
Identification of Antioxidants in Young Mango Leaves by LC-ABTS and LC-MS
Trakul Prommajak, Sang Moo Kim, Cheol-Ho Pan, Sang Min Kim, Suthat Surawang and Nithiya Rattanapanone*Published Date : 2019-08-28
DOI : 10.12982/CMUJNS.2014.0038
Journal Issues : Number 3, September - December 2014
ABSTRACT
Thai eat the young leaves of mango as vegetables. Antioxidants in young leaves of mango cultivars ‘Talapnak’, ‘Chok Anan’ and ‘Nam Dok Mai’ were identified by high-performance liquid chromatography coupled with an online ABTS assay (HPLC-ABTS) and electrospray ionization mass spectrometer (HPLC-ESI-MS). Young leaves of mango cv. ‘Nam Dok Mai’ had the highest antioxidant capacity. Major antioxidants in young mango leaves were mangiferin and benzophenones (maclurin and iriflophenone derivatives). Mangiferin presented in higher quantities than other compounds in each cultivar, with cv. ‘Talapnak’ containing the most (37.92±0.98 mg/g DW). The compound with the highest antioxidant capacity in all cultivars was mangiferin pentoside (from 1.19±0.25 mmol TE/g DW in cv. ‘Chok Anan’ to 2.13±0.04 mmol TE/g DW in cv. ‘Talapnak’). The compound with the highest Trolox equivalent antioxidant capacity was maclurin galloyl glucoside (1.75±0.62 mol TE/mol).
Keywords: Young mango leaf, Antioxidant, HPLC-ABTS, Xanthone, Benzophenone
INTRODUCTION
Mango (Mangifera indica L.) is a native plant in South Asia, and distributed across tropical and subtropical regions of the world. It is usually grown for its fruit, which is recognized as the ‘king of fruits’ or ‘superfruit’ (Mukherjee and Litz, 2009; Noratto et al., 2010). Besides the fruit, other parts of the mango tree provide value-added products. Mango peel and kernel are by-products of mango processing. Mango peel can be used as a dietary fiber, and mango kernel is used to manufacture mango kernel oil or mango kernel flour (Masibo and He, 2009). In Thailand, the young leaves of mango are consumed as vegetables. Mango leaves have traditionally been used to treat diseases, including dysentery and flatulence. In Indonesia, reddish-colored young leaves are also eaten as a salad or side dish (Flach et al., 1993). In India, young mango leaves are used as ethnomedicine for treatment of diabetes (Sarmah and Hazarika, 2012). Mangiferin is the most important phenolic compound in mango leaves, bark, peels and kernels, and is present in particularly high quantities in young leaves (Barreto et al., 2008). Mangiferin has many biological activities, including anticancer, antimicrobial, anti-allergenic, anti-inflammatory, analgesic, immunomodulatory and hypolipidemia, as well as antioxidant activity (Masibo and He, 2008).
HPLC-ABTS is an analysis system developed for simultaneous purification, identification and capacity determination of the antioxidant compounds. After separating by HPLC column, the compounds were reacted with ABTS radical, which had an absorption maximum at 734 nm. In the presence of an antioxidant, ABTS radical loses its color and causes a negative peak in the chromatogram. Trolox equivalent antioxidant capacity (TEAC) value, a specific antioxidant capacity of individual compounds, can be calculated by this method (Koleva et al., 2001). This technique has been used to determine antioxidants in many plants, including gardenia fruit, pomegranate seed and green and black tea (Stewart et al., 2005; He et al., 2010; He et al., 2011).
Antioxidants in the young leaves of Thai mango have not been reported. In this study, HPLC-ABTS, in combination with HPLC-ESI-MS, was used to analyze the antioxidants in the young leaves of three different mango cultivars. The results provide qualitative and quantitative information about the antioxidants present, as well as the specific antioxidant activities of young mango leaves.
MATERIALS AND METHODS
Plant Materials and Chemicals
Young leaves of three different mango (Mangifera indica L.) cultivars, ‘Talapnak’, ‘Chok Anan’ and ‘Nam Dok Mai’, were obtained from a local farm in Chiang Mai, Thailand in November 2012. Young mango leaves were reddish-brown in color, while mature leaves were dark green.
2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and potassium persulfate were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of vegetable extracts
Fresh young mango leaves were selected and washed in tap water for 1 min. The leaves were extracted with 60% ethanol at the ratio of 1 g per 5 ml using a blender (Model MR 4050 CA, Braun, Spain) for 30 s. The extracts were then filtered through a double layer of muslin cloth and centrifuged at 2,500g for 20 min. The supernatant was kept at -80°C before analysis. Extraction was performed in duplicates.
Identification and quantification of antioxidants
HPLC analysis was performed with Agilent 1200 (Agilent Technologies, Santa Clara, CA, USA) according to the method of He et al. (2010) with slight modification. The sample (10 μl) was separated by Prevail C18 (250×46 mm) column (Alltech Associates, Lokeren, Belgium) with gradient elution (A: 100% methanol, B: methanol:water:formic acid at the ratio of 20:80:0.25 by volume) at a flow rate of 0.7 ml/min and 30°C. Gradient elution began with 100% B, then changed to 90% B at 6.25 min, 80% B at 12.5 min, 55% B at 50 min, 20% B at 68.75 min and returned to 100% B at 75 min. The compounds were detected at 280, 330 and 520 nm.
The compounds escaping from the UV-Vis detector were subjected to online-ABTS analysis. ABTS reagent (2 mM ABTS and 3.5 mM potassium persulphate) was prepared and kept in the dark at room temperature for 16 h to stabilize the radical before use. The reagent, at a flow rate of 0.5 ml/min, reacted with the eluted compounds and was detected at 734 nm. In the presence of antioxidants, it produced a negative peak in the chromatogram. Antioxidant capacity was calculated from the area of the negative peak and expressed as Trolox equivalent (TE). Specific antioxidant capacity of the compounds, or Trolox equivalent antioxidant capacity (TEAC), was obtained from the molar ratio between antioxidant capacity (TE) and the quantity of the compounds.
