
Clusterin as a Blood Biomarker for Diagnosis of Mild Cognitive Impairment and Alzheimer's Disease
Natcha Panachamnong, Pised Methapatara, Somporn Sungkarat, Khanittha Taneyhill and Nutjeera Intasai*Published Date : 2019-08-28
DOI : 10.12982/CMUJNS.2014.0039
Journal Issues : Number 3, September - December 2014
ABSTRACT
With increasing global life expectancy, Alzheimer’s disease will become an increasingly prevalent health problem. The development of biomarkers that predict risk for both Alzheimer’s disease and mild cognitive impairment will be useful for early diagnosis of dementia. To date, no surrogate blood biomarker exists to classify between Alzheimer’s disease/mild cognitive impairment and normal controls or Alzheimer’s disease and mild cognitive impairment/normal control as a diagnostic parameter. In this study, we analyzed serum levels of amyloid-β 40 (Aβ40), amyloid-β 42 (Aβ42), clusterin (CLU) and p97 using ELISA kits from 157 subjects with normal cognition, mild cognitive impairment and Alzheimer’s disease. We found a significant increase in serum levels of Aβ42 (P<0.05) and serum clusterin (P<0.001) between normal and Alzheimer’s disease subjects and between normal and mild cognitively impaired subjects. In contrast, serum Aβ40 and p97 levels did not differ significantly between all groups. We also used receiver operating characteristic curves to determine the cut-off point of Aβ42 and clusterin to differentiate either cognitively normal from cognitively impaired subjects (both Alzheimer’s disease and mild cognitive impairment) or cognitively normal and mild cognitively impaired subjects from those with Alzheimer’s disease. Only clusterin with 84% sensitivity, 75% specificity and good accuracy of diagnosis showed promise for diagnosing patients with cognitive impairment (Alzheimer’s disease and mild cognitive impairment).
Keywords: Alzheimer’s disease, Mild cognitive impairment, Biomarker, Clusterin, Aβ42
INTRODUCTION
With increasing global life expectancy, Alzheimer’s disease (AD) will become an increasingly prevalent health problem. Alzheimer’s disease is the most common type of dementia; approximately one in eight people over the age of 65 years are at risk of developing Alzheimer’s disease (Kidd, 2008). Currently, accurate diagnosis of Alzheimer’s disease is based on medical history, neurological tests and clinical examination criteria (McKhann et al., 1994; APA, 1994). To date, there is no reliable diagnostic test for Alzheimer’s disease. In addition, no adequate treatments to cure or prevent disease progression exist. Thus, early detection of preclinical Alzheimer’s disease among mild cognitive impaired (MCI) patients may provide greater opportunity for early treatment and monitoring of disease progression (Morris, 2005).
The majority of the studies of accepted Alzheimer’s disease biomarkers to date have been carried out using samples of cerebrospinal fluid obtained by lumbar puncture (Blennow and Hampel, 2003). This is an invasive procedure, particularly in elderly people, which is unsuitable for routine laboratory testing. Recently, development of diagnostic tests for Alzheimer’s disease and mild cognitive impairment has begun to focus on blood biomarkers (Ray et al., 2007; German et al., 2007). There is evidence that Alzheimer’s disease-associated amyloid-β (Aβ) proteins, and in particular amyloid-β 42 (Aβ42), are the main components of amyloid senile plaques in Alzheimer’s disease pathogenesis (Selkoe, 1994). Aβ42 is initially deposited in the Alzheimer’s patient’s brain instead of the more predominant amyloid-β 40 (Aβ40) (Younkin, 1995). Mayeux and colleagues found that plasma Aβ42 may be elevated many years before the onset of sporadic Alzheimer’s disease (Mayeux et al., 1998). Another study suggested that an increased level of Aβ42 in the plasma of Alzheimer’s patients may be involved in the pathology of Alzheimer’s disease in the brain (Abdullah et al., 2009). Besides Aβ, other plasma/serum proteins have been reported to be associated with Alzheimer’s disease. For example, proteins in the apolipoprotein family, such as apolipoprotein E (ApoE) and apolipoprotein J (ApoJ), or clusterin, are expressed in both brain and plasma, and bind to Aβ with high affinity (Wilson et al, 2008; Bell et al., 2007). Reports have also linked melanotransferin (MTf), or p97, with Alzheimer’s disease. Levels of p97 in the serum of Alzheimer’s patients are often higher than those in non-demented elderly controls or in patients with other dementias (Kim et al., 2001; Ujiie et al., 2002).
Unfortunately, to date, there is no surrogate blood biomarker for classification between Alzheimer’s disease, mild cognitive impairment and controls as a diagnostic parameter. The aim of this study was to compare the serum protein biomarkers, including Aβ40, Aβ42, clusterin and p97, between subjects with normal cognition, mild cognitive impairment and Alzheimer’s disease for diagnosis and monitoring the progression in Alzheimer’s disease by using an enzyme-linked immunosorbent assay (ELISA). This may then prove useful as a diagnostic tool for Alzheimer’s disease and improve diagnostic accuracy of early Alzheimer’s disease. An ideal biomarker should reflect the neuropathology of Alzheimer’s disease and can be used in monitoring the progression of the disease and the efficacy of therapy for Alzheimer’s disease. Moreover, the focus of this interest is not only limited to patients with Alzheimer’s disease, but also extends to those individuals at increased risk for Alzheimer’s disease, mild cognitive impairment.
