
Anticancer Activity of Metal Complexes with Acesulfame Mixed with Triphenylphosphine Ligands Bussaba Boonseng*, Teerawat Khudkham, Sutthida Wongsuwan, and Jaruwan Chatwichien
Published Date : 2019-09-20
DOI : https://doi.org/10.12982/CMUJNS.2019.0029
Journal Issues :
Number 4 , October-December 2019
ABSTRACT
Keywords: Complexes, Acesulfame, Anticancer, Triphenylphosphine
INTRODUCTION
Anticancer activity is one of the most attractive applications involved the examination in capability of coordination compounds in biological studies. Silver(I) complexes was found to be the most effective substrate in antibacterial and antifungal studies for several years (Kumar et al.,2010; Chandra and Ruchi, 2013; Charef et al., 2015. Our recent research also revealed that silver(I) complexes incorporated with the mixed ligands of triphenylphosphine and acesulfame exhibit excellent activity against A549 lung cancer cells (Boonseng et al., 2018). Herein, we would like to expand the scope of metals to the first-row transition metals; nickel(II) and copper(II) which has been prevalently used for numerous catalysis and biological studies (Chandra et al., 2015; Ghobadi et al., 2018; Mo et al., 2018; Saha et al., 2018).
Acesulfame-K is a commercially artificial sweetener in which its anionic form is considered to be an excellent ligand according to the containing of several donor atoms. Apart from conveniently and commercially available, the compound was also found to provide low toxicity at low concentration leading to the practically using for living organism (Cong et al., 2013). Owing to the prior advantage of acesulfame-K, the recent research by Cavicchioli and co-workers (2010) was revealed that the using of acesulfame as the ligand for platinum(II) and silver(I) complexes exhibited exceptional antimycobacterial and antibacterial activity. Moreover, in order to improve the ability of metal complexes in biological activities regarding the inhibition of cancer cells growing, several researches have been attracted to the structural modification of transition metal complexes. The study by the Nawaz group (2011) revealed that the introduction of triphenylphosphine (PPh3) into the heterocyclic thiones silver(I) complexes could perform more excellent activity against cancer cells than using only thione compounds. Thereafter, Kaplan and co-workers reported the activity of silver(I) complex against lung cancer A549 cells via MTT assay in which the better reactivity of such complex than its coordinated ligands were discovered (Kaplan, 2017). Lung cancer has become our desired target according to the report by World Health Organization (WHO) which indicated that lung cancer is the most common cause of cancer-related death of people and more than 50 percent of lung cancer cases occurred in less developed countries (Gazdar et al., 2010).
Therefore, in this study, the complexes of silver(I), nickel(II) and copper(II) incorporated with the mixed ligands of acesulfame and PPh3 were synthesized and their structures were determined by FT-IR, 1H and 31P NMR spectroscopy, mass spectrometry including X-Ray powder diffraction. The products were examined in their inhibition of lung cancer cells growing (A549) and their abilities were presented and compared in term of IC50 values.
MATERIALS AND METHODS
RESULTS
Synthesis of metal complexes with acesulfame and mixed ligands of acesulfame and triphenylphosphine
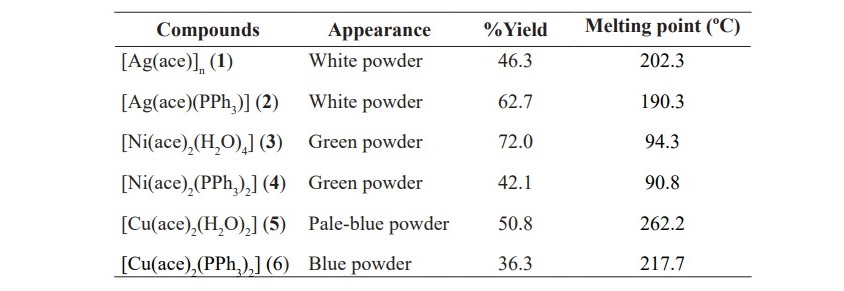
The complexes of silver(I), nickel(II) and copper(II) were prepared as aforementioned in material and methods section. Melting points of complexes were determined at the heating rate of 5 °C/min using GALLENKAMP Melting Point Apparatus. Appearance and percentage yield of each compound are shown in Table 1.
