
Quality Changes in Ripened Mango and Litchi Flesh After Cryogenic Freezing and During Storage
Julaluk Tangtua, Noppol Leksawasdi and Nithiya Rattanapanone*Published Date : 2019-08-25
DOI : 10.12982/CMUJNS.2014.0036
Journal Issues : Number 3, September - December 2014
ABSTRACT
This study investigated a methodology to cease the activity of polyphenol oxidase (PPO) enzyme and peroxidase (POD) enzyme on ripened mango flesh cv. Maha Chanok and litchi flesh cv. Hong Huay before storage using liquid nitrogen. The immersion of halved-ripened mango flesh in a mixture solution of 1% citric acid and 1% calcium chloride for 2 min resulted in the optimal activity decrease of PPO and POD at 64.0 and 48.3%, respectively. Compared to the control, which was soaked in the previously stated mixture solution before freezing by liquid nitrogen and kept at -24°C for 6 months, the PPO and POD enzyme activities of the frozen ripened mango flesh only slightly increased during the storage period. The average enzyme activities in the control group were lower by 53.4 and 40.8%, respectively. In case of litchi flesh, the implementation of 0.5% calcium chloride solution for 5 min inactivated PPO and POD enzyme activities by 39.9 and 34.2%, respectively, whereas the texture of the flesh was improved, without leaving a bitter taste. In addition, the measured compression force of the litchi flesh texture, which belonged to the experimental set, was higher than the control set and differed significantly (p ≤ 0.05) from each other. When the presoaked litchi flesh in 0.5% calcium chloride solution was frozen in the liquid nitrogen and maintained at -24°C for 6 months, the firmness of frozen litchi flesh after thawing was relatively stable throughout the storage period. The activity of PPO enzyme dropped slightly, whereas POD activity only decreased during the first month and the activities increased afterwards. The activities of enzymes in the experimental set were significantly lower than the control set (p ≤ 0.05).
Keywords: Ripened mango, Litchi flesh, Citric acid, Calcium chloride, Polyphenol oxidase, Peroxidase, Cryogenic, Liquid nitrogen.
INTRODUCTION
PPO and POD enzymes of fresh-cut fruits are inhibited, along with the browning reaction, by certain chemicals (Salcini and Massantini, 2005). The immersion of fruit flesh in the solution also assists in decreasing the quantity of enzyme, as well as released substrate from cells after slicing, which is responsible for the forming of brown color at the surface (Salcini and Massantini, 2005). The implementation of calcium chloride solution also improves the firmness preservation of strawberry fruit (Torreggiani and Maestrelli, 2006) and sliced mango flesh (Souza et al., 2006) during frozen storage. This storage method mitigates the rate of various intracellular biochemical reactions and inactivated microbial growth (Delgado and Sun, 2000). In fact, food quality after thawing depends on the rate at which it is first frozen. If food is frozen slowly, larger-sized ice crystals form and damage the cell structure. The seepage of water from cells occurs and eventually leads to weight and firmness losses (Kondratowicz and Matusevicius, 2002). Cryogenic freezing technology has been adopted for food preservation by employing cryogens, such as liquid nitrogen (Agnelli and Mascheroni, 2002). It provides a rapid rate of heat absorption from food, and quickly brings food temperatures below the freezing point (Khadatkar et al., 2004). The size of ice crystals is also minimized, therefore avoiding damage to cell structure, decreasing water loss and leakage of intracellular liquid after thawing. In addition, taste, texture, flavor, nutritional values and external appearance are preserved (Kondratowicz and Matusevicius, 2002). Weight loss is also minimal after thawing as is seen in the cryogenic freezing of strawberry fruit with drip loss of only 4% after storage for six months (Lee and Smith, 1979; Kidmose and Martens, 1999; Khadatkar et al., 2004).
This research focused on the implementation of citric acid and/or calcium chloride solutions for the inhibition of PPO and POD activities, texture improvement of ripened mango and litchi flesh before cryogenic freezing, as well as the quality changes of frozen fruits during six-month storage periods.
MATERIALS AND METHODS
Materials
The mango fruit cv. Maha Chanok and litchi fruit cv. Hong Huay used in the preliminary enzyme inhibition study were purchased from a local market, Muang District, Chiang Mai Province in April 2008. The fruits to be processed by freezing in the factory were kindly provided from the fruit orchard of Mr. Pradondej Kanlayanamitr, Tah Torn Subdistrict, Mae Ai District, Chiang Mai Province. The harvested fruits were obtained in May 2008, with the following selection criteria for mango and litchi fruits:
Mango fruit cv. Maha Chanok: Commercially mature stage and the fully yellow peel colored more than 80%. Weight of fruit was 350-400 g.
Litchi fruit cv. Hong Huay: Commercially harvested stage and fruit were selected for uniformity of size and lack of symptoms of damage and injury caused by insects. Weight of fruit was 20-25 g.
Effects of Citric Acid and/or Calcium Chloride on PPO and POD Activities in Ripened Mango Flesh.
Ripened mangoes were peeled and cut in two halves, and each half was divided into two pieces, resulting in four longitudinal cutlets of mango flesh p er fruit. Each cutlet of mango flesh was dipped in the solutions of citric acid at 0 (control), 0.5, 1.0 and 1.5% (w/v) for 2 min. In the same way, samples were also dipped in the solutions of calcium chloride at 0 (control), 0.5, 1.0 and 1.5% (w/v) for 2 min. The most effective treatment of inhibiting enzyme activities was selected. Then, the cutlets of fresh-cut mango were dipped in the mixture solution between citric acid and calcium chloride at the best concentration of each. PPO and POD activities in mango flesh were measured and reported by percent reduction of enzymes activities in comparison to the control. Five replicates per treatment were used, with one fruit per replicate.
