
Influences of Cultivation Conditions on Microbial Profiles of Pacific White Shrimp (Litopenaeus vannamei) Harvested from Eastern and Central Thailand
Matichon Lokkhumlue and Cheunjit Prakitchaiwattana*Published Date : 2019-08-27
DOI : 10.12982/cmujns.2014.0022
Journal Issues : Number 1, January-april 2014
ABSTRACT
This study investigated influences of cultivation conditions on microbial profiles of Pacific White Shrimp (Litopenaeus vannamei) from three different cultivation environments: location, season and cultivation system (sanitation control farm and semi-natural farm). The first shrimp samples were collected from a sanitation control farm in Rayong (Eastern Thailand) in January 2011 (S1). Average temperature of water in the ponds was 27.1±0.2 °C with salinity 22.8±2.5 ‰ and pH 8.50±0.16. After harvesting, the samples were immediately shocked by ice before subjecting to microbiological analysis. Under these conditions, total plate counts (TPCs) of shrimps were generally below 4.00 Log CFU/g. Vibrio spp. was observed in 25 g of all samples. Vibrio parahaemolyticus was observed in terms of most probable number (MPN) values ranging from 3.6 to 11 MPN/g. The second shrimp samples were collected from the same farm in June 2011 (S2). Average temperature and salinity of waters were significantly higher than S1 (30.6±2.1°C and 30.4±0.4 ‰, respectively), whereas pH value was not significantly different (8.37±0.17). The TPCs and occurrence of Vibrio spp. of this batch were also similar to S1, whereas MPN values of V. parahaemolyticus were significantly higher (240 to >1100 MPN/g). The third shrimp samples were collected from a semi-natural farm in Samut Songkram (Central Thailand) in August 2011 (S3). In this farm, salinity was dramatically lower than the sanitation control farms (0.6±0.5 ‰), whereas the temperature and pH value were relatively similar (29.1±0.3°C and 8.27±0.27, respectively). TPCs observed were significantly higher than the control system farms (5.14 to 5.86 Log CFU/g). Vibrio spp. was detected in all samples. Interestingly, MPN values of Vibrio parahaemolyticus were significantly lower than the first two samples (<0.3 to 3.6). According to the results, microbial load on shrimp could be influenced by salinity and farming operation systems. The V. parahaemolyticus populations correlated positively with increasing temperature. In addition, salinity increase seems to be a key factor influencing contamination levels of V. parahaemolyticus on shrimps.
Keywords: Vibrio Parahaemolyticus, Salinity, Temperature, Shrimp, Cultivation
INTRODUCTION
In Thailand, the Pacific White Shrimp (Litopenaeus vannamei) is an important aquaculture product that is exported to several countries, including the United States, Japan, Canada, European Union, Australia and Korea (OAE, 2010). The average annual production was 153,737 tons and average annual export income was over THB 34,000 million, respectively, over the 5-year period from 2006 to 2011 (OAE, 2011). Cultivation farms are mainly located in coastal areas and estuaries bordering the Gulf of Thailand and Andaman Sea, including Rayong and Samut Songkhram provinces (OAE, 2011).
The Genus Vibrio is a gram-negative curved rod and facultative anaerobic bacteria that are prevalent in estuaries and marine environments (Jakšic et al., 2002; Hosseini et al., 2004; Thongchankaew et al., 2011). Some Vibrio spp. are halophilic bacteria (tolerance up to 10% NaCl), with sodium ions stimulating growth. The pH and temperature conditions for their growth range from 8 to 8.80 and 20 to 37°C, respectively (Bhunia, 2008). Several Vibrio spp., including Vibrio parahaemolyticus, are reported pathogens, mainly found in seafood imported from Asian countries (Wong et al., 1999; Nishibuchi, 2003). V. parahaemolyticus infection from consuming raw and undercooked seafood causes acute gastroenteritis (Nolan et al., 1984; Lozano-León et al., 2003; Harth et al., 2009).
