
In Vitro Antioxidant Activity and In Vivo Antidepressant-like Effect in Mice of the Ethanolic Extract from Leaves of Ocimum gratissimum Linn.
Amit Upadhyay* and Deepak BhagwatPublished Date : 2019-08-25
DOI : 10.12982/CMUJNS.2014.0037
Journal Issues : Number 3, September - December 2014
ABSTRACT
This paper investigated the antioxidant activity and antidepressant-like effect of ethanolic leaf extract of Ocimum gratissimum (OGE). In vitro antioxidant activity of OGE was assessed in three complimentary test systems: DPPH (1, 1-Diphenyl-2-picrylhydrazyl), nitric oxide scavenging activity and reducing power assay. The antidepressant-like effect of OGE was investigated in mice using the forced swim test (FST) and tail suspension test (TST). Additionally, the mechanisms involved in the antidepressant-like effect of OGE were investigated using TST in four receptor systems: serotonergic, noradrenergic, dopaminergic and GABAergic. The extract showed potent antioxidant activity in all three in vitro test systems. OGE (100-400 mg/kg, p.o.) decreased the immobility time of mice in both FST and TST in a dose-dependent manner, without accompanying changes in locomotor activity in the digital photoactometer. The anti-immobility effect of the extract (400 mg/kg, p.o.) in the TST was significantly reversed by prazosin (1 mg/kg, i.p., an α1-adrenoceptor antagonist), levosulpiride (50 mg/kg, i.p., a dopamine D2 receptor antagonist) and p-chlorophenylalanine (p-CPA, 100 mg/kg, i.p., an inhibitor of serotonin synthesis) but not by yohimbine (1 mg/kg, i.p., an α2-adrenoceptor antagonist) and baclofen (10 mg/kg, i.p., a GABAB agonist). OGE produced a specific antidepressant-like effect that was dependent on the interaction with serotonergic, noradrenergic (α1-adrenoceptor) and dopaminergic (D2 receptor) systems. The results suggested that O. gratissimum possessed potent antioxidant and antidepressant-like activities and this plant deserved further investigation as an alternative therapeutic tool for treatment of depression.
Keywords: Antidepressant, Antioxidant, Forced swim test, Monoaminergic, Ocimum gratissimum, Tail suspension test
Introduction
Depression is a chronic and potentially life-threatening heterogeneous disorder that manifests with various behavioural, psychological and physiological symptoms, such as depressed mood, feeling of guilt or worthlessness, decreased appetite and libido, insomnia, and recurrent thoughts of suicide (American Psychiatry Association, 1994; DSM-IV, 2003; Posser et al., 2009). According to the World Health Organisation, depression is a medical and social problem that affects about 340 million people worldwide (WHO, 2001). It has a high worldwide prevalence rate, ranging from 2-15%, (Moussavi et al., 2007), and is predicted to be the second leading cause of death by the year 2020 (Rosenzweig-Lipson et al., 2007).
Although a number of antidepressant drugs are available in allopathic medicine, they produce only a partial remission and prolonged utilization may cause potential adverse effects (Guadarrama-Cruz et al., 2008 and Kiss, 2008). Therefore, more effective therapies with fewer or no adverse effects need to be developed. Herbal medicines have been used as an effective alternative treatment of affective disorders for many years and numerous medicinal plants have been investigated for their uses in mental disorders over the past decade (Zhang, 2004). For example, Hypericum perforatum (St John’s wort), Crocus sativus (saffron), Rhodiola rosea (roseroot), Albizia julibrissin (mimosa), Panax ginseng (Korean ginseng), Lavandula supp. (levender), Echium amoenum (borage) and Ginkgo biloba have been proven clinically to possess antidepressant-like effects (Sarris et al., 2011).
Ocimum gratissimum Linn. (Lamiaceae) is an erect, small, aromatic shrub originally from Asia and Africa (Martins et al., 2008). It is popularly known in India as “Ram Tulshi”. The plant is commonly used in folk medicine to treat various diseases, including diarrhoea, upper respiratory tract infections, fever, headache, skin diseases, ophthalmic disorders, pneumonia, cough, conjunctivitis, sunstroke, influenza, gonorrhoea and mental illness (Akinmoladun et al., 2007; Mahapatra et al., 2010). Pharmacological studies carried out on O. gratissimum showed that this plant exerted several biological effects, including antibacterial (Nakamura et al., 1999), antidiarrhoeal (Offiah and Chikwendu, 1999), antifertilty (Obianime et al., 2010), antifungal (Dubey et al., 2000; Faria et al., 2006; Okigbo and Ogbonnaya, 2006), antioxidant (Akinmoladun et al., 2007), anthelmintic (Pessoa et al., 2002), antiviral (Ayisi and Nyadedzor, 2003), hepatoprotective (Arhoghro et al., 2009), hypoglycaemic (Egesie et al., 2006), anti-carcinogenic (Ekunwe et al., 2010), antinociceptive (Rabelo et al., 2003), anticonvulsant and anxiolytic activities (Okoli et al., 2010).
Traditionally O. gratissimum has been used to treat mental illness in folk medicine. Other species of Ocimum like O. sanctum have been reported previously for their putative antidepressant-like activity (Chatterjee et al., 2011). Previous research on other plants have revealed the relationship between antidepressant-like effects and various antioxidant compounds, such as phenolics (proenthocyanidine) (Xu et al., 2010), flavonoids (liquiritin and isoliquiritin) (Wang et al., 2008) and phenylpropanoids (syringin and rosavin) (Kurkin et al., 2006). O. gratissimum has many active constituents, such as phenolics, flavonoids, tannins, alkaloids and phenylpropanoids. The GC/MS study of the essential oil of O. gratissimum presented 14 compounds; the major constituents were eugenol (43.7 %) and 1,8-cineole (32.71%). Other minor constituents were α-pinene, β-pinene, β-myrcene, cis-ocimene, linalool, α-terpineol, β-elemene, trans-caryophyllene, α-humulene, germacrene-D, α- selinene and β-selinene (Pessoa et al., 2002). Therefore, the present research was designed to investigate the possible in vitro antioxidant activity and in vivo antidepressant-like activity of the ethanolic leaf extract of O. gratissimum (OGE) in FST and TST, two predictive animal models for antidepressant activity. Additionally, possible mechanisms underlying its antidepressant- like effect were investigated pharmacologically using TST.
Materials and Methods
Plant material and preparation of the ethanolic extract from O. gratissimum
The leaves of Ocimum gratissimum Linn. (Lamiaceae) were collected from the herbal garden of the Indian Institute of Integrative Medicine (IIIM), CSIR, Jammu, India in September 2011 and were authenticated by Dr. H. B. Singh, Chief Scientist & Head, Raw Material Herbarium & Museum, National Institute of Science Communication And Information Resources, New Delhi (Ref. No. NISCAIR/RHMD/CONSULT/-2012-13/2009/17).
The fresh leaves of O. gratissimum were chopped, cleaned and air dried in a shaded area for two weeks. The dried leaves were reduced to coarse powder, which was then extracted by cold percolation method to prevent degradation of any heat labile phytoconstituents presented in the leaves. The coarsely powdered dried leaves (300 g) were packed in a percolator, soaked with 95% ethanol (2.5 L) and kept overnight. The extract was drained next morning and marc was resoaked in the fresh solvent and the extraction process was repeated three times for complete extraction of phytochemicals. Finally, all extracts were mixed, filtered and concentrated under reduced pressure (40-50°C) using a rotary evaporator (Heidolph HeiVapAdvantage, ML/G3, Germany) and dried to yield 36.2 g of a dark green colored semisolid. This dry ethanolic crude extract (yield 12.06 % w/w) was then stored in a vacuum desiccator until further use.
Animals
Adult Balb/C mice (either sex), weighing 20-30 g, were procured from the Central Animal House Facility of the National Institute of Pharmaceutical Education and Research, Mohali, Punjab, India. Animals had free access to water and food and were maintained at constant room temperature (22-25°C) under a 12:12 h light : dark cycle. Mice were allowed to acclimatize to the holding room for 48 h before the behavioural procedure. Animals were randomly distributed into specified experimental groups. All experiments were carried out between 10:00 and 16:00 h, with each animal used only once (n=5 animals per group). The experimental protocol was duly approved (Approval no. ASCB/IAEC/03/11/048) by the Institutional Animals Ethics Committee and the procedures in this study were performed in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals of the Government of India. All efforts were made to reduce the number of animals used in the study and to minimize the suffering of animals.