Effects of Calcium Chloride on PPO and POD Activities and Firmness in Litchi Flesh.
Litchi flesh, after removal of the seed and pericarp, were divided into four groups. Each group was dipped in calcium chloride solution at 0 (control), 0.5, 1.0 and 1.5% (w/v) for 5 min. Three replicates per treatment were used, with six fruits per replicate. Firmness and PPO and POD activities were measured and reported by percent in comparison to the control.
Freezing of Mango and Litchi Fleshes by Cryogenic Method Using Liquid Nitrogen.
Preparation of Ripened Mango Flesh
The mango fruit were washed in clean water and immersed in 100 mg/L peroxyacetic acid at 4°C for 5 min (Narciso and Plotto, 2005) and drained. After peeling and slicing in half longitudinally, one half side of the mango flesh was selected for the control sample (not dipped in any solution) and another half side was treated (dipped in the mixture solution of 1.0% (w/v) citric acid and 1.0% (w/v) calcium chloride for 2 min).
Preparation of Litchi Flesh
Litchi fruit were washed in clean water and immersed in 100 mg/L peroxyacetic acid at 4°C for 5 min (Narciso and Plotto, 2005) and drained. Litchi flesh, after removal of seed and peel, were divided into two experiments. The first experiment, or control, was not dipped in any solution. In the second experiment, the flesh was dipped in 0.5% (w/v) calcium chloride solution for 5 min.
Cryogenic Freezing Method
The flesh of both fruits were immersed in 50 mg/L peroxyacetic acid at 4°C for 3 min (Narciso and Plotto, 2005) and placed on the mechanical belt with the mango flesh facing downward and the attached seed side facing upward. The fruits were then frozen cryogenically using liquid nitrogen. The process was carried out in two rounds for the mango flesh and one round for the litchi flesh. They were then immediately packed in polyethylene bags and stored at -24°C for 6 months. Samples were randomly selected in each month for evaluating the quality changes after thawing.
Fruit Quality Evaluation During Frozen Storage
Physical Analysis
The color at three points of the sample with attached mango peel after thawing was measured using the C.I.E L*, a* and b* color space by Minolta chromameter (TA-XT2i/50, Stable Micro Systems, Ltd., Godalming, UK). Values were shown in L*, Chroma and Hue angle.
Mango and litchi flesh in polyethylene bags were thawed by using a water-bath at 25°C for 90 min. A texture analyzer was used to evaluate the firmness of the mango and litchi flesh. During the puncture test, a 6.0 mm diameter stainless steel cylindrical probe for mango flesh and a 2 mm diameter stainless steel cylindrical probe for litchi flesh were used to penetrate the middle of the longitudinal axis of each sample (10 and 5 mm depth, respectively) at a speed of 5 mm s-1. Maximum force at rupture (or firmness) was recorded in Newtons (Narciso and Plotto, 2005).
Chemical Analysis
pH: The flesh of mango and litchi were homogenized in a commercial blender (Moulinex, Barcelona, Spain). Then, 50 ml of distilled water was added into 5 g of homogenized flesh. The pH was measured with a pH-meter (Model C831, Consort, Turnhout, Belgium).
Total titratable acidity: Titratable acidity was determined by homogenizing 5 g of pulp in 50 mL of distilled water (after boiling and cooling) and then titrated by auto-titrator (Model 230, TitroLine easy, SCHOTT, Belgium) with 0.1 M NaOH, to an end point of pH 8.1. The results were expressed as grams of citric acid/100 g pulp for mango and malic acid/100 g pulp for litchi (Ranganna, 1986).
Total soluble solids: Total soluble solids of fruit juices were measured with a Digital refractometer (Model PR-101, Atago, Atago Co. Ltd, Tokyo, Japan, 0-45%) and the results were expressed as percentages (%).
Reducing sugar content: Reducing sugar content was measured according to the method of Miller (1959). This method used a spectrophotometer (Thermo Spectronic) and its absorbance was measured at 540 nm. Values were expressed as mg per 100 g fresh weight.
Total phenolic compounds: Total phenols were determined according to the method of Singleton and Rossi (Souza et al., 2006). Three grams of homogenized pulp was extracted by 80% ethanol. Then, the solutions were reacted with Folin-Ciocalteu reagent. Total phenols were determined colorimetrically at 765 nm with a UV/VIS spectrophotometer (Analytik Jena, SPE Cord 40, Germany). The results were expressed as milligram of gallic acid per g of fresh weight.
Total Carotenoid: Five grams of homogenized mango flesh was extracted with a mixture of 10% acetone and hexane solution. Carotenoid concentration was determined by spectrophotometer and absorbance values recorded at 450 nm. Beta-carotene was used as the standard (AOAC, 2000). The total carotenoids were expressed as milligrams of beta-carotene per g of fresh weight.
Biochemical Analysis
Determination of PPO and POD activities.
Frozen mango and litchi pulp were homogenized. One gram of homogenized pulp was blended in a cold mortar with 10 ml 0.05 M sodium phosphate buffer (pH
6.2) containing 0.1 M sodium chloride and then centrifuged at 3,000xg for 30 min at 4°C. The supernatant was collected for the crude enzyme assay (Flurkey and Jen, 1978).