Vibrio spp. and V. parahaemolyticus in seafood are most common in summer, followed by spring, autumn and winter, respectively (Zarei et al., 2012). Environmental conditions – including temperature, pH, dissolved oxygen, turbidity and salinity – affect microbial diversity and population in sediment and seawater (Parveen et al., 2008). In particular, warm temperatures (above 20°C) were found to be a primary factor that increased the prevalence of Vibrio spp. (Maeda et al., 2003; Thompson et al., 2004). The combination of high temperature and salinity was reported as an important factor to support growth of the bacteria, particularly Vibrio spp. and V. parahaemolyticus (DePaola et al., 2003; del Refugio Castañeda Chávez et al., 2005; Parveen et al., 2008; Thongchankaew et al., 2011).
The microbiological quality of seafood products for export must meet the the standards of exporting countries. Contamination levels of V. parahaemolyticus in frozen and chilled crustacean products for export to the United States (fresh- raw: for consumption without further cooking), Australia and New Zealand (cookedready to eat: for consumption without further cooking) and the EU (cooked-ready to eat: for consumption without further cooking) must be less than 104, 103 and 3 MPN/g, respectively. For export to Japan (fresh-raw: for consumption without further cooking), China (fresh: cooked before consumption) and Korea (cooked: food that is consumed without cooking), V. parahaemolyticus must not be be detected in any 25 grams of product (FIQD, 2011). However, seafood manufacturers, particularly frozen Pacific White Shrimp industries (a top-five export product of Thailand), still experience problems with V. parahaemolyticus contamination. Contamination with this bacteria is difficult to control with levels varying, depending upon where cultivated.
This study therefore aims to investigate the influences of cultivation conditions on the microbiological profile of Pacific White Shrimp (Litopenaeus vannamei) from the main cultivation areas of Eastern and Central Thailand.
MATERIALS AND METHODS
Sampling sites
The first batch of Pacific White Shrimp (Litopenaeus vannamei) samples was collected from five cultivation ponds of a sanitation control farm located in Rayong, Eastern Thailand in January 2011 (S1). In sanitation control farms, personal hygiene and equipment sanitation are controlled and aquaculture chemicals applied. The second batch of samples was collected from the same farm in June 2011 (S2). The third batch of samples was collected from a semi-natural farm located in Samut Songkhram, Central Thailand in August 2011 (S3). This farm used a semi-natural cultivation technique in which only commercial feed was used.
Samples collection and preparation
Approximately 500 g of shrimp were collected from each pond as samples. Shrimp were then placed in a sterile plastic bag, which was immediately shocked by sterile ice, before subjecting to microbiological analysis on location.
Water samples were collected along with the shrimp from the five cultivation ponds. Two hundred and fifty milliliter water samples were collected at approximately 50 cm below the surface of the water and transferred into sterile 250 ml screw cap bottles. The water samples were transported to a laboratory for further physical analysis.
Microbiological analysis of shrimp
The total plate counts (TPCs) and Vibrio spp. was determined by following the methods of the Bacteriological Analytical Manual (USFDA, 2004).
Vibrio parahaemolyticus was determined by use of the most probable number method (MPN) with partial modification from the Bacteriological Analytical Manual (USFDA, 2004). Twenty-five grams of samples were placed in stomacher bag with 225 ml of alkaline peptone water (APW) (Himedia, India) then shaken for 30 s. The APW was then serially diluted and incubated at 35±2°C 24 hr before streaking onto Thiosulfate Citrate Bile Sucrose agar (TCBS agar) (Merck, Germany) plate and incubated at 35±2°C 24 hr. The suspect colonies of V. parahaemolyticus were evaluated with screening and confirmation test following the Bacteriological Analytical Manual (USFDA, 2004).
The physical and chemical properties analysis of water
The temperature of pond water at three sites (1 m from the edge of the pond and 50 cm below the surface) was measured with a thermometer. The salinity was also measured on location with a reflecto-salinometer (N.O.W., Japan). The pH of the water was further determined at the laboratory using a pH meter (Cyberscan, USA).
RESULTS
Pond water properties and microbial profiles of shrimp from S1
The conditions of water collected from three different environments are shown in Table 1.