Table 1. General information of the synthesized complexes.
Characterization of metal complexes with acesulfame and mixed ligands of acesulfame and triphenylphosphine
The obtained complexes were analyzed in compositions and structures by IR and NMR spectroscopic methods, mass spectrometry including X-Ray powder diffraction to acquire molecular structures of the synthesized products.
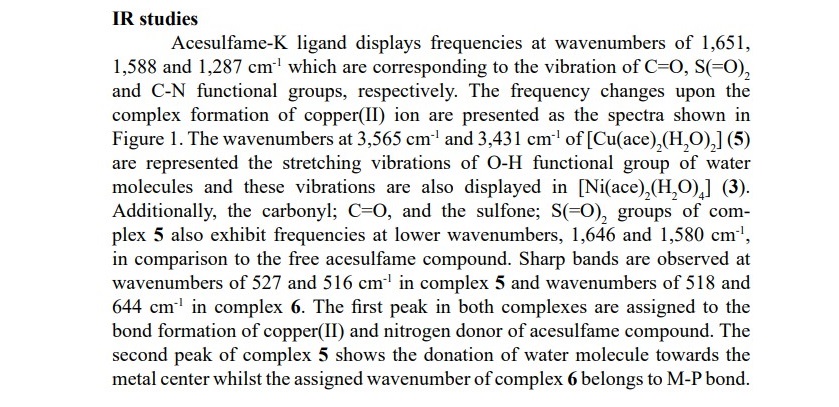
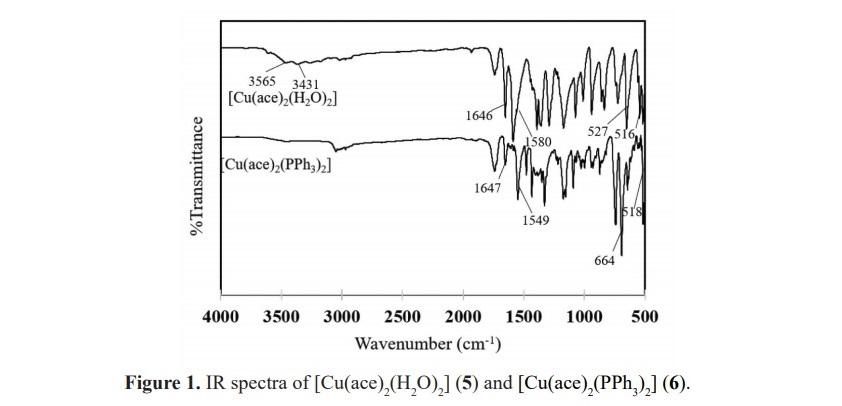
In order to compare the different types of metal centers affected the vibration frequencies, complexes 2, 4 and 6 are chosen and their spectra are presented in Figure 2. A high frequency shift in υ(C–N) bands in all complexes indicate the coordination of ligand to the metal ions. M-N bond was observed in the region of 559-516 cm-1 whilst M-O bond was also observed according to the metal-oxygen of water or metal-oxygen of acesulfame molecule. Moreover, the frequencies involved M-P bond formation in complexes 2, 4 and 6 were exhibited at the wavenumbers about 660-640 cm-1. In addition, an unexpected signal was observed in complex 4 at the frequencies range of 3,400-3,050 cm-1 which might be attributed to the moisture contained in the substrate. IR data of free acesulfame ligand and all complexes are shown in Table 2.