Analysis of Polyphenol Oxidase (PPO) Activity
PPO activity was assayed using the procedure, with slight modification, of Lee and Smith (1979). An aliquot of 0.25 ml of crude enzyme extraction was added to 0.25 ml of 0.25 M catechol and 2 ml of 0.2 M sodium phosphate buffer (pH 6.5). Then, enzyme activity was determined by measuring the increase in absorbance at 420 nm with a spectrophotometer at 25°C.
Analysis of Peroxidase (POD) Activity
POD activity was assayed using the procedure, with slight modification, of Flurkey and Jen (1078). An aliquot (0.10 ml) of crude enzyme extraction was added to 2.4 ml of 0.1 M sodium acetate buffer solution (pH 6.0) containing 0.5% guaiacol and 0.1% H2O2. Enzyme activity was determined by measuring the increase in absorbance at 470 nm with a spectrophotometer at 25°C.
Protein content was determined according to the method of Bradford (1976) and enzyme activity was measured with three replicates per treatment and bovine serum albumin was used as standard protein. One unit of enzyme activity was defined as the amount of enzyme that caused a change of 0.001 in absorbance at 420 or 470 nm per minute, pH 6.5 or 6.0, respectively. The PPO and POD activities were expressed as unit/mg total protein/minute.
Microbial Analysis
The number of bacteria in the frozen mango and litchi flesh were quantified using the spread plate technique (AOAC, 2000). Ten grams of frozen fruit fresh were cut by sterilized scissors and placed in sterilized bags. After weighing, 90 ml of 1% sterilized phosphate buffer (pH 7.5) was added to the bags. Then, the cut fruit was agitated by stomacher. These aliquots of buffer from each sample were analyzed in sequential dilution steps of 10-1 and 10-2.
An aliquot of 0.1 ml of diluted liquid from each bag or tube was pipetted onto the center of an agar plate and spread evenly over the surface by means of a sterilized bent-glass-rod and left standing for 10-15 min. The medium was inoculated at 35±1°C for 48 hours. The number of microbes was determined and the results were expressed as colonies per 1 g fresh weight.
Sensory Evaluation
Frozen mango and litchi flesh were thawed after storage for six months. Both experiments of mango flesh were cut into two longitudinal sections and cut again into six cross sections with a sterile blade. Each sample in a white plastic cup was evaluated in terms of color, texture, sweet taste, sour taste and overall acceptability. For litchi flesh, one fruit per cup was evaluated shape, texture, sweet taste, sour taste and overall acceptability. Fifty members of an untrained sensory panel, using a hedonic nine-point scale, assessed the sensory quality of the fresh processed fruit.
STATISTICAL ANALYSIS
Data were tested by analysis of variance using SPSS version 16 with significance level P ≤ 0.05. Means of each treatment were compared using T-test (Two-sample T-test) and results from storage for six months in each treatment were compared using Tukey’s test.
RESULTS
Effects of Citric Acid and/or Calcium Chloride on PPO and POD Activities in Ripened Mango Flesh.
Dipping the cutlet of fresh-cut mango in 1% (w/v) citric acid or 1% (w/v) calcium chloride solutions mitigated PPO and POD activities (Table 1).
The mixture solution between 1% citric acid and 1% calcium chloride resulted in the most optimal activity decrease of PPO (42.4±7.3%) and POD (40.3±7.6%) compared with the control (Table 1).
Table 1. Reduction of PPO and POD activities (%) and firmness (Newtons) in mango flesh cv. Maha-Chanok after dipping in varying concentrations of citric acid and/or calcium chloride solutions for 2 min.
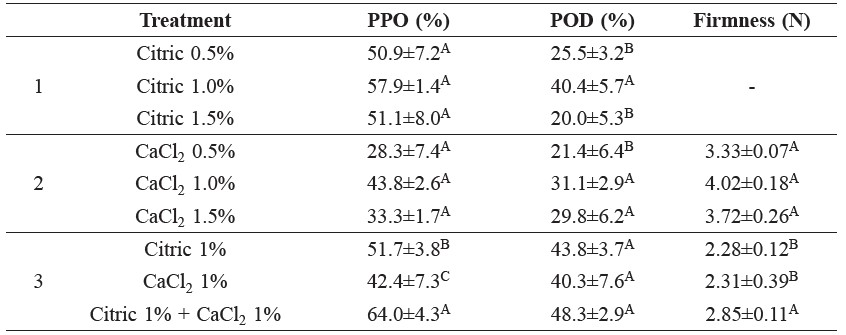
Note: Values are means ± standard deviation. A, B and C superscripts indicate significant difference (P≤0.05) in each column of treatments.
Effects of Calcium Chloride on PPO and POD Activities and Firmness in Litchi Flesh.
Immersion of litchi flesh in the calcium chloride solution at 0.5, 1.0 or 1.5% (w/v) for 5 min decreased PPO activity to 39.8, 34.4 and 40.1% and POD activity to 34.2, 30.7 and 32.8%, respectively, in comparison to the control.Although dipping the litchi flesh in the 1.5% (w/v) calcium chloride solution was more effective at mitigating PPO and POD activities than 0.5% (w/v), the 1.5% solution created an undesirable bitter flavor. In addition, the texture of litchi flesh dipped in all treatments did not show any significant differences (P≤0.05). Dipping litchi flesh in the 0.5% (w/v) calcium chloride solution was chosen, as it retained the natural flavor qualities.