Drugs and Treatments
The drugs used in the present study were: Imipramine hydrochloride, prazosin, yohimbine, p-chlorophenylalanine, baclofen (all from Sigma-Aldrich, St. Louis, USA), fluoxetine hydrochloride (Wockhardt Ltd., Aurangabad, India) and levosulpiride injection (Lesuride; Sun Pharma, India). All drugs were administered by intraperitoneal (i.p.) route in a constant volume of 10 ml/kg body weight. All drugs were dissolved in normal saline (0.9% NaCl solution). Control animals received appropriate vehicle (5% Tween 80) by oral route. The extract was freshly prepared by emulsifying in 5% Tween 80 and orally administered daily for 7 days, 60 min before the FST, TST and locomotor activity. Imipramine (15 mg/kg, i.p.) and fluoxetine (20 mg/kg, i.p.) were used as standard drugs in FST and TST, respectively. Prazosin (1 mg/kg, i.p.), yohimbine (1 mg/kg, i.p.), levosulpiride (50 mg/kg, i.p.), p-CPA (100 mg/kg, i.p.) and baclofen (10 mg/kg, i.p.) were used as the standard drugs in groups to investigate possible mechanisms involved in the antidepressant-like effect of OGE. The administration schedule and doses of the drugs used were chosen on the basis of data from the literature (Dhingra and Sharma, 2006; Machado et al., 2007 and Dhingra and Goyal, 2008) and the dose of extract for study was selected on the basis of an acute oral toxicity study.
In vitro assays
Preliminary phytochemical screening
The freshly prepared ethanolic extract was tested for the presence of phytoconstituents like carbohydrates, proteins, glycosides, alkaloids, phenolics, flavonoids, terpinoids, saponins and eugenol, using the standard procedures (Trease and Evans, 1978).
Total phenolic content
The total phenolic contents were determined by Folin-Ciocalteu reagent. A solution of OGE (0.5 ml of 1:10 g/ml) or gallic acid (standard phenolic compound) was mixed with Folin-Ciocalteu reagent (5 ml, 1:10 diluted with distilled water) and aqueous Na2CO3 (4 ml, 1.0 M). The mixtures were allowed to stand for 15 min and the total phenols were determined at 765 nm using a spectrophotometer (Shimadzu, 1700, Singapore). The standard curve was prepared using 10, 20, 30, 40 and 50 μg/ml solutions of gallic acid in ethanol : water (50:50, v/v). Total phenolic contents are expressed in terms of mg/g gallic acid equivalent (mg/g GAE) of dry mass of OGE (McDonald et al., 2001).
Total flavonoid content
Aluminium chloride colorimetric method was used for total flavonoid content determination in OGE. Plant ethanolic extract (0.5 ml of 1:10 g/ml) were separately mixed with 1.5 ml of ethanol, 0.1 ml of 10% aluminium chloride, 0.1 ml of 1.0 M potassium acetate and 2.8 ml of distilled water. It remained at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm spectrophotometrically. Standard stock solution of quercetin (100 μg/ml) was prepared by dissolving 10 mg quercetin in 100 ml of ethanol and standard calibration curve was prepared by using 10, 20, 30, 40 and 50 μg/ml quercetin solutions in ethanol. Total flavonoid contents are expressed in terms of mg/g quercetin equivalent of dry mass of OGE (Chang et al., 2002).
DPPH (1, 1-Diphenyl-2-picrylhydrazyl) radical scavenging activity
In vitro antioxidant activity of OGE was carried out by DPPH radical scavenging activity (Mensor et al., 2001). Sample stock solution (1.0 mg/ml) of OGE was diluted to final concentrations of 250, 125, 50, 25, 10 and 5 μg/ml in ethanol. DPPH ethanol solution (0.3 mM, 1 ml) was added to 2.5 ml solution of the extract or standard and the mixture was allowed to react at room temperature for 30 min. Gallic acid served as a standard compound. The absorbance of the resulting mixture was measured at 518 nm using spectrophotometer and converted to percentage antioxidant activity (% AA) using the formula:
% AA = 100 – [(A sample – A blank) × 100]/A control
where, Acontrol is the absorbance of 0.3mM DPPH (1 ml) + ethanol (2.5 ml), Ablank is the absorbance of ethanol (1.0 ml) + extract solution (2.5 ml) and Asample is the absorbance of DPPH + extract solution/Gallic acid solution.
Nitric oxide radical scavenging activity
Nitric oxide free radical scavenging activity of OGE was estimated using Griess reagent (Nabavi et al., 2009). Sodium nitroprusside (10 mM in phosphate buffered saline) was mixed with different concentrations of extract dissolved in water and incubated at room temperature for 150 min. The same reaction mixture, but with an equivalent amount of water instead of the extract, served as a control. After the incubation period, 0.5 ml of Griess reagent (1% sulfanilamide, 2% H3PO4 and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride) was added. The absorbance of the chromophore formed was read at 546 nm spectrophotometrically. Quercetin was used as a positive control. The percentage inhibition was calculated by using the formula:
% inhibition = A Control – A Test /A Control × 100
Reducing power assay
Extract (2.5 ml, 50-800 mg/ml) in water was mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1%). The mixture was incubated at 50°C for 20 min. Trichloroacetic acid (2.5 ml, 10%) was added to the mixture to stop the reaction. The mixture was then centrifuged at 3000 rpm for 10 min. The upper layer of solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 solution (0.5 ml, 0.1%) and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was used as positive control (Ebrahimzadeh et al., 2009).
In vivo activities
Acute oral toxicity study
Acute oral toxicity study was performed according to the Organization for Economic Co-operation and Development (OECD) guideline No. 423 for the testing of chemicals. Three animals (n=3) were used in each step and animals were kept on fasting for 3-4 h before administration of the extract. The extract was administered to each mouse in a single dose at a starting dose of 2000 mg/kg body weight and observed individually for its gross behavioural parameters and for any sign of acute toxicity, morbidity or mortality during the first 24 h and daily thereafter, for a total of 14 days. The oral doses of extract for study were selected on the basis of this acute oral toxicity study (Singhal and Gupta, 2012).
Forced swim test (FST) in mice
For the development of immobility the individual mouse was placed in an inescapable, clear rectangular glass cylinder (25 x 12 x 25 cm3) filled with water at 24 ± 1°C to a depth of 15 cm. Two swimming sessions were conducted between 10:00 and 16:00: an initial 15 min pre-test session (training session) followed by a 6 min test session 24 h later (Porsolt et al., 1977; Yu et al., 2002). After an initial few minutes of vigorous activity, each animal gained a typical immobile posture. A mouse was considered immobile when it made no further attempts to escape and remained floating passively in the water without struggling, apart from the minimum movement of its limbs necessary to keep its head above water. The duration of immobility during the last 4 min was measured. After both swimming sessions, the animals were removed from the water, completely dried with a towel and placed in their cages (Herrera-Ruiz et al., 2006). Fresh water was used for each animal to minimize the effect of pheromones in soiled water from the previous animal, which may induce agitation and affect motility (Abel and Bilitzke, 1990).
Tail suspension test (TST) in mice
The TST was carried out according to the method described by Steru et al., (1985) with a few modifications as a facile mean of evaluating potential antidepressants. For development of immobility in tail suspension test, mice were individually suspended on the edge of a table about 58 cm above the bottom for 6 min by using adhesive tape placed approximately 1 cm from the tip of the tail. A mouse was considered immobile when it hanged passively and completely motionless. The duration of immobility period was recorded for a total of 6 min, because in the TST, animals become immobile much quicker, but they fail to remain immobile for periods as long as in the FST, hence immobility scores are calculated for the entire duration of the test (Cryan et al., 2005).
Measurement of locomotor activity
To assess the possible effects of the OGE on locomotor activity, an individual mouse was placed in a digital photoactometer (INCO, Ambala, India) for a period of 10 min as previously described by Dhingra and Goyal (2008). The photoactometer consisted of a square arena (30 x 30 x 25 cm) with wire mesh bottom. Six lights and six photocells were placed in the outer periphery of the bottom in such a way that a single mouse can block only one beam at a time. The photocell is activated when the light beam falling on the photocells are cut off by animals crossing the beam and a count is recorded in an electronic counting meter that counts the number of ‘cut offs’.
