
Physiological Changes of Fruit and Vegetable Carving
Jomkhwun Suwannarak, Putkrong Phanumong, Nithiya Rattanapanone*Published Date : 2019-08-27
DOI : 10.12982/cmujns.2014.0023
Journal Issues : Number 1, January-april 2014
ABSTRACT
This study investigated the physiological responses of carving fruits and vegetables on respiration rate, ethylene production and electrolyte leakage. Pumpkin, carrot, radish and cantaloupe were carved into the shape of rose or carnation flowers, while Japanese cucumber was carved into lotus flower. All five plants were also carved into leaf shapes. The physiological changes were investigated during each stage of the carving process. The results showed that respiration and ethylene production rates were affected by carving, increased significantly during carving steps for all styles and shapes. Carving into leaf shapes induced higher respiration and ethylene production rates than carving into flower shapes. Carving pumpkin into a rose flower shape caused higher respiration and ethylene production rates than a carnation flower due to differences in intensity of the wound stress. High electrolyte leakage occurred in all samples, regardless of plant or shape. The physiological changes identified here affected both quality and shelf life of carved plant.
Keywords: Carving, Physiological changes, Pumpkin, Carrot, Radish, Cantaloupe, Japanese cucumber
INTRODUCTION
Vegetable and fruit carving is a traditional Thai art that originated in the Royal Thai courts, although no official recorded evidence on the origin exists. When Sukhothai province was the capital of Thailand (A.D. 1238-1438) during the reign of King Phra Ruang, one of his female retinues, Miss Nopamas, wrote a book entitled, Tao Sri Chulalak or Nang Nopamas, which mentioned various traditional rites. One of the rites involved floating bowls that were elaborately decorated with beautifully carved flowers and plant materials of many kinds. This is considered the official commencement of the art of vegetable and fruit carving in Thailand (Fine Arts Department, 1971).
Today, fruit and vegetable carving has become popular across the globe – found in a variety of restaurants, hotels, catering halls, exhibitions and cruise ships (Siam Carving Academy, 2011). Fruits and vegetables are carved to decorate plates of food, enhancing their beauty and edibility (Suwannaruk, 2004). In addition, they are used to decorate the dining table and rooms on special occasions. The carved fresh fruits and vegetables are usually left at room temperature for a relatively long period for presentation or before serving. As they are living tissues, they deteriorate, suffering tissue damage, softening and microbial decay. The wounding caused by carving (peeling, cutting, and slicing), can modify plant metabolism, including respiration and ethylene production rates (Toivonen and Brummell, 2008). As respiration rates drive key biological, physiological and chemical processes, they are inversely related to the quality and subsequent shelf life of a product (Zagory, 1999). However, no reports exist on the physiological changes of carved fruits or vegetables, of value to potentially extending the shelf life of carved plants – a high demand export product. The objective of this study was to determine the physiological changes in fruits and vegetables when carved, including respiration rate, ethylene production and electrolyte leakage.
MATERIALS AND METHODS
Plant materials
Pumpkin (Cucurbita moschata Decne.) cv. Kangkok, Japanese cucumber (Cucumis sativas.), cantaloupe (Cucumis melo L. var. cantaloupensis), radish (Raphanus sativus Linn.) and carrot (Daucuscarota Linn.) of unknown cultivar were purchased from a local market in Muang District, Chiang Mai province, Thailand in August, 2012. Fruits and vegetable were transferred to laboratory immediately and washed with tap water and air dried prior to the experiment.
Sample preparation
Pumpkin was carved into three shapes: a carnation flower, rose flower and leaf. Carrot, radish and cantaloupe were carved into two shapes: a rose flower and leaf. Japanese cucumber was carved into two shapes: a lotus flower and leaf.
Carving carnation and rose flower shapes: After washing with tap water, whole pumpkin and cantaloupe were separated into eight sections. Each section was cut into a trapezoid shape before removal of seeds and hand peeling with a sharp knife. Carrot and radish were cut crosswise into pieces. Then the samples were trimmed into a semi-circle shape. The semi-circle piece was carved manually into a rose and carnation flower shape with a sharp carving knife.
Carving lotus shapes: The Japanese cucumber was cut crosswise into one to three parts. The pieces were separated from the top by carving knife into eight sections to create the first petal. The second petal followed and the seeds were removed.