Table 1. Chemical and physical properties of pond water of Pacific White Shrimp cultivation farms located in Rayong determined in January 2011 (S1) and June 2011 (S2) and Samut Songkhram in August 2011 (S3).
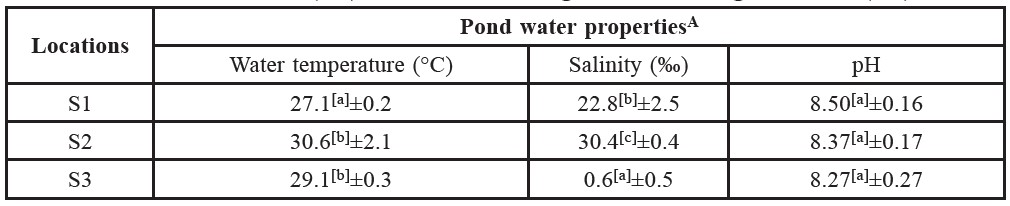
Note: AThe values are means ± SD of three replications. [a], [b], [c] The mean values with significant difference at P≤0.05 in each column are indicated by superscript letter.
The average temperature of S1 water at sampling time was 27.1±0.2°C with salinity 22.8±2.5 ‰ and pH 8.50±0.16. The microbial counts in TPC values are displayed in Table 2. The TPC values of S1 samples ranged from 2.78 to 4.03 Log CFU/g. The detection of Vibrio spp. and the MPN/g values of V. parahaemolyticus are shown in Table 3. Vibrio spp. was observed in every S1 sample. The contamination level of V. parahaemolyticus as indicated by MPN values ranged from 3.6 to 11 MPN/g.
Table 2. Total plate counts (TPCs) of Pacific White Shrimp collected from Rayong in January (S1) and June 2011 (S2) and Samut Songkhram in August 2011 (S3).
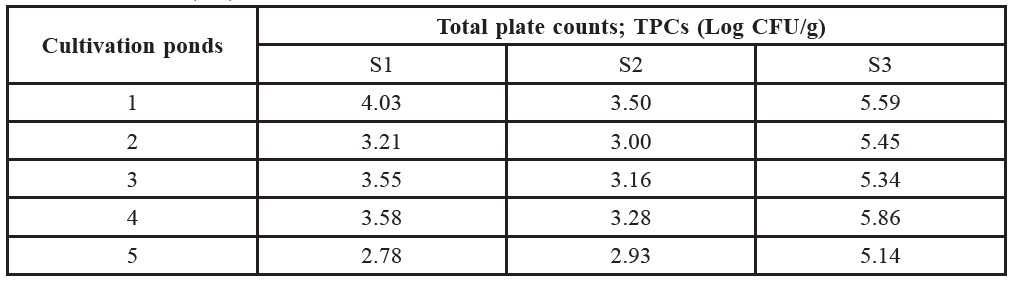
Table 3. The Vibrio spp. and MPN/g values of Vibrio parahaemolyticus of Pacific White Shrimp collected from Rayong in January (S1) and June 2011 (S2) and Samut Songkhram in August 2011 (S3).
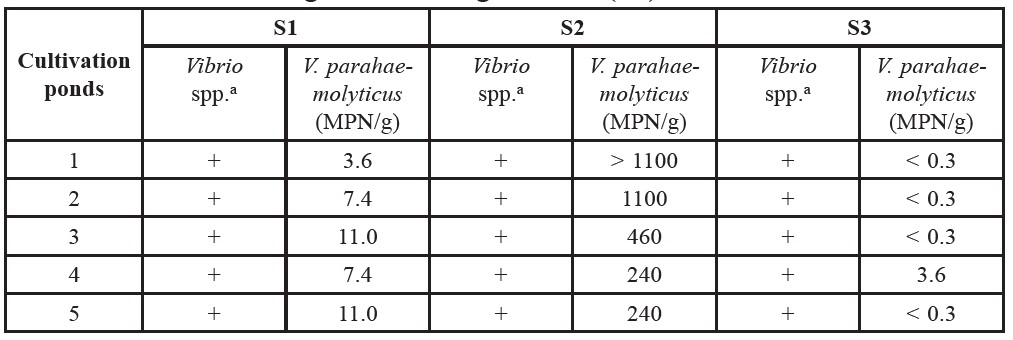
Note: a + = Detected, - = Not detected.
