
Elevated Ambient PM10 Levels Affecting Respiratory Health of Schoolchildren in Chiang Mai, Thailand
Waraphan Phornwisetsirikun, Tippawan Prapamontol*, Somrak Rangkakulnuwat, Somporn Chantara and Prasak TavornyutikarnPublished Date : 2019-08-28
DOI : 10.12982/CMUJNS.2014.0040
Journal Issues : Number 3, September - December 2014
ABSTRACT
The health effects from exposure to particulate air pollution during the dry season (February-March) in northern Thailand, a longstanding problem, have become particularly acute since 2007. Although several studies have reported the effect of ambient PM10 levels on airway oxidative stress and pulmonary function among respiratory disease patients, few studies have focused on healthy children, especially in Thailand. We conducted a prospective follow-up study to assess the respiratory health among schoolchildren exposed to different levels of ambient PM10 by comparing exhaled malondialdehyde (MDA) concentrations and pulmonary function indices. Exhaled breath condensate (EBC) samples were obtained from individual participants. MDA in EBC has been proposed as a biomarker of airway oxidative stress. The participants were 54 healthy schoolchildren with median (min-max) age of 11 (10-12) years old from a primary school in Chiang Mai City, Thailand. Questionnaires and EBC samples were collected twice, in the rainy season (July 2011, low PM10 level) and the dry season (March 2012, high PM10 level). It required about 10 minutes to collect EBC samples of 1-3 mL from individual participants, using an EBC-collecting apparatus that was modified specifically for this research from a standard hospital-use apparatus. MDA concentration in EBC was analyzed using HPLCUV detection. Trained public health personnel performed the pulmonary function tests. The median of exhaled MDA concentrations in the rainy and dry seasons was 0.17 and 0.22 μM, respectively. Mean ± SD of forced expiratory volume in one-second/forced vital capacity ratios (FEV1/FVC) in the rainy and dry seasons was 94.6 ± 4.4 and 91.3 ± 4.7 percent predicted, respectively. Exhaled MDA concentration significantly increased and the FEV1/FVC ratio significantly decreased in the dry season (p<0.05). This results shows that elevated ambient PM10 levels affect the respiratory health of schoolchildren. Further, exhaled MDA could be used as a biochemical marker of airway oxidative stress due to ambient PM10 among healthy schoolchildren.
Keywords: Ambient PM10, Chiang Mai, exhaled breath condensate (EBC), malondialdehyde (MDA), pulmonary function, schoolchildren
INTRODUCTION
Airborne particulate pollution is a longstanding public health problem worldwide. Animal and human studies have reported that exposure to ambient particulate matter was associated with adverse health effects, including increased mortality rates (Lee et al., 1998; Samet et al., 2000) and an increased number of hospital visits due to respiratory and cardiovascular diseases (Ware et al., 1986; Burnett et al., 1997; Peters et al., 1999; Peng et al., 2008). Ambient particulate matter (PM), such as those less than 10 micrometers (μm) in aerodynamic diameter (PM10), contains a complex mixture of pollutants. Many studies have demonstrated that ambient PM10 can produce oxidative stress at both the cellular and living systems level, and appear to initiate responses particularly dangerous to children, a population believed to have enhanced vulnerability to ambient PM10 (Kelly, 2003; Li et al., 2003; Schwart, 2004). Exhaled breath condensate (EBC) is a new tool for determining pulmonary inflammation and oxidative stress markers implicated in the pathogenesis of various respiratory conditions. EBC collection is relatively simple to perform, inexpensive, non-invasive and safely applied to children and patients with severe diseases; it is also suitable for repeated measurements (Mutlu et al., 2001; Hunt, 2002). Some chemical components in EBC can be used as biomarkers of airway diseases, such as oxidative stress in the lungs (Chan et al., 2009). Oxidative stress can arise for many reasons, including air pollutant exposure. Oxidative stress can be assessed and monitored through the determination of the levels of biomarkers, such as hydrogen peroxide, lipid peroxidation-derived products, namely aldehydes and isoprostanes, and protein carbonyl groups (Taylor, 2011).
