
Effect of Physical Therapy Training on Gait Initiation in Patients with Moderate Parkinson's Disease
Fuengfa Khobkhun, Sunee Bovonsunthonchai*,Roongtiwa Vachalathiti and Apichart PisarnpongPublished Date : 2019-08-24
DOI : 10.12982/cmujns.2014.0019
Journal Issues : Number 1, January-april 2014
ABSTRACT
The present study investigated the effects of physical therapy treatment on gait initiation in patients with Parkinson’s disease (PD). Thirteen patients with PD were randomized into a treatment (n = 7) and control (n = 6) group. Participants were assessed for their severity level, using the Modified Hoehn and Yahr scale, and motor evaluation, using the Unified Parkinson’s Disease Rating Scale (UPDRS), items III and IV. At pre- and post-assessments, gait initiation was assessed using a gait mat, synchronized with a video camera. The treatment group received a physical therapy training program based on the TrainingBIG™ technique and task-specific concepts, three times per week for four weeks. The control group received no physical therapy treatment. From analysis of the pre- and post-assessment variables, only the treatment group showed a significant decrease in preparatory phase time (p = 0.043) and increase in step length (p = 0.018). In addition, the treatment group had a significant increase in step length (p = .022) at post-assessment when compared to the control group. The present findings demonstrated that physical therapy treatment would be beneficial for patients with PD experiencing gait initiation problems.
Keywords: Parkinson’s disease, Physical therapy, Gait initiation
INTRODUCTION
Parkinson’s disease (PD) is a movement disorder disease, commonly found in the elderly (Guttman et al., 2003). Four clinical features of signs and symptoms are resting tremor, rigidity, bradykinesia and postural instability (Jankovic and Tolosa, 2007). In addition, patients with PD may have abnormalities in speaking, writing, facial expression, gait, posture and eyeball movement as well as in the autonomic nervous system and mental and emotional states (Jankovic and Tolosa, 2007). A previous study described postural control and balance impairment in patients with PD as a deficit in equilibrium response that affects the abilities of movement initiation, termination and turning (Morris, 2000). Gait initiation, the transitional state between standing and walking, is one of the functional tasks involved in locomotor performance. It is a common skill for changing direction, requiring postural control and balance between static and dynamic movements (Hass et al., 2005). Previous findings suggested that the anticipatory postural adjustment deficit led to delay in stepping initiation (Halliday et al., 1998; Hass et al., 2005). Gait in patients with PD are characterized by an increase in cadence but a decrease in step length, step width and gait velocity (Halliday et al., 1998).
Prior to the discovery of L-dopa therapy, physical therapy played a crucial role in patients with PD, helping to control and alleviate disease symptoms (Morris, 2000). Many studies (Baatile et al., 2000; de Goede et al., 2001; Hirsch et al., 2003) have demonstrated that medication and physical therapy in combination were more fruitful than medication alone in patients with PD. Several treatment techniques and concepts have been used for improving performance in patients with PD (Schenkman et al., 1998; de Goede et al., 2001; Scandalis et al., 2001). The task-specific principle is a popular technique frequently used for improving individual performance in patients with PD and other neurological conditions (Schenkman et al., 1998; Scandalis et al., 2001; Sidaway et al., 2006). A novel technique, TrainingBIG™ provides standardized behavioral intervention for motor system recovery in patients with PD (Farley and Koshland, 2005). This training is based on multiple repetitions, intensity and complexity. The cornerstone of the principle is derived from a speech therapy treatment – the Lee Silverman Voice Treatment™ (LSVT™) (Farley and Koshland, 2005).
However, evidence for the efficacy of physical therapy treatment on gait initiation in patients with PD remains limited. The present study therefore investigated the effects of physical therapy treatment on gait initiation characteristics in patients with PD based on the TrainingBIG™ technique and task-specific concepts.
MATERIALS AND METHODS
Participants
Thirteen participants diagnosed with idiopathic PD voluntarily participated in the study. They were recruited from the Movement Disorder Clinic, Siriraj Hospital, Bangkok, Thailand. The selected participants met the following studyinclusion criteria:
• Taking PD medication for at least one month, with no signs of the wearing-off phenomena.
• Not clinically diagnosed with dementia or musculoskeletal conditions that might interfere with test performance.
• Modified Hoehn and Yahr Stages scores of 2.5.
• Able to walk independently without using an assistive device and able to follow commands and instructions.
In addition, during the study period, the participants did not attend any other exercise programs.