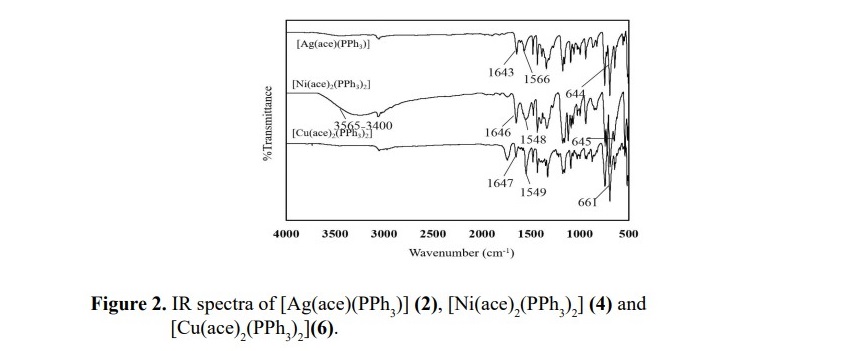
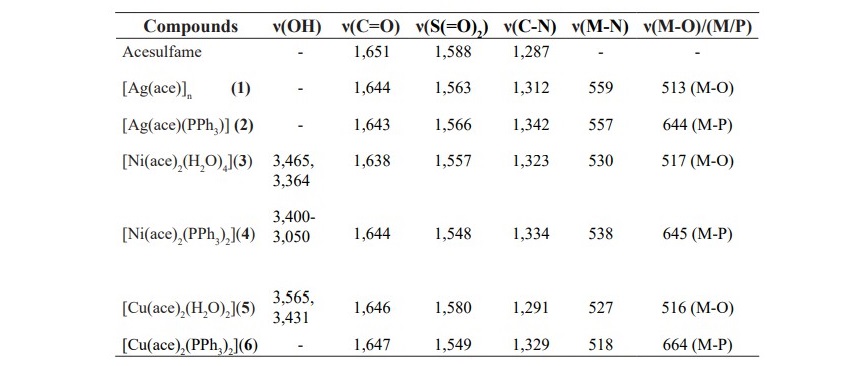
Table 2. IR data of free ligands and complexes 1-6.
NMR studies
The synthesized complexes were observed in 1H and 31P NMR spectra to examine the change of proton and phosphorus signals upon the coordination of ligands to the metal ions.
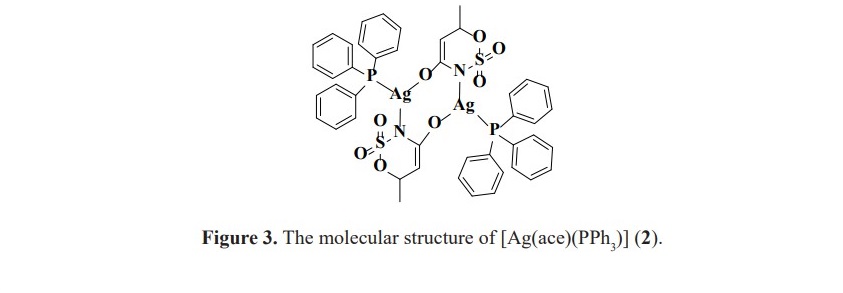
Generally, free PPh3 shows the 31P signal at the chemical shift about -5.25 ppm. For the synthesized complexes 2, 4 and 6, the observed phosphorus atom of each one was found to resonate at the chemical shift of 15.81, 25.62 and 25.50 ppm, respectively. Complex 2 has been already known in its molecular structure from X-ray single crystal diffraction previously reported by our group. Structure of [Ag(ace)(PPh3)] (2) is shown in Figure 3.
However, complexes 4 and 6 still cannot be affirmed the exact position of PPh3 coordinated to nickel(II) and copper(II) ions. To the extent of our knowledge including the steric bulk of PPh3, the proposed structures of the two complexes are presented in Figure 4. Moreover, the spectra also present the 31P NMR signals at -5.18 ppm for complex 4 and -5.05 ppm for complex 6 which are attributed to the presence of unreacted PPh3. Such the peak was not observed in complex 2 according to the purified single crystal collected for the analysis by 31P NMR spectroscopic technique. The 31P spectra of complexes 2, 4 and 6 are presented in Figure 5.
