
Karyological Studies on Hammerhead Flatworm, Bipalium kewense (Tricladida, Terricola) from Thailand
Nuntaporn Getlekha*Published Date : 2020-05-12
DOI : https://doi.org/10.12982/CMUJNS.2020.0029
Journal Issues : Number 3, July-September 2020
ABSTRACT Species of the Bipalium genus (bipaliid land planarian) are widely distributed in the Southeast Asia, around greenhouses and gardens. However, taxonomy and cytogenetic data in this genus are restricted to few species. In this way, the present study includes the chromosomal investigation, using conventional (Giemsa staining) approaches in Bipalium kewense from Northeast Thailand. The specimen, B. kewense with two dorsal stripes and a blackish brown head crescent, a lunate head moderately developed (40–150 mm long and 3–5 mm wide); light yellowish brown with one broad mid-dorsal and two marginal stripes; without stripes on the ventral side. The results showed that B. kewense had 2n=10, and the fundamental number (NF) was 20. The types of chromosomes are 2 large metacentric, 2 medium metacentric, 2 medium submetacentric and 4 medium acrocentric chromosomes. The karyotype formula of Bipalium kewense is as follows: 2n (10) = Lm2+Mm2+Msm2+Ma4 or 2m+2a+2a+2m+2sm
Keywords Land planarian, Terricola, Platyhelminthes, chromosome
INTRODUCTION
Land planarians are one of the most unusual and interesting creatures in and around greenhouses and gardens. Its 54 described species exhibit a complex taxonomy with cryptic lineages across their extensive distribution showing typical characteristics of the distinctive shape of their head region and stripes on the body. This animal belongs to one of the most ancient animal groups, the Phylum Platyhelminthes–also known as flatworms. Originally from southeast Asia, Bipalium kewense has been dispersed by man to more than 50 countries around the world (Winsor, 1983; Jean-Lou et al., 2018). Bipalium is a genus of large predatory land planarians. They are often loosely called "hammerhead worms" or "broadhead planarians" because of the distinctive shape of their head region. Land planarians are unique in that they possess a "creeping sole" on their ventral side (Curtis et al., 1983). Several species are considered as invasive to the United States and to Europe (Filella-Subira, 1983; Ogren, 1985; Justine et al., 2018).
The karyology, cytogenetics and cytotaxonomy of freshwater planarians have been extensively studied and there has been a developing interest in the chromosomes of marine species (Ball, 1976, 1979; Gallen and Pucci-Nelli, 1979). The terrestrial planarians, however, have been almost totally neglected in this respect apart from brief notes by Minelli (1977), who reported a probable chromosome complement of 2n=10 from a species of Microplana (Rhynchodemidae) from the Jura Mountains, France. Karyotypes reported for species of the genus Bipalium (Bipaliidae) show some variety both among and within its recognized species. Seo et al. (1988) found that Bipalium nobile and B. multilineatum, from Japan both have a diploid chromosome number of 2n=10 while B. kewense from Japan had 2n=18. Other specimens of B. kewense from Australia were found by Winsor (1981) to have a diploid chromosome number of 2n=16. Oki et al. (1991) found three additional, undescribed species of Bipalium from Sanjo, Japan, and Chichijima Island, and karyological studies of two of these show a diploid chromosome number of 2n=10. In addition, Yamamoto et al. (2001) reported that B. kewense from Bunkyo and Kakido have 2n=18 while B. nobile, B. multilineatum and one undescribed species of Bipalium from Nameshi, Bunkyo, Kinkai, Nagayo and Nagasaki, all of them have a diploid chromosome number of 2n=10 and the last undescribed species of Bipalium from Benten have 2n=12 (Table 1).
The work was undertaken not only to fill some gaps in our empirical knowledge but also in the hope of providing comparative information that may throw light on the phylogenetic relationships between the three principal suborders of the Tricladida, relationships which at the moment are rather unclear (Ball, 1981). This study was aim to report here on the karyotypes and idiogram of Thailand specimens of B. kewense, and record their external appearance.