Table 1. Demographic characteristics of the subject groups.
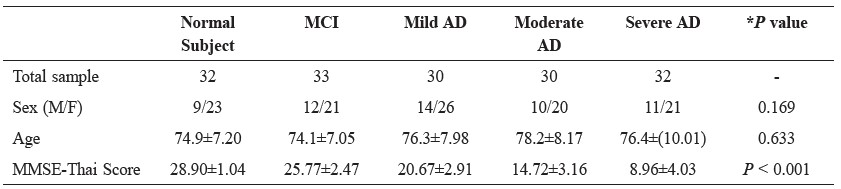
Note: Values are expressed as mean ± SD, M = male, F = female, *P < 0.05 was considered statistically significant
MATERIALS AND METHODS
Subjects
The study population consisted of 157 subjects in three groups: 92 with Alzheimer’s disease (mild AD, n= 30; moderate AD, n=30; severe AD, n=32), 33 with mild cognitive impairment and 32 cognitively normal subjects. Clinically diagnosed patients with Alzheimer’s disease and mild cognitive impairment were recruited from Suanprung Psychiatric Hospital, Chiang Mai, Thailand. All patients underwent a standard clinical assessment, including neurological, physical and psychiatric examinations. Criteria for controls were absence of memory complaints or other cognitive symptoms, preservation of general cognitive functioning and no active neurological or psychiatric disease. The Mini-Mental State Examination-Thai Version 2002 score (MMSE-Thai score) was added for investigation and classification of cognitive function in Alzheimer’s disease, mild cognitive impairment and normal subjects. The Ethics Committees at Suanprung Psychiatric Hospital and the Faculty of Associated Medical Sciences, Chiang Mai University approved the study. Demographic characteristics of the subjects are shown in Table 1.
Sample collection
Five milliliters of venous blood samples were collected from all participants. After clotting, blood was centrifuged at 1,800 g for 10 minutes. Serum samples were aliquoted into microcentrifuge tubes and stored at -70ºC before analysis.
Serum protein analysis of Aβ40, Aβ42, clusterin and p97 by ELISA
A concentration of Aβ40, Aβ42, clusterin and p97 were measured by ELISA techniques in Alzheimer’s disease, mild cognitive impairment, and normal subject serum samples, as instructed by the manufacturer. Samples were measured in duplicate. All samples had undergone only one freeze-thaw cycle and were analyzed within 12 months of collection.
Table 2. Concentration of Aβ40, Aβ42, clusterin (CLU) and p97 levels in subject groups.
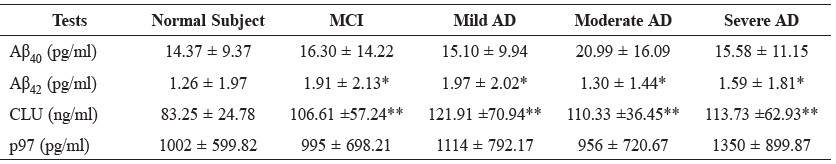
Note: Values are expressed as mean ± SD, * P<0.05, ** P<0.00
Aβ40 & Aβ42
Serum Aβ40 and Aβ42 levels were measured using sandwich ELISA kit (Invitrogen, Camarillo, USA). The ELISA plates were pre-coated with monoclonal antibody specific for the NH2-terminus of Hu Aβ40 and Aβ42. Briefly, 50 μl of Aβ peptide standards, controls or serum samples were added into the microplate wells. Fifty microliters of Hu Aβ40 or Aβ42 detection antibody were then pipetted into the wells. After incubating for 3 hours at room temperature with shaking, a solution of horseradish peroxidase (HRP)-conjugated anti-rabbit IgG was added and incubated for 30 min at room temperature with shaking. Finally, 100 μl of substrate was added and the absorbance was read at 450 nm on a microplate reader.
Clusterin
Serum clusterin levels were detected using a human clusterin competitive ELISA Kit (Adipogen, Incheon, Korea). The wells of a 96-well ELISA plate were coated with human clusterin recombinant protein. Briefly, 50 μl of serum samples, human clusterin standard or control sample were added into microplate wells followed by the addition of 50 μl of clusterin detection antibody. After 1 hour incubation at 37°C, a solution of HRP-conjugated anti-rabbit IgG was added and incubated for 1 hour at 37°C. Finally, 100 μl of substrate was added and the absorbance was read at 450 nm on a microplate reader.
p97
Serum p97 levels were estimated using sandwich ELISA kit (USCN Life Science Inc., China). The 96-well ELISA plates were pre-coated with anti-MTf (p97) antibodies. Briefly, 100 μl of standard blank or serum sample were added into microplate wells. Then 100 μl of p97 biotin-conjugated detection antibody were pipetted into the wells. After 1 hour incubation at 37°C, a solution of avidin conjugated to HRP was added and incubated for 30 min at 37°C. Finally, 90 μl of substrate was added and the absorbance was read at 450 nm on a microplate reader.