Table 2. Reduction of PPO and POD activities (%) and firmness (Newtons) in litchi flesh cv. Hong Huay after dipping in varying concentrations of calcium chloride solutions for 5 min.
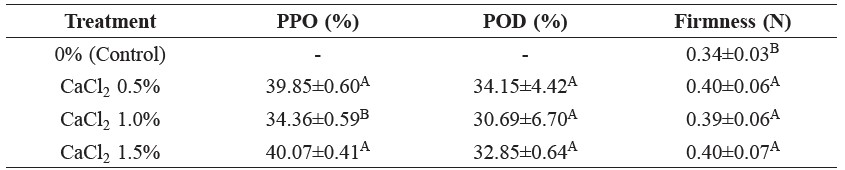
Note: Values are means ± standard deviation. A, B and C superscripts indicate significant difference (P≤0.05) in each column of treatments.
Quality of mango and litchi flesh during frozen storage for six months
Physical analysis
The results showed that the average L* value in both experiments slightly increased while C* and Ho values did not change significantly during six months of storage. The firmness of mango flesh slightly increased during six months of storage, from 2.8 to 3.1 Newtons for the control and 3.9 to 4.2 Newtons for the treated sample (Table 3). The firmness of litchi flesh also slightly increased during six months of storage, from 0.6 to 0.7 Newtons for the control and from 0.7 to 0.8 Newtons for the treated sample (Table 4). The average firmness value of both mango and litchi flesh in the treated sample were not significantly higher than control throughout the six months of storage (P≤0.05).
Table 3. Physicochemical evaluation and microbial counts of the immersion of fresh-cut mango initially and during storage at -24°C for six months.
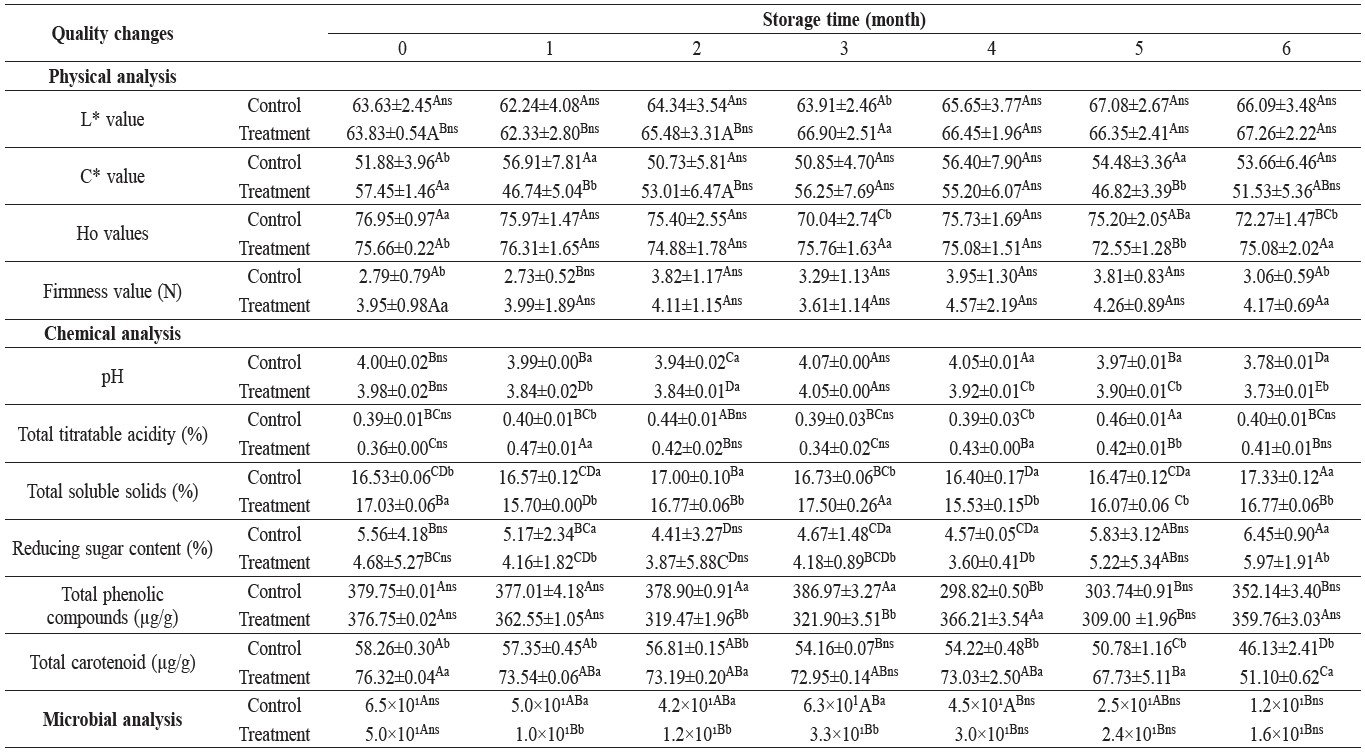
Note: Values are means ± standard deviation. The number with the same alphabet (A-E) and letters of superscript (a, b and ns) indicate no significant difference (p > 0.05) for comparison between different columns of the same row and differences between control and treatment, respectively.
Table 4. Physicochemical evaluation and microbial counts of litchi flesh dipped in 1.0% (w/v) calcium chloride solution initially and during storage at -24°C for six months.
