3D7 strain of P. falciparum parasites for 48 hours. Parasites were thereafter fixed and strained, and parasitemias of treated and control cultures were determined. Results are means, compared to untreated controls from 3 experiments. Error bars represent standard deviations of results (Bagavan et al., 2011).
RESULTS
Extraction and isolation
The milled fresh of marine sponge, Xestospongia sp (38 kg), were extracted with ethanol (30 L) at room temperature for 3 days. The ethanol extract was evaporated in vacuum to give a semisolid residue (376 g). After being suspended in water (2.0 L), the solution was successively fractionated with n-hexane, EtOAc and n-BuOH. Evaporation in vacuum resulted in the crude extracts of n-hexane (10.3 g), EtOAc (11.4 g), and n-BuOH (19.6 g), respectively. All extracts were evaluated for their antiplasmodial activity against P. falciparum 3D7 and showed antiplasmodial activity with IC50 values of 1.95, 0.41, and 1.68 µg/mL, respectively.
The EtOAc extract (11.4 g) was applied to silica gel column chromatography, eluted with n-hexane-EtOAc (100:1 to 1:100, v/v) to produce eight fractions (F.1-8). Fraction F.4 (420 mg) was subjected to silica gel column chromatography eluted with n-hexane-acetone (100:1 to 1:100, v/v) to produce six subfractions, 4A-4F. Subfraction 4C (130 mg) was applied to silica gel column chromatography using CHCl3-MeOH (9.5:0.5, v/v), then octa desyl silane (ODS) eluting with MeOH-H2O (2:3, v/v) to afford 1 (12.3 mg). F.6 (370 mg) was subjected to silica gel column chromatography eluted with n-hexane-acetone (100:1 to 1:100, v/v) to produce seven subfractions, 6A-4G. Subfraction 6E (130 mg) was applied to silica gel column chromatography using n-hexane-acetone (8:2, v/v) to afford 2 (14.6 mg).
Epoxysarcovagenine-D (1). Yellowish solid, m.p. 113-115 oC; [α]20D +16.5o (c = 0.1, CHCl3); UV λmax (MeOH) nm (log ɛ) 280 (5.2); IR (KBr) nmax cm-1: 3440, 3240, 2920, 2880, 1670, 1645, 1146, 1056; 1H-NMR (CDCl3, 600 MHz): Table 1; 13C-NMR (CDCl3, 125 MHz): Table 1; HR-TOFMS m/z 441.3040 [M+H]+, calcd. for C27H4ON2O3 m/z 440.3039.
Epoxynepapakistamine-A (2). White crystals; m.p 118-120 oC; IR (KBr) nmax cm-1: 3446, 2945, 1728, 1660, 1150, 847; 1H-NMR (CDCl3, 600 MHz): Table 1; 13C-NMR (CDCl3, 125 MHz): Table 1; HR-TOFMS m/z 559.3670 [M+H]+, calcd. for C32H5ON2O6 m/z 558.3669.
DISCUSSION
The ethanolic extract from the milled fresh of Xestospongia sp was concentrated and extracted successively with n-hexane, ethyl acetate and n-butanol. All of the extracts were evaluated for their antiplasmodial activity against P. falcifarum 3D7 in vitro and the ethyl acetate extract exhibited strongest antiplasmodial activity with an IC50 value of 0.41 µg/mL. By using antiplasmodial assay to guide separations, the ethyl acetate fraction was separated by combination of column chromatography on silica gel and octadesylsilane to afford two antiplasmodial steroidal alkaloids, 1 and 2 (Figure 1).