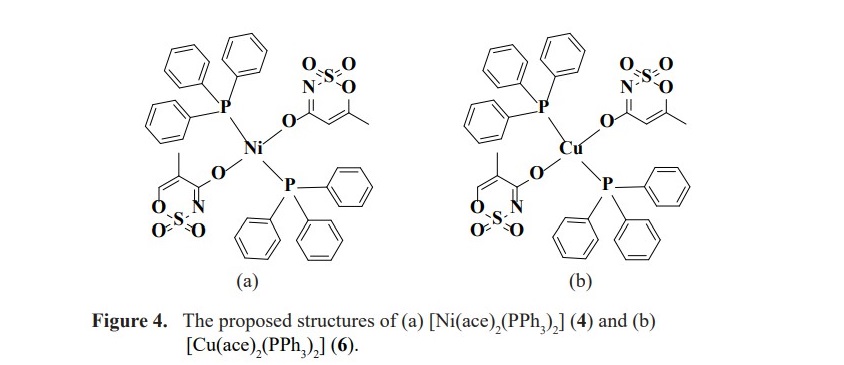
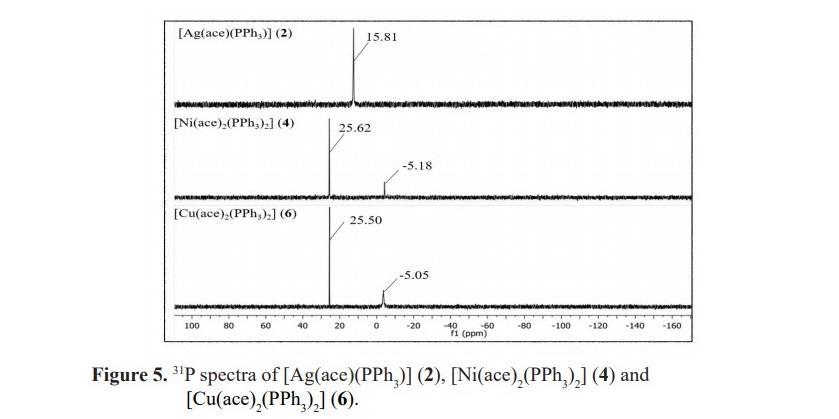
The orientation of PPh3 on the nickel(II) and copper(II) metal centers is supposed to be similar according to the comparable in their chemical shifts. Moreover, these values are also different from silver(I) complex in which the structure is in a dimer fashion with trigonal planar around the metal center (Figure 3). Unfortunately, for 1H NMR study, the spectra could not be obtained to verify the complex formation as the signals of protons in acesulfame are all obscured by abundant protons of phenyl rings in PPh3. For the complexes contained only acesulfame ligand; complexes 1, 3 and 5, 1H NMR study could be constructed. The 1H spectra of the novel complex 5 revealed the signals at the chemical shifts of 5.26 and 1.89 ppm belonged to the proton at para position of sulfone functional group (HA) and the protons of methyl substituent (HB) with the integration ratio of 1:3 (Figure 6). These peaks exhibited upfield shifted from 6.04 and 2.20 ppm in free acesulfame ion. Additionally, 1H NMR of known complexes 1 and 3 also provided similar results to the literature (Icbudak et al., 2006; Cavicchioli et al., 2010).