Identification of the compounds was performed by HPLC coupled with electrospray ionization mass spectrometer (HPLC-ESI-MS) under the same elution condition as described above. Quadrupole mass spectrometer was operated in both positive and negative mode. Mass spectra were recorded in the range of m/z 100-1500.
Statistical analysis
Multiple linear regression of TEAC value was performed by R version 2.15.2 (http://cran.r-project.org/) to study the effects of the number and position of the hydroxyl group on TEAC values of the compounds identified from young mango leaves. The number of OH groups at different positions was used as explanatory variables, while the TEAC value of the compound was used as the response variable. Predicted TEAC values from regression analysis were plotted against the actual TEAC value. Regression coefficients were used to determine the effect of the chemical structure of the compounds on antioxidant capacity.
RESULTS
Identification and quantification of antioxidants
HPLC-ABTS chromatograms of young leaves extracts of three mango cultivars are shown in Figure 1. Positive peaks were used for detection of phenolic compounds, while negative peaks were used for detection of antioxidant capacity. Identification of antioxidants in young leaves of three mango cultivars are shown in Table 1. The major antioxidants in young mango leaves were derivatives of xanthone and benzophenones. Xanthones including mangiferin (compound 11) and mangiferin pentoside (compound 13) were present at the quantities of 29.11-37.92 mg/g DW and 6.50-14.20 mg/g DW, respectively (Table 2). Compounds 2, 3 and 8 were maclurin derivatives, while compounds 4, 5, 9, 12 and 15 were iriflophenone derivatives.
Galloylquinic acid (compound 1), trigalloylglucose (compound 6) and syringic acid (compound 7) are hydroxybenzoic acids with antioxidant activity found in young mango leaves. Flavonoids, including epicatechin gallate (compound 10) and quercetin hexoside (compound 17), antioxidants as well, were also found in young mango leaves.
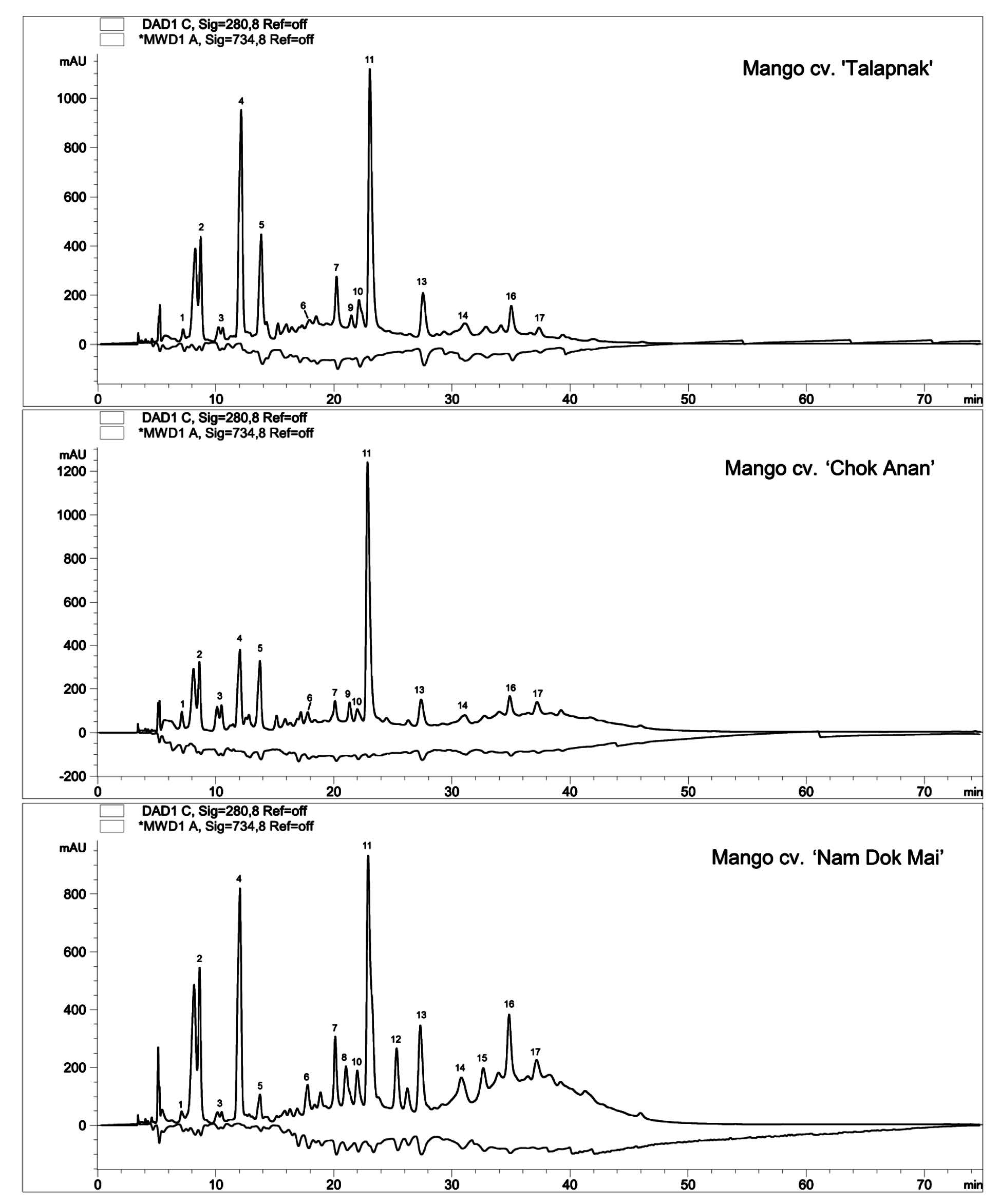
Figure 1. HPLC-ABTS chromatograms of young mango leaves extracts. Positive peaks were UV signal at 280 nm for the detection of phenolic compounds. Negative peaks were Vis signal at 734 nm for the detection of ABTS radical scavenging activity.
Table 1. Identification of antioxidants in young mango leaves.