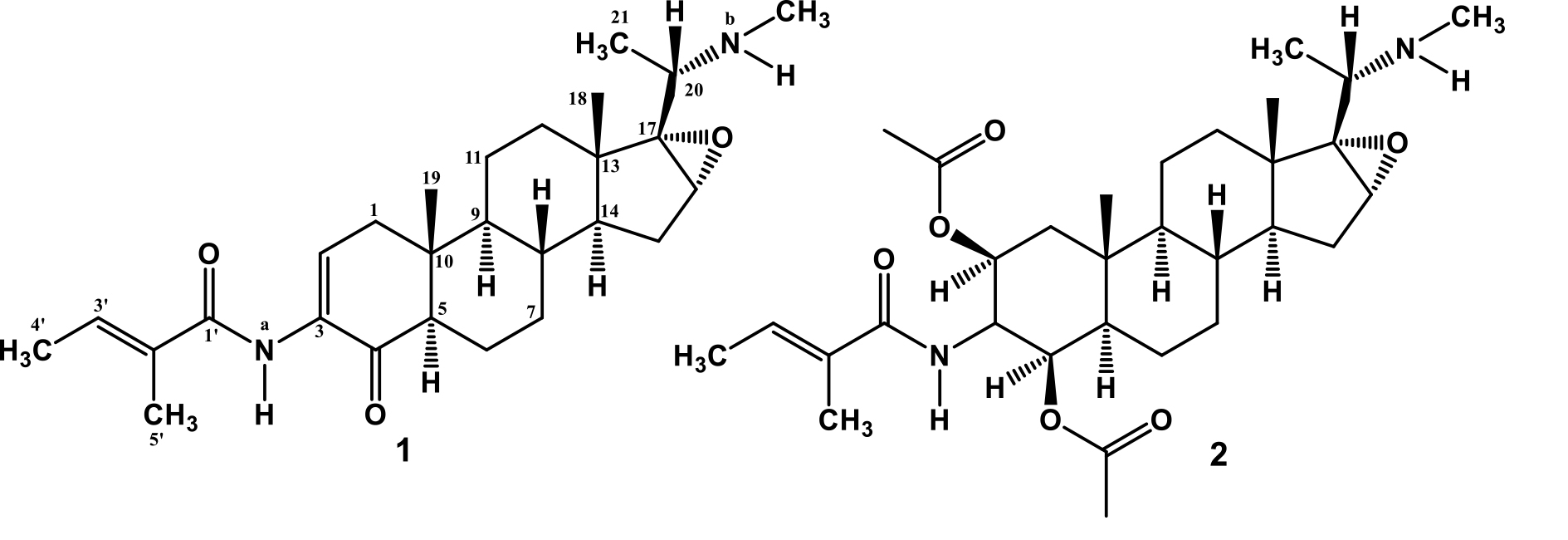
Figure 1. The chemical structures of 1 and 2 isolated from Xestospongia sp.
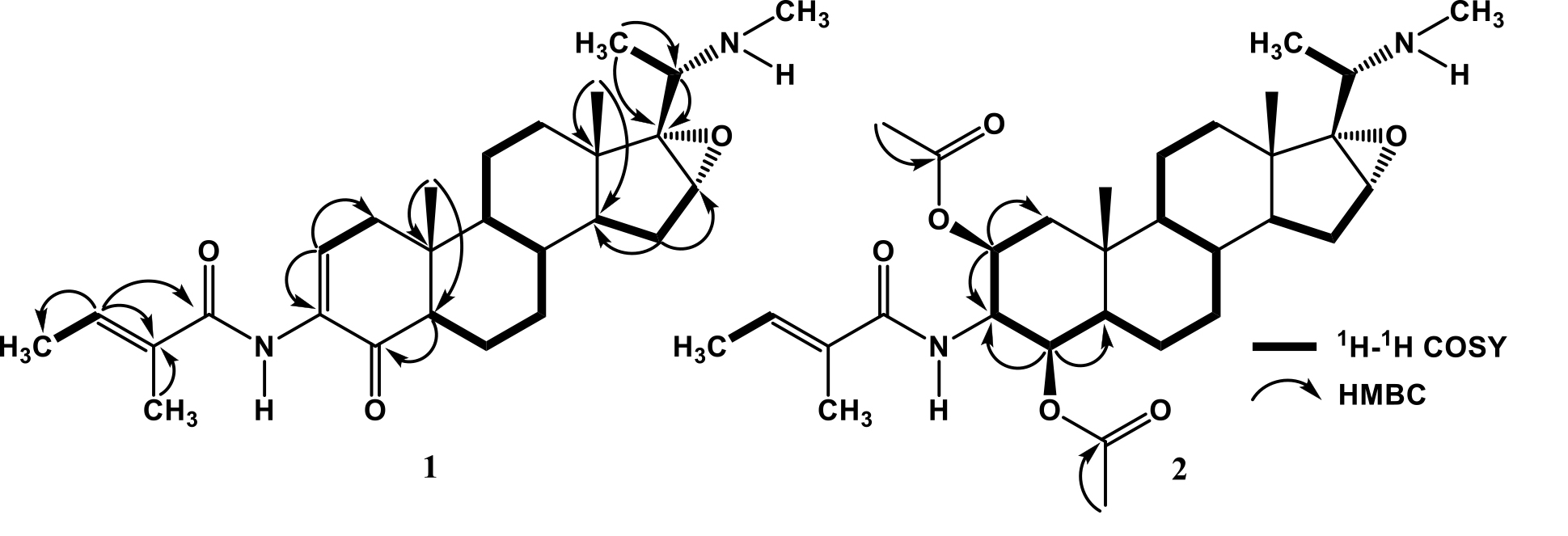
Figure 2. Selected 1H-1H COSY and HMBC Correlations for Compounds 1 and 2.
Compound 1 was isolated as yellowish solid; m.p 113-115 oC; [α]20D +16.5o (c = 0.1, CHCl3). Its molecular formula was determined as C27H4ON2O3 (nine indices of hydrogen deficiency) by HR-TOFMS m/z 441.3040 [M+H]+ and NMR spectral data (Table 1). The UV spectrum showed an absorption maximum at 280 nm (log ɛ 5.2), indicating the presence of an enone system (Supratman et al., 2000). Infrared (IR) absorption bands due to amine, conjugated carbonyl, amide and ether groups were observed at 3440, 1670, 1645 and 1146 cm-1, respectively.
The 1H-NMR spectrum of 1, displayed the presence of two tertiary methyl signals at δH 0.82 and 0.87 (each 3H, s, Me-18 and Me-19), a secondary methyl at [δH 1.12 (3H, d, J=6.5 Hz, Me-21), two methyl proton of tigloyl group at δH [2.40 (3H, d, J=6.9 Hz, Me-3¢) and 2.53 (3H, d, J=1.2 Hz, Me-5¢)] and a Nb-Me at δH 3.26 (3H, s). Proton signals of olefinic protons at δH [7.55 (1H, dd, J=6.9, 2.6 Hz, H-2) and 6.34 (1H, dd, J=6.9, 1.2 Hz, H-3¢)], an oxygenated sp3 methine at δH 3.10 (1H, dd, J=6.7, 2.4 Hz, H-16) and two N-H protons at δH [8.03 (1H, br.s, Na-H) and 2.10 (1H, br.s, Nb-H) were also observed in the H-NMR spectrum. The 13C NMR spectrum showed 27 signals, which are clarified by their chemical shifts and the DEPT spectra, consisting of six methyls, six methylenes, eight methines (including two olefinic carbons at δC 125.9 and 131.2), three sp3 quaternary carbons, two sp2 quaternary carbons at δC 132.1 and 131.5, a carbonyl ketone at δC 196.2 and a carbonyl amide at δC 168.0. These functionalities accounted as four out of the total nine indices of hydrogen deficiency. The remaining five indices of hydrogen deficiency were consistent to the pregrane-type steroidal skeleton with a tigloyl amino group, N-methylaminoethane