Chiang Mai City is located in northern Thailand, approximately 700 km from Bangkok, in the Ping River valley, surrounded by mountains. The Pollution Control Department (PCD), Ministry of Natural Resources and Environment provides information on air quality parameters, including PM10, NOX, O3, SO2, and CO from two air quality monitoring stations in Chiang Mai Province. In recent years, average PM10 levels in Chiang Mai City have been elevated in the dry season, especially January to March, exceeding Thailand’s standard limit, set at 120 microgram/cubic meter (μg/m3).
This study analyzed the effects of exposure to ambient PM10 on respiratory health among schoolchildren in Chiang Mai City by comparing pulmonary function indices and exhaled MDA concentration between the rainy and dry seasons.
MATERIALS AND METHODS
Study subjects
The study subjects consisted of 54 primary schoolchildren, 10 to 12 years of age, who resided for at least one year within two kilometers of the air quality monitoring station at Yupparaj Wittayalai School, Chiang Mai City. Subjects diagnosed with asthma, chronic respiratory disease or long-term medication use were excluded from the study. The present study protocol was approved by the Human Experimentation Committee of the Research Institute for Health Sciences (RIHES), Chiang Mai University (HEC approval No. 1/2010). Children and parents gave written informed consent before participating in the study.
Study time period
Data was collected in two periods: 1) the rainy season (July 2011, a month with low PM10 levels) and 2) the dry season (March 2012, a month with high PM10 levels). These periods were chosen based on the monthly mean concentration of PM10 at the air quality monitoring station, Yupparaj Wittayalai School, Chiang Mai City. The PM10 concentration varied from 14.1 to 33.8 μg/m3 and 40.3 to 195.0 μg/m3 in the rainy and dry seasons, respectively. The monthly mean concentration of PM10 was 21.3 μg/m3 in the rainy season (July 2011) and 106.2 μg/m3 in the dry season (March 2012), as shown in Figure 1.
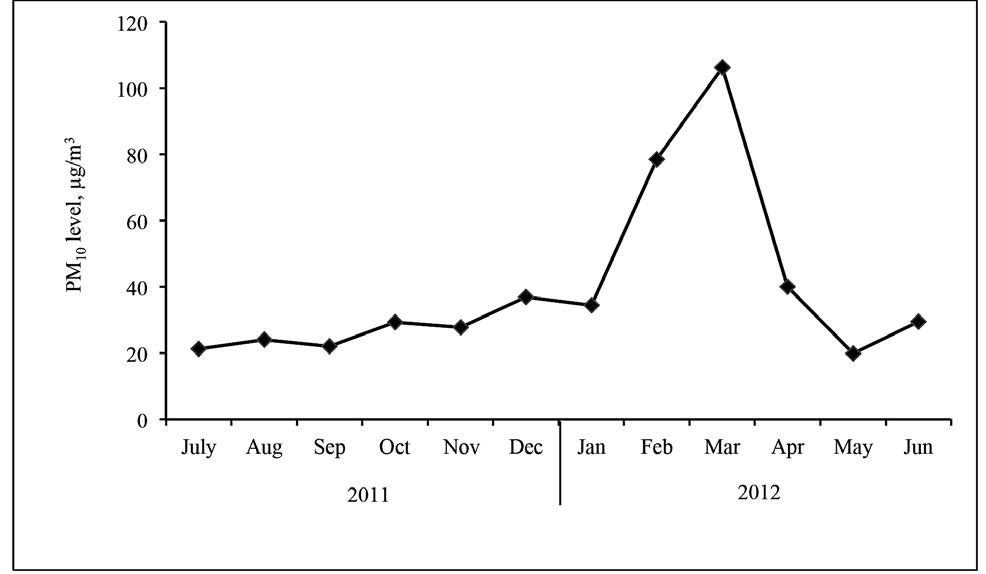
Figure 1. Monthly mean concentration of PM10 during the study period at the air monitoring station at Yupparaj Wittayalai School, Chiang Mai City.