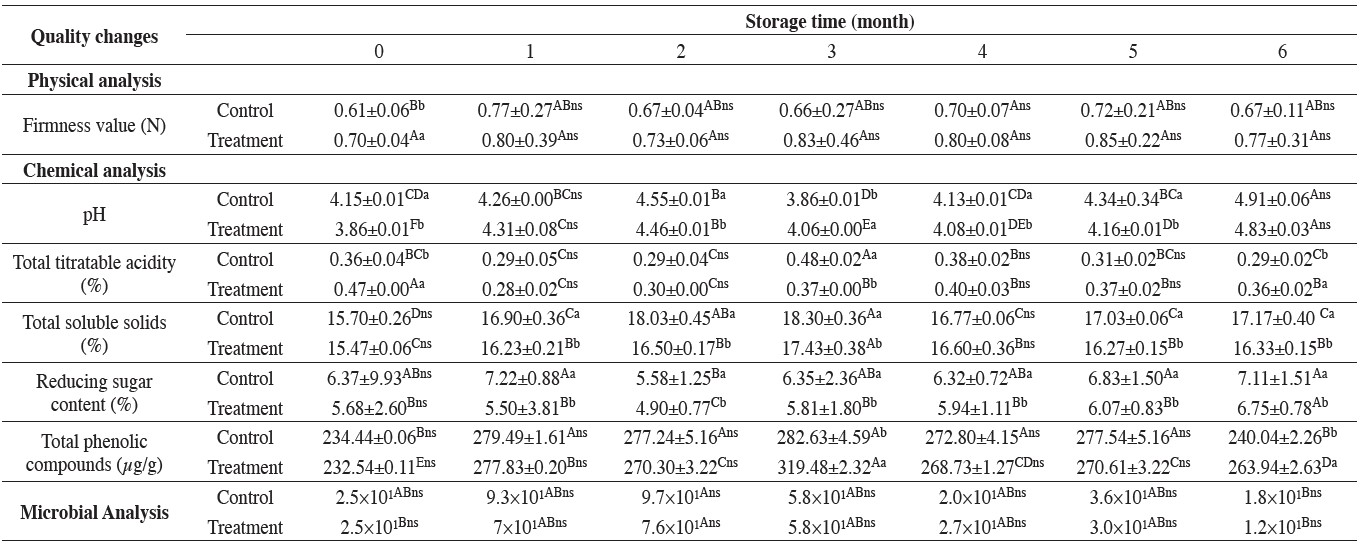
Note: Values are means ± standard deviation. The number with the same alphabet (A-F) and letters of superscript (a, b and ns) indicate no significant difference (p > 0.05) for comparison between different columns of the same row and different between control and treatment, respectively.
Chemical analysis
pH, total titratable acidity, total soluble solids and reducing sugar content
The pH and total soluble solids in frozen mango and litchi flesh increased slightly. The increase in titratable acidity was consistent with the decrease in pH. The reducing sugar in frozen mango and litchi flesh in both experiments throughout the six-month storage period increased to 5.2 and 6.5% in the control. This was
higher than the treated sample (4.5 and 4.5%, respectively). In addition, dipping the treated sample in the solution increased the loss of sugar and total soluble
solids progressively, compared to the control sample.
Total phenolic compounds
The initial contents of total phenolic compounds in the control and treated mango flesh were 379.8 and 376.8 μg/g of fresh weight, respectively, and decreased
gradually during the six months of storage to 7.3 and 4.5%, respectively (Table 3).
The initial contents of total phenolic compounds in the control and treated litchi flesh were 234.4 and 234.4 μg/g of fresh weight, respectively, and slightly increased throughout the six months of storage (Table 4).
Total Carotenoid
Treated samples had higher total carotenoid content in comparison with the control and showed significant difference (P≤0.05). Carotenoid contents in the control and treated samples decreased as the storage period increased, from 58.3 and 76.3 μg/g of fresh weight at the beginning of storage to 46.1 and 51.1 μg/g of fresh weight after six months of storage, respectively (Table 3).
Biochemical analysis
The PPO of the frozen mango flesh decreased during the first four months of storage, and then increased. The PPO activity in the control samples increased more rapidly than the treated sample. The average PPO activity in the control and treated samples during the six-month storage period was 852.5 and 397.6 unit/mg protein/min, respectively (Figure 1).
The average POD activity in the control and treated samples during the six-month storage period was 1,596.2 and 885.3 unit/mg protein/min, respectively. The PPO and POD activity in the treated samples relative to the control throughout the six-month storage period decreased to 53.4 and 40.8%, respectively (Figure 1). The PPO activity of the frozen litchi flesh in both experiments decreased and the average PPO activity in the control and treated samples during the six-month storage was 1,492.1 and 973.2 unit/mg protein/min, respectively. In contrast, POD activity decreased during the first two months of storage, and then increased thereafter. The average POD activity in the control and treated during the six-month storage period was 7,090.0 and 4,594.4 unit/mg protein/min, respectively.
The PPO and POD activity of the treated samples in relation to the control decreased 34.6 and 35.3%, respectively, during the storage period (Figure 2).