substituent and additional an epoxide ring (Kaulanai et al., 2002; Farabi et al., 2018; Wu et al., 2019). To clarify the position of functional group in 1, 2D NMR experiments were carried out and the results as shown in Figure 2. In the 1H-1H COSY spectrum of 1 displayed correlations in C1-C2, C5-C6-C7-C8-C9, C11-C12, C14-C15-C16 and C4'-C3' supporting the presence of pregnane-type steroidal structure with a tigloyl group and additional an epoxide ring. The HMBC correlations from tertiary methyl signals at δH 0.82 and 0.87 to C-10 (δC 38.0), C-1 (δC 38.9), C-5 (δC 54.2) and C-13 (δC 41.6), C-12 (δC 32.8), C-14 (δC 45.2) was enabled to assign two tertiary methyls were attached at C-10 and C-13, respectively. An olefinic signal at δH 7.55 was correlated to C-1 (δC 38.9), C-3 (δC 132.1) and C-4 (δC 196.2), whereas H-5 (δH 2.28) was correlated to C-4 (δC 196.2), suggest the position of α,β-unsaturated ketone was located at C-2, C-3 and C-4. Another olefinic signal at δH 6.34 was correlated to Me-4'(δC 12.20), Me-5' (δC 13.9) and carbonyl amide (δC 168.0), suggested a tigloyl amino group was attached at C-3. An oxygenated proton at δH 3.10 was correlated to C-17 (δC 72.9) and C-15 (δC 27.0), whereas methine proton at δH 1.36 was correlated to C-16 (δC 59.4), indicated that epoxy ring was located at C-16 and C-17. A methine proton at δH 2.51 was mutually coupled to methyl at δH 1.12 and were correlated to oxygenated carbon at C-17 (δC 72.9), indicated N-methylaminoethane substituent was attached at C-17. The relative stereochemistry of 1 was determined on the basis of coupling constant in the 1H-NMR spectrum and a biogenetic point of view the occurrence of pregnane-type steroidal structure in natural (Rahman et al., 2002; Farabi et al., 2018). These observations along with the similarity of spectral data and physicochemical properties between 1 and previously reported (20S)-20-(N-methylamino)-3β-(tigloyl-amino)-5α-pregnan-16α,17α-epoxy-4-one, isolated from the leaves Sarcococca coriacea (Kaulani et al., 2002; Wu et al., 2019), let us identify 1 as (20S)-20-(N-methylamino)-3β-(tigloyl-amino)-5α-pregnan-16α,17α-epoxy-4-one, which isolated from marine sponge Xestospongia sp for the first time.