Pond water properties and microbial profiles of shrimp from S2
The average temperature, salinity and pH value of S2 water at sampling time were 30.6±2.1°C, 30.4±0.4‰ and 8.37±0.17, respectively (Table 1). The microbial counts in TPC values of S2 samples ranged from 2.93 to 3.50 Log CFU/g (Table 2). Vibrio spp. was detected in all S2 samples. The contamination
levels of V. parahaemolyticus ranged from 240 to >1100 MPN/g (Table 3).
Pond water properties and microbial profiles of shrimp from S3
The average temperature of S3 water at sampling time was 29.1±0.3°C with salinity 0.6±0.5 ‰ and pH 8.27±0.27. The TPCs values shown in Table 2 ranged from 5.14 to 5.86 Log CFU/g. In Table 3, Vibrio spp. was observed in all S3 samples and the contamination level of V. parahaemolyticus ranged from <0.3 to 3.6 MPN/g.
DISCUSSION
According to the results in Table 1, the significant differences of pond water conditions are noted. These variations, such as temperature and salinity level, are significantly related to seasonal and geographical factors as demonstrated in several previous observations (DePaola et al., 2003; Parveen et al., 2008; Thongchankaew et al., 2011). From the Annual Weather Summary of Thailand in 2011 (TMD,2011), mean area temperature and rainfall in Eastern Thailand in January 2011 (S1 sample collection period) were 26.4°C and 0.0 mm, respectively. In June 2011 (S2 sample collection period), they were 28.4°C and 277.5 mm, respectively. For S3 farm, in August 2011, mean temperature was approximately 28.1°C and rainfall was around 211.8 mm. Interestingly, temperature of shrimp cultivation water of each farm as observed in this study was relatively close to the area temperature. Thus, water temperature of S1 collected in winter was accordingly lower (27.1±0.2°C) than S2 and S3 (30.6±2.1 and 29.1±0.3, respectively). In the same way, salinity levels of pond water from different parts of Thailand were also notably different, which could be affected by geographical factors. Such factors can relate to particular physical and chemical conditions of the soil and water in the ponds. Consequently, these different conditions could affect the occurrence of microbes in the shrimp cultivation ecosystems (DePaola et al., 2003; Maeda et al., 2003; Parveen et al., 2008; Thongchankaew et al., 2011).
As observed in this study (Tables 2 and 3), the TPC occurrence of Vibrio spp. and V. parahaemolyticus in shrimp from three different conditions were relatively different. In particular, the contamination levels (MPN/g) of V. parahaemolyticus in shrimps were found to be significantly different in samples from Rayong (S1 and S2) and Samut Songkhram (S3). This could be an effect of the high salinity of the pond water. Based on the nature of V. parahaemolyticus, this microorganism needs salt (NaCl) for growth (Bhunia, 2008). Salt concentrations of 0.5 to 8% stimulates the growth of V. parahaemolyticus, with an optimum concentration of approximately 3% (Lake et al., 2003). As shown in Table 1, the salt concentration of S3 water (0.06%) was lower than the minimum threshold (0.5%) for V. parahaemolyticus growth, whereas the salt levels in S1 and S2 were in an appropriate range (2 and 3%, respectively). In accordance with these salinity values, the MPN levels were significantly higher in S1 and S2 shrimp compared to S3. This observation reflected the significant influence of salinity on the occurrence of V. parahaemolyticus in shrimp.