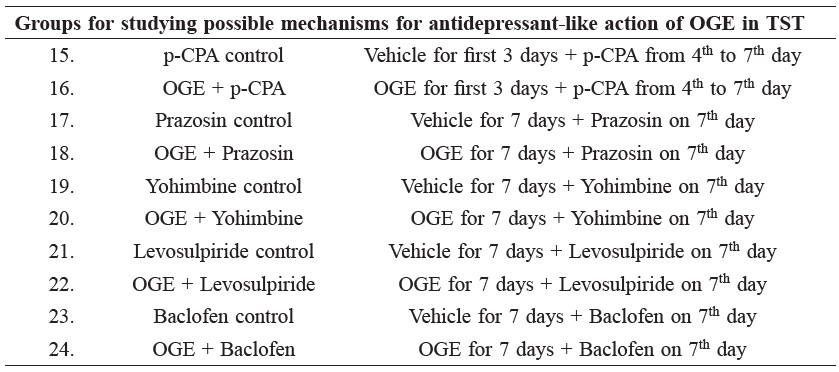
Investigation of possible mechanism(s) underlying the antidepressant-like effect of OGE in TST.
Involvement of serotonergic system
To investigate whether the antidepressant-like effect of OGE involved the serotonergic system, p-CPA (an inhibitor of serotonin synthesis, 100 mg/kg, i.p.) was injected once a day for 4 consecutive days (Redrobe et al., 1998).
Involvement of noradrenergic system
The involvement of noradrenergic system in the antidepressant-like effects of OGE was investigated by treatment of separate groups of animal with prazosin (an α1-adrenoceptor antagonist, 1 mg/kg, i.p.) and yohimbine (an α2-adrenoceptor antagonist, 1 mg/kg, i.p.) (Elhwuegi, 2004; Taylor et al., 2005).
Involvement of dopaminergic system
To rule out the involvement of the dopaminergic system in the antidepressant-like effects of OGE, mice were treated with levosulpiride (a dopamine D2 receptor antagonist, 50 mg/kg, i.p.) (Yamada et al., 2004; Hirano et al., 2007).
Involvement of GABAergic system
To investigate whether the antidepressant-like effects of OGE involved the GABAergic system, mice were treated with baclofen (a GABAB agonist, 10 mg/kg, i.p.) (Dhingra and Goyal, 2008).
Experimental design
Mice were divided into 24 separate groups and each group contained a minimum of 5 animals.
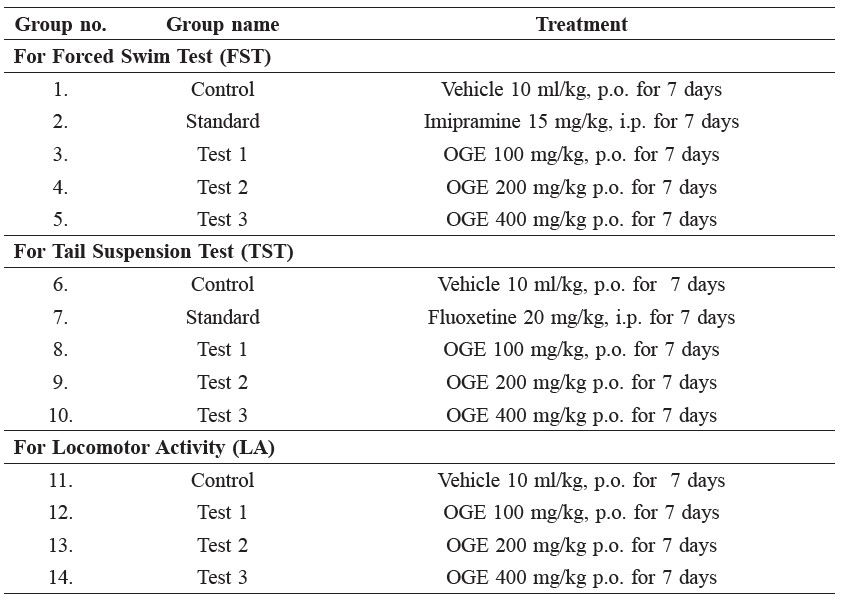
Table 1. Experimental design for the study.
Statistical analysis
All the experimental results are expressed as the mean ± S.E.M. (except total phenolic and flavonoid content determination, where results are expressed as the mean ± S.D.). Comparisons between experimental and control groups were performed by one-way ANOVA followed by Dunnett's test, while groups for study of the mechanism of action were analysed by one-way ANOVA followed by Tukey's test. A value of P<0.05 was considered statistically significant.
Results
Preliminary phytochemical screening
The results of phytochemical screening of OGE revealed the presence of carbohydrates (non-reducing sugars), alkaloids, tannins, phenolics, cardiac glycosides,
flavonoids and terpenoids, while reducing sugars, proteins and saponins were absent in the extract.
Total phenolic and flavonoid contents
The results suggested that OGE contains high phenolic and flavonoid compounds that were measured spectrophotometrically, as shown in Figure 1. The total phenolic content estimated in the OGE was 78.89 ± 0.04 mg/g gallic acid equivalent (GAE) of dry mass of O. gratissimum extract, as determined by reference to the standard curve of gallic acid (y=0.012x-0.021, R2=0.998). Total flavonoid content estimated in OGE was 164.67 ± 2.347 mg/g quercetin equivalent of dry mass of O. gratissimum extract, as determined by reference standard curve of quercetin (y=0.004x+0.007, R2=0.997).
DPPH radical scavenging activity
A dose-response curve of DPPH radical scavenging activity of OGE compared to that of standard antioxidant gallic acid is shown in Figure 2. Both OGE and gallic acid showed a dose-dependent free radical scavenging activity and the percentage antioxidant activity of OGE and gallic acid reached upto a comparable level of 69.54% and 88.72%, respectively, at a concentration of 250 μg/ml. Further, the IC50 value of OGE and gallic acid were estimated to be 86 μg/ml and 12 μg/ml, respectively.
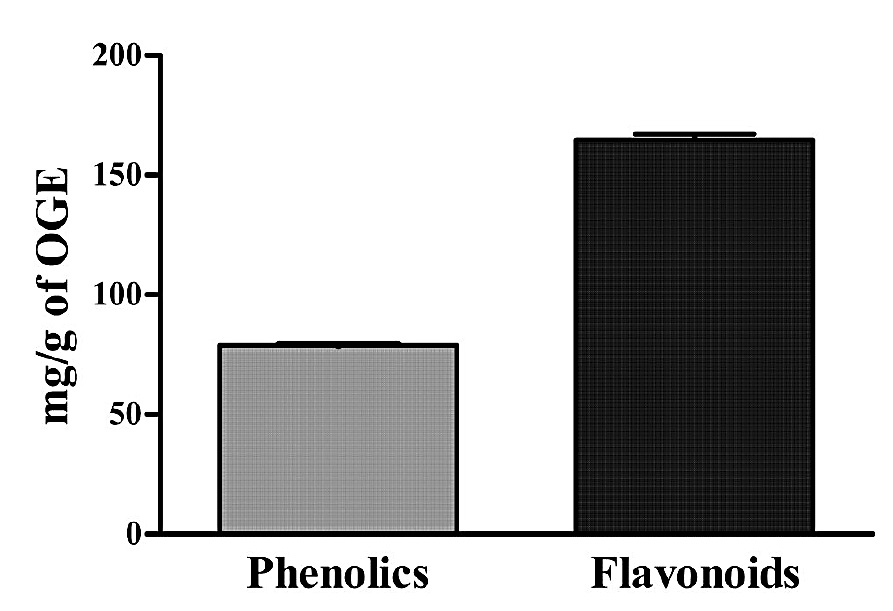
Figure 1. Total phenolic and flavonoid contents of OGE. Values are expressed as mean ± S.D. (n=3).