Compound 2 was isolated as white crystal; m.p 113-115 oC; [α]20D +20.5o (c = 0.2, CHCl3). Its molecular formula was determined as C32H5ON2O6 (nine indices of hydrogen deficiency) by HRTOFMS m/z m/z 559.3670 [M+H]+ and NMR spectral data (Table 1). The UV spectrum showed an absorption maximum at 285 nm (log ε 5.1) Shiono et al., 2016, indicating the presence of an enone system. Infrared (IR) absorption bands due to amine, conjugated carbonyl, amide and ether groups were observed at 3,420, 1,720, 1,670 and 1,146 cm-1, respectively. NMR spectral of 2 was very similar to those of 1, except the absence of α,β-unsaturated ketone at C-2, C-3 and C-4 at [δH 7.55 (1H, d, J=6.9, 2.6 Hz), δC 125.9, 132.1 and 196.2] and appeared the oxygenated carbons at [δH 4.53 (1H, d, J=9.3), δC 71.7 and 4.52 (1H, dd, J=8.7, 2.4 Hz), δC 74.1,)] and two acetyl groups [δH 2.05 and 2.00 (each 3H, s), δC 20.9, 21.3, δC 170.1 and 170.4], suggesting that 2 was an acetyl derivative of 1. An oxygenated proton at δH 4.53 was correlated to C-3 (δC 50.0), C-1 (δC 40.2) and carbonyl ester at C-1'' (δC 170.1), whereas another an oxygenated proton at δH 4.52 was correlated to
C-3 (δC 50.0), C-5 (δC 48.7) and carbonyl ester at C-1''' (δC 170.4), suggested two acetyl groups was located at C-2 and C-4, respectively.
These observations along with the similarity of spectral data and physicochemical properties between 1 and previously reported (20S)-20-(N-methylamino)-3β-(tigloyl-amino)-5α-pregnan-16α,17α-epoxy-2β,4β-di-O-acetate, isolated from the leaves Sarcococca coriacea (Kaulani et al., 2002; Wu et al., 2019), let us identify 2 as (20S)-20-(N-methylamino)-3β-(tigloyl-amino)-5α-pregnan-16α,17α-epoxy-2β,4β-di-O-acetate, which also isolated from marine sponge Xestospongia sp for the first time.