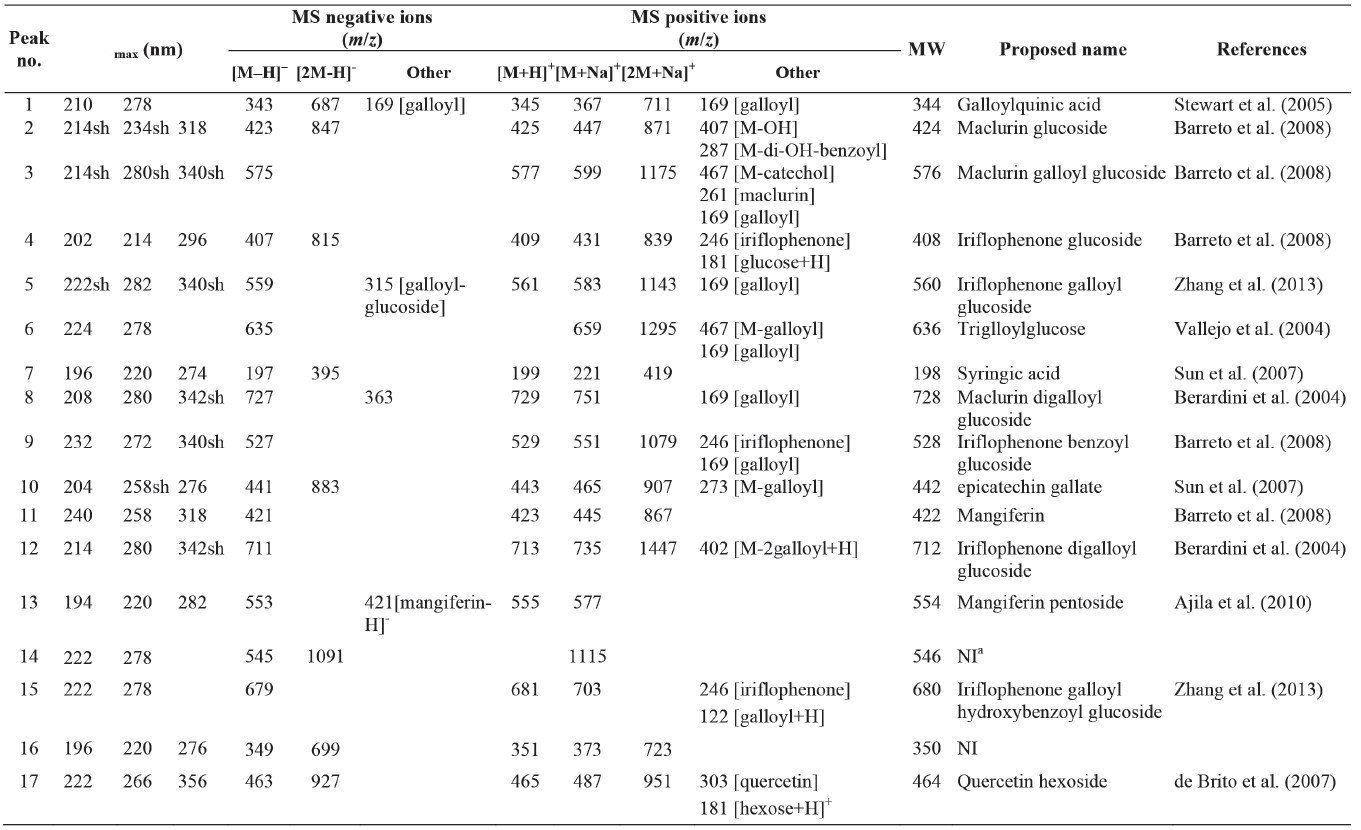
Table 2. Quantity of antioxidants in young leaves of three mango cultivars.
Note: aMean±SD of duplicate analyses; flavonoids were quantified as molar equivalent of quercetin; other phenolic compounds were quantified as molar equivalent of caffeic acid. bND, not detected.
Antioxidant capacity of phenolic compounds
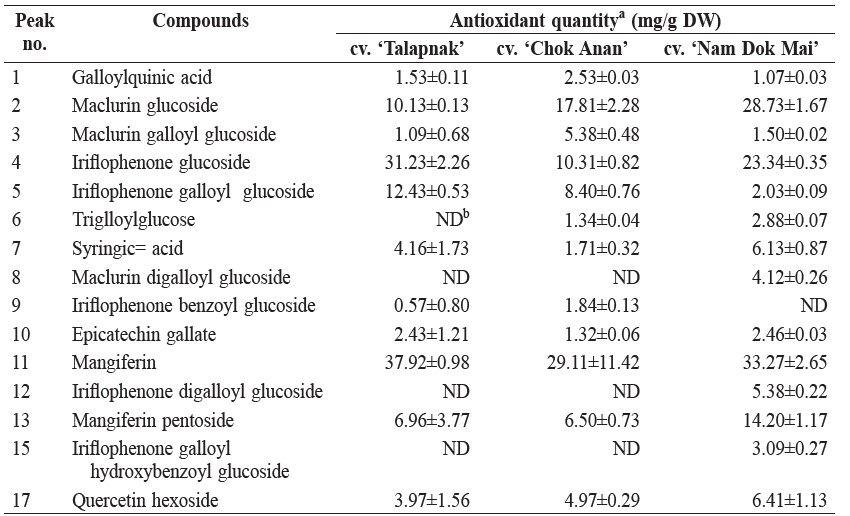
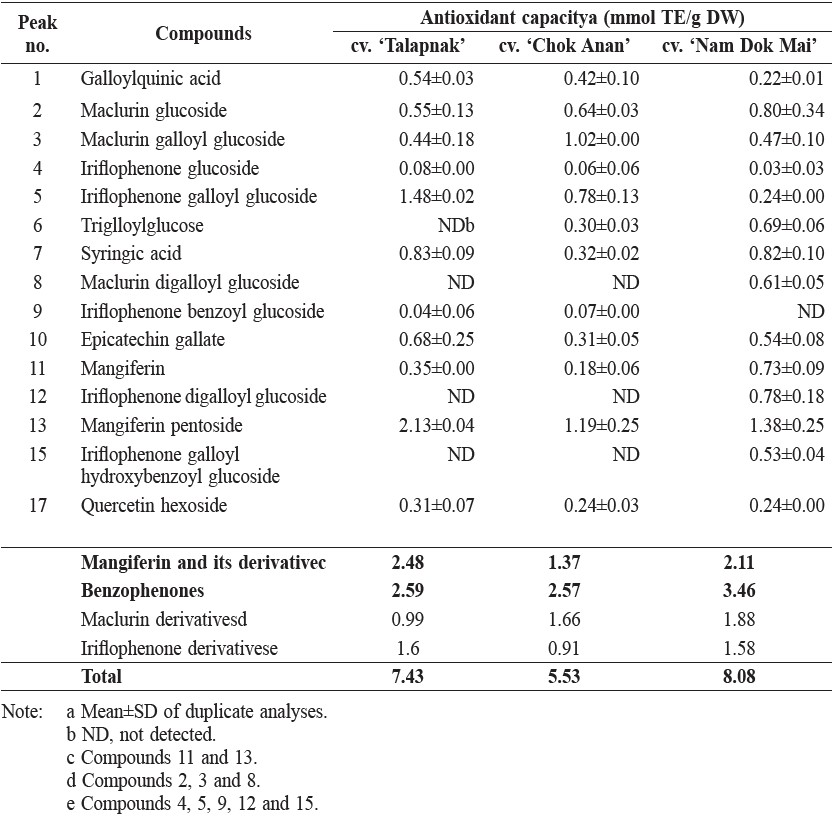
The compound with the highest antioxidant capacity in the young leaves of three different mango cultivars was mangiferin pentoside, with the highest value in cv. ‘Talapnak’ (2.13 mmol TE/g DW) and the lowest value in cv. ‘Chok Anan’ (1.19 mmol TE/g DW). However, the antioxidant activities of all of the benzophenone derivatives were higher than those of mangiferin and mangiferin pentoside in all cultivars (Table 3).
The compound with the highest TEAC value in young mango leaves was maclurin galloylglucoside (1.75 mol TE/mol), followed by trigalloylglucose (1.47 mol TE/mol) and iriflophenone galloyl hydroxybenzoyl glucoside (1.17 mol TE/mol) (Table 4).
Table 3. Antioxidant capacity of phenolic compounds in young leaves of 3 mango cultivars.
DISCUSSION
HPLC-ABTS chromatograms of the ethanol extracts from young leaves of three different mango cultivars showed a similar pattern, indicating that the antioxidant activity of young mango leaves derived from either similar or the same compounds. Most of the antioxidants were hydrophilic compounds, which were eluted from the column within 40 min.