Participants were randomly allocated into a treatment (n = 7) and control (n = 6) group. Six males and one female participated in the treatment group and five males and one female participated in the control group. The mean age of the treatment and control groups was 67.7 ± 5.0 years and 68.8 ± 5.5 years, respectively. PD onset of both groups was similar (5.3 ± 2.1 years for the treatment group and 4.7 ± 4.0 years for the control group). The UPDRS score items III and IV in the treatment and the control groups were 21.4 ± 10.6 scores and 19.0 ± 3.2 scores, respectively. Average number of freezing during walking over the one-week period prior to participating in the study for the treatment and the control groups was 2.6 ± 0.8 times/day and 2.3 ± 0.8 times/day, respectively. The Ethical Committee of the University Institutional Review Board at Mahidol University approved this study.
Gait characteristics were tested before training and after the 4-week treatment period, for both the treatment and control groups. For gait initiation assessment, participants stood at approximately the middle part of the walkway (The Zebris Force Distribution Measurement-System-Gait Analysis), synchronized with a video camera. The video camera was placed perpendicular to the right side of participants and a light signal was placed in front of the participants. Participants initiated their step with the right leg and walked through the end of the walkway with the instruction, “Start walking after seeing the light signal and continue walking to the end of walkway”. Gait initiation time and step length were averaged from three walking trials.
The treatment group received an exercise program based on the Training-BIG™ technique and task-specific concepts three times per week for four weeks with a physical therapist. The exercise protocol was composed of: a breathing exercise and chest mobilization; a stretching exercise; and training of alternative arm swing, balance, gait initiation and walking. Participants performed the exercises in the same series and at approximately the same time of day. Both groups continued their medical treatment with a neurological specialist.
Statistical analysis
Given the small number of patients with PD meeting the inclusion criteria, the Mann-Whitney U test was used to compare the variables between the treatment and control groups and the Wilcoxon Signed-Rank test was used to compare the variables between pre- and post-assessments. All data were analyzed using SPSS version 18.0, S/N 5082368 NY, US. The statistical significance level was set at p-value less than 0.05.
RESULTS
Within group comparison
Table 1 shows the comparisons of gait initiation times (preparatory phase time and stepping phase time) and step length between pre- and post-assessments in the treatment and control groups. Significant differences of the preparatory phase time and step length were found between pre- and post-assessments in the treatment group (p = 0.043 and p = 0.018, respectively), but not in the control group (p = 0.345 and p = 0.249, respectively). There was no significant difference of the stepping phase time between pre- and post-assessments in either the treatment group (p = 1.000) or the control group (p = 0.917).
Table 1. Comparisons of gait initiation times and step length between pre- and post-assessments in the treatment and the control groups.
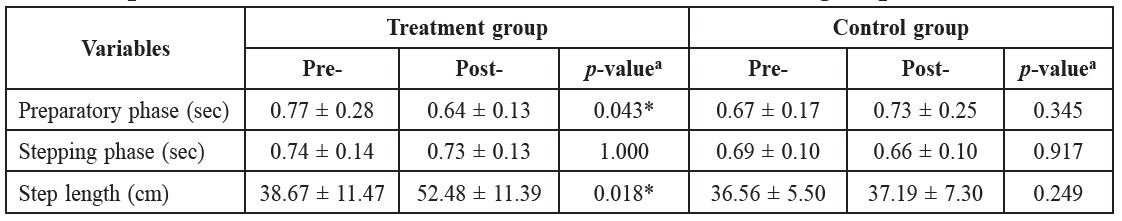
Note: a = p-value Wilcoxon Signed-Rank test. * = significant difference (p-value < 0.05).
Between groups comparison
Table 2 shows the comparisons of gait initiation times (preparatory phase and stepping phase times) and step length between the treatment and the control groups at pre- and post-assessments. At pre-assessment, no significant differences of the gait initiation time and step length were found between the treatment and control groups. At post-assessment, only the step length demonstrated significant difference (p = 0.022) between the treatment and control groups.
Table 2. Comparisons of gait initiation times and step length between the treatment and the control groups at pre- and post-assessments.