The antiplasmodial activity of the two isolated compounds 1 and 2 against P. falciparum was conducted according to the method described in previous paper (Frӧhlich et al., 2016) and were used an artemisinin (5.20 x 10-6 µM) as a positive control (Ravikumar et al., 2012; Fröhlich et al., 2016). Compounds 1 and 2 showed strong antiplasmodial activity with IC50 values of 0.013 and 0.158 µM, respectively, suggesting the presence of acetyl groups can decrease antiplasmodial activity, in contras with the presence of an enone group can increase antiplasmodial activity. Both compounds, (20S)-20-(N-methylamino)-3β-(tigloyl-amino)-5α-pregnan-16α,17α-epoxy-4-one (1) and (20S)-20-(N-methylamino)-3β-(tigloyl-amino)-5α-pregnan-16α,17α-epoxy-2β,4β-di-O-acetate (2) have been isolated as a potent cholinesterase inhibiting compounds from Sarcococca coriacea (Kaulani et al., 2002), but the antiplasmodial activity was shown in this study for the first time.
Table 1. NMR Data (600 MHz for 1H and 150 MHz for 13C, in CDCl3) for 1 and 2.
|
Position |
1 |
2 |
|||||||
|
13C NMR δc (mult) |
1H NMR δH (Integral, mult, J=Hz) |
13C NMR δc (mult) |
1H NMR δH (Integral, mult, J=Hz) |
|
|||||
|
1
2 3 4 5 6
7
8 9 10 11
12
13 14 15
16 17 18 19 20 21 N-Me Na-H Nb-H 1' 2' 3' 4' 5' 1'' 2'' 1''' 2''' |
38.9 (t)
125.9 (t) 132.1 (s) 196.2 (s) 54.2 (d) 20.4 (t)
20.5 (t)
33.1 (d) 54.9 (d) 38.0 (s) 30.2 (t)
32.8 (t)
41.6 (s) 45.2 (d) 27.0 (t)
59.4 (t) 72.9 (s) 15.9 (q) 14.1 (q) 51.0 (d) 17.8 (q) 34.6 (q) - - 168.0 (s) 131.5 (s) 131.2 (d) 12.2 (q) 13.9 (q) - - - - |
2.00 (1H, d, 6.9) 1.75 (1H, d, 2.6) 7.55 (1H, dd, 6.9, 2.6) - - 2.28 (1H, m) 1.64 (1H, m) 1.39 (1H, m) 1.50 (1H, m) 1.27 (1H, m) 1.41 (1H, m) 1.40 (1H, m) - 1.52 (1H, m) 1.25 (1H, m) 1.56 (1H, m) 1.30 (1H, m) - 1.36 (1H, m) 1.65 (1H, m) 1.55 (1H, m) 2.51 (1H, dd, 6.7, 2.4) - 0.82 (3H, s) 0.87 (3H, s) 3.10 (1H, q, 6.5) 1.12 (1H, d, 6.5) 3.26 (3H, s) 8.03 (1H, br.s) 2.10 (1H, br.s) - - 6.34 (1H, dd, 6.9, 1.2) 2.40 (3H, d, 6.9) 2.53 (3H, d, 1.2) |
40.2 (t)
71.7 (d) 50.0 (d) 74.1 (d) 48.7 (d) 20.4 (t)
25.0 (t)
33.2 (d) 65.0 (d) 35.0 (s) 31.4 (t)
32.9 (t)
41.6 (s) 45.7 (d) 27.0 (t)
59.4 (d) 72.9 (s) 16.0 (q) 15.3 (q) 51.0 (d) 17.9 (q) 34.6 (q) - - 168.0 (s) 131.5 (s) 131.2 (d) 12.2 (q) 13.9 (q) 170.1 (s) 20.9 (q) 170.4 (s) 21.3 (q) |
1.73 (1H, m) 1.48 (1H, d, 9.3) 4.53 (1H, d, 9.3) 4.72 (1H, d, 8.7) 4.52 (1H, dd, 8.7, 2.4) 2.00 (1H, m) 1.64 (1H, m) 1.39 (1H, m) 1.52 (1H, m) 1.27 (1H, m) 1.41 (1H, m) 1.40 (1H, m) - 1.52 (1H, m) 1.27 (1H, m) 1.56 (1H, m) 1.38 (1H, m) - 1.40 (1H, m) 1.65 (1H, m) 1.55 (1H, m) 2.51 (1H, m) - 0.82 (3H, s) 1.12 (3H, s) 3.10 (1H, q, 6.5) 1.13 (3H, d, 6.5) 3.22 (3H, s) 5.95 (1H, d, 8.7) 2.20 (1H, br.s) - - 6.34 (1H, q, 6.8) 2.20 (3H, d, 6.8) 2.53 (3H, s) - 2.05 (3H, s) - 2.00 (3H, s) |
|||||
CONCLUSIONS