Benzophenones, including maclurin and iriflophenone, are also important antioxidants in young mango leaves. The highest quantity of mangiferin was found in the young leaves of mango cv. ‘Talapnak’ (37.92 mg/g DW), while the lowest quantity appeared in cv. ‘Chok Anan’ (29.11 mg/g DW). However, the quantities were lower than those found in the young leaves of mango cv. ‘Van Dyke’ (58.12 mg/g DW) and ‘Embrapa-141-Roxa’ (67.20 mg/g DW) from Brazil (Barreto et al., 2008). Mangiferin is a compound found in many parts of mango, including peels, kernel, bark and pulp. The quantity in mango pulp was lower than in other parts (Ribeiro et al., 2008). Mangiferin content varied among mango cultivars (Berardini et al., 2005). Although mango is a primary source of mangiferin, it has also been identified from other plant families, including honeybush (Clyclopia spp.) and African mango (Irvingia gabonensis) (Sun and Chen, 2012; Matkowski et al., 2013).
Table 4. Trolox equivalent antioxidant capacity (TEAC) of phenolic compounds in young leaves of 3 mango cultivars.
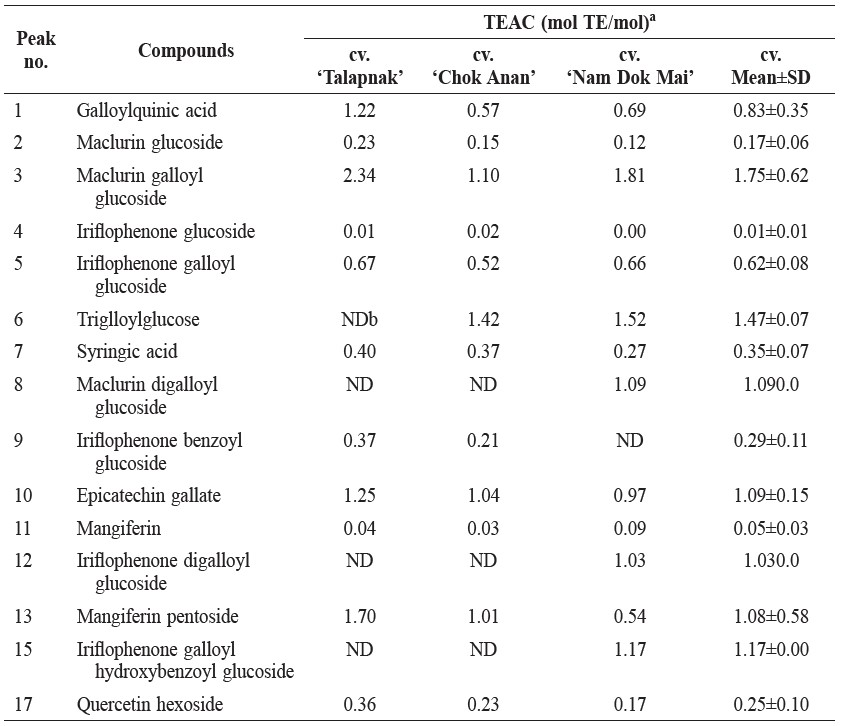
Note: aMolar of Trolox with equivalent antioxidant capacity of a 1 molar substance. bND, not detected.