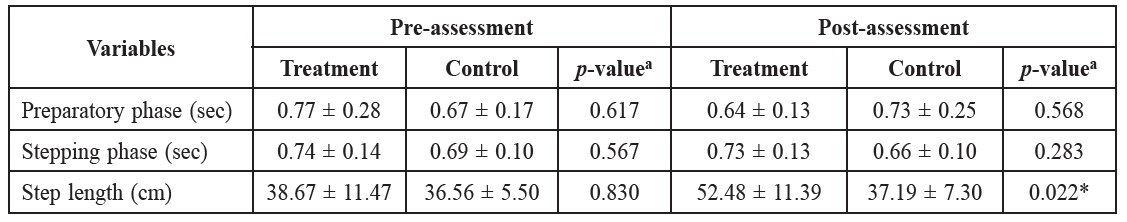
Note: a = p-value Mann-Whitney U test. * = significant difference (p-value < 0.05).
DISCUSSION
Significant improvement of the preparatory phase time was found only in the treatment group at post-assessment. Several factors may explain this finding. First, the stretching exercises that included the upper trunk, lower trunk, hip flexors and ankle plantarflexors may facilitate the changes of strategy during gait initiation. This is supported by a previous study (Kerrigan et al., 2003) that found that stretching of the hip flexors and plantarflexors improved walking speed and suggested that stretching of the hip flexors might improve muscle flexibility and balance in the elderly. In addition, stretching exercises may reduce freezing of the gait, resulting in improved gait initiation time (Schenkman et al., 1998; Morris, 2000). Second, the TrainingBIG™ concept used in the study may assist the patients to correct their movement pattern through new programming in the brain, improving movement calibration and developing neural plasticity (Farley and Koshland, 2005; Farley et al., 2008). Third, gait initiation recognition could be improved by task specificity, which was trained using auditory cues. This specific training resulted in patients learning and memorizing their movements (Shumway-Cook and Woollacott, 2001; Ford et al., 2007).
Unlike the preparatory phase time development, the treatment group demonstrated no improvement of the stepping phase time after training. This may come from different regulatory mechanisms in the brain. The preparatory phase time involves anticipatory postural adjustment (Hass et al., 2005; Rocchi et al., 2006) and postural correction during movement, which requires controlling the center of gravity within the base of support (Henriksson and Hirschfeld, 2005; Rocchi et al., 2006). In contrast, the stepping phase time or the executive movement time, occurs after the preparatory period to the end of movement and relies on commands from the brain to maintain the stability accompanying executive movement (Hass et al., 2005; Rocchi et al., 2006). During this period, it is very difficult to control both stability and mobility simultaneously for gait function, especially in patients with PD.
The treatment group increased step length over the control group at post-assessment. This corresponds to previous studies (Morris, 2000; de Goede et al., 2001; Sidaway et al., 2006). Improvement of step length in patients with PD may be due to the visual cues’ effect (Sidaway et al., 2006). Visual cues provide external cues, which stimulate and reprogram movement. The effect of visual cues during gait training demonstrated that it improved step length, gait speed and the joint angles of lower extremity (Sidaway et al., 2006). Moreover, the improvement in step length may result from the TrainingBIG™ concept, which assisted in the quality of movement by increasing movement amplitude. The patients with PD were trained to focus on movement bigness and learned how to use more effort to initiate their gait (Farley and Koshland, 2005). In addition, stretching exercises for the trunk and lower extremity muscles may assist the muscles to prompt and increase flexibility, acting as a strategy to generate longer step length during gait initiation (Schenkman et al., 2000; Kerrigan et al., 2003).
CONCLUSION
This study found improved gait initiation after physical therapy treatment in patients with PD. Thus, the exercise program is recommended for patients with PD to increase their gait initiation performance and may assist in the prevention of freezing characteristics and the risk of falling. The exercise program was designed so that the patients, with the help of relatives, could continue the program on their own. Continuous exercise, specific physical therapy treatment and other medical treatment remain critical components in the care of patients with PD.
Assessment of the treatment effect in the follow-up period and collecting data in other severity stages of the PD population should be carried out in future research.
ACKNOWLEDGEMENTS
This study is partially supported by the Graduate Studies of Mahidol University Alumni Association and the Faculty of Physical Therapy, Mahidol University.
REFERENCES
Baatile, J., W.E. Langbein, F. Weaver, C. Maloney, and M.B. Jost. 2000. Effect of exercise on perceived quality of life of individuals with Parkinson’s disease. Journal of Rehabilitation Research and Development 37(5): 529-534.
de Goede, C.J.T., S.H.J. Keus, G. Kwakkel, and R.C. Wagenaar. 2001. The effects of physical therapy in Parkinson's disease: a research synthesis. Archives of Physical Medicine and Rehabilitation 82(4): 509-515. 10.1053/apmr.2001.22352
Farley, B.F., and G.F. Koshland. 2005. Training BIG to move faster: the application of the speed- amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Experimental Brain Research 167(3): 462-467.