Carving leaf shapes: The wedged sections of pumpkin and cantaloupe were first sliced into discs of approximately 1.0-1.5 mm in thickness. Further trimming and slicing with the carving knife into leaf shapes followed. Carrot and radish were cut along the length into three sections of approximately 1.0-1.5 mm in thickness and then cut out from the two outer sides of the slice before carving into leaf shapes. For cucumber, a slice was cut from the side of cucumber to form the preliminary leaf shape before detailed carving (Suwannaruk, 2004).
Determination of respiration and ethylene production rates
Respiration – the rate of carbon dioxide production or oxygen consumption – and ethylene production rates were studied at each step of the carving process. Four plant samples were carved into flower shapes in a three-step process: wedged- or piece- section (first step), semi-circle piece (second step) and carnation or rose flowers (third step). Three plant samples were carved into leaf shapes in a threestep process: slice piece, leaf shapes and carved-leaves.
Respiration and ethylene production rates can be measured using the closed-system method described by Haggar et al. (1992). All samples were placed into a one-liter glass jar and sealed with a cap. After standing for 3-4 h at 25°C, 1 ml of headspace gas was withdrawn using a syringe through a rubber septum. Carbon dioxide and ethylene concentrations were measured by injecting a 1 ml headspace gas sample into gas chromatography (Model No. 6890N, Agilent Technologies, USA) equipped with a flame ionization detector. Respiration and ethylene production rates were calculated as mgCO2· kg-1· h-1 and μlC2H4·kg-1· h-1, respectively.
Electrolyte leakages
Three replications were used in this experiment. One sample each during flower carving and two samples each during leaf carving per replication were dipped in double-deionized water. Conductivity of the surrounding solution was measured at the beginning of 1 min (C1) and after standing for 60 min (C60) at room temperature (25°C). The samples were then sterilized at 121°C for 25 min, and total conductivity (CT) of the bathing solution was measured after cooling, using a conductivity meter (Sartorius Model No. PP-20, Goettingen, Germany). Electrolyte leakage was calculated from the following equation: E = (C60 −C1)/CT ×100 and expressed as a percentage of total electrolytes in the tissue (Fan and Sokorai, 2005).
Statistical analysis
The experiment was based on completely randomized design. Four or three replicates were used in each experiment. ANOVA was performed using SPSS Version 13 at p ≤ 0.05. Duncan’s multiple range test compared the mean values.
RESULTS
Respiration and ethylene production rates
Carving into rose, carnation and tulip flowers shapes. The respiration and ethylene production rates during carving of pumpkin, carrot, radish, cantaloupe and Japanese cucumber into rose, carnation and lotus flower shape are shown in Table 1. The carving significantly (p ≤ 0.05) affected respiration rates. In addition, the respiration of the second step (peeling and carving into semi-circle shapes) and the third step (carving into rose or carnation flowers) significantly increased (p ≤ 0.05) from the first step (piece or wedged-shape cutting) for all plant types. This was probably due to tissue wound response. Carving pumpkin into a rose flower shape had a significantly higher respiration rate (p ≤ 0.05) than carving into a carnation shape. The CO2 concentration increased 1.1 fold between the first and second carving steps, and 2.4 and 2.1 fold between the first and third steps (for the rose and carnation shapes, respectively). The ethylene production rates showed similar trends with the CO2 production rates. Ethylene concentration increased 2.4 fold between the first and second steps. Carving into rose and carnation flower shapes increased ethylene concentrations by 8.5 and 6.9 fold, over the first step, respectively.
Carving cantaloupe into a rose flower showed the highest respiration rate, followed by pumpkin, radish and carrot. The CO2 concentration of cantaloupe, pumpkin, radish and carrot rose flower increased by 3.4, 2.4, 2.4 and 2.4 fold over the first step. The ethylene production rates of carrot and radish were under the detectable limits of the gas chromatograph for all carving steps. This was probably due to the low ethylene synthesis in root crops. Carving Japanese cucumber into a lotus flower shape induced respiration and ethylene production increases of 1.8 and 3.2 fold over the first step.