Table 1. Cytogenetic data of the genus Bipalium (Bipaliidae).
|
No. |
Species |
2n |
NF |
Formula |
Locality |
Reference |
|
1 |
Bipalium kewense |
16 |
– |
– |
Australia |
Winsor (1981) |
|
|
|
18 |
– |
– |
Japan |
Seo et al. (1988) |
|
|
|
18
|
–
|
2m+2m+2m+2sm+2st+2sm +2sm+2sm+2sm |
Chichijima Island |
Oki et al. (1991) |
|
|
|
18
|
–
|
2m+2m+2m+2st+2sm+2sm +2sm+2sm+2sm |
Bunkyo and Kakido |
Yamamoto |
|
|
|
10 |
20 |
4m+2sm+4a or 2m+2a+2a+2m+2sm |
Thailand |
Present study |
|
2
|
B. nobile
|
10 |
–
|
–
|
Japan |
Seo et al. (1988) |
|
|
|
10
|
–
|
2m+2sm+2sm+st&sm+2sm
|
Yokohama
|
Oki et al. (1991) |
|
|
|
10
|
–
|
2m+2sm+sm&st+2st+m&sm
|
Toyonaka
|
Oki et al. (1991) |
|
|
|
10
|
–
|
2m+2sm+2sm+st&sm+2sm
|
Nameshi
|
Yamamoto |
|
3 |
B. multilineatum |
10 |
–
|
–
|
Japan |
Seo et al. (1988) |
|
|
|
10 |
–
|
2m+sm&st+2sm+2sm+2sm&st |
Bunkyo |
Yamamoto |
|
|
|
10 |
–
|
2m+2sm+2st+2sm+2sm |
Kinkai |
Yamamoto |
|
|
|
10 |
–
|
2m+sm&st+2st+sm&st |
Nagayo |
Yamamoto |
|
4 |
Bipalium sp. |
10 |
– |
2m+2sm+2sm+2sm+2sm |
Sanjo |
Oki et al. (1991) |
|
5 |
Bipalium sp.
|
10
|
–
|
2m+2sm+2sm+2sm+2m
|
Chichijima Island |
Oki et al. (1991) |
|
6 |
Bipalium sp.
|
10
|
–
|
2m+2m+2m+2m+2sm
|
Nagasaki |
Yamamoto |
|
7 |
Bipalium sp.
|
10
|
–
|
2m+2sm+2sm+2m+m&sm
|
Nagasaki |
Yamamoto |
|
8 |
Bipalium sp.
|
10
|
–
|
2m+2m+m&sm+2m+2m
|
Nagasaki |
Yamamoto |
|
9 |
Bipalium sp.
|
10
|
–
|
2m+m&sm+2sm+2m+2m
|
Bunkyo |
Yamamoto |
|
10 |
Bipalium sp.
|
12
|
–
|
2m+2st+2m+2sm+2m+2m
|
Benten |
Yamamoto |
Note: 2n=diploid chromosome number, NF=fundamental number (number of chromosome arms), m=metacentric, sm=submetacentric, st=subtelocentric, a=crocentric chromosome and – = not available
MATERIAL AND METHODS
Chromosome preparation
Firstly, A single specimen, tentatively identified as Bipalium kewense on the basis of external features, was collected from Khon Kaen University, Khon Kaen Province, Thailand. A photograph of a live specimen was retained by Getlekha for taxonomic purposes. Secondly, the specimen was soaked into a solution of 0.02% colchicine and left for four h. Thirdly, Chromosomes were prepared from whole body by the squash technique (Chen and Ebeling, 1968, Nanda et al., 1995). Next, the tissues were cut into small pieces and suck (up and down) by syringe then mixed with hypotonic solution (0.075M KCl). After discarding all large pieces of tissues, 7 ml of cell sediments were transferred to a 15 ml centrifuge tube and incubated for 30 min at room temperature. Then, hypotonic solution was discarded from the supernatant after centrifugation at 1200 rpm for 8 min. After that, cells were fixed in a fresh cool fixative (3 absolute methanol:1 glacial acetic acid) to which up to 7 ml were gradually added before being centrifuged again at 1,200 rpm for 8 min, at which time the supernatant was discarded. Next, the fixation was repeated until the supernatant was clear and the pellet was mixed with 1 ml fixative. Finally, the mixture was dropped onto a clean and cold slide by a plastic pipette followed by air-dry technique (Kasiroek et al., 2017).