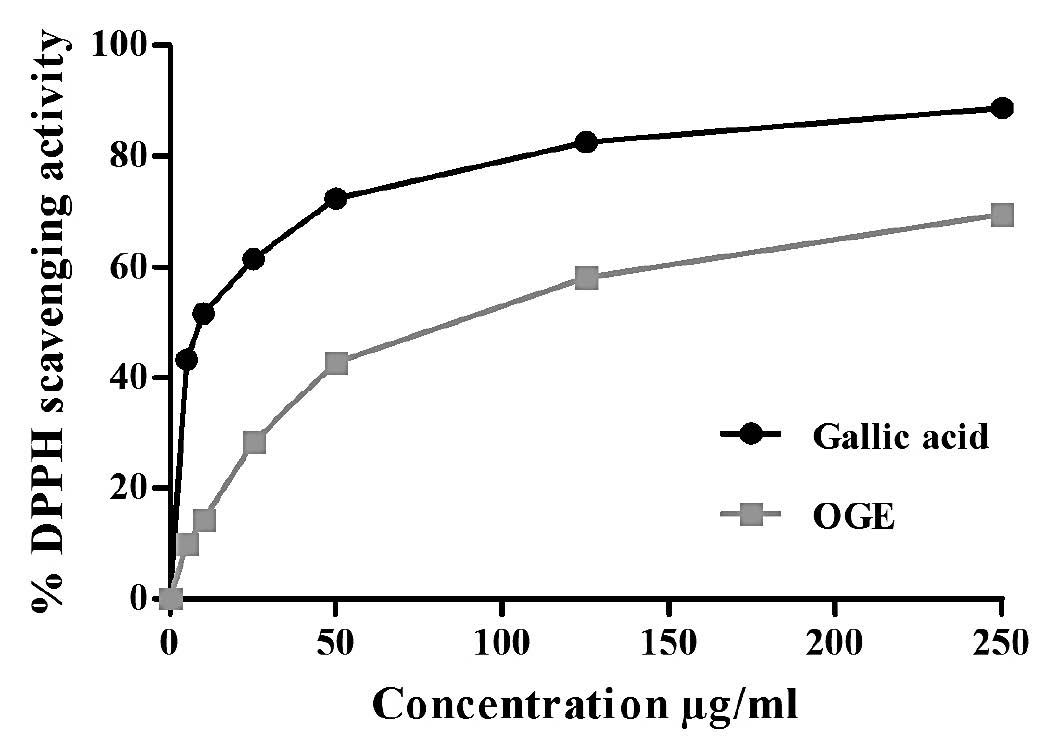
Figure 2. DPPH radical scavenging activity of the OGE.
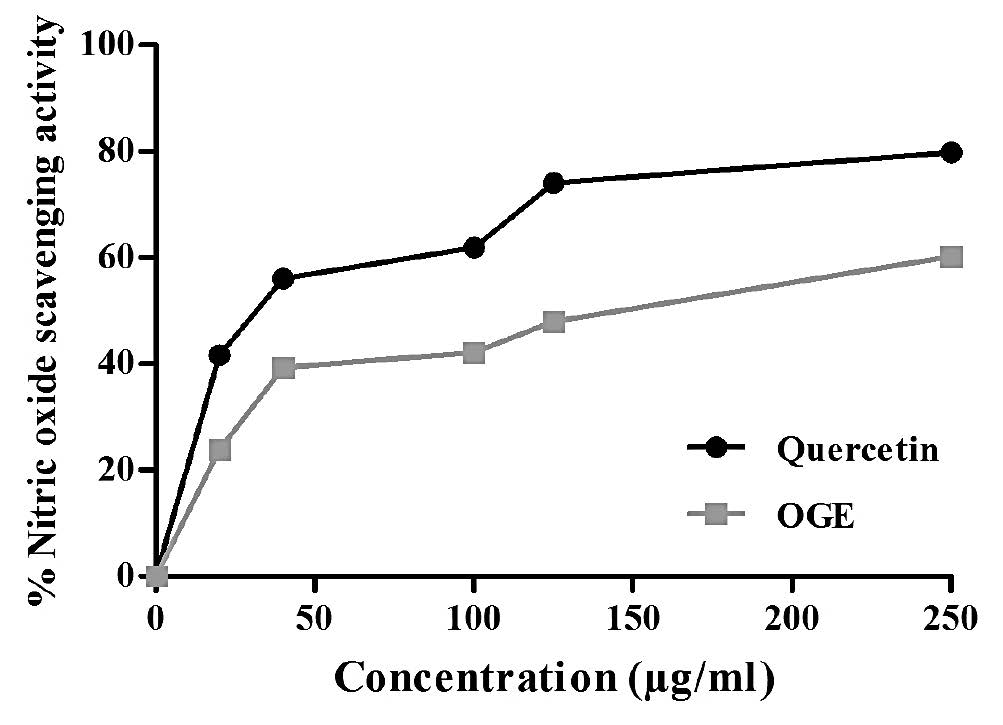
Figure 3. Nitric oxide radical scavenging activity of OGE.
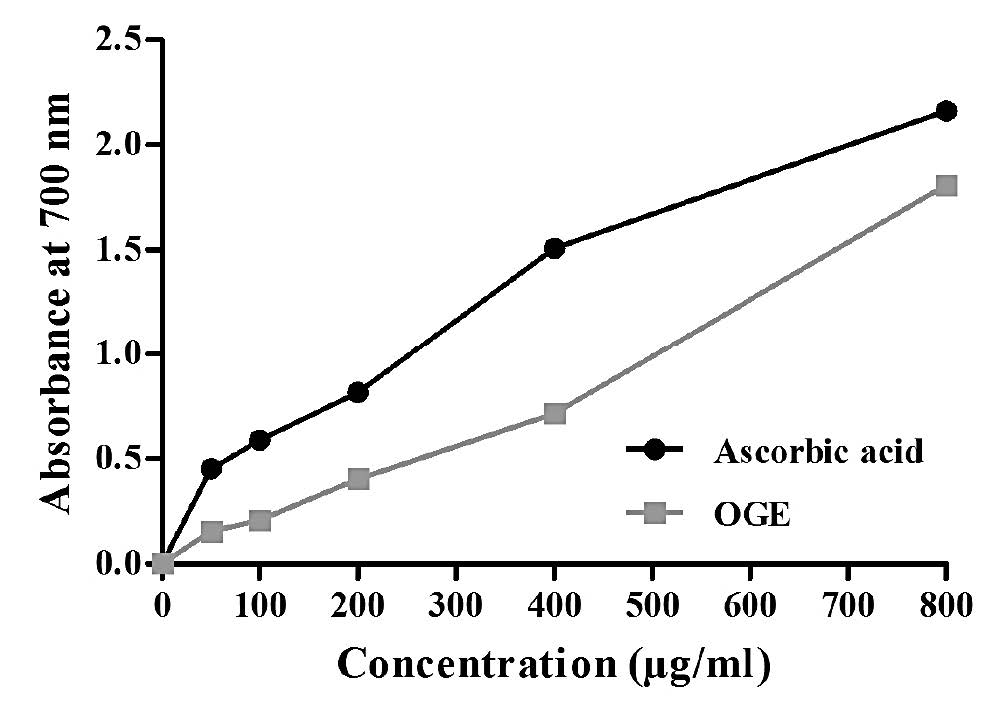
Figure 4. Reducing power assay of OGE.