These benzophenones are attached to glucose, galloyl or benzoyl moieties. The quantity of monoglucoside derivative was higher than that of more complex derivatives for both maclurin and iriflophenone. A similar trend was also found in the young leaves of mango cv. ‘Van Dyke’ and ‘Embrapa-141-Roxa’. However, the ratio between maclurin glucoside and iriflophenone glucoside varied among cultivars. The ratio of maclurin glucoside per iriflophenone glucoside was higher than found in the ‘Chok Anan’ and ‘Nam Dok Mai’ cultivars, while less than found in the ‘Talapnak’ cultivar. The quantity of iriflophenone glucoside was much higher than that of maclurin glucoside in the ‘Van Dyke’ and ‘Embrapa-141-Roxa’ cultivars (Barreto et al., 2008). Benzophenone glycosides with double substitution (from galloyl or benzoyl moieties) were found only in cv. ‘Nam Dok Mai’. Maclurin is an intermediate in biosynthesis of mangiferin and isomangiferin (Berardini et al., 2004). In some literature, benzophenones from mango were named as foliamangiferosides (Zhang et al., 2011). The chemical structures of some antioxidants found in young mango leaves are shown in Figure 2.
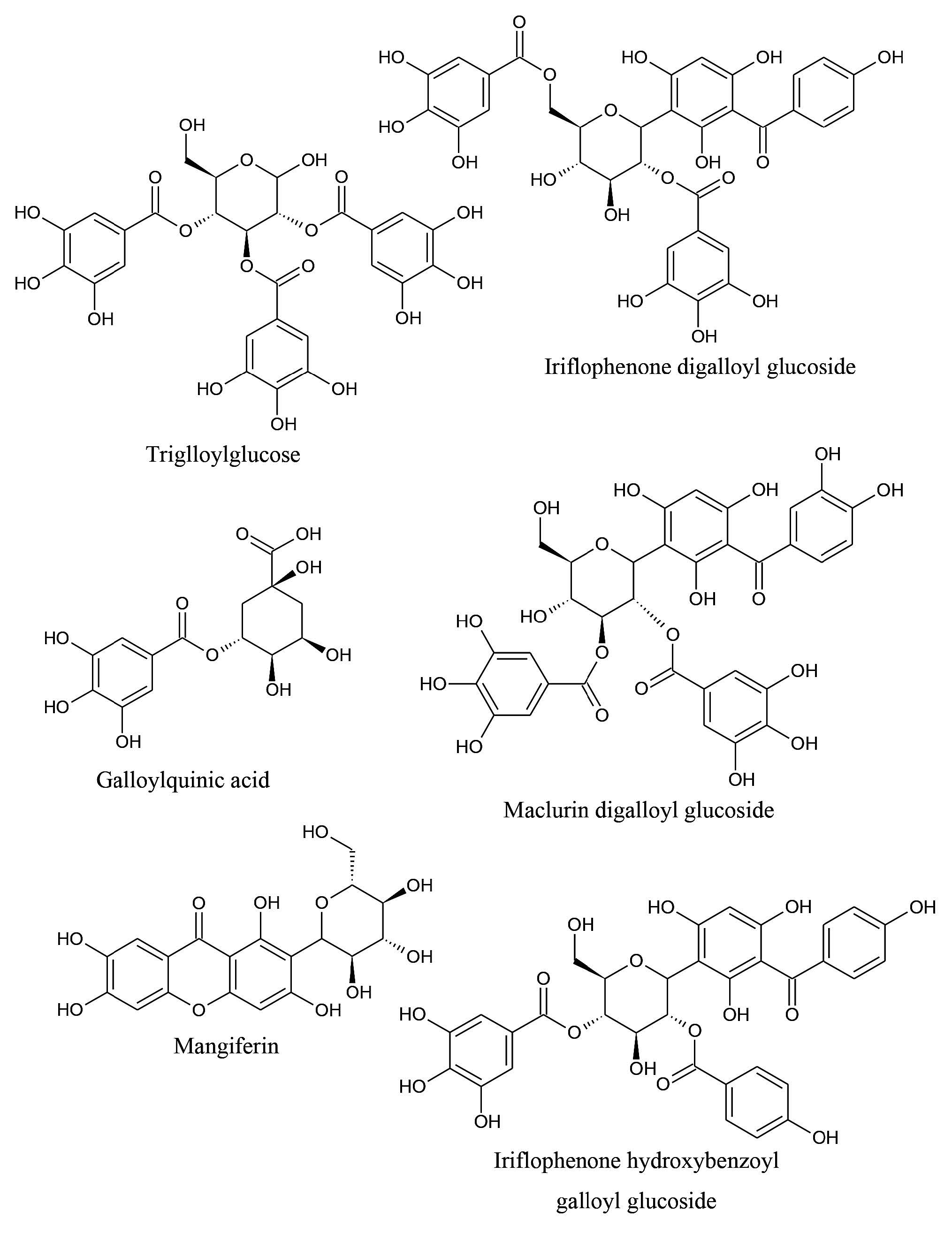
Figure 2. Some antioxidants found in young mango leaves.
Mono- and di-galloyl glucosides of maclurin were found in young Thai mango leaves. However, these compounds were not detected in both old and young Brazillian mango leaves. In Brazillian mango cultivars, gallic acid and gallate esters of methanol, glucose, mangiferin, maclurin and iriflophenone were identified. The number of galloyl moieties could be up to five in esterification with glucose, while benzophenones estirified with up to two galloyl moieties. Mangiferin was found in most parts of the mango tree. Benzophenones were absent in mango kernel, while isomangiferin was found only in mango bark (Barreto et al., 2008; Ribeiro et al., 2008). In this study, isomangiferin was also not detected in young Thai mango leaves.
Galloylquinic acid, trigalloylglucose, syringic acid, epicatechin gallate and quercetin hexoside were previously reported in many parts of mango (Gu et al., 2003; Elzaawely and Tawata, 2010; Ramirez et al., 2013). Kaempferol glucoside was identified from the peel of mango cv. ‘Tommy Atkins’ (Berardini et al., 2004). However, this compound was not detected in this study.