Chromosome staining
The slide was conventionally stained with 20% stock Giemsa’s solution for 30 min. After that, the slides were soaked in distilled water (Sangpakdee et al., 2017).
Chromosome checking, Karyotyping and Idiograming
Standardized karyotypes and idiograms of B. kewense were constructed. Then, chromosome checking was performed on mitotic metaphase cells under a light microscope. Next, the frequencies of chromosome number per cell were counted. After that, the maximum frequency of chromosome number per cell is the diploid chromosome number of B. kewense. Finally, twenty cells with clearly observable and well-spread chromosome were selected for karyotyping.
The length of short arm chromosome (Ls) and long arm chromosome (Ll) were measured and calculated to the length of total arm chromosome (LT, LT=Ls+Ll). The relative length (RL), the centromeric index (CI) and standard deviations (S.D.) of RL and CI were calculated. Chromosomal nomenclature follows Chaiyasut (1989) by the CI (q/p+q) between 0.50–0.59, 0.60–0.69, 0.70–0.89 and 0.90–0.99 were representing the metacentric, submetacentric, acrocentric and telocentric chromosomes, respectively. The fundamental number (number of chromosome arms, NF) was obtained by assigning a value of two to metacentric, submetacentric and acrocentric chromosomes and one to telocentric chromosome. All parameters were used in karyotyping. The idiogram was constructed using a model drawing of karyotype and accomplished by a computer program (Chooseangjaew et al., 2017).
RESULTS
The B. kewense had 2n=10, and the fundamental number (NF) was 20. The types of chromosomes are 2 large metacentric, 2 medium metacentric, 2 medium submetacentric and 4 medium acrocentric chromosomes (Figure 1). The karyotype formula of B. kewense. is as follows: 2n (10) = Lm2+Mm2+Msm2+Ma4 (4m+2sm+4a or 2m+2a+2a+2m+2sm)
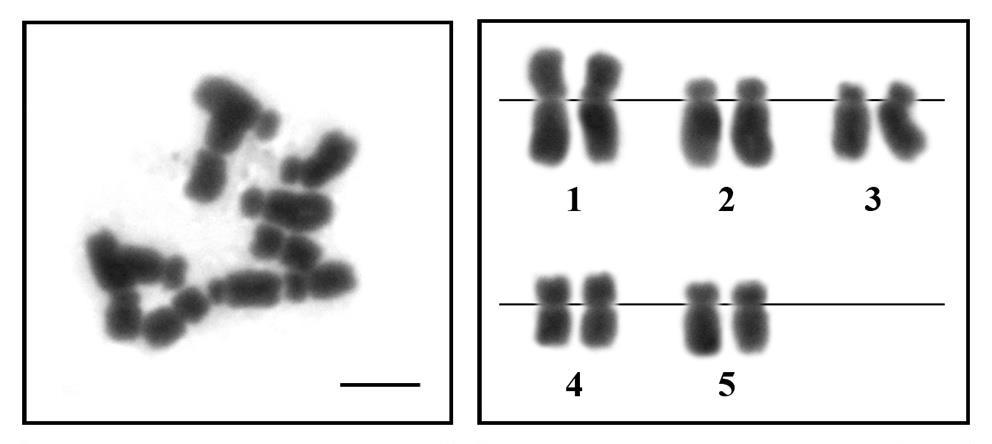
Figure 1. Metaphase chromosome plate and karyotype of the Bipalium kewense (2n=10) by conventional staining technique (scale bar indicate 5 μm).
From an analysis of twenty particularly clear metaphase plates precise karyometric data and an idiogram have been prepared. The size and type of chromosomes were showed in the Tables 2. An idiogram is a model of each pair of chromosome presumed that the large chromosome size (LT>5.20 μm), the medium size (3.14–5.20 μm) and the small size (LT<3.14 μm) (Figure 2).