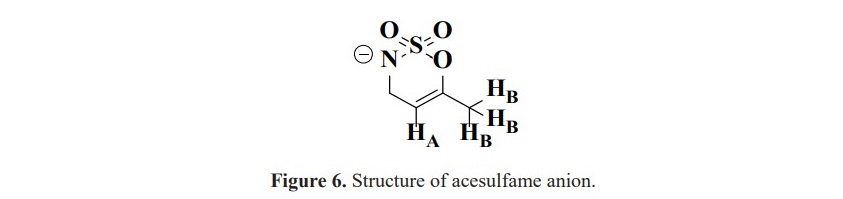

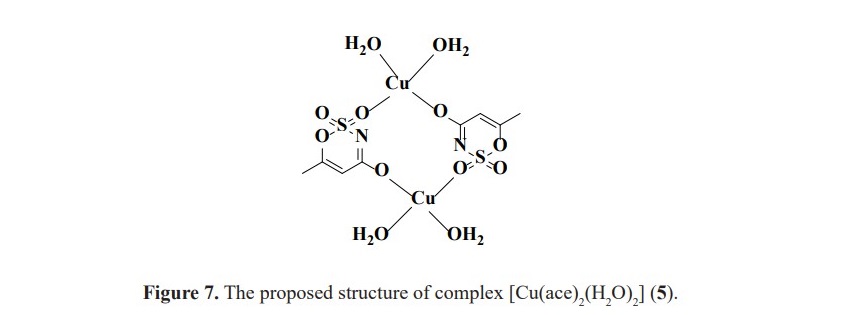
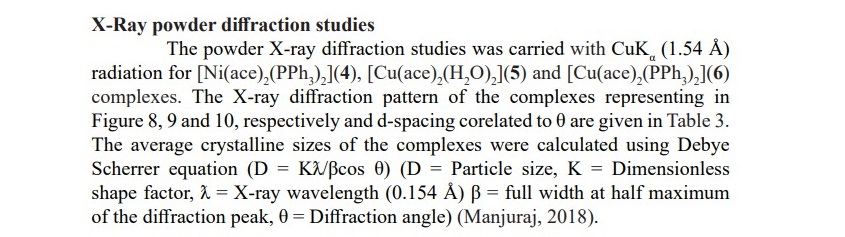
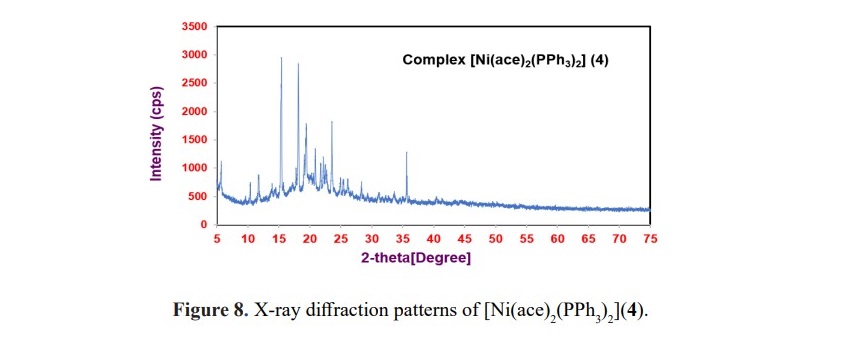
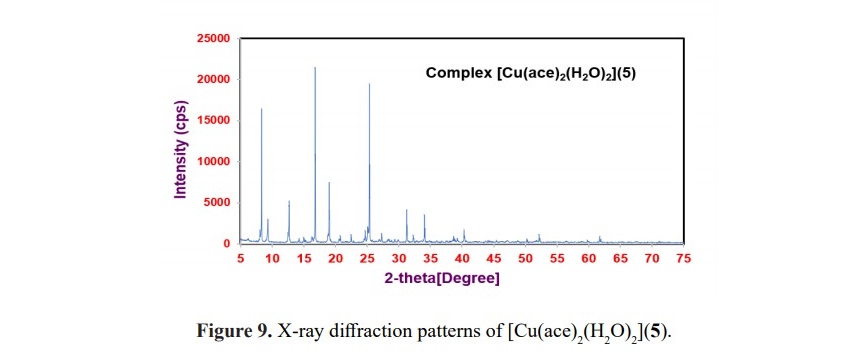
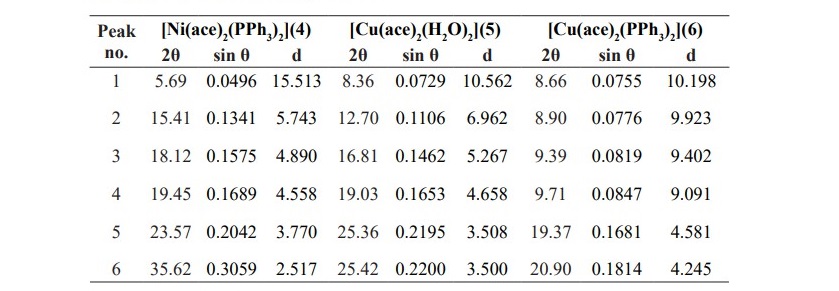
Table 3. XRD data of complexes 4-6.