The ratio of total antioxidant capacity of maclurin derivatives to iriflophenone derivatives varied among cultivars, and was dependent on their contents. Antioxidant activity of maclurin derivatives was higher than those of iriflophenones in the ‘Chok Anan’ and ‘Nam Dok Mai’ cultivars, but was lower in the ‘Talapnak’ cultivar.
TEAC value measured the specific antioxidant capacity of the compounds. TEAC value of mangiferin was 0.05 mol TE/mol (Table 4), which was similar to the 133.07 mol TE/g (equivalent to 0.056 mol TE/mol) reported previously (Petrova et al., 2011).
Antioxidant activity of phenolic compounds was influenced by the number and position of hydroxyl (OH) groups (Arts et al., 2003). Multiple linear regression was performed on the antioxidants identified from young mango leaves. The number of OH groups at different position was used as explanatory variables, while the TEAC value of the compound was used as the response variable. Correlation between actual and predicted TEAC (Multiple R2 = 0.7465) confirmed the structure-activity relationship of the OH groups (Figure 3). From the regression coefficient, OH groups substituted at the para position of aromatic compound influenced the antioxidant capacity of phenolic compounds the most (Figure 4). OH group of maclurin also had a positive effect on antioxidant activity, comparable to meta-substituted OH of phenolic compounds. On the other hand, OH attached to alkane had a negative effect on antioxidant activity. In most cases, the alkane OH was located in glucose moiety. Glycosides of phenolic compounds had lower antioxidant activity than their corresponding aglycone. Sugar moiety reduced antioxidant activity of phenolic compound by substituting free OH group, which was responsible for radical scavenging. Glycosylation also decreased coplanarity of the B-ring in flavonoids and increased hydrophilicity of the compounds (Heim et al., 2002).
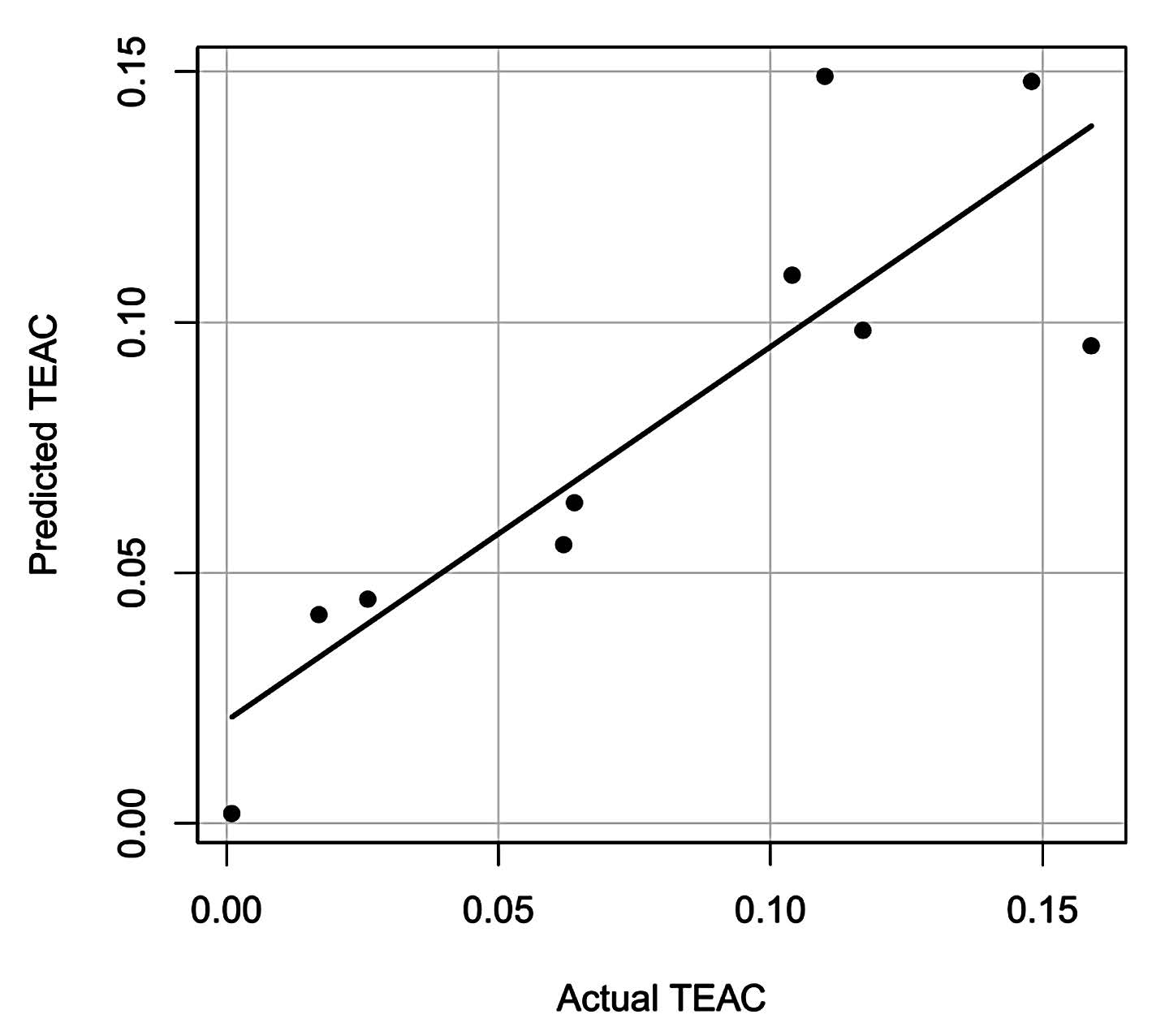
Figure 3. Actual and predicted TEAC values of antioxidants from young mango leaves.