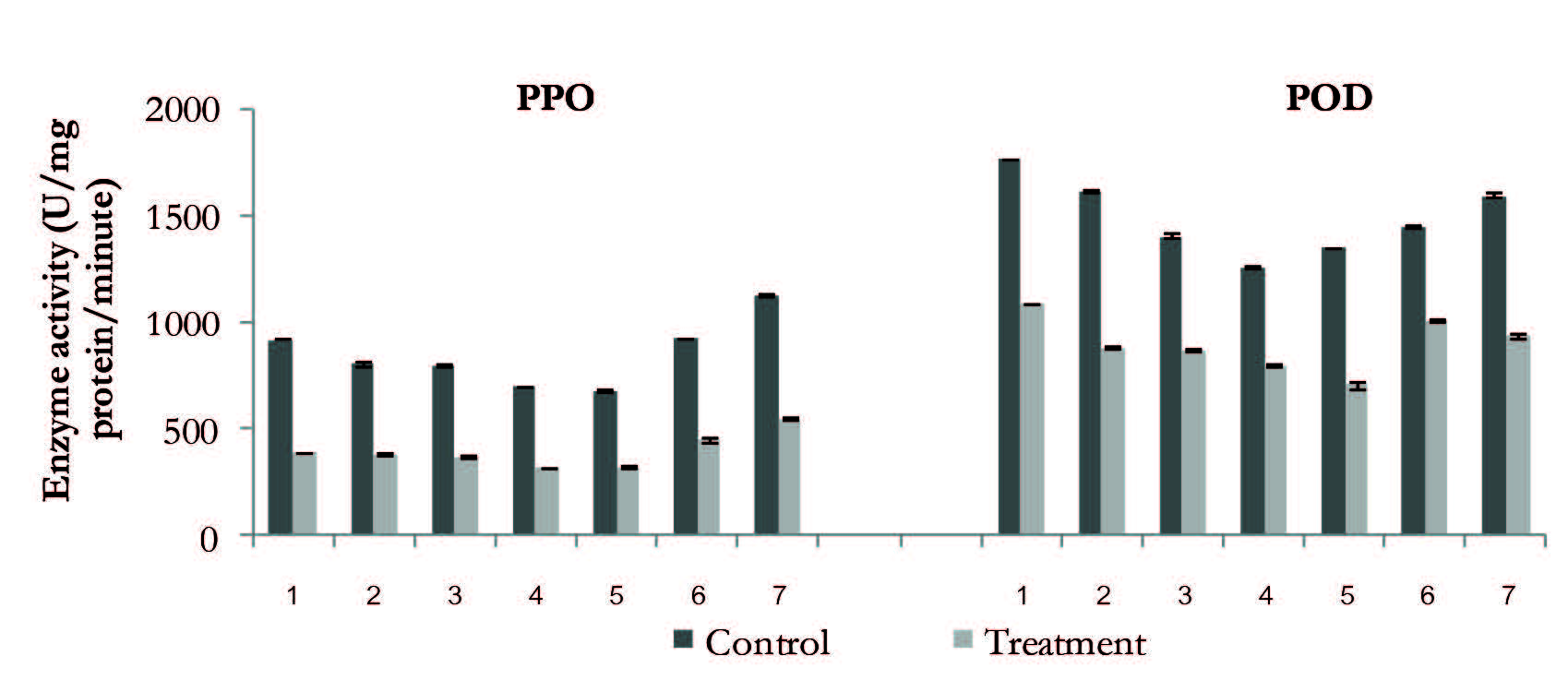
Figure 1. Changes in PPO and POD of ripened mango flesh during storage at -24°C for six months.
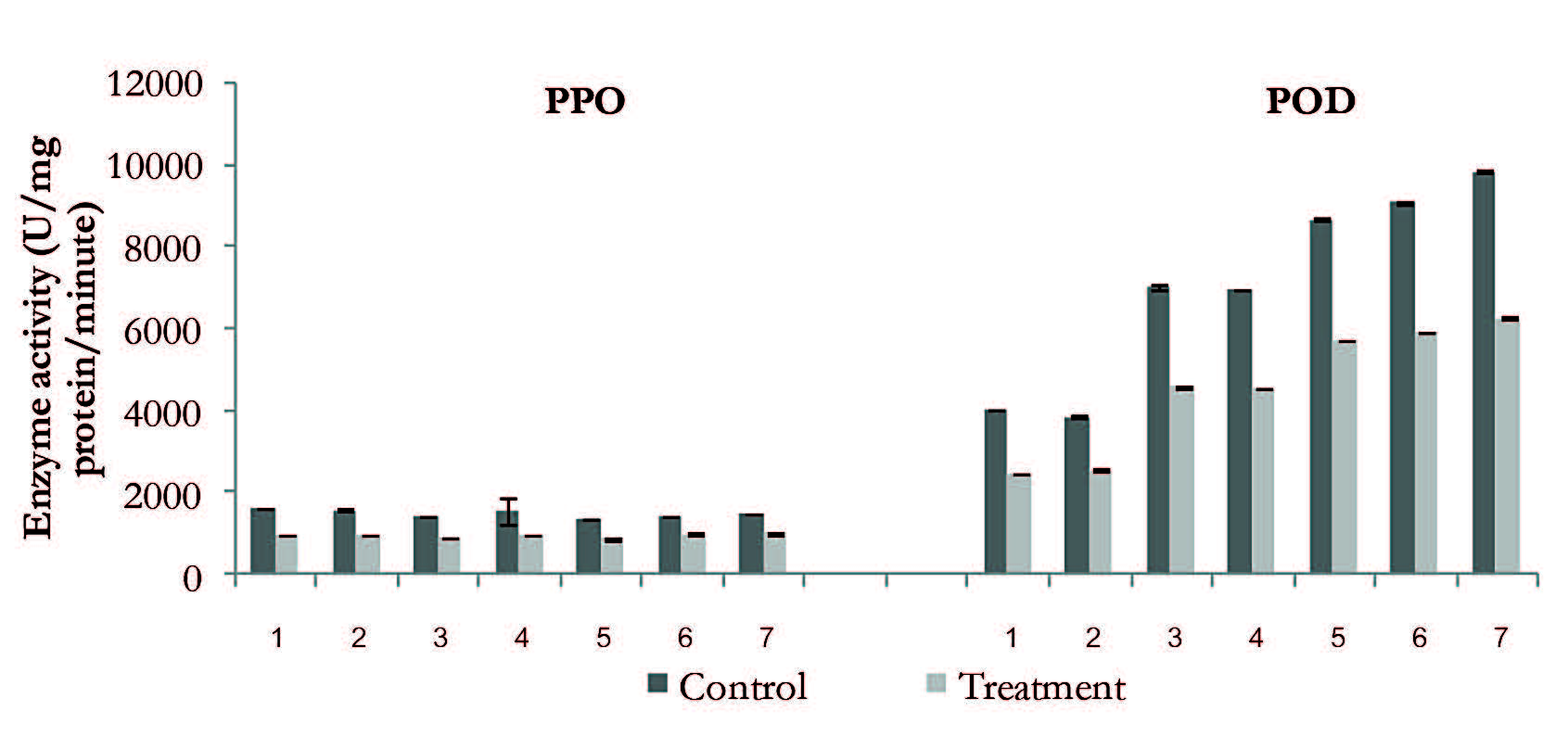
Figure 2. Changes in PPO and POD activities of litchi flesh during storage at -24°C for six months.