Anticancer studies
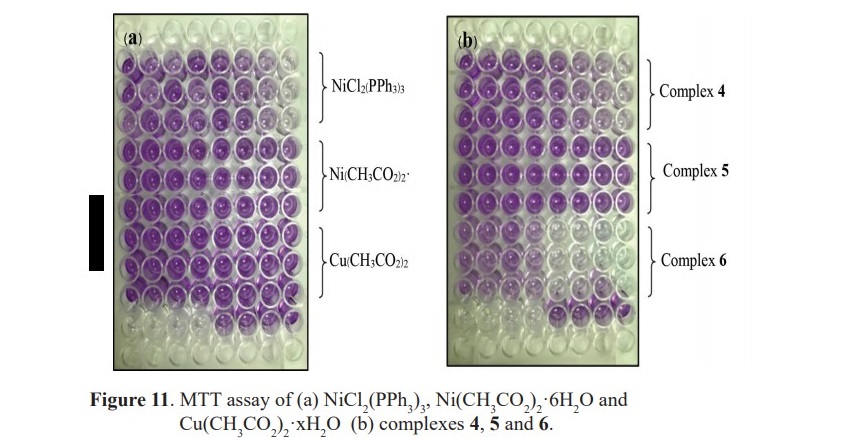
The prepared complexes were examined in the anticancer activity against A549 lung cancer cells and compared with starting materials and the standard Etoposide. The MTT assay of some examined substrates from this study is presented in Figure 11.
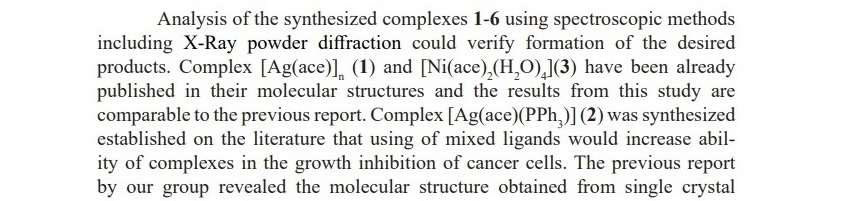
The IC50 values of complexes 1, 3 and 5 which contained only acesulfame ligand are 42.04, 50.79 and 222.40, respectively. The results indicate deficient ability of such complexes in comparison to the standard compound, however; these values are still superior than their starting materials. Therefore, this exper- iment could be confirmed that the synthesized complexes with the introduction of acesulfame performed better activity than non-coordinated compounds and their metal precursors. The excellent results were achieved by complexes with the mixed ligands of acesulfame and triphenylphosphine compounds. Complexes 2, 4 and 6 provided the IC50 values of 1.65, 13.45 and 1.06, respectively. Amongst the studied metal centers, copper(II) of complex 6 exhibited the best inhibition against the growth of A549 cancer cells. The obtained results are proposed to be involved the influence of metal type and also the proportions of the mixed ligands on the prepared complexes. However, the effect of mixed ligands ratio and structural geometry on biological activity should be further investigated. The results of all biological studies are shown in Table 4.