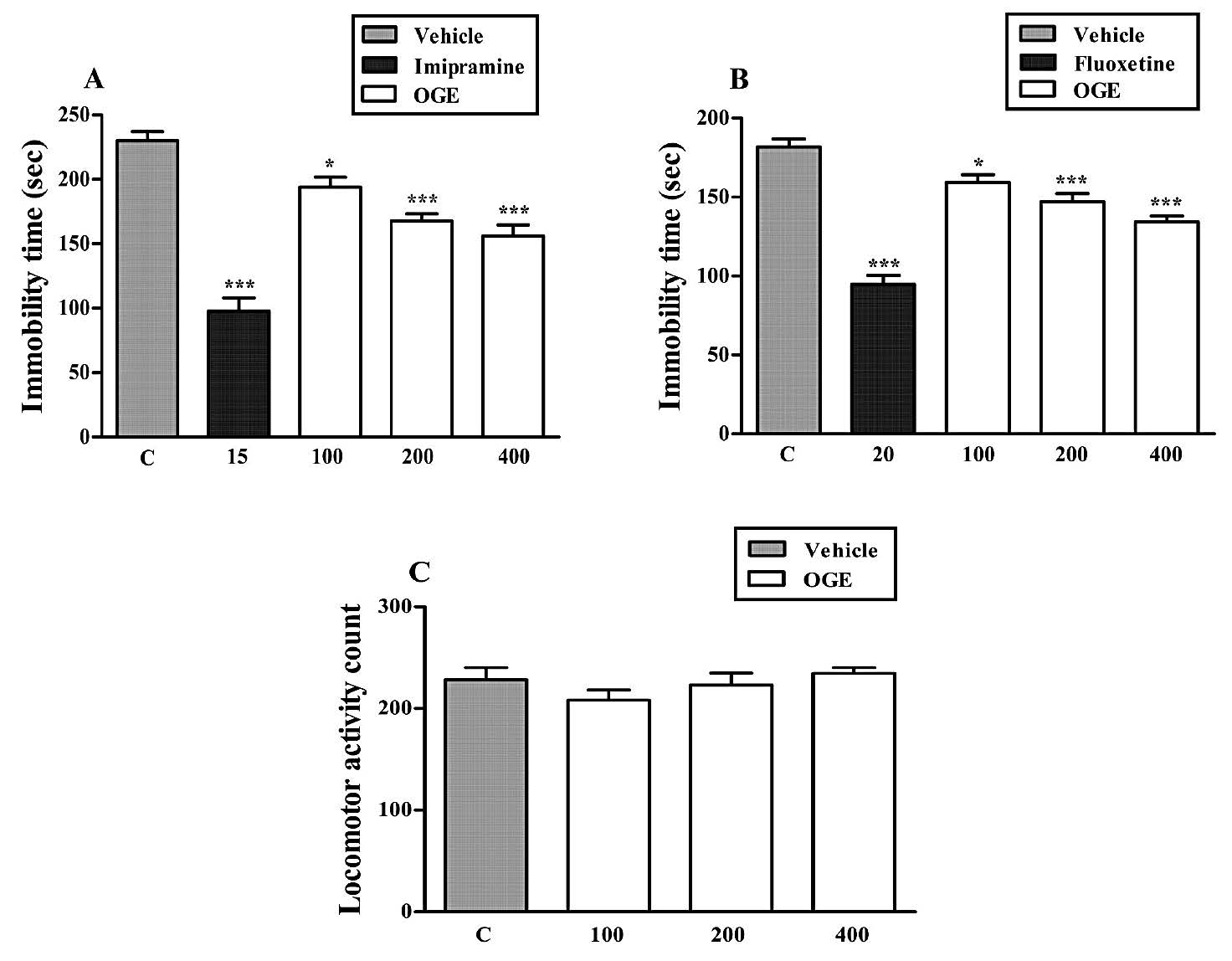
Figure 5. Effect of the repeated treatment (7 days) with the OGE (100-400 mg/kg, p.o.) and imipramine (15 mg/kg, i.p.) in the FST (Panel A), with the OGE (100-400 mg/kg, p.o.) and fluoxetine (20 mg/kg, i.p.) in the TST (Panel B) and with the OGE (100-400 mg/kg, p.o.) in locomotor activity (Panel C). Each column represents the mean ± S.E.M. (n=5). *P<0.05 and ***P<0.001 compared with the vehicle-treated control group (C).
Nitric oxide radical scavenging activity
The results of nitric oxide radical scavenging activity is illustrated in the dose-response curve of OGE compared to that of standard antioxidant quercetin (Figure 3). It showed that both OGE and quercetin scavenged the nitric oxide free radicals in a dose-dependent manner. The percentage antioxidant activity of OGE and quercetin reached upto a comparable level of 60.19% and 79.75%, respectively, at a concentration of 250 μg/ml. The IC50 value of OGE and quercetin was estimated to be 160 μg/ml and 22.6 μg/ml, respectively.
Reducing power assay
The dose-response curve for reducing power of ascorbic acid and OGE is shown in Figure 4. Similar to ascorbic acid, absorbance of OGE also increased in a dose-dependent manner. The reductive potential of ascorbic acid was remarkably higher than that of OGE at all concentrations, but the differences seemed to be less significant at higher concentration (800 μg/ml). This suggested that OGE has good reducing power activity at higher concentrations, reflecting its potential antioxidant property.
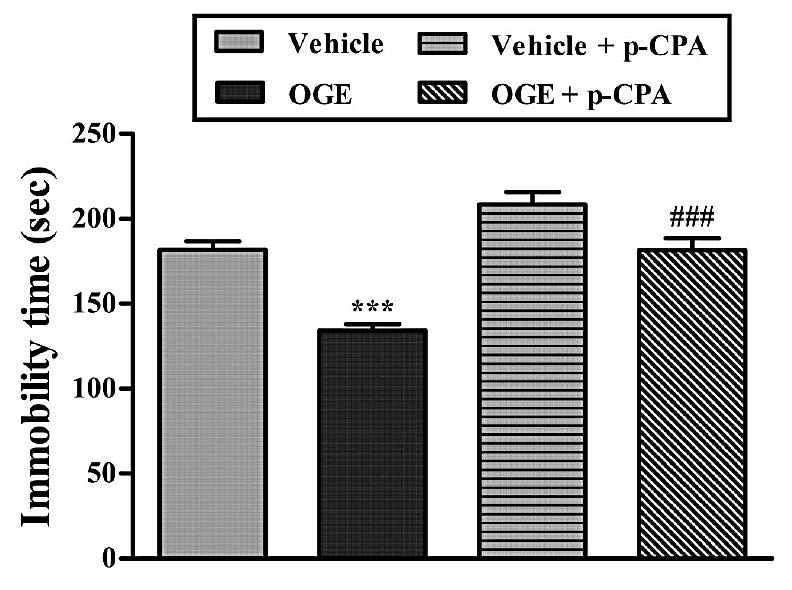
Figure 6. Effect of the treatment of mice with p-CPA (100 mg/kg, i.p. once a day for 4 consecutive days) on the anti-immobility effect of OGE (400 mg/kg, p.o.) in the TST. Each column represents the mean ± S.E.M. (n=5). ***P<0.001 as compared with the vehicle-treated control and ###P<0.001 as compared with the extract (OGE) alone.
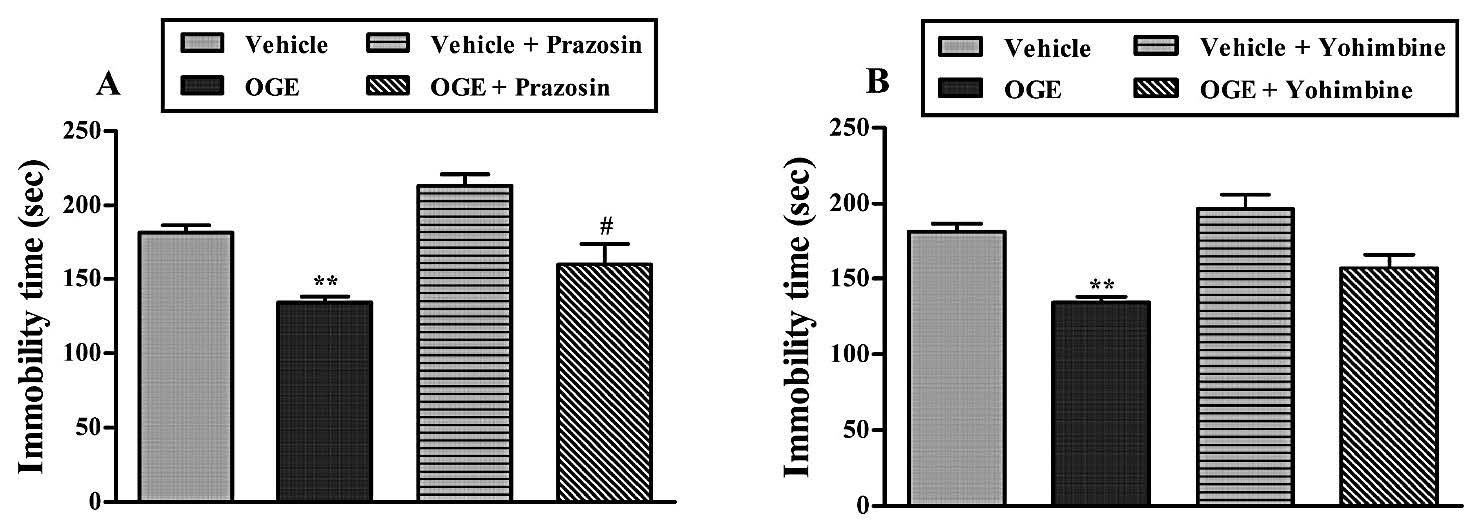
Figure 7. Effect of the treatment of mice with prazosin (1 mg/kg, i.p., panel A) or with yohimbine (1 mg/kg, i.p., panel B) on the anti-immobility effect of OGE (400 mg/kg, p.o.) in the TST. Each column represents the mean ± S.E.M. (n=5). **P<0.01 as compared with the vehicle-treated control and #P<0.05 as compared with the extract (OGE) alone.