Carving into leaf shapes. The respiration and ethylene production rates during the carving of pumpkin, carrot, radish, cantaloupe and Japanese cucumber into leaf shapes are shown in Table 2. The respiration and ethylene production rates of the five plants for the slicing (first step), trimming into leaf shapes and non-carving (second step), and carved-leaves (third step) increase progressively. The climacteric fruit of cantaloupe showed the highest respiration rate in carved leaf of 281.44±3.31 mg CO2·kg-1·h-1, an increase of about 2.2 fold over the first step.
Leaf carving creates relatively larger wounding than flower carving, with each step resulting in larger increases in respiration rates. For pumpkin, the second and third steps showed an increase of 1.6 and 2.2 fold for CO2 production rates and 15.2 and 25.7 fold in ethylene production rates, when compared with the first step, respectively. Similar results were also observed in carrot and radish leaves, with increases of 2.3 and 1.9 fold in CO2, production rates, and 2.8 and 2.6 fold in ethylene production rates.
Carved-leaf cucumber, with the peel not removed, showed the lowest CO2 and ethylene production rates, increases of 1.2 and 1.7 fold over the first step. A similar result was reported by Agar et al. (1999), who observed that CO2 and ethylene production rates of peeled kiwi fruit slices were about 2 to 4 times higher than those of unpeeled slices when stored at 20°C.
Electrolyte leakage
Electrolyte leakage of carved plant tissues is shown in Table 1-2. Percentage of electrolyte leakage significantly increased (P ≤ 0.05) with the higher CO2 and ethylene production rates during the carving steps of pumpkin, carrot, radish, cantaloupe and Japanese cucumber into three types of flower shapes. The carving operation of the piece or wedged-section into semi-circle shapes resulted in a small change in percentage electrolyte leakage. However, carving pumpkin into rose and carnation flowers significantly increased (P ≤ 0.05) electrolyte leakage by 4.4 and 3.9 fold over the first step. A similar trend was observed with carving carrot, radish, cantaloupe and Japanese cucumber into rose or lotus flower shapes. The percentage of electrolyte leakage increased from the first step by 5.0, 8.4, 7.9 and 9.6 fold, respectively.
Carved leaves showed the same trend of increasing electrolyte leakage during carving. The carving tended to induce higher electrolyte leakage in all carved-leaf plants than the slice and non-carved plant. Leaves carved of cantaloupe showed the highest percentage of electrolyte leakage (31.04±3.03). Percentage of electrolyte leakage in leaves carved of pumpkin (27.31±5.81) followed. Leaves carved from carrot, radish and Japanese cucumber showed the percentage electrolyte leakage increased by 2.6, 2.5 and 2.7 fold. This result was consistent with the CO2 and ethylene production rates, in which carved-leaf shapes of cantaloupe showed the highest value compared to the other plants.
Table 1. Respiration and ethylene production rates and electrolyte leakage during carving of pumpkin, carrot, radish, cantaloupe and Japanese cucumber into rose, carnation and lotus flower shapes.
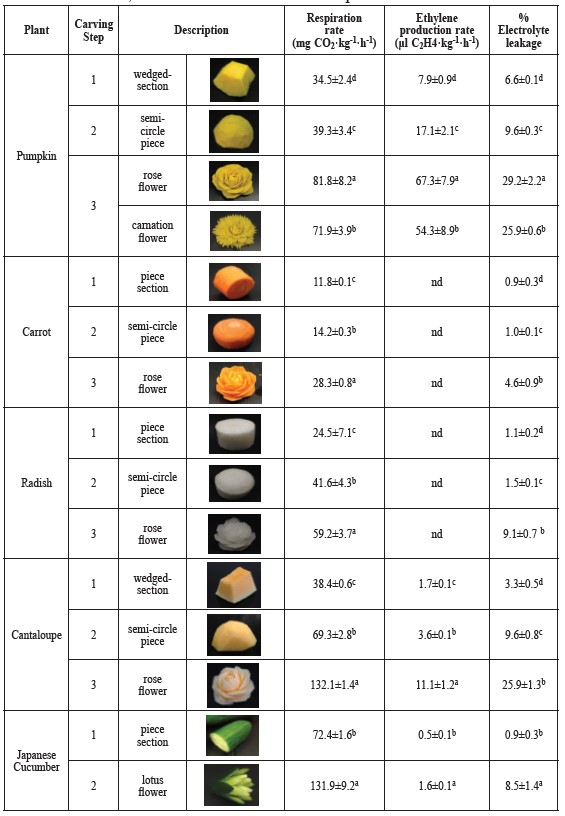
Note: nd = not detected. Data expressed as mean of n = 4. Values in each column with distinct lower case letters represent the significantly different results (p ≤ 0.05).