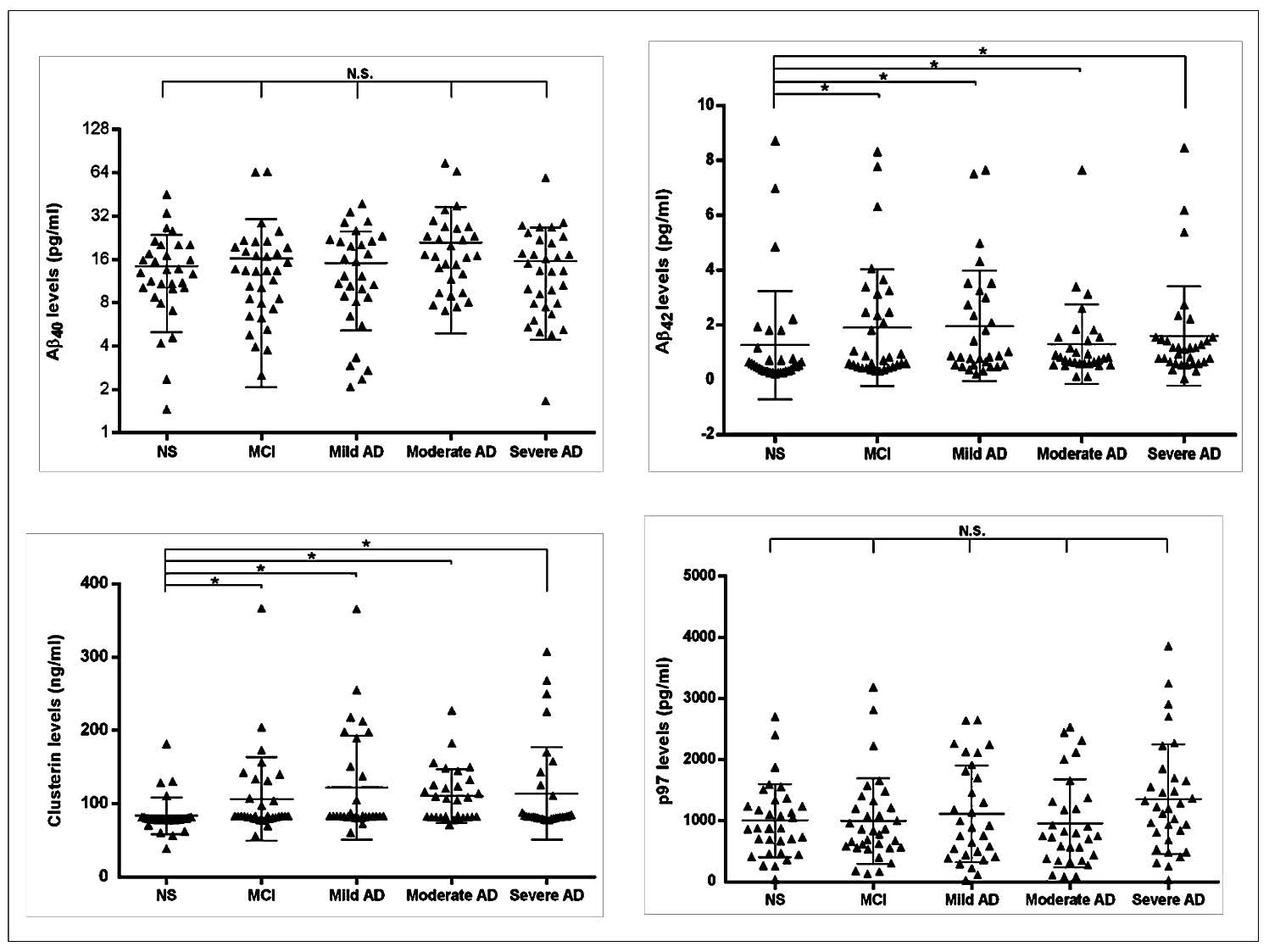
Note: All data are shown. Horizontal bars represent the mean ± SD values.
Figure 1. Scatter plot comparison of serum Aβ40, Aβ42, clusterin and p97 levels in Alzheimer's disease (AD), mild cognitive impairment (MCI) and cognitive normal subject (NS) groups as measured by ELISA. (A) No significant difference of serum Aβ40 was observed among all five groups. (B) Significant difference in serum Aβ42 in Alzheimer's and mild cognitive impairment patients was demonstrated compared to normal cognitive subjects (P<0.05). (C) Significant difference in serum clusterin in Alzheimer's and mild cognitive impairment patients was demonstrated compared to normal cognitive subjects (P<0.001). (D) No significant differences of serum p97 was observed among all five groups.