Two antiplasmodial steroidal alkaloids have been isolated from marine sponge, Xestospongia sp and identified by spectroscopic methods as epoxysarcovagenine-D (1) and epoxyepapakistamine-A (2). Compounds 1 showed stronger antiplasmodial activity than compound 2 with an IC50 value of 0.013 µM, suggesting the presence of acetyl groups can decrease antiplasmodial activity, whereas the presence an enone group can increase antiplasmodial activity.
ACKNOWLEDGMENTS
This investigation was financially supported by the Domestic Postgraduate Education Scholarship (BPPDN Grant) from Directorate General of Higher Education, Ministry of Research, Technology and Higher Education, Indonesia (Period: 2014-2017 granted for Murtihapsari). We are extremely grateful to Regis Hocde, Laurent Pouyaud, Amir M. Suruwaky and the Team of Expedition Lengguru 2014 (LIPI, Politeknik KP Sorong, IRD-France) who kindly helped authors during sampling marine sponges in Kaimana. Authors also express thank to Jonathan M. Marbun for the kind support during antiplasmodial assay work at the Eijkman Institute for Molecular Biology in Jakarta.
REFERENCES
Aguinaga, N.M., Mercado, I.E.S., and Williams, P. 2010. Xestosaprol D and E from Indonesian marine sponge Xestospongia sp. Tetrahedron Letters. 51(4): 751-753. https://doi.org/10.1016/j.tetlet.2009.11.132
Armstrong, R.A., Dingh, H., Torres, J., Nemeth, R.S., Can, A., Roman, C., Eustice, R., Riggs, L., and Moliner, G.G. 2006. Characterizing the deep insular shelf coral reef habitat of the Hind Bank Marine conservation district (US virgin Islands) using the seabed autonomous underwater vehicle. Continental Shelf Research. 26(2): 194-205. https://doi.org/10.1016/j.csr.2005.10.004
Ashok, P., Ganguly, S., and Murugesan, S. 2014. Manzamine alkaloids: isolation, cytotoxicity, antimalarial activity and SAR studies. Drug Discovery Today. 19(11): 1781-1791. https://doi.org/10.1016/j.drudis.2014.06.010
Bagavan, A., Rahuman, A.A., Kaushik, N.K., and Sahal, D. 2011. In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitology Research. 108(1): 15-22. https://doi.org/10.1007/s00436-010-2034-4
Bell, J.J., and Smith, D. 2004. Ecology of sponge assemblages (Porifera) in the Wakatobi region, south-east Sulawesi, Indonesia: richness and abundance. Journal of the Marine Biological Association of the United Kingdom. 84(3): 581-591. https://doi.org/10.1017/S0025315404009580h
Calcul, L., Longeon, A., Mourabit, A.A., Guyot, M., and Bourguet, K.M.I. 2003. Novel alkaloid of the aaptamine class from an Indonesian marine sponge of the genus Xestospongia. Tetrahedron. 59(34): 6539-6544. https://doi.org/10.1016/S0040-4020(03)01069-X
Dai, J., Sorribas, A., Yoshida, W.Y., Kelly, M., and Williams, P.G. 2010. Xestosaprols from Indonesian Marine Sponge Xestospongia sp. Journal of Natural Products. 73(6): 1188-1191. https://doi.org/10.1021/np100203x
Darumas, U., Chavanich, S., and Suwanborirux, K. 2007. Distribution patterns of the renieramycin-producing sponge, Xestospongia sp., and its association with other reef organisms in the Gulf of Thailand. Zoological Studies. 46(6): 695-704.