DISCUSSION
Carving induced physiological changes in the respiration rates, ethylene production rates and percentage electrolyte leakage in all five types of fruits and vegetables used in this study. The plants also showed signs of product deterioration. The two different carving styles – into flower and leaf shapes – induced different physiological changes in the carved product, depending on the wounding size, species, maturity and variety of plant. Carving into a rose flower shape induced higher respiration rates than a carnation shape, which might indicate more serious tissue damage occurs in carving a rose flower shape, given the increased surface area of the shape allows for a more rapid diffusion of oxygen into the internal cell compartments. Wounding plant cells increased metabolic activity (Zagory, 1999). In addition, the high ethylene production rate during carving could induce increased respiration in cantaloupe and pumpkin-carved product. Baskaran et al. (2001) also reported that cutting fully mature pumpkin into pieces of 1 1/4" in size induced a high respiration rate of 155.8 mg CO2· kg-1· h-1 at 28±2°C.
Carving carrots into leaf shapes induced the lowest CO2 and ethylene production rates of 63.86 mg CO2· kg-1· h-1 and 78.83 μl C2H4·kg-1· h-1 compared to the other plants, however, they still increased significantly (p ≤ 0.05) during carving. Barry-Ryan and O’Beirne (2000) observed that hand-peeling carrot increased the respiration rate by 18%, and slicing carrot into disks (6 mm thick) increased the respiration rate by 40% compared to unpeeled-whole carrot. Izumi et al. (1996) has reported on the effect of cutting styles on the physiological changes of carrot. The respiration of carrot slices (20-40 mm diameter and 5 mm thick), carrot sticks (ca. 5 mm wide, 50 mm long and 4 mm thick) and carrot shreds (ca. 4 mm wide, 50 mm long and 2 mm thick) were studied during storage at 0, 5 and 10°C. Cutting styles affected the respiration rate in the following order: carrot shreds > sticks > slices. The rate subsequently decreased when the samples were stored at lower temperatures. The respiration rate of carrot slices, sticks and shreds were in the range of 2.5-13.0, 7.6-24.3 and 5.7-22.1 ml CO2· kg-1· h-1 at 0-10°C, respectively, which was lower than our study. The discrepancy may depend on the species, cultivar and post-harvest conditions, such as pretreatment (washing and chemical treatment), cutting style (size, shape and wounding area) and storage temperature (Soliva-Fortuny and Martín-Belloso, 2003). For other plants, fresh-cut radish into shreds (2 mm thick) showed higher CO2 production rates than sliced radish (2 mm thick) at storage temperatures of 1 and 5°C (Aguila et al., 2006).
Table 2. Respiration and ethylene production rates and electrolyte leakage during carving step of pumpkin, carrot, radish, cantaloupe and Japanese cucumber into leaf shapes.
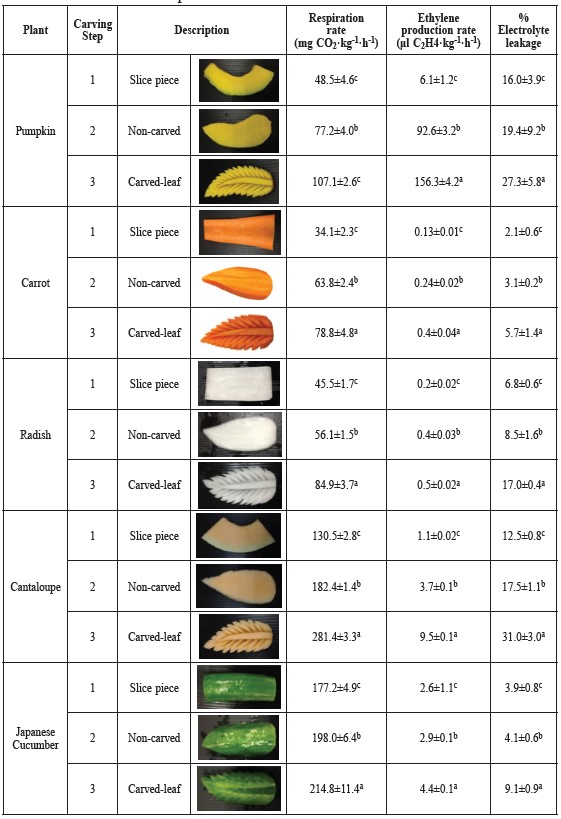
Note: Data expressed as mean of n=4. Values in each column with distinct lower case letters represent the significantly different results (p ≤ 0.05).