Source: The Pollution Control Department, Ministry of Natural Resources and Environment.
Pulmonary function test and exhaled MDA analysis
Pulmonary function test was performed with portable electric mini-spirometer (Datospir, Sibel SA, Barcelona, Spain) administered by a trained technician according to the recommendation of the American Thoracic Society (American Thoracic Society, 1995). The best of three consecutive spirometer recordings was used. The measured parameters were forced vital capacity (FVC), forced expiratory volume in one second (FEV1) and forced expiratory ratio (FEV1/FVC %).
EBC samples were collected from the schoolchildren using a device we developed (Figure 2). The device consists of a mouthpiece with two way non-rebreathing valve. The mouthpiece was connected to a condensation chamber by flexible plastic tubing and the condensation chamber was immersed in a liquid nitrogen slurry. After rinsing their mouth, subjects were instructed to form a complete seal around the mouthpiece and maintain a dry mouth during collection by periodically swallowing excess saliva. The subjects sat comfortably and wore a nose clip. They were instructed to breathe tidally and very slowly expire through the mouthpiece for 10 minutes to collect approximately 1-3 mL of condensate. The collected EBC samples were frozen immediately in dry ice (–70°C) and stored in the freezer (–20°C) until analysis.

Figure 2. Photograph of a subject using the developed EBC collecting set.
The concentration of MDA in EBC was determined by measuring MDA–thiobarbituric acid (TBA) adducts according to the method previously described by Wong et al. (1987) with slight modifications. Briefly, 50 μL of EBC sample was mixed to 0.44 mol/L of H3PO4 (750 μL) and 42 mmol/L of TBA solution (250 μL) and the mixture was heated at 95°C for 60 minutes. Then, the mixture was cooled in ice water for 5 minutes and kept at room temperature for an additional 20 minutes. Three hundred μL of the mixture were transferred into another centrifuge tube with 300 μL of Methanol-NaOH solution and centrifuged at 13000 rpm for 10 minutes. The absorbance was measured at 532 nm after separation with an HPLC system equipped with a mobile phase of 50 mmol/L of KH2PO4 (pH 6.8): methanol (60:40, v/v). MDA concentration was calculated from the standard calibration curve and expressed as μmol/L of EBC. The determination of MDA in EBC samples was performed with an eight-point calibration curve based on water blank measurement and seven concentrations (0.08, 0.15, 0.3, 0.6, 1.2 and 2.4 μM). The detection limit was approximately 0.08 μM. For readings that gave results below the method sensitivity, the MDA concentration in EBC was set as LOD/√2, which was 0.28 (Hornung and Reed, 1990).
Data analyses
The Kolmogorov-Smirnov test was used for the fitness of the variables to the normal distribution. Mean or median values were reported according to described central tendencies. The results were expressed as mean ± SD for normally distributed data and median and range for non-normally distributed data. Comparisons of exhaled MDA concentrations between rainy and dry seasons were performed by the Mann-Whitney test and paired t-test for pulmonary function indices. A statistical software package (SPSS Version 17; SPSS Thailand) was used. Statistical significance was defined as p<0.05.
RESULTS
General characteristics of the study subjects
Table 1 shows the general characteristics of the schoolchildren participating in the study. Sex distribution of the subjects was homogenous (male: female = 1: 1). Median age of the subjects at the start of the study was 11 years (range 10-12 years). The median height was 144 cm (range 130-164 cm) and median weight was 34 kg (range 25-59 kg).
Table 1. The demographic characteristics and anthropometric measurements of study subjects.