However, although the salinity values of S1 and S2 waters were relatively similar, the MPN levels of S2 (240 to >1,100 MPN/g) observed were higher than S1 (3.6 to 11 MPN/g). Other factors, in particular temperature, might also be influencing the occurrence of V. parahaemolyticus in combination with salinity. V. parahaemolyticus is a mesophilic bacteria preferring to grow in temperatures of 10 to 44°C, with an optimum temperature of 37°C (Nishibuchi, 2003). The temperature of S2 water (30.6°C), as shown in Table 1, was close to the optimum temperature. At this temperature, V. parahaemolyticus would grow well, and consequently were more prevalent in S2 shrimp. This observation also corresponds with previous reports, which demonstrated that the occurrence of V. parahaemolyticus tended to increase at temperatures close to 30°C (DePaola et al., 2003; Parveen et al., 2008).
In this study, Vibrio spp. was found in all shrimp samples. The occurrence of these bacteria do not appear to be related to any cultivation condition, since Vibrios are marine bacteria which occur naturally in the estuaries, coasts and marine environments. Thus, they are commonly found in seafood, especially in shrimp, regardless of season (Zarei et al., 2012).
Total plate count (TPC) is an important criterion for indicating microbiological quality of seafood. The International Commission on Microbiological Specifications for Foods (ICMSF) recommends that the TPC value of frozen raw crustaceans should be not over 106 CFU per gram (ICMSF, 1986). From the results in Table 2, TPCs of shrimp cultivated under different conditions also differed. The TPCs of shrimp cultivated in higher salinity water (S1 and S2) were lower than in lower salinity water (S3). In this case, salt could adversely affect microorganisms growing in pond ecosystems (Hotchkiss, 1923).
However, as found in this study, farming operation systems seemed to be another influential factor. According to the results, the TPCs of shrimps from the sanitation control farm were significantly lower than the semi-natural farm (Table 2). This demonstrated that sanitation controls and/or the addition of aquaculture chemicals in the sanitation control farm helped reduce microbial contamination. Consequently, shrimp from the sanitation control farm showed relatively better microbiological quality in terms of TPC level.
ACKNOWLEDGEMENTS
We appreciate the assistance of Graduate School, Chulalongkron University, The Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (FW1015B) and National Research Council of Thailand (Graduate research scholarship 2012) for financial support.
REFERENCES
Bhunia, A.K. 2008. Foodborn microbial pathogens: mechanisms and pathogenesis. West Lafayette, Indiana: Springer. p. 241-252. 10.1007/978-0-387-74537-4
del Refugio Castañeda Chávez, M., V.P. Sedas, E. Orrantia Borunda, and F.L. Reynoso. 2005. Influence of water temperature and salinity on seasonal occurrences of Vibrio cholerae and enteric bacteria in oyster-producing areas of Veracruz, México. Marine Pollution Bulletin 50: 1641-1648. 10.1016/j.marpolbul.2005.06.036
DePaola, A., J.L. Nordstrom, J.C. Bowers, J.G. Wells, and D.W. Cook. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Applied and Environmental Microbiology 69: 1521-1526. 10.1128/AEM.69.3.1521-1526.2003
FIQD. 2011. Standard of Microbiological Quality. Fish Inspection and Quality Control Division Department of Fisheries (FIQD), Thailand.
Harth, E., L. Matsuda, C. Hernández, M.L. Rioseco, J. Romero, N. González-Escalona, J. Martínez-Urtaza, and R.T. Espejo. 2009. Epidemiology of Vibrio parahaemolyticus Outbreaks, Southern Chile. Emerging Infactious Diseases 15: 163-168. 10.3201/eid1502.071269
Hosseini, H., A.M. Cheraghali, R. Yalfani, and V. Razavilar. 2004. Incidence of Vibrio spp. in shrimp caught off the south coast of Iran. Food Control 15: 187-190. 10.1016/S0956-7135(03)00045-8
Hotchkiss, M. 1923. Studies on salt action vi. the stimulating and inhibiting effect of certain cations upon bacterial growth. Journal of Bacteriology 8: 141-162.
ICMSF. 1986. Sampling for microbiological analysis: Principles and specific application. Toronto: International Commission on Microbiological Specifications for Foods (ICMSF).