DISCUSSION
CONCLUSION
In summary, metal complexes with acesulfame in the absence and in the presence of triphenylphosphine were prepared and characterized. The biologi- cal study with cancer cells revealed great activity of the synthesized complex- es in comparison to their starting substrates. The capability is increased when triphenylphosphine was introduced to the metal complexes. Anyhow, the effect of the proportions of acesulfame and triphenylphosphine including the exact molecular structures of the synthesized complexes should be further investigated.
ACKNOWLEDGEMENTS
This research was supported by grants funded by Naresuan University. The authors also would like to thank Faculty of Science, Naresuan University and Chiang Mai University for providing instruments.
REFERENCES
Boonseng, B., Chatwichien, J., Chotima, R., Khudkam, T., and Pila, T. 2018. Synthesis, characterization and anticancer studies of acesulfame mixed with triphenylphosphine silver(I) complexes. Proceedings of the Pure and Applied Chemistry International Conference 2018 (PACCON 2018); 2018 Febuary 7-9; Songkhla: The Chemical Society of Thailand under The Patronage of Professor Dr. HRH Princess Chulabhorn. IN6-IN10.
Cavicchioli, M., Massabni, A.C., Heinrich, T.A, Costa-Neto, C.M., Abrao, E.P., Fonseca, B.A.L., Castellano, E.E., Corbi, P.P., Lustri, W.R., and Leite, C.Q.F. 2010. Pt(II) and Ag(I) complexes with acesulfame: crystal structure and a study of their antitumoral, antimicrobial and antiviral activities. Journal of Inorganic Biochemistry. 104(5): 533-540. https://doi.org/10.1016/j.jinorgbio.2010.01.004
Chandra, S., and Ruchi. 2013. Synthesis, spectroscopic characterization, mo- lecular modeling and antimicrobial activities of Mn(II), Co(II), Ni(II), Cu(II) complexes containing the tetradentate aza Schiff base ligand. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy. 103: 338-348. https://doi.org/10.1016/j.saa.2012.10.065
Chandra, S., Vandana, and Kumar, S. 2015. Synthesis, spectroscopic, anticancer, antibacterial and antifungal studies of Ni(II) and Cu(II) complexes with hydrazine carboxamide, 2-[3-methy1-2-thienyl methylene]. Spec- trochimica Acta Part a-Molecular and Biomolecular Spectroscopy. 135: 356-363. https://doi.org/10.1016/j.saa.2014.06.143
Charef, N., Sebti, F., Arrar, L., Djarmouni, M., Boussoualim, N., Baghiani, A., Khennouf, S., Ourari, A., AlDamen, M.A., Mubarak, M.S., et al. 2015. Synthesis, characterization, X-ray structures, and biological activity of some metal complexes of the Schiff base 2,2 ‘-(((azanediylbis (propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))diphenol. Polyhedron. 85: 450-456. https://doi.org/10.1016/j.poly.2014.09.006
Cong, W.N., Wang, R., Cai, H., Daimon, C.M., Scheibye-Knudsen, M., Bohr, V.A., Turkin, R., Wood, W.H., Becker, K.G., Moaddel, R., et al. 2013. Long-term artificial sweetener acesulfame potassium treatment alters neurometabolic functions in C57BL/6J mice. Plos One. 8(8): 1-18. https://doi.org/10.1371/journal.pone.0070257
Gazdar, A.F., Girard, L., Lockwood, W.W., Lam, W.L., and Minna, J.D. 2010. Lung cancer cell lines as tools for biomedical discovery and research. Jnci-Journal of the National Cancer Institute. 102(17): 1310-1321. https://doi.org/10.1093/jnci/djq279