Microbial Analysis
The total microbial count of frozen mango flesh in the control and treated samples when initially stored at the dilution 10-1 was 6.5×101 and 5.0×101 colonies/g of fresh weight and for frozen litchi flesh in the control and treated samples was 2.5×101 and 2.5×101 colonies/gram of fresh weight, respectively. The number of microorganisms in both types of fruit slightly declined during the six months of storage.
Sensory evaluation
Sensory evaluations of frozen mango and litchi flesh were based on estimations of color (mango flesh) or shape (litchi flesh), texture, sweet taste, sour taste and overall acceptability, after six months of storage and thawing. Panelists scored the mango and litchi flesh in both experiments more than 6 and 5, respectively, implying the flesh after thawing was acceptable to the panelists. The sensory scores for the treated mango and litchi flesh were significantly higher than the control (P ≤0.05).
DISCUSSION
The effectiveness of 1% (w/v) citric acid in inhibiting enzymatic browning of the cut surface of fresh-cut mango was evaluated and compared, using various concentrations. The results showed that dipping the cutlet of fresh-cut mango in 1% (w/v) citric acid reduced the activity of both enzymes. This might be due to the low pH (less than 5) due to the citric acid solution, given the optimum pH for PPO and POD enzymes in mango pulp is in the range 5-7 (Garcia and D.M. Barrett, 2002). These results were consistent with the immersion of apple slices in a solution of 1.0% citric acid, which inhibited and decreased the activity of PPO to 20% (Suttirak and Manurakchinakorn, 2010). However, the reduction of PPO in all treatments did not show any significant differences (P≤0.05). This might be due to the minor differences in pH in the citric acid solution at 0.5, 1.0 and 1.5% (w/v) (2.9, 2.9 and 2.8, respectively). Lu et al. (2007) reported no significant differences (P> 0.05) in L* values (%) from apple slices among different citric acid concentrations (0.6-6.0 g/l) at 4 h.
A citric acid solution not only inhibited the activity of PPO, but also minimized the loss of chemical composition and inhibited discoloration on the surface of the fresh-cut chestnut (Jiang et al., 2003). Moreover, no brown spots formed in the treatments with antioxidant solutions, suggesting that peroxidase was not related to enzymatic browning. Although POD might also contribute to enzymatic browning, its function is still questionable (Garcia and Barrett, 2002).
Our results also revealed that weight loss was greater in non-treated fruit, while calcium treated fruit lost less weight during storage compared to fruit stored in the open (Figure 3 and 4). The dipping of litchi flesh in a solution of calcium chloride preserved its texture. Since calcium ions react with pectic acid, the formation of calcium pectates could help retain fruit firmness and provide resistance to softening (Goncalves et al., 2000). In addition, the calcium ion is an important element not only in stabilizing cells wall, but also in maintaining membrane structure. Therefore, the addition of calcium ions improved the firmness of fresh-cut fruit and retarded the softening of the flesh during storage. According to Mahmud et al. (2008), who dipped cutlets of fresh-cut papaya in 2.5% (w/v) calcium chloride and stored for 21 days at 13±1°C, calcium chloride inhibited firmness loss, extended shelf- life, prevented ripening and delayed senescence.