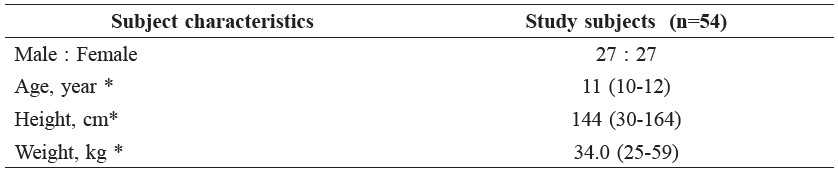
Note: * median (range)
Pulmonary function indices and exhaled MDA concentration
In Table 2, the mean FVC and FEV1 predicted in the rainy season was 94.4% and 104.5%, respectively, and in the dry season 96.3% and 102.4%, respectively. The means of the predicted FVC and FEV1 predicted were not significantly different between the rainy and dry seasons. The mean FEV1/FVC ratios in the rainy and dry seasons were 94.6 and 91.3%, respectively. We found the FEV1/FVC ratio decreased significantly in the dry season (p<0.05). The median concentrations of exhaled MDA in the rainy and dry seasons were 0.17 and 0.22 μM, respectively. The MDA concentrations significantly increased in the dry season (p<0.05).
Table 2. The comparison of pulmonary function indices and exhaled MDA concentration of the subjects between the rainy and dry season.
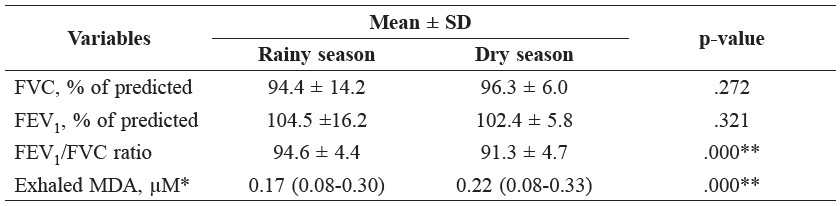
Note: *median (range); **significant difference between seasons.
DISCUSSION
The results from the present study support our hypothesis that elevated ambient PM10 level is positively associated with FEV1/FVC ratio and exhaled MDA concentration.
In contrast to some earlier results, we did not find differences in the predicted values of FVC and FEV1 between the rainy and dry seasons. In our study, the FEV1 predicted value tended to be lower in the dry season than in rainy season; however, the difference was not statistically significant. Other studies have shown that PM10 levels adversely affect the pulmonary function of children (Kim et al., 2005; Kasamatsu et al., 2006). Our findings agree with several previous studies of ambient air pollution from Helsinki, Athens, Amsterdam and Burmingham (Hartog et al., 2010), Bangkok (Lungkulsen et al., 2006) and New Zealand (Epton et al., 2008). Researchers of those studies concluded that elevated PM10
levels had no relationship with pulmonary function of schoolchildren, but a small effect on respiratory symptoms. Several possible reasons may explain the lack of relationship between PM10 and pulmonary function observed in our study. Firstly, in the rainy season, respiratory infections are common and may confound the relationship with PM10, making effects difficult to detect. Secondly, the present study had a rather small sample size of 54 participants; therefore, we did not find a statistically significant adverse change in pulmonary function between the two seasons. We might need a larger sample to detect significant changes in pulmonary function. Thirdly, the present study collected data in March 2012 (dry season), when the PM10 level exceeded the national standard of 120 μg/m3 for only 10 days. Moreover, based on their anthropometric measurements, the subjects in our study were rather healthy schoolchildren; therefore, the effects of PM10 may not be detected by pulmonary function testing. The finding may also be confounded by some other environmental factor, such as environmental tobacco smoke and/or cooking smoke at home. In addition, other potential confounders may play a role, including socio-economic factors of household crowding, number of smokers in the family, type of fuel used for cooking, air-conditioned bedroom, indoor pets, traffic levels in the neighborhood or diets. Eroshina et al. (2004) showed that environmental and social factors affected the health, respiratory dysfunction and impaired lung function of schoolchildren in Moscow. To decrease the influence of these confounders, this study was designed to follow up the health outcomes of the same group of schoolchildren in different seasons.