Farley, B.G., C.M. Fox, L.O. Ramig, and D. McFarland. 2008. Intensive amplitude specific therapeutic approaches for Parkinson’s disease: Toward a neuroplasticity-principled rehabilitation model. Topics in Geriatric Rehabilitation 24(2): 99-114. 10.1097/01.TGR.0000318898.87690.0d
Ford, M.P., R.C. Wagenaar, and K.M. Newell. 2007. The effects of auditory rhythms and instruction on walking patterns in individuals post stroke. Gait and Posture 26(1): 150-155. 10.1016/j.gaitpost.2006.08.007
Guttman, M., S. Kish, and Y. Furukawa. 2003. Current concepts in the diagnosis and management of Parkinson’s disease. Canadian Medical Association Journal 168(3): 293-301.
Halliday, S.E., D.A. Winter, J.S. Frank, A.E. Patla, and F. Prince. 1998. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait and Posture 8(1): 8-14. 10.1016/S0966-6362(98)00020-4
Hass, C.J., D.E. Waddell, R.P. Fleming, J.L. Juncos, and R.J. Gregor. 2005. Gait initiation and dynamic balance control in Parkinson’s disease. Archives of Physical Medicine and Rehabilitation 86(11): 2172-2176. 10.1016/j.apmr.2005.05.013
Henriksson, M., and H. Hirschfeld. 2005. Physically active older adults display alterations in gait initiation. Gait and Posture 21(3): 289-296. 10.1016/j.gaitpost.2004.03.001
Hirsch, M.A., T. Toole, C.G. Maitland, and R.A. Rider. 2003. The effect of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Archives of Physical Medicine and Rehabilitation 84(8): 1109-1117. 10.1016/S0003-9993(03)00046-7
Jankovic, J., and E. Tolosa. (2007). Parkinson’s disease and movement disorders. Philadelphia: Lippincott Williams and Wilkins. 10.1136/jnnp.2007.131045
Kerrigan, D.C., A. Xenopoulos-Oddsson, M.J. Sullivan, J.J. Lelas, and P.O. Riley. 2003. Effect of a hip flexor–stretching program on gait in the elderly. Archives of Physical Medicine and Rehabilitation 84(1): 1-6. 10.1053/apmr.2003.50056
Morris, M.E. 2000. Movement disorders in people with Parkinson’s disease: a model for physical therapy. Physical Therapy 80(6): 578-597.
Rocchi, L., L. Chiari, M. Mancini, P. Carlson-Kuhta, A. Gross, and F.B. Horak. 2006. Step initiation in Parkinson's disease: influence of initial stance condition. Neuroscience Letters 406(1-2): 128-132. 10.1016/j.neulet.2006.07.027
Scandalis, T.A., A. Bosak, J.C. Berliner, L.L. Helman, and M.R. Wells. 2001. Resistance training and gait function in patients with Parkinson’s disease. American Journal of Physical Medicine and Rehabilitation 80(1): 38-43. 10.1097/00002060-200101000-00011
Schenkman, M., T.M. Cutson, M. Kuchibhatla, J. Chandler, C.F. Pieper, L. Ray, and K.C. Laub. 1998. Exercise to improve spinal flexibility and function for people with Parkinson’s disease: a randomized, controlled trial. Journal of the American Geriatrics Society 46(10): 1207-1216.
Schenkman, M., M. Morey, and M. Kuchibhatla. 2000. Spinal flexibility and balance control among community-dwelling adults with and without Parkinson’s disease. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 55A(8): 441-445.
Shumway-Cook, A., and M.H. Woollacott. (2001). Motor control: theory and practical applications. Philadelphia: Williams and Wilkins.
Sidaway, B., J. Anderson, G. Danielson, L. Martin, and G. Smith. 2006. Effects of Long-term gait training using visual cues in an individual with Parkinson disease. Physical Therapy 86(2): 186-194.
Fuengfa Khobkhun1, Sunee Bovonsunthonchai1*, Roongtiwa Vachalathiti1 and Apichart Pisarnpong2
1 Faculty of Physical Therapy, Mahidol University, Nakhon Phatom 73170, Thailand
2 Movement Disorder Clinic, Division of Neurology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand
*Corresponding author. E-mail: sunee.bov@mahidol.ac.th
Total Article Views