Statistical analysis
Statistical comparison of age, sex and MMSE-Thai score were evaluated by one-way analysis of variance (ANOVA). The significance of differences
between Alzheimer’s disease groups, mild cognitive impairment group and cognitive normal subject group were evaluated using non-parametric Kruskal-Wallis and Mann-Whitney U tests. Results of the analyses were considered significant at P-value less than 0.05. The relationship between sensitivity and specificity for the different groups of patients (Alzheimer’s disease and mild cognitive impairment) versus cognitive normal subject or Alzheimer’s disease versus mild cognitive impairment and cognitive normal subject was described using receiver operating characteristic curve analysis (Greiner, 1996). The receiver operating characteristic curve was calculated with 95% confidence intervals.
Table 3. Receiver operating characteristic curve analysis of Aβ42 and clusterin in serum for discriminating patients with cognitive impairment (Alzheimer’s disease and mild cognitive impairment) and cognitive normal subjects.

Table 4. Receiver operating characteristic curve analystis of Aβ42 and clusterin in serum for discriminating patients with Alzheimer’s disease compare to mild cognitive impairment and cognitive normal subjects.

RESULTS
Sample characteristics
A summary of the demographic data is given in Table 1. According to their clinical assessment, individuals were grouped as either Alzheimer’s disease (mild, moderate and severe), mild cognitive impairment or cognitively normal. All groups were age- and sex- matched. Interestingly, a significant decrease in MMSE-Thai score was found in Alzheimer’s disease and mild cognitive impairment groups compared with cognitive normal subject group (P<0.001).
Concentrations of serum Aβ40, Aβ42, clusterin and p97 in Alzheimer’s disease, mild cognitive impairment and cognitive normal subject
Mean ± SD values for serum Aβ40, Aβ42, clusterin and p97 levels in Alzheimer’s disease, mild cognitive impairment and cognitive normal subject groups are summarized in Table 2. The Alzheimer’s disease groups were divided into three subgroups, including mild Alzheimer’s disease, moderate Alzheimer’s disease and severe Alzheimer’s disease. There were no significant differences among all five groups in serum Aβ40 and p97 levels. On the other hand, there was a significant increase in serum levels of Aβ42 (P<0.05) and clusterin (P<0.001) between Alzheimer’s disease groups and cognitive normal subjects and between mild cognitive impairment and cognitive normal subjects (Table 2 and Figure 1).
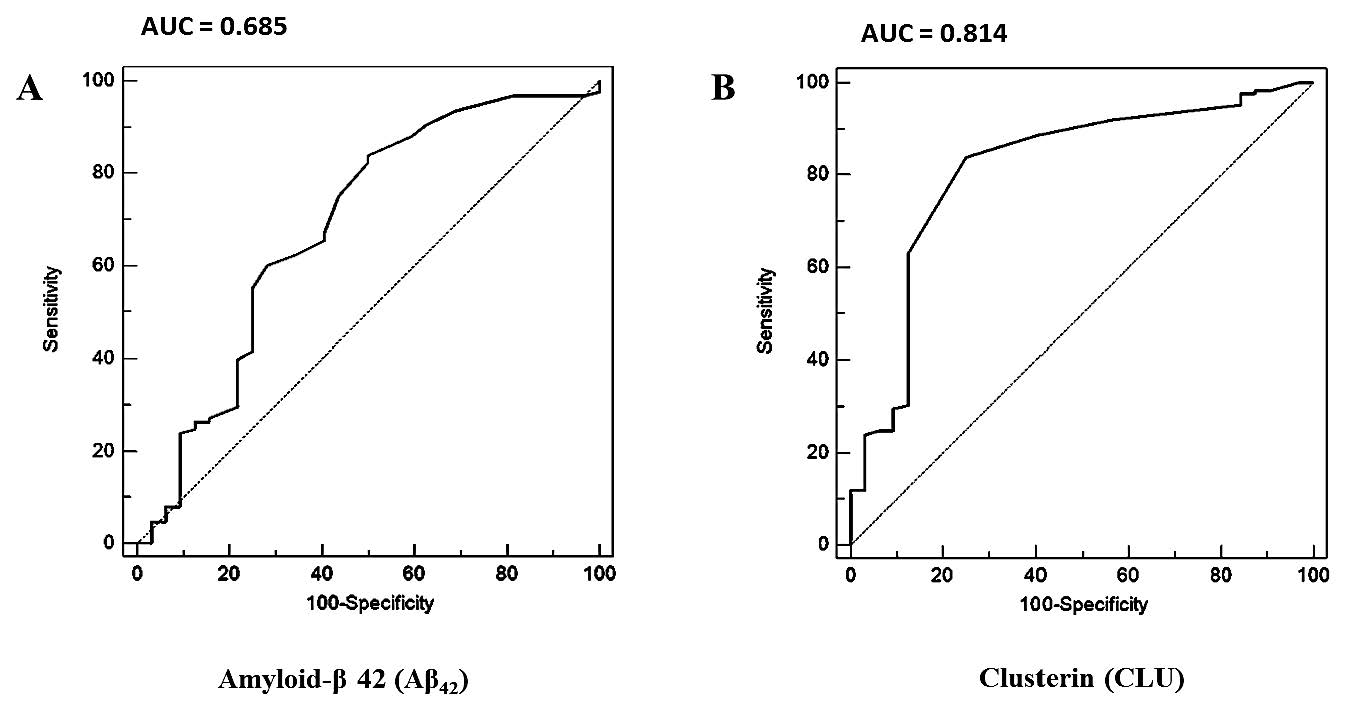
Figure 2. Receiver Operating Characteristic (ROC) curves analysis of Aβ42 and clusterin for distinguishing between cognitive impairment subjects (Alzheimer’s disease and mild cognitive impairment) and cognitive normal subjects. (A) ROC curve analysis of serum Aβ42. Cut-off point
and area under ROC curve (AUC) of serum Aβ42 was > 0.49 pg/ml and 0.685, respectively. (B) ROC curve analysis of serum clusterin. Cutoff point and AUC of serum clusterin was > 80.23 ng/ml and 0.814, respectively.