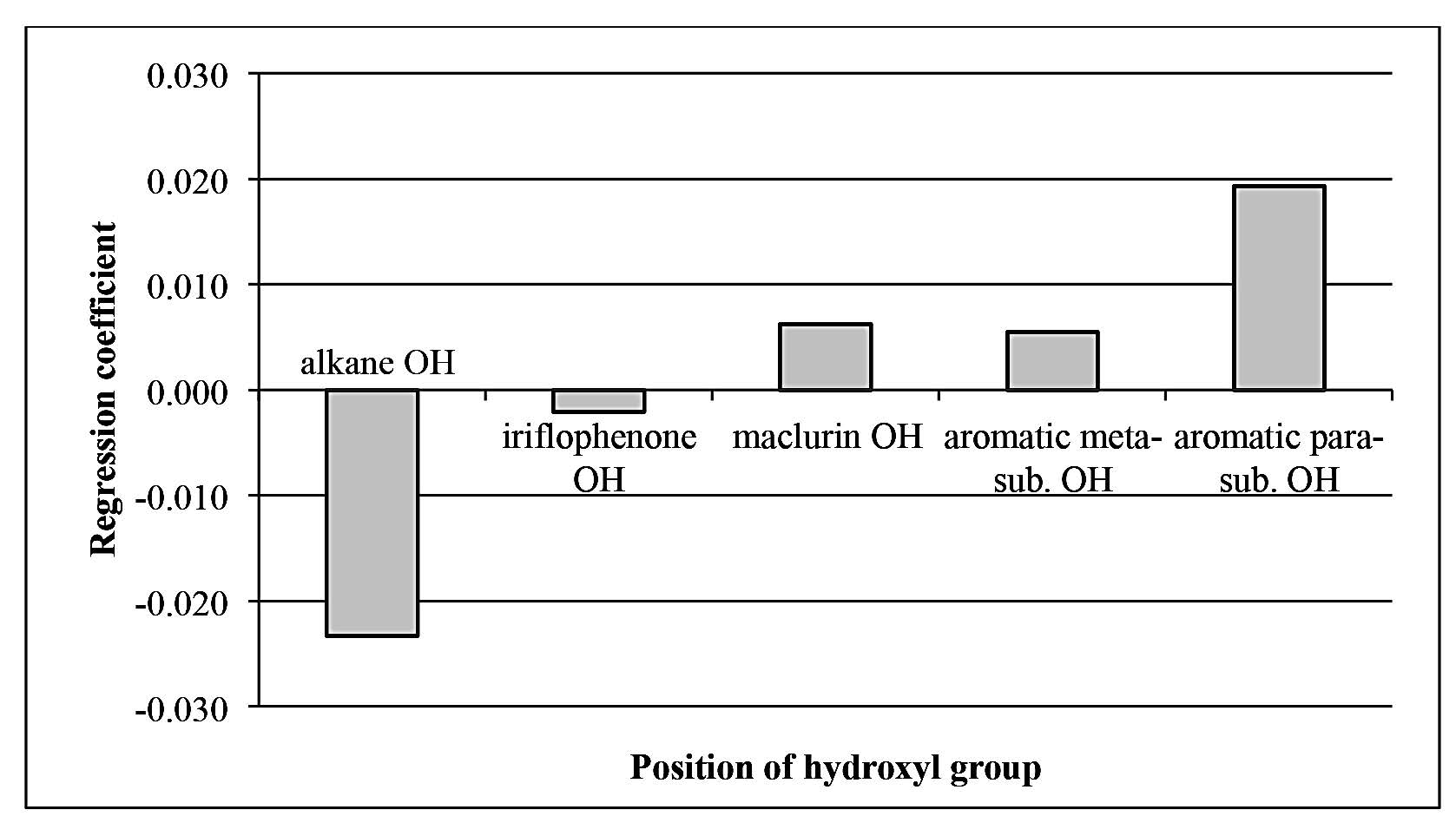
Figure 4. Regression coefficient of multiple linear regression of TEAC value as influenced by number and position of OH group in antioxidants from mango leaves.
CONCLUSIONS
Major antioxidants in young leaves of mango cv. ‘Talapnak’, ‘Chok Anan’ and ‘Nam Dok Mai’ were the derivatives of xanthone mangiferin and benzophenones (maclurin and iroflophenone). The content of mangiferin was higher than that of other compounds in each cultivar. The compound with the highest antioxidant activity was mangiferin pentoside, while the compound with the highest TEAC value was maclurin galloylglucoside. Further work should investigate antioxidantsin the young leaves of other mango varieties and the effect of the growth period on the antioxidative compounds.
ACKNOWLEDGEMENTS
This work was supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0260/2551). C. Pan and S. Kim were supported by a KIST Gangneung Institute Intramural Grant (2Z04220).
REFERENCES
Ajila, C., L. Jaganmohan Rao, and U. Prasada Rao. 2010. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food and Chemical Toxicology 48: 3406-3411. DOI: 10.1016/j.fct.2010.09.012
Arts, M.J., J. Sebastian Dallinga, H.P. Voss, G.R. Haenen, and A. Bast. 2003. A critical appraisal of the use of the antioxidant capacity (TEAC) assay in defining optimal antioxidant structures. Food Chemistry 80: 409-414. DOI: 10.1016/S0308-8146(02)00468-5
Barreto, J.C., M.T. Trevisan, W.E. Hull, G. Erben, E.S. de Brito, B. Pfundstein, G. Würtele, B. Spiegelhalder, and R.W. Owen. 2008. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). Journal of Agricultural and Food Chemistry 56: 5599-5610. DOI: 10.1021/jf800738r
Berardini, N., R. Carle, and A. Schieber. 2004. Characterization of gallotannins and benzophenone derivatives from mango (Mangifera indica L. cv.‘Tommy Atkins’) peels, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Communications in Mass Spectrometry 18: 2208-2216. DOI: 10.1002/rcm.1611
Berardini, N., R. Fezer, J. Conrad, U. Beifuss, R. Carle, and A. Schieber. 2005. Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol O- and xanthone C-glycosides, anthocyanins, and pectin. Journal of Agricultural and Food Chemistry 53: 1563-1570. DOI: 10.1021/jf0484069
de Brito, E.S., M.C. Pessanha de Araújo, L.Z. Lin, and J. Harnly. 2007. Determinationof the flavonoid components of cashew apple (Anacardium occidentale) by LC-DAD-ESI/MS. Food Chemistry 105: 1112-1118. DOI: 10.1016/j.foodchem.2007.02.009
Elzaawely, A.A., and S. Tawata. 2010. Preliminary phytochemical investigation on mango (Mangifera indica L.) leaves. World Journal od Agricultural Sciences 6: 735-739.