Table 2. Mean length of short arm chromosome (Ls), length long arm chromosome (Ll), length total arm chromosome (LT), relative length (RL), centromeric index (CI) and standard deviation (SD) of RL, CI from 20 metaphase cells of the Bipalium kewense (2n=10).
|
Chromosome pair |
Ls |
Ll |
LT |
RL±SD |
CI±SD |
Chromosomes size |
Chromosomes type |
|
||||
|
1 |
2.82 |
3.46 |
6.27 |
0.2643±0.0249 |
0.5512±0.0027 |
large |
metacentric |
|||||
|
2 |
1.29 |
3.58 |
4.87 |
0.2052±0.0768 |
0.7345±0.0026 |
medium |
acrocentric |
|||||
|
3 |
1.11 |
3.22 |
4.33 |
0.1823±0.1659 |
0.7443±0.0062 |
medium |
acrocentric |
|||||
|
4 |
1.85 |
2.29 |
4.14 |
0.1745±0.0753 |
0.5521±0.0045 |
medium |
metacentric |
|||||
|
5 |
1.45 |
2.68 |
4.12 |
0.1737±0.0388 |
0.6492±0.0025 |
medium |
submetacentric |
|||||
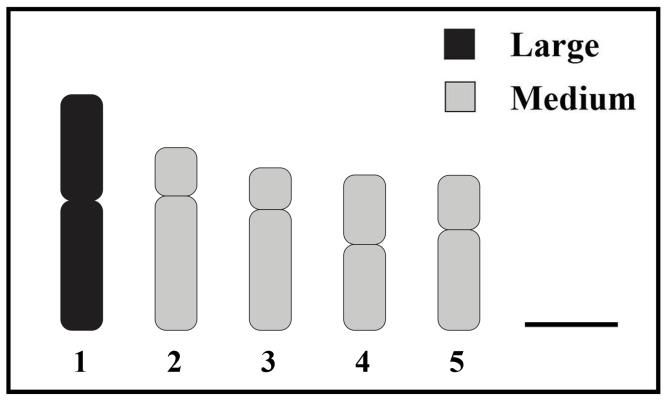
Figure 2. Standardized idiogram showing lengths and morphology of chromosomes of the Bipalium kewense (2n=10) (scale bars indicate 2 μm).
DISCUSSIONS
The specimen, B. kewense have external morphology with two dorsal stripes and a blackish brown head crescent, a lunate head moderately developed (40–150 mm long and 3–5 mm wide); light yellowish brown with one broad mid–dorsal and two marginal stripes; without stripes on the dorsal side that show a little bit different external appearance from B. kewense from Australia, Japan and Island. The diploid chromosome number of 2n=10 of B. kewense found in this study is consistent with almost all previous reports of genus Bipalium except some reported such as Bipalium kewense (2n=16, 18) and Bipalium sp. No. 10 (2n=12) in Table 1 (Winsor, 1981; Seo et al., 1988; Oki et al., 1991; Yamamoto et al., 2001) revealing that characteristic of the genus Bipalium. Regarding chromosomal morphology, this study is in agreement with others in the genus Bipalium, with karyotypes predominantly formed by biarmed chromosomes. However, there are differences number of metacentric, submetacentric, acrocentric or subtelocentric chromosomes in karyotype among the reports (see Table 1), which in part might be caused by condition of pretreatment (concentrations or duration of colchicine treatment) (Leitao et al., 1999) and nomenclature of the chromosomes adopted. Moreover, some variation might be attributable to chromosome rearrangements among different Bipalium species (Ahmed, 1973, Pereira et al., 2011).