The receiver operating characteristic curve analysis for determination of serum Aβ42 and serum clusterin cut-off value
To identify the optimal serum Aβ42 and serum clusterin concentration for discriminating patients with cognitive impairment and cognitive normal subjects, a cut-off value was determined. Using receiver operating characteristic curve analysis, the area under the curve of serum Aβ42 is 0.685 and serum clusterin is 0.814 (Figure 2). The optimal cut-off value of serum Aβ42 for discriminating Alzheimer’s disease and mild cognitive impairment patients with cognitive normal subjects was 0.49 pg/ml (84% sensitivity and 50% specificity), while that of serum clusterin was 80.23 ng/ml (84% sensitivity and 75% specificity), as summarized in Table 3. In addition, we determined a cut-off point of 0.49 pg/ml for serum Aβ42 to differentiate Alzheimer’s disease from mild cognitive impairment and cognitive normal subjects with 88% sensitivity and 38.5% specificity, and 80.23 ng/ml for serum clusterin with 87% sensitivity and 49.2% specificity (Table 4). The area under the curve of serum Aβ42 is 0.603 and serum clusterin is 0.687, as summarized in Figure 3. The diagnosis accuracy of serum Aβ42 and serum clusterin was sufficient and very good, respectively. Although mild cognitive impairment and Alzheimer’s disease subjects showed good sensitivity of serum Aβ42 and serum
clusterin more than 80%, at this point the specificity is relatively low.
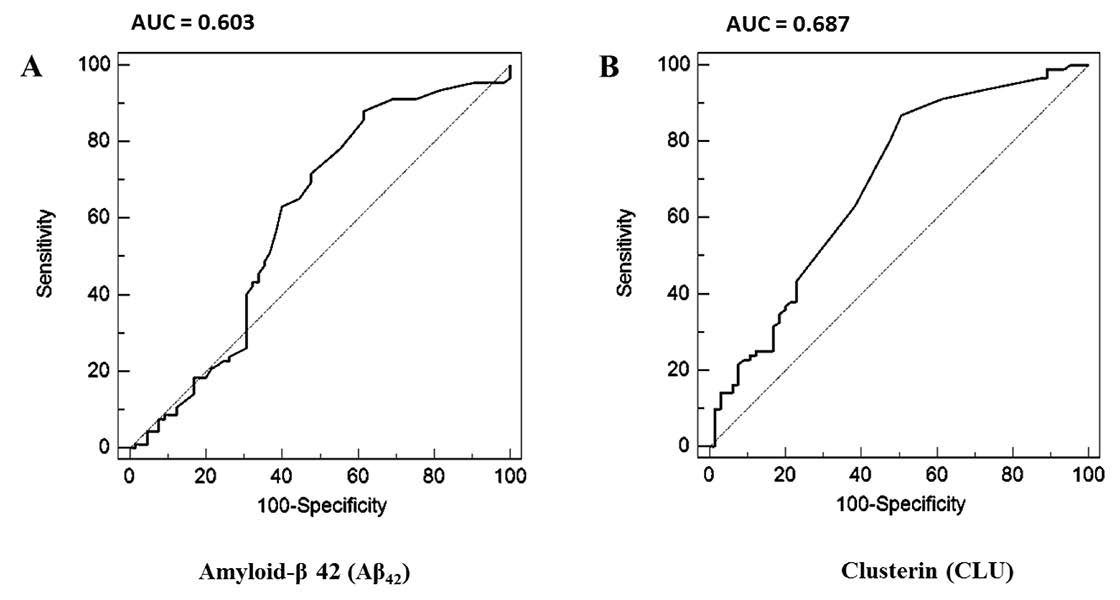
Figure 3. Receiver Operating Characteristic (ROC) curves analysis of Aβ42 and clusterin for distinguishing Alzheimer's patients from mild cognitive impairment and cognitive normal subjects. (A) ROC curve analysis of serum Aβ42. Cut-off point and area under ROC curve (AUC) of serum Aβ42 was > 0.49 pg/ml and 0.603, respectively. (B) ROC curve analysis of serum clusterin. Cut-off point and AUC of serum clusterin > 80.23 ng/ml and 0.687, respectively.