Acute oral toxicity study
The administration of OGE at limit dose of 2000 mg/kg did not cause any mortality in the mice during 14 days. Further, the animals did not show any sign of acute toxicity, i.e., no changes in alertness, spontaneous motor activity, reactivity to sound and touch, body and limb tone, respiration, urination, pupil size, pinnal reflex, corneal reflex, righting reflex were found, for all 14 days of study. Abnormal signs pertaining to toxicity such as ataxia, body tremors, convulsions, diarrhoea, writhing, pilo erection, sedation, coma and cyanosis were not observed in any mice during 14 days. However, mild sluggishness and discomfort were observed during the first few hours. Therefore, LD50 of the extract was assumed to be greater than the limit dose tested and 2000 mg/kg was considered as a cut-off dose. On the basis of these observation, one tenth of the cut off dose, i.e., 200 mg/kg, was considered as the therapeutic dose for further study and, therefore, three oral test doses (100, 200 and 400 mg/kg) were selected to explore the antidepressant-like activity of OGE.
Effect of repeated treatment of the OGE in the immobility time in FST and TST
The effects of the repeated oral administration (7 days) of OGE on the immobility time of mice in the FST and TST are shown in Figures 5A and 5B, respectively. The extract given by oral route at doses of 100, 200 and 400 mg/kg significantly decreased the immobility time of mice in the FST (Figure 5A) and TST (Figure 5B) in a dose dependent manner as compared to the control group. The maximum anti-immobility effects of OGE were seen at the dose level of 400 mg/kg, p.o. in both FST and TST. The results obtained in this experiment, analyzed by one-way ANOVA, revealed a significant effect in the FST [F (4, 20)
=37.99, P<0.001] and TST [F (4, 20) =41.92, P<0.001].
Effect of repeated treatment of the OGE on the locomotor activity in the digital photoactometer
In order to rule out the possibility that the anti-immobility effect of the OGE was due to a psychostimulant effect, the OGE was administered to independent groups of mice that were subsequently tested in the digital photoactometer. Results showed that the treatment of mice with OGE at doses of 100, 200 and 400 mg/kg, p.o. produced no significant locomotor effect in the mice in the photoactometer as compared with the control group, as shown in Figure 5C. The results obtained in this experiment, analyzed by one-way ANOVA, revealed a significant effect [F (3, 16) =1.221, P>0.05].
Investigation of possible mechanism(s) underlying the antidepressant-like effect of OGE in the TST
Since 400 mg/kg, p.o. dose of the OGE was the most effective dose in both FST and TST, all experiments investigating the mechanisms underlying the antidepressant-like effect of the extract were performed in the TST using this dose.
Involvement of the serotonergic system
The result, illustrated in Figure 6, showed that the treatment of mice with the p-CPA significantly reversed the anti-immobility effect elicited by the extract (400 mg/kg, p.o.). The results obtained in this experiment, analyzed by one-way ANOVA, showed a significant effect of p-CPA x OGE interaction [F (3, 16) =26.55, P<0.001].
Involvement of the noradrenergic system
The result, depicted in Figure 7A, suggested that treatment of mice with prazosin was able to reverse the antidepressant-like effect of the OGE (400 mg/kg, p.o.) in the TST. The results obtained in this experiment, analyzed by oneway ANOVA, revealed a main effect of the prazosin x OGE interaction [F (3, 16) =18.05, P<0.05]. Figure 7B shows that treatment of mice with yohimbine did not reverse the antidepressant-like effects of the OGE (400 mg/kg, p.o.) in the TST. The results obtained in this experiment, analyzed by one-way ANOVA, revealed a main effect of the yohimbine x OGE interaction [F (3, 16) =14.61, P>0.05].
Involvement of the dopaminergic system
The results, illustrated in Figure 8, suggested that treatment of mice with levosulpiride was able to reverse the antidepressant-like effect of the OGE (400 mg/kg, p.o.) in the TST. The results obtained in this experiment, analyzed by one-way ANOVA, revealed a main effect of the levosulpiride x OGE interaction
[F (3, 16) =38.42, P<0.001].
Involvement of the GABAergic system
Figure 9 shows that the treatment of mice with baclofen was not able to reverse the antidepressant-like effects of the OGE (400 mg/kg, p.o.) in the TST. The results obtained in this experiment, analyzed by one-way ANOVA, revealed a main effect of the baclofen x OGE interaction [F (3, 16) =3.255, P>0.05].
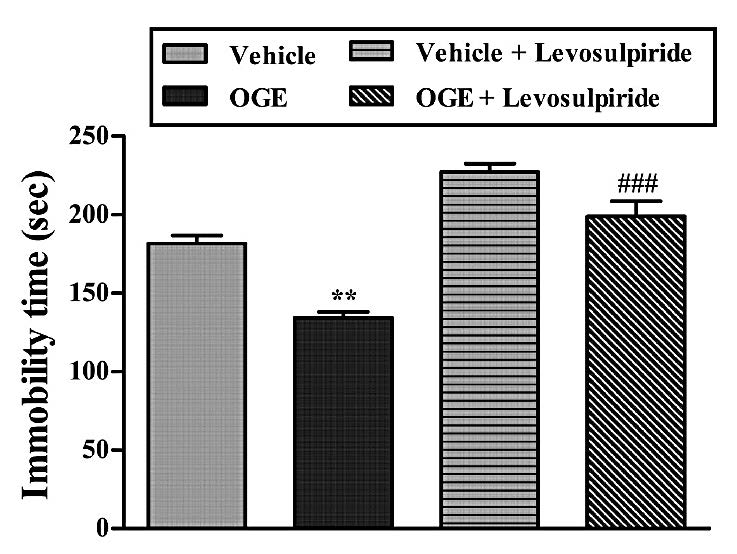
Figure 8. Effect of the treatment of mice with levosulpiride (50 mg/kg, i.p.) on the anti-immobility effect of OGE (400 mg/kg, p.o.) in the TST. Each column represents the mean ± S.E.M. (n=5). **P<0.01 as compared with the vehicle-treated control and ###P<0.001 as compared with the extract (OGE) alone.
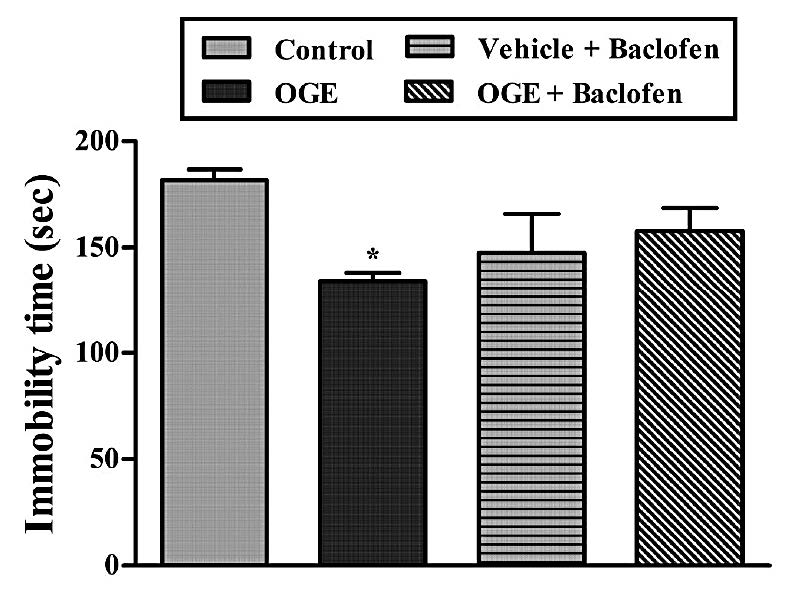
Figure 9. Effect of treatment of mice with baclofen (10 mg/kg, i.p.) on the anti-immobility effect of OGE (400 mg/kg, p.o.) in the TST. Each column represents the mean ± S.E.M. (n=5). *P<0.05 as compared with the vehicle-treated control.
Discussion
In the present study, the ethanolic extract of leaves of O. gratissimum exhibited good in vitro antioxidant activities, as revealed in DPPH radical scavenging activity, nitric oxide radical scavenging activity and reducing power assay. The presence of antioxidant compounds may play important roles in the treatment of neurological disorders associated with stress-like depression. OGE showed considerable amount of phenolic and flavonoid contents in spectrophotometric method. So it may be suggested that one of such active component(s) presented in the OGE could be responsible for the in vitro antioxidant activity and in vivo antidepressant-like activity.