However, the previous report of cytogenetics in genus Bipalium shown many of an undescribed species, Bipalium sp. that see in the Table 1 but they described about the detail of characteristic features of the specimen for example an undescribed species from Sanjo, Bipalium sp. No. 4, with two dorsal stripes and a yellow head crescent, an undescribed species from Chichijima Island, Bipalium sp. No. 5, with five dorsal stripes, an undescribed species from Nagasaki, Bipalium sp. No. 6, a lunate or semilunate head moderately large (70 mm long and 4 mm wide); dark brown above with one mid-dorsal and two lateral stripes; with a pair of indistinct, dark lateral stripes on the ventral side, Bipalium sp. No. 7, a lunate head well developed (70 mm long and 6 mm wide); dark grayish brown above with one mid-dorsal and two marginal stripes; with a pair of indistinct, lateral stripes on the ventral side, Bipalium sp. No. 8, a semilunate head well developed (80 mm long and 5–7 mm wide); light yellowish brown above with one broad mid-dorsal and two thin marginal stripes; with a pair of indistinct, lateral stripes on the ventral side, an undescribed species from Bunkyo, Bipalium sp. No. 9, a lunate head moderately developed (30 mm long and 2–4 mm wide); dark brown above with one blackish mid-dorsal stripe; without stripes on the ventral side, an undescribed species from Benten, Bipalium sp. No. 10, a semilunate head moderately large (200 mm long and 10–15 mm wide); blackish brown to black above; with a pair of dark, indistinct stripes on each lateral side of the creeping sole (Table 1) (Oki et al., 1991; Yamamoto et al., 2001).
CONCLUSION
In this report, B. kewense from Thailand resembles in external morphology to B. kewense from Australia, Japan and Island but the chromosome number is clearly different (10 vs. 16, 18 and 18 respectively) show that they might be different species similar to the cause of the damselfish (Dascyllus abudafur and Dascyllus aruanus), both of them are similar morphology but clearly different on the diploid chromosome number (48 vs. 30, respectively) (Getlekha, 2017). According to some specimens have not yet been identified because of a few number reported about taxonomy were not sufficient to classify the specimens. Therefore, they give additional descriptions about the characteristic of the specimen along with the karyological data and the collected location for fill some gaps in their works. In addition, this research is apply for a basis data for classification of land planarian in the future and the phylogenetic relationships between the three principal suborders of the Tricladida, relationships which at the moment are rather unclear.
ACKNOWLEDGEMENTS
This research supported by Department of Biology, Faculty of Science and Technology, Muban Chombueng Rajabhat University. I would like to thank the cytogenetic research group for the accuracy check of the report and valuable help.
REFERENCES
Ahmed, M. 1973. Cytogenetics of oysters. Cytologia. 38: 337–346.
Ball, I.R. 1976. Les caryotypes de trois planaires marines nord-americaines: contribution a la phylogenie et a la classification du groupe (Platyhelminthes, Turbellaria, Tricladida). Canadian Journal of Zoology. 54: 644–651. https://doi.org/10.1139/z76-076
Ball, I.R. 1979. The karyology of Procerodes lobata (Turbellaria, Tricladida, maricola). Canadian Journal of Zoology. 57: 1820–1821. https://doi.org/10.1139/z79-237
Ball, I.R. 1981.The phletic status of the Paludicola. Hydrobiologia. 84: 7–12. https://doi.org/10.1007/BF00026156
Chaiyasut, K. 1989. Cytogenetics and cytotaxonomy of the family zephyranthes. Bangkok: Chulalongkorn University.
Chen, T.R., and Ebeling, A.W. 1968. Karyological evidence of female heterogamety in the mosquito fish, Gambusia affinis. Copeia. 1: 70–75. https://doi.org/10.2307/1441552
Chooseangjaew, S., Tanyaros, S., Maneechot, N., Buasriyot, P., Getlekha, N., and Tanomtong, A. 2017. Chromosomal characteristics of the tropical oyster, Crassostrea belcheri Sowerby, 1871 (Ostreoida, Ostreidae) by conventional and Ag-NOR banding techniques. Cytologia. 82: 3–8. https://doi.org/10.1508/cytologia.82.3
Curtis, S.K., Cowden, R.R., Moore, J.D., and Robertson, J.L. 1983. Histochemical and ultrastructural features of the epidermis of land planarian Bipalium adventitium. Journal of Morphology. 175: 171–194. https://doi.org/10.1002/jmor.1051750206
Filella-Subira, E. 1983. Nota sobre la presència de la planària terrestre Bipalium kewense Moseley, 1878 a Catalunya. Butlletí de la Institució Catalana d’História. Natural. 49: 151.