DISCUSSION
Blood-based biomarkers for Alzheimer’s disease diagnosis have been widely investigated. However, blood-based biomarkers for Alzheimer’s disease diagnosis presented contrary results and it is unclear whether changes in the peripheral blood reflect pathology within the brain (Zetterberg et al, 2010; Mehta, 2007; Irizarry, 2004). In this study, the concentrations of Aβ40, Aβ42, clusterin and p97 in serum were investigated in Alzheimer’s disease, mild cognitive impairment, and cognitive normal subjects who were grouped base on neurological, physical and MMSE-Thai score.
Concordant with previous reports, Aβ42 and clusterin in serum of Alzheimer’s disease and mild cognitive impairment was significantly elevated compared to normal subjects (Mehta et al., 2000; Assini et al., 2004; Schrijvers et al., 2011; Xing et al., 2012). Several studies claimed that the increase of Aβ42 and clusterin in Alzheimer’s disease could be useful in improving the progression of Alzheimer’s patients (Chiu et al., 2012; Thambisetty et al, 2010; 2012). It has been reported that subjects with normal cognition having a high-plasma Aβ42 level were twice as likely to develop Alzheimer’s disease as subjects having a low-plasma Aβ42 level; and plasma Aβ42 levels were elevated in Alzheimer’s disease patients compared to controls (Schupf et al., 2007). It has been reported that an increase in the plasma Aβ42 level predicted the conversion from normal cognition to mild cognitive impairment, but did not predict conversion from normal cognition to Alzheimer’s disease (Blasko et al., 2008). In addition, an increased Aβ42 level was detected in women, but not men, with mild cognitive impairment (Assini et al., 2004).
Peripheral concentration of clusterin is associated with changes in patients with Alzheimer’s disease and mild cognitive impairment. Our current observations provided evidence linked to high clusterin levels in Alzheimer’s disease and mild cognitive impairment which is related to previous study. Thambisetty et al. (2010) showed that high concentrations of plasma clusterin were correlated with the development of entorhinal cortex atrophy, and severity and progression of Alzheimer’s disease. Recently, they found that high plasma clusterin levels in mild cognitive impairment were associated with slow rates of brain atrophy (Thambisetty et al, 2012). In addition, association of plasma clusterin with Alzheimer’s disease has been recently reported (Schrijvers et al., 2011). Among patients with Alzheimer’s disease, higher clusterin levels were associated with more severe disease. Plasma clusterin levels were not related to the risk of incident Alzheimer’s disease during total follow-up (Schrijvers et al., 2011). Thus, Aβ42 and clusterin in serum were considerably higher in Alzheimer’s disease and mild cognitive impairment patients than in cognitive normal subjects. This may be an indication of increased risk for developing Alzheimer’s disease.
In contrast, serum Aβ40 levels in Alzheimer’s disease and mild cognitive impairment were not significantly different compared to cognitive normal subject. Although previous studies have reported elevated plasma Aβ40 concentrations in patients with Alzheimer’s disease (Mehta et al., 2000; van Oijen et al., 2006), some reports suggested that Aβ40 levels were not altered in Alzheimer’s disease and mild cognitive impairment patients, which is similar to our study. In a longitudinal study, it appeared that Alzheimer’s disease patients at baseline and those who developed Alzheimer’s disease during follow-up had significant higher plasma Aβ42, but not Aβ40 levels, and plasma Aβ42, but not Aβ40 levels, declined over time in newly acquired Alzheimer’s patients (Mayeux et al., 2003). In addition, no difference in plasma Aβ40 levels between patients with mild cognitive impairment who later developed Alzheimer’s disease and patients with stable mild cognitive impairment or healthy subjects has been reported. Therefore, plasma Aβ40 level was not an optimal biomarker in Alzheimer’s disease (Hansson et al., 2010)
Kennard et al. (1996) have demonstrated that p97 concentrations are consistently elevated in the serum of Alzheimer’s patients, compared with controls. There was no overlap between the groups, and the correlation between age and p97 serum concentration was not significant. Kim DK et al. (2001) showed that serum p97 concentrations were elevated three to four fold in Alzheimer’s disease as compared to non-Alzheimer’s disease dementia and normal controls. These results support the significance of high serum p97 levels in Alzheimer’s disease and its potential utility as a biological marker in Alzheimer’s disease. However, we found that p97 levels in serum of Alzheimer’s disease and mild cognitive impairment patients were not different from cognitive normal subjects. Two of the limitations of our study were its small sample size and many participants in Alzheimer’s disease and mild cognitive impairment groups were not newly diagnosed cases. Thus, the effect of dementia treatment may affect the level of some serum proteins in subjects, including p97.