The assessment of antidepressant activity was conducted using the rodent forced swim test (FST) and the tail suspension test (TST). The immobility behaviour displayed in rodents when subjected to an unavoidable and inescapable stress has been hypothesized to reflect behavioural despair, which in turn may reflect depressive disorders in humans. There is, indeed, a significant correlation between clinical potency and effectiveness of antidepressants in both models (Cryan et al., 2002). The results demonstrated that the administration of OGE for seven consecutive days significantly decreased the time of immobility of mice in a dose-dependent manner in both FST and TST, when compared with the control group. These results indicated the antidepressant-like activity of O. gratissimum. The antidepressant-like effect of OGE was not associated with any motor effects, since it did not produce any significant change in locomotor activity of mice as compared to control.
To unmask the possible mechanism by which the extract produced antidepressant- like effect, further investigations were carried out using TST, since the monoaminergic system is one of the most important targets in the pathophysiology and treatment of depression (Elhwuegi, 2004). We investigated the involvement of serotonergic, noradrenergic, dopaminergic and GABAergic systems in the anti-immobility effect of OGE in the TST by using specific agonist/antagonist.
The serotonergic receptor system is considered a valuable target for antidepressant drugs, like selective serotonin reuptake inhibitors (SSRIs). P-CPA (an inhibitor of serotonin synthesis) inhibits the enzyme tryptophan hydroxylase and depletes nearly 60% of the endogenous stores of serotonin on its administration for four consecutive days in mice (Redrobe et al., 1998). This study showed that treatment of mice with p-CPA (an inhibitor of serotonin synthesis) for four consecutive days caused significant inhibition of the anti-immobility effect of the extract in the TST by increasing the duration of immobility of mice. These results clearly suggested that an intact serotonergic system plays a critical role in the effect of the OGE in the TST.
The noradrenergic system has long been implicated in the pathophysiology of depression, since depression seems to be associated with a hypofunction of the noradrenergic system and some antidepressants act by increasing the synaptic availability of norepinephrine (Elhwuegi, 2004; Taylor et al., 2005). In the present study, prazosin was able to reverse the anti-immobility effect of the OGE, whereas yohimbine was ineffective in reversing the immobility period in mice. These results indicated that the extract may exert its effect by interacting with α1-adrenoceptors, but not with α2-adrenoceptors.
The dopaminergic system is an important target implicated in the regulation of mood disorders (Dailly et al., 2004). Studies indicated that dopamine receptor antagonist prevented the antidepressant-like effect of some antidepressant agents in the FST and TST (Yamada et al., 2004; Hirano et al., 2007). In the present study, levosulpiride (a dopamine D2 receptor antagonist) decreased the antidepressant-like effect of the OGE in the TST. These results suggested that the effect of OGE in the TST may depend on activation of dopamine D2 receptors.
Some previous studies also revealed the involvement of GABAergic system in the antidepressant effect of some plants (Dhingra and Goyal 2008). Thus, the present study investigated whether the treatment of mice with baclofen (a GABAB agonist) affects the antidepressant-like effect of OGE in the TST. The result indicated that baclofen did not significantly affect the anti-immobility effect of OGE. Therefore, OGE exhibits its antidepressant activity without interacting with the GABAergic system.
Conclusions
The ethanolic extract of O. gratissimum possessed a considerable amount of antioxidant compounds, both flavonoids and phenolics. The extract exhibited a specific antidepressant-like effect in mice that was proven to be mediated by interaction with the monoaminergic systems, including the serotonergic, noradrenergic (only α1-adrenoceptors) and dopaminergic systems. The active principle(s) responsible for the antidepressant-like effects of the OGE is/are, so far, not known. But the phytochemical studies revealed the presence of phenols, flavonoids, alkaloids and antioxidants that might be responsible for the observed effect. Further studies are necessary to correlate the active compound(s) responsible for the antidepressant-like effects of the extract of O. gratissimum.
REFERENCES
Abel, E.L., and P.J. Bilitzke. 1990. A possible alarm substance in the forced swimming test. Physiol Behav 48: 233-239.
Akinmoladun, A.C., E.O. Ibukun, A. Emmanuel, E.M. Obuotor, and E.O. Farombi. 2007. Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Sci Res Essay 2: 163-166.
American Psychiatric Association. 1994. The diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Press.
Arhoghro, E.M., K.E. Ekpo, and G.O. Ibeh. 2009. Effect of aqueous extract of scent leaf (Ocimum gratissimum) on carbon tetrachloride (CCl4) induced liver damage in albino Wister rats. Afr J Pharm Pharmacol 3: 562-567.
Ayisi, N.K., and C. Nyadedzor. 2003. Comparative in vitro effects of AZT and extracts of Ocimum gratissimum, Fícus polita, Clausena anisata, Alchornea cordifolia and Elaeophorbia drupifera against HIV-1 and HIV-2 infections. Antivir Res 58: 25-33. DOI: 10.1016/S0166-3542(02)00166-3
Chang, C., M. Yang, H. Wen, and J. Chern. 2002. Estimation of total flavonoid content in propolis by two complimentary colorimetric methods. J Food Drug Anal 10: 178-182.
Chatterjee, M., P. Verma, R. Maurya, and G. Palit. 2011. Evaluation of ethanol leaf extract of Ocimum sanctum in experimental models of anxiety and depression. Pharm Biol 49: 477-483.
Cryan, J.F., A. Markou, and I. Lucki. 2002. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23: 238-245. DOI: 10.1016/S0165-6147(02)02017-5
Cryan, J.F., C. Mombereau, and A. Vassout. 2005. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29: 571-625. DOI: 10.1016/j.neubiorev.2005.03.009
Dailly, E., F. Chenu, C.E. Renard, and M. Bourin. 2004. Dopamine, depression and antidepressants. Fundam Clin Pharmacol 18: 601-607.
Dhingra, D., and A. Sharma. 2006. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog Neuropsychopharmacol Biol Psychiatry 30: 449-454. DOI: 10.1016/j.pnpbp.2005.11.019
Dhingra, D., and P.K. Goyal. 2008. Inhibition of MAO and GABA: Probable mechanisms for antidepressant-like activity of Nardostachys jatamansi DC in mice. Indian J Exp Biol 46: 212-218.
DSM-IV. 2003. Manual Diagnóstico y Estadístico de los Trastornos Mentales. Texto revisado. Masson S.A. Barcelona.
Dubey, N.K., T.N. Tiwari, D. Mandin, H. Andriamboavonjy, and J.P. Chaumont. 2000. Antifungal properties of Ocimum gratissimum essential oil (ethyl cinnamate chemotype). Fitoterapia 71: 567-569. DOI: 10.1016/S0367-326X(00)00200-8
Ebrahimzadeh, M.A., S.M. Nabavi, S.F. Nabavi, and B. Eslami. 2009. Antioxidant activity of aqueous extract of Pyrus boissieriana fruit. Pharmacologyonline 1: 1318-1323.
Egesie, U.G., A.B. Adelaiye, J.O. Ibu, and O.J. Egesie. 2006. Safety and hypoglycaemic properties of aqueous leaf extract of Ocimum gratissimum in streptozotocin induced diabetic rats. Niger J Physiol Sci 21: 31-35.
Ekunwe, S.I., M.S. Thomas, X. Luo, H. Wang, Y. Chen, X. Zhang, and G.B. Begonia. 2010. Potential cancer-fighting Ocimum gratissimum (OG) leaf extracts: increased anti-proliferation activity of partially purified fractions and their spectral fingerprints. Ethn Dis 20: 12-16.
Elhwuegi, A.S. 2004. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry 28: 435-451. DOI: 10.1016/j.pnpbp.2003.11.018
Faria, T.J., R.S. Ferreira, L. Yassumoto, J.R.P. Souza, N.K. Ishikawa, and A.M. Barbosa. 2006. Antifungal activity of essential oil isolated from Ocimum gratissimum L. (eugenol chemotype) against phytopathogenic fungi. Braz Arch Biol Technol 49: 867-871.