Ghobadi, K., Zare, H.R., Khoshro, H., Gorji, A., and Benvidi, A. 2018. Effect of molecular structure of the N, N’-bis(2-hydroxy-1-naphthal- dehyde)-1,3-phenylenediimine ligand on the electrocatalytic properties of its Ni(II) complex for reduction of CO2. Journal of Molecular Structure. 1171: 466-470. https://doi.org/10.1016/j.molstruc.2018.06.023
Icbudak, H., Adiyaman, E., Cetin, N., Bulut, A., and Buyukgungor, O. 2006. Synthesis, structural characterization and chromotropism of a Ni(II) and a Co(II) compound with acesulfamate as a ligand. Transition Metal Chemistry. 31(5): 666-672. https://doi.org/10.1007/s11243-006-0045-x
Kaplan, A., Ciftci, G.A., and Kutlu, H.M. 2017. The apoptotic and genomic studies on A549 cell line induced by silver nitrate. Tumor Biology. 39(4): 1-12. https://doi.org/10.1177/1010428317695033
Khan, M.I., Khan, A., Hussain, I., Khan, M.A., Gul, S., Iqbal, M., Rahman, I.U., and Khuda, F. 2013. Spectral, XRD, SEM and biological properties of new mononuclear Schiff base transition metal complexes. Inorganic Chemistry Communications. 35: 104–109. https://doi.org/ 10.1016/j.inoche.2013.06.014
Kumar, G., Kumar, D., Devi, S., Johari, R., and Singh, C.P. 2010. Synthesis, spectral characterization and antimicrobial evaluation of Schiff base Cu (II), Ni (II) and Co (II) complexes. European Journal of Medicinal Chemistry. 45(7): 3056-3062. https://doi.org/10.1016/j.ejmech.2010.03.036
Manjuraj, T., Krishnamurthy, G., Bodke, Y.D., Naik, H.S.B., and Kumar, H.S.A. 2018. Synthesis, XRD, thermal, spectroscopic studies and biological- evaluation of Co(II), Ni(II) Cu(II) metal complexes derived from 2-benzimidazole. Journal of Molecular Structure. 1171: 481-487. https:// doi.org/10.1016/j.molstruc.2018.06.055
Mo, Q.Y., Deng, J.G., Liu, Y.N., Huang, G.D., Li, Z.W., Yu, P., Gou, Y., andbYang, F. 2018. Mixed-ligand Cu(II) hydrazone complexes designed to enhance anticancer activity. European Journal of Medicinal Chemistry. 156: 368-380. https://doi.org/10.1016/j.ejmech.2018.07.022
Nawaz, S., Isab, A.A., Merz, K., Vasylyeva, V., Metzler-Nolte, N., Saleem, M., and Ahmad, S. 2011. Synthesis, characterization and antimicrobial studies of mixed ligand silver(I) complexes of triphenylphosphine and heterocyclic thiones: crystal structure of bis[{(mu(2)-diazinane-2- thione) (diazinane-2-thione) (triphenylphosphine)silver(I) nitrate}]. Polyhedron. 30(9): 1502-1506. https://doi.org/10.1016/j.poly.2011.02.054
Saha, D., Gayen, S., and Koner, S. 2018. Cu(II)/Cu(II)-Mg(II) containing pyridine-2,5-dicarboxylate frameworks: Synthesis, structural diversity, inter-conversion and heterogeneous catalytic epoxidation. Polyhedron. 146: 93-98. https://doi.org/10.1016/j.poly.2018.02.023
Yurdakul, Ö., and Köse, D.A. 2014. Mixed ligand complexes of acesulfame/ nicotinamide with earth alkaline metal cations MgII, CaII, BaII and SrII: synthesis and characterization, Hittite Journal of Science and Engineering. 1(1): 51-57. https://doi.org/10.17350/HJSE19030000008
Bussaba Boonseng*, Teerawat Khudkham, Sutthida Wongsuwan, and Jaruwan Chatwichien
Faculty of Science, Naresuan University, Phitsanulok 65000, Thailand
*Corresponding author. E-mail: bussabab@nu.ac.th
Total Article Views
Article history:
Received: November 6, 2018;
Revised: March 1, 2019;
Accepted: March 18, 2019