Farabi, K., Harneti, D., Nurlelasari., Maharani, R., Hidayat, A.T., Awang, K., Supratman, U., and Shiono, Y. 2018. New cytotoxic pregnane-type steroid from the stem bark of Aglaia elliptica (Meliaceae). Record of Natural Products. 12(2): 121-127. https://doi.org/10.25135/rnp.21.17.07.118
Frӧhlich, T., Karagӧz, A.C., Reiter, C., and Tsogoeva, S.B. 2016. Artemisinin-derived dimers: potent antimalarial and anti-cancer agents. Journal of Medicinal Chemistry. 59: 7360-7388. https://doi.org/10.1021/acs.jmedchem.5b01380
Fromont, J. 1991. Descriptions of species of the Petrosida (Porifera: Demospongiae) occurring in the tropical waters of the Great Barrier Reef. Beagle Records Northern Territory Museum of Art and Sciences. 8: 73-96.
Girard, E., Jaimovitch, E., Vergne, C., Almourabit, A., Laurent D., and Molgo, J. 2004. In rencontres en toxinologie. Envenimations, intoxications. Lavoisier. 141–152.
Kerr, R.G., and Borges, M.K. 1994. Biochemical and morphological heterogeneity in the Caribbean sponge Xestospongia muta (Pterosida: Petrosiidae). Sponges in Time and Space. Proceedings of the 4th International Porifera Congress. 4: 65-73.
Kaulani, S.K., Choudhary, M.I., Khalid, A., Manandhar, M.D., Shaheen, F., Rahman, A-ur., and Gewali, M.B. 2002. New cholinesterase inhibiting steroidal alkaloids from the leaves of Sarcococca coriacea of Nepalese origin. Chemical and Pharmaceutical Bulletin. 50(11): 1423-1426. https://doi.org/10.1248/cpb.50.1423
Kritsanapuntu, S.N., Chaitanawisuti, T., Yeemin, S., and Putchakan, S. 2001. First investigation on biodiversity of marine sponges associated with reef coral habitats in the eastern Gulf of Thailand. Asian Marine Biology. 18: 105-115.
Laurent, D., Valerie J., Arnaud P., Martine K., Dominique D., Sophie S., Olivier L., Nicolas L., Maryvonne F., Frederic A., et al. 2006. Antimalarial potential of xestoquinone, a protein kinase inhibitor from a vanuatu marine sponge Xestospongia sp. Bioorganic & Medicinal Chemistry. 14(13): 4477-4482. https://doi.org/10.1016/j.bmc.2006.02.026
Lambros, C., and Vanderberg, J.P.J. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. The Journal of Parasitology. 65(3): 418-420. https://doi.org/10.2307/3280287
Moon, S.S., MacMillan, J.B., Olmstead, M.M., Ta, T.A., and Molinski, T.F. 2012. (+)-7S-Hydroxyxestospongin A from the marine sponge Xestospongia sp. and absolute configuration of (+)-xestospongin D. Journal of Natural Products. 65(3): 249-254. https://doi.org/10.1021/np010427z
Murtihapsari, M., Salam, S., Kurnia, D., Darwati, D., Kadarusman, K., Abdullah, F.F., Herlina, T., Husna, M.H., Awang, K., Shiono, Y., et al. 2019. A New Antiplasmodial Sterol from Indonesian marine sponge, Xestospongia sp. Natural Product Research. 18: 1-8. https://doi.org/10.1080/14786419.2019.1611815
Rahman, A-ur., Ul-Haq, Z., Khalid, A., and Choudhary, I.M., 2002. Pregnane-type steroidal alkaloids of Sarcococca saligna: a new class of cholinesterase inhibitors. Helvetica Chimica Acta. 85(2): 678-688. https:// doi.org/10.1002/1522-2675(200202)85:2<678::AID-HLCA678>3.0.CO; 2-2
Ravikumar, S., Inbaneson, S.J., and Suganthi, P. 2012. In vitro antiplasmodial activity of chosen terrestrial medicinal plants against Plasmodium falciparum. Asian Pacific Journal of Tropical Disease. 2(7): S252-180-256.