Guadarrama-Cruz, G., F.J. Alarcon-Aguilar, R. Lezama-Velasco, G. Vazquez-Palacios, and H. Bonilla-Jaime. 2008. Antidepressant-like effects of Tagetes lucida Cav. in the forced swimming test. J Ethnopharmacol 120: 277-281. DOI: 10.1016/j.jep.2008.08.013
Herrera-Ruiz, M., Y. Garcia-Beltran, S. Mora, G. Diaz-Veliz, G.S. Viana, and J. Tortoriello. 2006. Antidepressant and anxiolytic effects of hydroalcoholic extract from Salvia elegans. J Ethnopharmacol 107: 53-58. DOI: 10.1016/j.jep.2006.02.003
Hirano, S., S. Miyata, K. Onodera, and J. Kamei. 2007. Involvement of dopamine D1 and α1-adrenoceptors in the antidepressant-like effect of chlorpheniramine in the mouse tail suspension test. Eur J Pharmacol 562: 72-76. DOI: 10.1016/j.ejphar.2007.01.063
Kiss, P.J. 2008. Theory of active antidepressants: a nonsynaptic approach to the treatment of depression. Neurochem Int 52: 34-39. DOI: 10.1016/j.neuint. 2007.04.006
Kurkin, V.A., A.V. Dubishchev, V.N. Ezhkov, I.N. Titova, and E.V. Avdeeva. 2006. Antidepressant activity of some phytopharmaceuticals and phenylpropanoids. Khim Far Zh 40: 33-38. DOI: 10.1007/s11094-006-0205-5
Machado, D.G., M.P. Kaster, R.W. Binfare, M. Dias, A.R. Santos, M.G. Pizzolatti, I.M. Brighente, and A.L. Rodrigues. 2007. Antidepressant-like effect of the extract from leaves of Schinus molle L. in mice: evidence for the involvement of the monoaminergic system. Prog Neuropsychopharmacol Biol Psychiatry 31: 421-428. DOI: 10.1016/j.pnpbp.2006.11.004
Mahapatra, S.K., S.P. Chakraborty, and S. Roy. 2010. Aqueous extract of Ocimum gratissimum Linn and ascorbic acid ameliorate nicotine-induced cellular damage in murine peritoneal macrophage. Asian Pac J Trop Med 3: 775-782. DOI: 10.1016/S1995-7645(10)60186-1
Martins, J.F., A.A. Alvarenga, E.M. Castro, A.P.O. Silva, C. Oliveira, and E. Alves. 2008. Leaf anatomy of alfavaca-cravo plants cultivated under colored nets. Cienc Rural 39: 82-87. DOI: 10.5897/AJB2014.13675
Mcdonald, S., P.D. Prenzler, M. Autolovich, and K. Robards. 2001. Phenolic content and antioxidant activity of olive extracts. Food Chem 73: 73-84. DOI: 10.1016/S0308-8146(00)00288-0
Mensor, L.I., F.S. Menezes, G.G. Leitao, A.S. Reis, T. Dossantos, C.S. Coube, and S.G. Leito. 2001. Screening of Brazillian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15: 127-130. DOI: 10.1002/ptr.687
Moussavi, S., S. Chatterji, E. Verdes, A. Tandon, V. Patel, and B. Ustun. 2007. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370: 851-858. DOI: 10.1016/S0140-6736(07)61415-9
Nabavi, S.M., M.A. Ebrahimzadeh, S.F. Nabavi, M. Fazelian, and B. Eslami. 2009. In vitro Antioxidant and Free Radical Scavenging Activity of Diospyros lotus and Pyrus boissieriana growing in Iran. Pharmacogn Mag 5: 122-126.
Nakamura, C.V., T. Ueda-Nakamura, E. Bando, A.F. Melo, D.A. Cortez, and B.P. DiasFilho. 1999. Antibacterial activity of Ocimum gratissimum L. essential oil. Mem Inst Oswaldo Cruz 94: 675-678.
Obianime, A.W., J.S. Aprioku, and C.T.O. Esomonu. 2010. Antifertility effects of aqueous crude extract of Ocimum gratissimum L. leaves in male mice. J Med Plant Res 4: 809-816.
Offiah, V.N., and U.A. Chikwendu. 1999. Antidiarrhoeal effects of Ocimum gratissimum leaf extract in experimental animals. J Ethnopharmacol 68: 327-330.
Okigbo, R.N., and U.O. 2006. Ogbonnaya. Antifungal effects of two tropical plant leaf extracts (Ocimum gratissimum and Aframomum melegueta) on postharvest yam (Dioscorea spp.) root. Afr J Biotechnol 5: 727-731.
Okoli, C.O., A.C. Ezike, O.C. Agwagah, and P.A. Akah. 2010. Anticonvulsant and anxiolytic evaluation of leaf extracts of Ocimum gratissimum, a culinary herb. Pharmacognosy Res 2: 36-40. DOI: 10.4103/0974-8490.60580
Pessoa, L.M., S.M. Morais, C.M. Bevilaqua, and J.H. Luciano. 2002. Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus. Vet Parasitol 109: 59-63. DOI: 10.1016/S0304-4017(02)00253-4
Porsolt, R.D., A. Bertin, and M. Jalfre. 1977. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229: 327-336.
Posser, T., M.P. Kaster, S.C. Barauna, J.B.T. Rocha, A.L.S. Rodrigues, and R.B. Leal. 2009. Antidepressant-like effect of the organoselenium compound ebselen in mice: Evidence for the involvement of the monoaminergic system. Eur J Pharmacol 602: 85-91. DOI: 10.1016/j.ejphar.2008.10.055
Rabelo, M., E.P. Souza, P.M.G. Soares, A.V. Miranda, F.J.A. Matos, and D.N. Criddle. 2003. Antinoceptive properties of the essential oil of Ocimum gratissimum L. (Labiatae) in mice. Braz J Med Biol Res 36: 521-524. Doi:10.1590/S0100-879X2003000400016.
Redrobe, J.P., M. Bourin, M.C. Colombel, and G.B. Baker. 1998. Dose-dependent noradrenergic and serotonergic properties of Venlafaxine in animal models indicative of antidepressant activity. Psychopharmacol 138: 1-8. DOI: 10.1007/s002130050638
Rosenzweig-Lipson, S., C.E. Beyer, Z.A. Hughes, X. Khawaja, S.J. Rajarao, J.E. Malberg, Z. Rahman, R.H. Ring, and L.E. Schechter. 2007. Differentiating antidepressants of the future: Efficacy and safety. Pharmacol Ther 113: 134-153. DOI: 10.1016/j.pharmthera.2006.07.002
Sarris, J., A. Panossian, I. Schweitzer, C. Stough, and A. Scholey. 2011. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol 21: 841-860. DOI: 10.1016/j.euroneuro.2011.04.002
Singhal, K.G., and G.D. Gupta. 2012. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4- induced liver injury in rats. Asian Pac J Trop Med 5: 677-685. DOI: 10.1016/S1995-7645(12)60106-0
Steru, L., R. Chermat, B. Thierry, and P. Simon. 1985. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol 85: 367-370. DOI: 10.1007/BF00428203
Taylor, C., A.D. Fricker, L.A. Devi, and I. Gomes. 2005. Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell Signal 17: 549-557. DOI: 10.1016/j.cellsig.2004.12.007
Trease, G.E., and M.C. Evans. 1978. A textbook of Pharmacognosy. Tindall and Cassell, Baillier, London.
Wang,W., X. Hu, Z. Zhao, P. Liu, Y. Hu, J. Zhou, D. Zhou, Z. Wang, D. Guo, and H. Guo. 2008. Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog Neuropsychopharmacol Biol Psychiatry 32: 1179-1184. DOI: 10.1016/j.pnpbp.2007.12.021
WHO. 2001. The World Health Report. Mental health: New understanding new hope. Geneva.
Xu, Y., S. Li, R. Chen, G. Li, P.A. Barish, W. You, L. Chen, M. Lin, B. Ku, J. Pan, and W.O. Ogle. 2010. Antidepressant-like effect of low molecular proenthocyanidin in mice: Involvement of Monoaminergic system, Pharmcol Biochem Behav 94: 447-453. DOI: 10.1016/j.pbb.2009.10.007
Yamada, J., Y. Sugimoto, and S. Yamada. 2004. Involvement of dopamine receptors in the antiimmobility effects of dopamine re-uptake inhibitors in the forced swimming test. Eur J Pharmacol 504: 207-211. DOI: 10.1016/j.ejphar.2004.09.057
Yu, Z.F., L.D. Kong, and Y. Chen. 2002. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J Ethnopharmacol 83: 161-165. DOI: 10.1016/S0378-8741(02)00211-8
Zhang, Z.J. 2004. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci 75: 1659-1699. DOI: 10.1016/j.lfs.2004.04.014
Amit Upadhyay* and Deepak Bhagwat
Pharmacology & Toxicology Research Lab., Department of Pharmacology, ASBASJSM College of Pharmacy, Bela (Ropar)- 140111, Punjab, India
*Corresponding author. E-mail: amit.mph012@gmail.com
Total Article Views