Jakšic, S., S. Uhitil, T. Petrak, D. Bazulic, and L. Gumhalter Karolyi. 2002. Occurrence of Vibrio spp. in sea fish, shrimps and bivalve molluscs harvested from Adriatic sea. Food Control 13: 491-493. 10.1016/S0956-7135(02)00027-0
Lake, R., A. Hudson, and P. Cressey. 2003. Risk profile: Vibrio parahaemolyticus in seafood. Christchurch: Institute of Environmental Science & Research Limited.
Lozano-León, A., J. Torres, C.R. Osorio, and J. Martıínez-Urtaza. 2003. Identification of tdh-positive Vibrio parahaemolyticus from an outbreak associated with raw oyster consumption in Spain. FEMS Microbiology Letters 226: 281-284. 10.1016/S0378-1097(03)0064-9
Maeda, T., Y. Matsuo, M. Furushita, and T. Shiba. 2003. Seasonal dynamics in coastal Vibrio community examined by a rapid clustering method based on 16S rDNA. Fisheries Science 69: 385-394. 10.1046/j.1444-2906.2003.00633.X
Nishibuchi, M. 2003. Vibrio parahaemolyticus. In: Miliotis M.D. and Bier J.W., editors. International Handbook of Foodborne Pathogens. New York: CRC Press.
Nolan, C.M., J. Ballard, C.A. Kaysner, J.L. Lilja, L.P. Williams Jr, and F.C. Tenover. 1984. Vibrio parahaemolyticus gastroenteritis: An outbreak associated with raw oysters in the pacific northwest. Diagnostic Microbiology and Infectious Disease 2: 119-128.
OAE. 2010. Basis of Thai Agricultural Economics 2010. Bangkok: Office of Agricultural Economics Thailand (OAE), Thailand.
OAE. 2011. Agricultural Statistic 2011 for Export: Shrimp. Office of Agricultural Economics Thailand (OAE), Thailand.
Parveen, S., K.A. Hettiarachchi, J.C. Bowers, J.L. Jones, M.L. Tamplin, R. McKay, W. Beatty, K. Brohawn, L.V. DaSilva, and A. DePaola. 2008. Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in Chesapeake Bay oysters and waters. International Journal of Food Microbiology 128: 354-361. 10.1016/j.ijfoodmicro.2008.09.019
Thompson, J.R., M.A. Randa, L.A. Marcelino, A. Tomita-Mitchell, E. Lim, and M.F. Polz. 2004. Diversity and dynamics of a north atlantic coastal Vibrio community. Applied and Environmental Microbiology 70: 4103-4110. 10.1128/AEM.70.7.4103-4110.2004
Thongchankaew, U., P. Sukhumungoon, P. Mitraparp-arthorn, K. Srinitiwarawong, S. Plathong, and V. Vuddhakul. 2011. Diversity of Vibrio spp. at the Andaman Tarutao Island, Thailand. Asian Journal of Biotechnology 3: 530-539. 10.3923/ajbkr.2011.053.539
TMD. 2011. Annual Weather Summary of Thailand in 2011. Thai Meteorology Department (TMD).
USFDA. 2004. Bacteriological Analytical Manual Online [Internet]. US Food and Drug Administration (USFDA); [cited 2010 December 15]. Available from: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/default.htm
Wong, H.C., M.C. Chen, S.H. Liu, and D.P. Liu., 1999. Incidence of highly genetically diversified Vibrio parahaemolyticus in seafood imported from Asian countries. International Journal of Food Microbiology 52: 181-188. 10.1016/S0168-1605(99)00143-9
Zarei, M., M.P. Borujeni, A. Jamnejad, and M. Khezrzadeh. 2012. Seasonal prevalence of Vibrio species in retail shrimps with an emphasis on Vibrio parahaemolyticus. Food Control 25: 107-109. 10.1016/j.foodcont.2011.10.024
Matichon Lokkhumlue and Cheunjit Prakitchaiwattana*
Department of Food Technology, Faculty of Science, Chulalongkorn University, Bangkok 10300, Thailand
*Corresponding author. E-mail: pcheunjit@chula.ac.th
Total Article Views