In our study, exhaled MDA concentration in the dry season was significantly higher than in the rainy season. This suggests that PM10 contained oxidative activity properties and induced oxidation-dependent alterations in inflammatory cells that were involved in the production of reactive oxygen species (Gonzalez, 2004; Ohyama et al., 2007). Oxidative stress occurs when there is an imbalance between oxidant and antioxidant on a cellular or individual level. Oxidative stress can arise for many reasons, including consumption of alcohol, medications, trauma, toxins, radiation and air pollution (Kelly, 2003; Romieu et al., 2008). Exposure to PM10 pollutant gives rise to oxidative stress within the lung, and this initiates responses that are harmful to susceptible populations. The generation of reactive oxygen species can cause oxidative damage to DNA, proteins or lipids in the body. MDA is one of the major final products of lipid peroxidation. The respiratory tract presents a large surface area in contact with air pollution, and is the first target of PM10 pollutants, where they evoke an inflammatory response; PM10 pollution sis an important trigger of lung inflammation (Flavia et al., 2010). Our findings suggested that MDA concentration in EBC provided an early biological marker for PM10 exposure, before clinical symptoms appeared.
CONCLUSION
Elevated ambient PM10 levels increased exhaled MDA concentrations and decreased the FEV1/FVC ratio in schoolchildren, affecting their respiratory health, although the sample size was small. Ambient PM10 levels should be better controlled to protect the community.
ACKNOWLEDGEMENTS
This study was carried out with financial support from the Center of Excellence on Environmental Health and Toxicology (EHT), Faculty of Science, Chiang Mai University, Chiang Mai, Thailand. The authors would like to thank the Research Institute for Health Sciences (RIHES), Chiang Mai University, for laboratory support.
REFERENCES
American Thoracic Society. 1995. Standardization of spirometry. Am J Respir Crit Care Med (15): 1107-1136.
Burnett, R.T., S. Cakmak, J.R. Brook, and D. Krewski. 1997. The role of particulate size and chemistry in the association between summertime ambient air pollution and hospitalization for cardiorespiratory diseases. Environ Health Perspect 105: 614-620.
Chan, H.P., C. Lewis, P. Thomas. 2009. Exhaled breath analysis: Novel approach for early detection of lung cancer. Lung Cancer 63: 164-168.
de Hartog, J.J., J.G. Ayres, A. Karakatsani, A. Analitis, H. ten Brink, K. Hameri, R. Harrison, K. Katsouyanni, A. Kotronarou, I. Kavouras, C. Meddings, J. Pekkanen, and G. Hoek. 2010. Lung function and indicators of exposure to indoor and outdoor particulate matter among asthma and COPD patients. Occup Environ Med 67: 2-10.
Epton, M.J., R.D. Dawson, W.M. Brooks, S. Kingham, T. Aberkane, J.-A. E. Cavanagh, C. M. Frampton, T. Hewitt, J. M. Cook, S. McLeod, F. McCartin, K. Trought and L. Brown. 2008. The effect of ambient air pollution on respiratory health of schoolchildren: a panel study. Environmental Health7:16.
Eroshina, K., K. Danishevski, P. Wilkinson, and M. McKee. 2004. Environmental and social factors as determinants of respiratory dysfunction in junior schoolchildren in Moscow. J Public Health 26: 197-214.
González-Flecha, B. 2004. Oxidant mechanism in response to ambient air particles. Mol Aspects Med 25(1-2): 169-182.
Hornung, R.W., and L.D. Reed. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1): 46-51.
Hunt, J. 2002. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy clin Immunol 110 (1): 28-34.