Carving pumpkin into flower shapes resulted in an increase in percentage electrolyte leakage. Carving the cut wedged-shape into a semi-circle shape resulted in only a small change in percent electrolyte leakage. Picchioni et al. (1994) reported that calcium chloride treatment at 45 and 90 mM decreased the ion leakage from shredded-carrot during 5 days storage at 10°C. Calcium pre-treatments might increase membrane integrity and thus decrease the rate of senescence of cut and stored carrot tissues. Moreover, the application of high-pressure carbon dioxide (HPCD) treatment might also improve the damaged cell membranes as indicated by relative electrolyte leakage and hardness in fresh-cut carrot slices (Bi et al., 2011).
Physiological changes caused by cutting have been reported in several different types of fruits and vegetables, including cut kiwifruit (Mao et al., 2007), cut melon (Ergun et al., 2007), apple slices (Chung and Moon, 2009), mango slices (Dea et al., 2010), cut broccoli (Fan and Sokorai, 2005) and cut lettuce (Martínez-Sánchez, 2011). Percentage electrolyte leakage could thus be used as an indirect measurement of plant cell membrane damage from stress or mechanical injury (Bajji et al., 2002; Nyanjage et al., 1999).
CONCLUSION
Carving induced physiological changes in the respiration rates, ethylene production rates and percentage electrolyte leakage of the fruits and vegetables in this study. Carving induced the highest physiological response in cantaloupe, Japanese cucumber and pumpkin and a lower response in carrot and radish. This is consistent with natural plant cell respiration with fruit > stem > root. They tended to deteriorate quickly, leading to a short shelf life. For further study, the development of methods to minimize the physiological changes that occur in carved plants and to improve product quality using calcium salts and chitosan coating will be conducted, in order to extend shelf life and better maintain product quality.
ACKNOWLEDGEMENTS
The financial support for this research was provided by National Research Universities, Office of the Higher Education Commission. The authors thank Postharvest Technology Research Institute, Chiang Mai University for providing instruments.
REFERENCES
Agar, I.T., R. Massantini, B. Hess-Pierce, and A.A. Kader. 1999. Post harvest CO2 and ethylene production and quality maintenance of fresh-cut kiwifruit slices. Journal of Food Science 64 (3): 433-440. 10.1111/j.1365-2621.1999.tb15058.X
Aguila, J.S., F. F. Sasaki, L. S. Heiffig, E. M. M. Ortega, A. P. Jacomino, and R. A. Kluge. 2006. Fresh-cut radish using different cut types and storage temperatures. Postharvest Biology and Technology 40: 149-154. 10.1016/j.postharvbio.2005.12.010
Bajji, M., J. Kinet, and S. Lutts, 2002. The use of the electrolyte method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth. Regul 36: 61-70. 10.1023/A:1014732714549
Barry-Ryan, C., and D. O’Beirne, 2000. Effect of peeling methods on the quality of ready-to-use carrot slices. International Journal of Food Science and Technology. 35 (2): 243-254. 10.1046/j.1365-2621.2000.00335.X
Baskaran, R. , R. Prasad, K. M. Shivaiah, and Habibunnisa. 2001. Storage behaviour of minimally processed pumpkin (Cucurbita maxima) under modified atmosphere packaging conditions. European Food Research and Technology. 212 :165-169. 10.1007/S002170000211
Bi, X., J. Wu, Y. Zhang, Z. Xu, and X. Liao, 2011. High pressure carbon dioxide treatment for fresh-cut carrot slices. Innovative Food Science & Emerging Technologies 12 (3): 298-304. 10.1016/j.ifset.2011.04.001
Chung, H.S., and Moon, K.D. 2009. Browning characteristics of fresh-cut ‘Tsugaru’ apples as affected by pre-slicing storage atmospheres. Food Chemistry. 114: 1433-1437. 10.1016/j.foodchem.2008.11.027
Dea, S., J. K. Brecht, M. C. N. Nunes, and E. A. Baldwin, 2011. Quality of freshcut ‘Kent’ mango slices prepared from hot water or non-hot water-treated fruit. Postharvest Biology and Technology 56: 171-180.