To identify the optimal serum Aβ42 and serum clusterin concentration for discriminating patients with cognitive impairment and cognitive normal subjects, receiver operating characteristic curve analysis was used in this study. Our results found that a serum Aβ42 level at a cut-off point of 0.49 pg/ml in cognitive impairment patients (Alzheimer’s patient and mild cognitive impairment) against cognitive normal subjects had good sensitivity (84%) with low specificity (50%). However, the accuracy of diagnosis was poor (the area under the curve of 0.685). For clusterin, we defined serum clusterin 80.23 ng/ml as the best cut-off point, which gives the same sensitivity (84%) as Aβ42 and higher specificity (75%) than Aβ42. Interestingly, the accuracy of diagnosis was good (the area under the curve of 0.814). Additionally, we attempted to differentiate patients with Alzheimer’s disease from mild cognitive impairment and cognitive normal subjects. The same cut-off points of 0.49pg/ml for serum Aβ42 in Alzheimer’s disease patients against mild cognitive impairment and cognitive normal subject also had good sensitivity (88%), but low specificity (38.5%). The accuracy of diagnosis was poor (the area under the curve of 0.603). Likewise, the same cut-off points of 80.23 ng/ml for serum clusterin showed 87% sensitivity and 49.2% specificity. The accuracy of diagnosis was poor (the area under the curve of 0.687). In comparison with other studies, Chiu et al. (2012) identified a cut-off point of 16.1 pg/ml for Aβ42 to differentiate control subjects from patients (both Alzheimer’s disease and mild cognitive impairment) with 85.3% sensitivity and 88.5% specificity and found it a useful biomarker for Alzheimer’s disease. Differences between study results may be related to sample size, sampling or timing of the sample collection in relation to the clinical period or stage of disease progression. In accordance with our study, reports have shown significantly increased serum clusterin levels in subjects with mild cognitive impairment and Alzheimer’s disease (Schrijvers et al., 2011; Thambisetty et al, 2010; 2012). However, these studies have not addressed the optimal cut-off value, sensitivity and specificity of serum clusterin for distinguishing between cognitive impairment subjects and cognitive normal subjects, nor did they differentiate patients with Alzheimer’s disease from mild cognitive impairment and cognitive normal subjects. An ideal biomarker should have a sensitivity of greater than 80% in detecting Alzheimer’s disease and a specificity of greater than 80% for distinguishing from other dementias (Ronald and Nancy Reagan Research Institute, 1998). It is difficult to determine the exact normal range and cut-off points of blood biomarker levels that provide both high sensitivity and specificity. Many variable factors can change the numerical data, such as sample size, disease stage, race, therapeutic effects of disease and the analytical process.
CONCLUSIONS
In summary, significantly elevated Aβ42 and clusterin levels were observed in Alzheimer’s disease and mild cognitive impairment subjects compared to cognitive normal subjects. There were no significant differences in Aβ40 and p97 levels between Alzheimer’s disease, mild cognitive impairment and cognitive normal subjects. Receiver operating characteristic curve analyses revealed that only clusterin may be used for diagnosing patients with cognitive impairment with 84% sensitivity, 75% specificity and good accuracy of diagnosis. Although these markers cannot be currently used as diagnostic tests for Alzheimer’s disease, our results demonstrated an association between serum Aβ42 and serum clusterin concentrations in patients with Alzheimer’s disease. Sample size, sampling or timing of the sample collection in relation to the clinical period or stage of disease progression should be considered in future studies to improve sensitivity, specificity and accuracy of clinical diagnosis of Alzheimer’s disease among individuals and may also help identify individuals with mild cognitive impairment.
ACKNOWLEDGMENTS
We thank all participants for their dedication and the medical staff of Suanprung Psychiatric Hospital for their support and help with collecting blood samples. The National Research Council of Thailand and the Faculty of Associated Medical Sciences, Chiang Mai University supported this study.
REFERENCES
Abdullah, L., C. Luis, D. Paris, B. Mouzon, G. Ait-Ghezala, A.P. Keegan, D. Wang, F. Crawford, and M. Mullan. 2009. Serum Abeta levels as predictors of conversion to mild cognitive impairment/ Alzheimer disease in an ADAPT subcohort. Mol Med 15:432-7
[APA] American Psychiatric Association 1994. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). R. R. Donnelly & Sons Company, Washington, DC.
Assini, A., S. Cammarata, A. Vitali, M. Colucci, L. Giliberto, R. Borghi, M.L. Inglese, S. Volpe, S. Ratto, F. Dagna-Bricarelli, and others. 2004. Plasma levels of amyloid beta-protein 42 are increased in women with mild cognitive impairment. Neurology 63:828-31
Bell, R.D., A.P. Sagare, A.E. Friedman, G.S. Bedi, D.M. Holtzman, R. Deane, and B.V. Zlokovic. 2007. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27:909-18.
Blasko, I., K. Jellinger, G. Kemmler, W. Krampla, S. Jungwirth, I. Wichart, K.H. Tragl, and P. Fischer. 2008. Conversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging 29:1-11.
Blennow, K., and H. Hampel. 2003. CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2:605-13.
Chiu, M.J., S.Y. Yang, T.F. Chen, J.J. Chieh, T.Z. Huang, P.K. Yip, H.C. Yang, T.W. Cheng, Y.F. Chen, M.S. Hua, and others. 2012. New assay for old
markers-plasma beta amyloid of mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res 9:1142-8.
German, D.C., P. Gurnani, A. Nandi, H.R. Garner, W. Fisher, R. Diaz-Arrastia, P. O’Suilleabhain, and K.P. Rosenblatt. 2007. Serum biomarkers for Alzheimer’s disease: proteomic discovery. Biomed Pharmacother 61:383-9.
Greiner, M. 1996. Two-graph receiver operating characteristic (TG-ROC): update version supports optimisation of cut-off values that minimise overall misclassification costs. J Immunol Methods 191:93-4.