Kasamatsua, J., M. Shimab, S. Yamazakic, K. Tamura, and G. Sun. 2006. Effects of winter air pollution on pulmonary function of schoolchildren in Shenyang, China. Int J Hyg Environ Health 209 (5): 435-444.
Kelly, F.J. 2003. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 60: 612-616.
Kim, J.H., D.H. Lim, J.K. Kim, S.J. Jeong, and B.K. Son. 2005. Effects of Particulate Matter (PM10) on the pulmonary function of middle-schoolchildren. J Korean Med Sci 20 (1): 42-45.
Langkulsen, U., W. Jinsart, K. Karita, and E. Yano. 2006. Health effects of respirable particulate matter in Bangkok schoolchildren. Int Congr Ser 1294: 197-200.
Lee, J.T., S.I. Lee, D. Shin, and Y. Chung. 1998. Air particulate matters and daily mortality in Ulsan, Korea. Korean J Prev Med 31(1): 82-90.
Li, N., C. Sioutas, A. Cho, D. Schmitz, C. Misra, J. Sempf, M. Wang, T. Oberley, J. Froines, and A. Nel. 2003. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111(4): 455-460.
Mazzoli-Rocha, F., S. Fernandes, M. Einicker-Lamas, and W.A. Zin. 2010. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biol Toxicol 26 (5): 481-498.
Mutlu, G.M., K.W. Garey, R.A. Robbins, L.H. Danziger, and I. Rubinstein. 2001. Collection and analysis of exhaled breath condensate in humans. Am J Respir Crit Care Med 164 (5): 731-737.
Ohyama, M, T. Otake, S. Adachi, T. Kobayashi, and K. Morinaga. 2007. A comparison of the production of reactive oxygen species by suspended particulate matter and diesel exhaust particles with macrophages. Inhal Toxicol 19 (1): 157-160.
Peng, R.D., H.H. Chang, M.L. Bell, A. McDermott, S.L. Zeger, J.M. Samet, and F. Dominici. 2008. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA 299 (18) (299): 2172-2179.
Peters, J.M., E. Avol, W. Navidi, S.J. London, W.J. Gauderman, F. Lurmann, W.S. Linn, H. Margolis, E. Rappaport, H. Gong, Jr, and D.C. Thomas. 1999. A study of twelve southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med 159 (3): 760-767.
Romieu, I, A. Barraza-Villarreal, C. Escamilla-Nunez, A.C. Almstrand, D. Diaz-Sanchez, P.D. Sly, and A.C. Olin. 2008. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immunol 121 (4): 903-909.
Samet, J.M., F. Dominici, F.C. Curriero, I. Coursac, and S.L. Zeger. 2000. Fine particulate air pollution and mortality in 20 US cities, 1987-1994. N Engl J Med 343: 1742-1749.
Schwartz, J. 2004. Air pollution and children’s health. Pediatrics 113 (4 Suppl): 1037-1043.
Taylor, D.R. 2011. Using biomarkers in the assessment of airways disease. J Allergy Clin Immunol 128 (5): 927-934.
Ware, J.H., B.G. Ferris, Jr, D.W. Dockery, J.D. Spengler, D.O. Stram, and F.E. Speizer. 1986. Effects of ambient sulfur oxides and suspended particles on respiratory health of preadolescent children. Am Re Respir Dis 133 (5): 834-842.
Wong, S.H., J.A. Knight, S.M. Hopfer, O. Zaharia, C.N. Leach, Jr., and F.W. Sunderman, Jr. 1987. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem 33 (2 Pt 1): 214-220.
Waraphan Phornwisetsirikun1,2, Tippawan Prapamontol2*, Somrak Rangkakulnuwat3, Somporn Chantara4 and Prasak Tavornyutikarn4
1 Environmental Science PhD Program and Center of Excellence on Environmental Health, and Toxicology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
2 Environment and Health Research Unit, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
3 Department of Pediatrics, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
4 Department of Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding Author: E-mail: tprapamontol@gmail.com
Total Article Views