Renga, B., Mencarelli, A., D’Amare, C., Cipriani, S., D’Auria, M.V., Sepe, V., Chini, M.G., Monti, M.C., Bifulco, G., Zampella, A., et al. 2012. Discovery that theonellaasterol a marine sponge sterol is a highly selective FXR antogonist that protects against liver injury in cholestatis. PLOS ONE. 7(1): 1-12. https://doi.org/10.1371/journal.pone.0030443
Shiono, Y., Miyazani, N., Murayama, T., Koseki, T., Harizon, Katja, D.G., Supratman, U., Nakata, J., Kakihara, Y., Seaki, M., et al. 2016. GSK-3ß inhibitory activities of novel dichloresorcinol derivatives from Cosmospora vilior isolated from a mangrove plant. Phytochemestry Letters. 18: 122-127. https://doi.org/10.1016/j.phytol.2016.09.007
Singh, A., and Rosenthal, P.J. 2001. Comparison of efficacies of cysteine protease inhibitors against five strains of Plasmodium falciparum. Antimicrobial Agents and Chemotherapy. 45(3): 949-951. https://doi.org/10.1128/AAC.45.3.949-951.2001
Supratman, U., Fujita, T., Akiyama, K., and Hayashi, H. 2000. New insecticidal bufadienolide, bryophyllin C, from the leaves of Kalanchoe pinnata. Bioscience, Biotechnology, and Biochemistry. 64(6): 1310-1312. https://doi.org/10.1271/bbb.64.1310
Williams, D.E., Lassota, P. and Andersen, R.J. 1998. Motuporamines A-C, cytotoxic alkaloids isolated from the marine sponge Xestospongia exigua (Kirkpatrick). The Journal of Organic Chemistry. 63(14): 4838-4841. https://doi.org/10.1021/jo980355p
Wu, J.C., Huo, S.J., and Du, J. 2019. 4-dehydroxyepisarcovagine A, a new steroidal alkaloid from Sarcococca pruniformis Lindl. Natural Product Research. 33(2): 169-173. https://doi.org/10.1080/14786419.2018.14400226
Murtihapsari1,2, Dikdik Kurnia1, Tati Herlina1, Dewa gede Katja3, Kadarusman4,5, Khalijah Awang6 Yoshihito Shiono7, and Unang Supratman1,8*
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jatinangor 45363, Indonesia
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, Papua State University, Jl. Gunung Salju, Kampus Amban, Monokwari, West Papua 98314, Indonesia
3Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sam Ratulangi, Kampus Bahu, Manado, 95115, North Sulawesi, Indonesia
4Sorong Marine and Fisheries Polytechnic, KKD BP, SR SGK, Tanjung Kasuari, Kota Sorong 98401, West Papua, Indonesia
5Department of Aquatic Resources Management, Sekolah Tinggi Perikanan, KKD PSP. SR BE, Pasar Minggu, Jakarta 12520, Indonesia
6Department of Chemistry, Faculty of Science, University of Malaya, Kuala Lumpur 59100, Malaysia
7Department of Food, Life, and Environmental Science, Faculty of Agriculture, Yamagata University, Tsuruoka, Yamagata 997-8555, Japan.
8Central Laboratory, Universitas Padjadjaran, Jatinangor 45363, Indonesia
*Corresponding author. E-mail: unang.supratman@unpad.ac.id
Total Article Views
Article history:
Received: July 25, 2019;
Revised: September 25, 2019;
Accepted: October 25, 2019