Hansson, O., H. Zetterberg, E. Vanmechelen, H. Vanderstichele, U. Andreasson, E. Londos, A. Wallin, L. Minthon, and K Blennow. 2010. Evaluation of plasma Abeta (40) and Abeta (42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging 31:357-67.
Irizarry, M.C. 2004. Biomarkers of Alzheimer disease in plasma. NeuroRx 1:226-34.
Kennard, M.L, H. Feldman, T. Yamada, and W.A. Jefferies. 1996. Serum levels of the iron binding protein p97 are elevated in Alzheimer’s disease. Nat Med 2:1230-5.
Kidd, P.M. 2008. Alzheimer’s disease, amnestic mild cognitive impairment, and age-associated memory impairment: current understanding and progress toward integrative prevention. Altern Med Rev 13:85-115.
Kim, D.K., M.Y. Seo, S.W. Lim, S. Kim, J.W. Kim, B.J. Carroll, D.Y. Kwon, T. Kwon, and S.S. Kang. 2001. Serum melanotransferrin, p97 as a biochemical marker of Alzheimer’s disease. Neuropsychopharmacology 25:84-90
Mayeux, R., A.M. Saunders, S. Shea, S. Mirra, D. Evans, A.D. Roses, B.T. Hyman, B. Crain, M.X. Tang, and C.H. Phelps. 1998. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med 338:506-11.
Mayeux, R., L.S. Honig, M.X. Tang, J. Manly, Y. Stern, N. Schupf, and P.P. Mehta. 2003. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology 61:1185-90.
McKhann, G., D. Drachman, M. Folstein, R. Katzman, D. Price, and E.M. Stadlan. 1984. Clinical diagnosis of Alzheimer’s disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939-44.
Mehta, P.D., T. Pirttila, S.P. Mehta, E.A. Sersen, P.S. Aisen, and H.M. Wisniewski. 2000. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol 57:100-5.
Mehta, P.D. 2007. Amyloid beta protein as a marker or risk factor of Alzheimer’s disease. Curr Alzheimer Res 4:359-63.
Morris, J.C. 2005. Mild cognitive impairment and preclinical Alzheimer’s disease. Geriatrics Suppl:9-14.
Ray, S., M. Britschgi, C. Herbert, Y. Takeda-Uchimura, A. Boxer, K. Blennow, L.F. Friedman, D.R. Galasko, M. Jutel, A. Karydas, and others. 2007. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med 13:1359-62.
Schrijvers, E.M., P.T. Koudstaal, A. Hofman, and M.M. Breteler. 2011. Plasma clusterin and the risk of Alzheimer disease. JAMA 305:1322-6.
Schupf, N., B. Patel, D. Pang, W.B. Zigman, W. Silverman, P.D. Mehta, and R. Mayeux. 2007. Elevated plasma beta-amyloid peptide Abeta (42) levels, incident dementia, and mortality in Down syndrome. Arch Neurol 64: 1007-13.
Selkoe, D.J. 1994. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci 17:489-517.
Thambisetty, M., A. Simmons, L. Velayudhan, A. Hye, J. Campbell, Y. Zhang, L.O. Wahlund, E. Westman, A. Kinsey, A. Guntert and others. 2010. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry 67:739-48.
Thambisetty, M., Y. An, A. Kinsey, D. Koka, M. Saleem, A. Guntert, M. Kraut, L. Ferrucci, C. Davatzikos, S. Lovestone, and others. 2012. Plasma clusterin concentration is associated with longitudinal brain atrophy in mild cognitive impairment. Neuroimage 59:212-7.
The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group. 1998. Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”.. Neurobiol Aging 19:109-16.
Ujiie, M., D.L. Dickstein, and W.A. Jefferies. 2002. p97 as a biomarker for Alzheimer disease. Front Biosci 7:e42-7.
van Oijen, M., A. Hofman, H.D. Soares, P.J. Koudstaal, and M.M. Breteler. 2006. Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol 5:655-60.
Wilson, M.R., J.J. Yerbury, and S. Poon. 2008. Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol Biosyst 4:42-52
Xing, Y.Y., J.T. Yu, W.Z. Cui, X.L. Zhong, Z.C. Wu, Q. Zhang, and L. Tan. 2012. Blood clusterin levels, rs9331888 polymorphism, and the risk of Alzheimer’s disease. J Alzheimers Dis 29:515-9.
Younkin, S.G. 1995. Evidence that A beta 42 is the real culprit in Alzheimer’s disease. Ann Neurol 37:287-8.
Zetterberg, H., K. Blennow, and E. Hanse. 2 Amyloid beta and APP as biomarkers for Alzheimer’s disease. Exp Gerontol 45:23-9.
Natcha Panachamnong1, Pised Methapatara2, Somporn Sungkarat3,
Khanittha Taneyhill4 and Nutjeera Intasai4*
1 Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
2 Suanprung Psychiatric Hospital, Chiang Mai, Thailand
3 Department of Physical Therapy, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
4 Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: nutjeera@yahoo.com
Total Article Views