Gallen, L., and Pucci-Nelli, I. 1979. The karyology of the genus Procerodes (Tricladida: Maricola) in British waters. Journal of the Marine Biological Association of the United Kingdom. 59: 961–967. https://doi.org/10.1017/S002531540003695X
Justine, J.L., Winsor, L., Gey, D., Gros, P., and Thévenot, J. 2018. Giant worms chez moi Hammerhead flatworms (Platyhelminthes, Geoplanidae, Bipalium spp., Diversibipalium spp.) in metropolitan France and overseas French territories". Peer J. 6: e4672. https://doi.org/10.7717/peerj.4672
Kasiroek, W., Indananda, C., Luangoon, N., Pinthong, K., Supiwong, W., and Tanomtong, A. 2017. First chromosome analysis of the humpback cardinalfish, Fibramia lateralis (Perciformes, Apogo- nidae). Cytologia. 82: 9–15. https://doi.org/10.1508/cytologia.82.9
Leitao, A., Boudry, P., Labat, J., and Thiriot-Quievreux, C. 1999. Comparative karyological study of cupped oyster species. Malacologia. 41: 175–186.
Minelli, A. 1977. Microplana mahnerti n. sp., a new terrestrial planarian from Jura Mts. (Turbellaria, Tricladida, Rhynchodemidae). Revue Suisse de Zoologie. 84: 173–176.
Nanda, I., Schsrtl, M., Fiechtinger, W., Schlupp, I., Parzefall, J., and Schmid, M. 1995. Chromosomal evidence for laboratory syn- thesis of triploid hybrid between the gynogenetic teleost Poecilia Formosa and its host species. Journal of Fish Biology. 47: 619–623. https://doi.org/10.1111/j.1095-8649.1995.tb01928.x
Ogren, R.E. 1985. The human factor in the spread of an exotic land planarian in Pennsylvania. Proceeding of the Pennsylvania Academy of Science. 59: 117–118.
Oki, I., Tamura, S., Ogren, R.E., and Kawakatsu, M. 1991. Karyology of four land-planarian species of the genus Bipalium from Japan. Hydrobiologia. 227: 163–167. https://doi.org/10.1007/BF00027597
Pereira, J.C., Chaves, R., Batista, F.M., Guedes-Pinto, H., and Leltao, A. 2011. Cytogenetic characterization of the dwarf oyster Ostrea stentina (Mollusca: Bivalvia) and comparative karyological analysis within Ostreinae. Journal of Shellfish Research. 30: 211–216. https://doi.org/10.2983/035.030.0203
Sangpakdee, W., Phimphan, S., Tengjaroenkul, B., Pinthong, K., Neeratanaphan, L., and Tanomtong, A. 2017. Cytogenetic study of tree microhylid species (Anura, Microhylidae) from Thailand. Cytologia. 82: 67–74. https://doi.org/10.1508/cytologia.82.67
Seo, N., Makino N., and Shirasawa, Y. 1988. The karyotypes of the genus Bipalium (Platyhelminthes: Terricola) — Three species in the fission type and two species in the non-fission type. The Bulletin of Tokyo Dental College. 14: 13–41.
Winsor, L. 1981. The taxonomy, zoogeography and biology of Bipalium kewense Mosele 1878 (Tricladida, Terricola). Hydrobiologia. 84: 17 (Abstract only)
Winsor, L. 1983. A revision of the cosmopolitan land planarian Bipalium kewense Moseley, 1878 (Turbellaria: Tricladida: Terricola). Zoological Journal of the Linnean Society. 79(1): 61–100. https://doi.org/10.1111/j.1096-3642.1983.tb01161
Yamamoto, K., Takai, M., Ogren, R.E., and Kawakatsu, M. 2001. Chromosomes of bipaliid land planarians from the vicinity of Nagasaki in Kyushu, Southern Japan (Platyhelminthes, Tricladida, Terricola). Belgian Journal of Zoology. 131 (Supplement 1): 221–222.
Nuntaporn Getlekha
Department of Biology, Faculty of Science and Technology, Muban Chombueng Rajabhat University, Ratchaburi 70150, Thailand
*Corresponding author. E-mail: taapu_getlekha@hotmail.com
Total Article Views
Article history:
Received: June 6, 2019
Revised: August 29, 2019
Accepted: September 17, 2019