Flach A., C. Branch, and J. Branch. 1993. The Indonesian Mango [online]. Available at: http://rfcarchives.org.au/Next/Fruits/Mango/IndonesianMango9-93. htm [Accessed April 25, 2014].
Gu, L., M.A. Kelm, J.F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz, and R.L. Prior. 2003. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. Journal of Agricultural and Food Chemistry 51: 7513-7521. DOI: 10.1021/jf034815d
He, W., X. Liu, H. Xu, Y. Gong, F. Yuan, and Y. Gao. 2010. On-line HPLC-ABTS screening and HPLC-DAD-MS/MS identification of free radical scavengers in Gardenia (Gardenia jasminoides Ellis) fruit extracts. Food Chemistry 123: 521-528. DOI: 10.1016/j.foodchem.2010.04.030
He, L., H. Xu, X. Liu, W. He, F. Yuan, Z. Hou, and Y. Gao. 2011. Identification of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant capacities by HPLC–ABTS+ assay. Food Research International 44: 1161-1167. DOI: 10.1016/j.foodres.2010.05.023
Heim, K.E., A.R. Tagliaferro, and D.J. Bobilya. 2002. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. Journal of Nutritional Biochemistry 13: 572-584. DOI: 10.1016/S0955-2863(02)00208-5
Koleva, I.I., H.A.G. Niederländer, and T.A. van Beek. 2001. Application of ABTS Radical Cation for Selective On-Line Detection of Radical Scavengers in HPLC Eluates. Analytical Chemistry 73: 3373-3381. DOI: 10.1021/ac0013610
Masibo, M., and Q. He. 2008. Major mango polyphenols and their potential significance to human health. Comprehensive Reviews in Food Science and Food Safety 7: 309-319. DOI: 10.1111/j.1541-4337.2008.00047.x
Masibo, M., and Q. He. 2009. Mango bioactive compounds and related nutraceutical properties - a review. Food Reviews International 25: 346-370. DOI:10.1080/87559120903153524
Matkowski, A., P. Kus, E. Goralska, and D. Wozniak. 2013. Mangiferin - a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Reviews in Medicinal Chemistry 13: 439-455.
Mukherjee, S.K., and R.E. Litz. 2009. Introduction: botany and importance. In R.E. Litz (ed) Mango: Botany, Production and Uses. CABI, Cambridge. Noratto, G.D., M.C. Bertoldi, K. Krenek, S.T. Talcott, P.C. Stringheta, and S.U. Mertens-Talcott. 2010. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. Journal of Agricultural and Food Chemistry 58: 4104-4112. DOI: 10.1021/jf903161g
Petrova, A., L.M. Davids, F. Rautenbach, and J.L. Marnewick. 2011. Photoprotection by honeybush extracts, hesperidin and mangiferin against UVB-induced skin damage in SKH-1 mice. Journal of Photochemistry and Photobiology B: Biology 103: 126-139. DOI: 10.1016/j.jphotobiol.2011.02.020
Ramirez, J.E., R. Zambrano, B. Sepúlveda, and M.J. Simirgiotis. 2013. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of Chilean pica mango fruits (Mangifera indica L. cv. piqueño). Molecules 19: 438-458. doi:10.3390/molecules19010438
Ribeiro, S.M.R., L.C.A. Barbosa, J.H. Queiroz, M. Knödler, and A. Schieber. 2008. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chemistry 110: 620-626. DOI: 10.1016/j.foodchem.2008.02.067
Sarmah, P.C., and R. Hazarika. 2012. Evaluation of hypoglycemic effect of mangifera leaf. International Journal of Applied Biology and Pharmaceutical Technology 3: 98-102.
Stewart, A.J., W. Mullen, and A. Crozier. 2005. On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Molecular Nutrition & Food Research 49: 52-60. DOI: 10.1002/mnfr.200400064
Sun, J., F. Liang, Y. Bin, P. Li, and C. Duan. 2007. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 12: 679-693. DOI: 10.3390/12030679
Sun, J. and P. Chen. 2012. Ultra high-performance liquid chromatography with high-resolution mass spectrometry analysis of African mango (Irvingia gabonensis) seeds, extract, and related dietary supplements. Journal of Agricultural and Food Chemistry 60: 8703-8709. DOI: 10.1021/jf302703u
Vallejo, F., F. Tomás-Barberán, and F. Ferreres. 2004. Characterisation of flavanols in broccoli (Brassica oleracea L. var. italica) by liquid chromatography–UV diode-array detection–electrospray ionisation mass spectrometry. Journal of Chromatography A 1054: 181-193. DOI: 10.1016/j.chroma.2004.05.045
Zhang, Y., Q. Qian, D. Ge, Y. Li, X. Wang, Q. Chen, X. Gao, and T. Wang. 2011. Identification of benzophenone C-glucosides from mango tree leaves and their inhibitory effect on triglyceride accumulation in 3T3-L1 adipocytes. Journal of Agricultural and Food Chemistry 59: 11526-11533. DOI: 10.1021/jf2028494
Zhang, Y., L. Han, D. Ge, X. Liu, E. Liu, C. Wu, X. Gao, and T. Wang. 2013. Isolation, structural elucidation, MS profiling, and evaluation of triglyceride accumulation inhibitory effects of benzophenone C-glucosides from leaves of Mangifera indica L. Journal of Agricultural and Food Chemistry 61: 1884-1895. DOI: 10.1021/jf305256w
Trakul Prommajak1, Sang Moo Kim2, Cheol-Ho Pan3, Sang Min Kim3, Suthat Surawang1 and Nithiya Rattanapanone1,4*
1 Division of Food Science and Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
2 Department of Marine Food Science and Technology, Gangneung-Wonju National University, Gangneung 210-702, Korea
3 Functional Food Center, Korea Institute of Science and Technology, Gangneung 210-340, Korea
4 Postharvest Technology Research Institute, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: agfsi001@gmail.com
Total Article Views