Ergun, M., J. Jeong, D. J. Huber, and D. J. Cantliffe. 2007. Physiology of freshcut ‘Galia’ (Cucumis melo var. reticulatus) from ripe fruit treated with 1-methylcyclopropene. Postharvest Biology and Technology 44(3): 286-292. 10.1016/j.postharvbio.2006.08.019
Fan, X., and K.J.B. Sokorai. 2005. Assessment of radiation sensitivity of fresh-cut vegetable using electrolyte leakage measurement. Postharvest Biology and Technology 36: 191-197. 10.1016/j.postharvbio.2004.12.004
Fine Art Department. 1971. “Tao Sri Chulalak” or “Nang Nopamas”. Assumption Publishing. Bangkok, p. 97-98.
Haggar, P.E., D.S. Lee, and K.L. Yam. 1992. Application of an enzyme kinetics based respiration model to closed system experiments for fresh produce. Journal of. Food Processes. Engineering. 15:143-157.
Izumi, H., A. E. Watada, N. P. Ko, and W. Douglas. 1996. Controlled atmosphere storage of carrot slices, sticks and shreds. Postharvest Biology and Technology 9: 165-172. 10.1016/S0925-5214(96)00045-2
Mao, L., G. Wang, and F. Que. 2007. Application of 1-methylcyclopropene prior to cutting reduces wound responses and maintains quality in cut kiwifruit. Journal of Food Engineering. 78: 361-365. 10.1016/j.jfoodeng.2005.10.004
Martínez-Sánchez, A., J.A. Tudela, C. Luna, A. Allende, and M.I. Gil. 2010. Low oxygen levels and light exposure affect quality of fresh-cut Romaine lettuce. Postharvest Biology and Technology 59 (1): 34-42. 10.1016/j.postharvbio.2010.07.005
Nyanjage, M.O., H. Wainwright, and C.F.H. Bishop. 1999. Effects of hot-water treatment and storage temperature on electrolyte leakage of mangoes (Mangifera
indica Linn.). Journal of Horticultural Science and Biotechnology 74: 566-572.
Picchioni, G.A., A.E. Watada, S.Roy, B.D. Whitaker, and W.P. Wergin. 1994. Membrane lipid metabolism, cell permeability, and ultrastructural changes in lightly processed carrots. Journal of Food Science 59 (3): 597-601. 10.1111/j.1365-2621.1994.tb05571.X
Siam Carving Academy [Internet] The history of fruit carving; 2011 [cited 2013 May 31]. Available from: http://www.siamcarvingacademy.com/the-historyof-fruit-carving.
Soliva-Fortuny, R.C., and O. Martin-Belloso. 2003. New advances in extending the shelf-life of fresh-cut fruits: A review. Trends in Food Science and Technology 14: 341-353. 10.1016/S0924-2244(03)00054-2
Suwannaruk, J. 2004. Vegetable fruit carving and banana-leaf arts. Odiean Store Publishing, Bangkok. p.112.
Toivonen, P.M.A., and D.A. Brummell. 2008. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetable. Postharvest Biology and Technology 48: 1-14. 10.1016/j.postharvbio.2007.09.004
Zagory, D. 1999. Effects of post-processing handling and packaging on microbial populations. Postharvest Biology and Technology 15: 313-321. 10.1016/S0925-5214(98)00093-3
Jomkhwun Suwannarak1, Putkrong Phanumong2, Nithiya Rattanapanone2,3*
1 Faculty of Home Economices Technology, Rajamangala University of Technology Phra-Nakhon, Bangkok 10300, Thailand
2 Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
3 Postharvest Technology Research Institute, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: agfsi001@gmail.com
Total Article Views