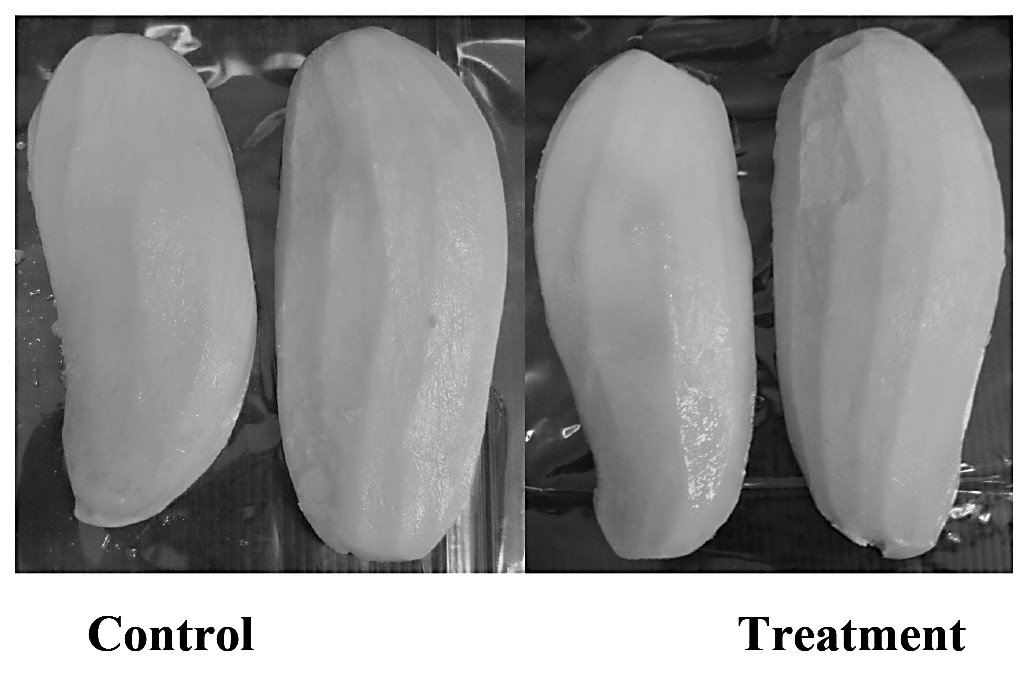
Figure 3. Thawed mango flesh after storage at -24°C for six months.
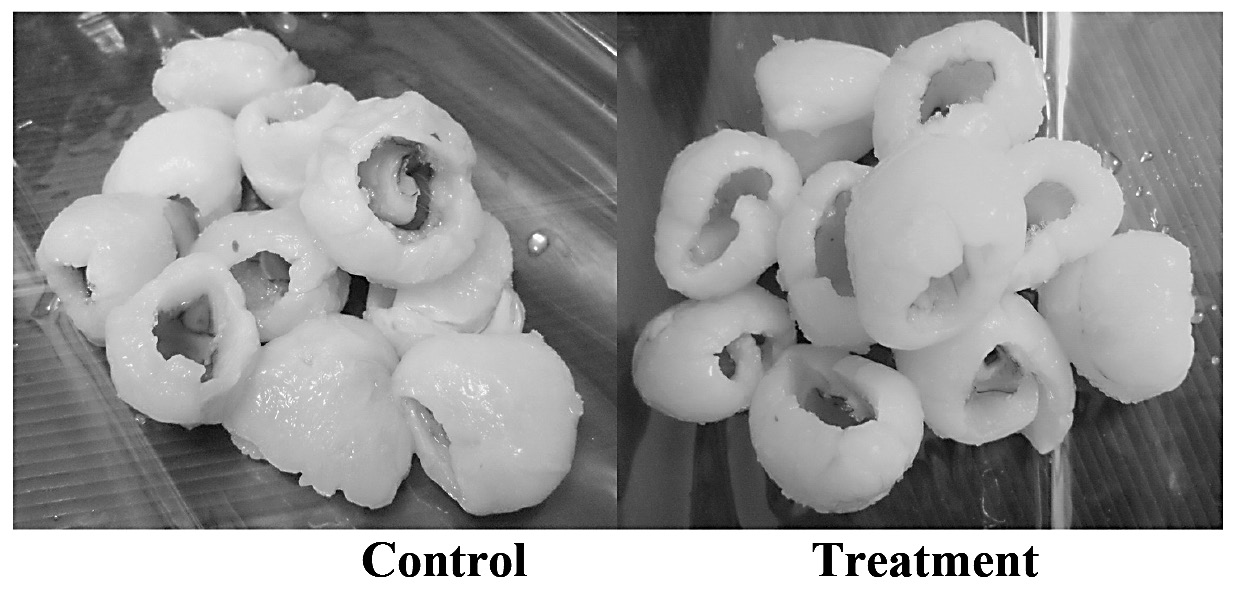
Figure 4. Thawed litchi flesh after storage at -24°C for six months.
However, the texture obtained from dipping the mango and litchi flesh in calcium chloride was not significantly different (p > 0.05) from the control. This might be due to the low level of pectin in litchi and mango flesh (0.4-0.5% and 0.5%, respectively), which results in low calcium pectin interaction (Mahattanatawee et al., 2006).
The cutlets of mango flesh after cryogenic freezing were evaluated for the quality changes during frozen storage. The flesh lost carotenoids. This could be due to the oxidative reaction on exposure to oxygen from packing the mango flesh in non-vacuum sealed polyethylene bags. Ramakrishnan et al. (2004) reported that the preservation of fruits by freezing is one of the most important methods for retaining high quality in agricultural products over long-term storage. Frozen storage produced a slight decrease in chemical content because low temperature effectively retarded biochemical changes in cells. Marin et al. (2005) found that frozen cutlets of mango flesh did not change significantly in amounts of reducing sugar and total sugar content during storage at -18°C for four months (P≥0.05). Dipping the cutlets of fresh-cut mango in the solution of citric acid and calcium chloride retarded PPO and POD activity. The frozen storage of mango could also inactivate enzymatic activity because the optimum temperature of PPO is 25°C (Sun et al., 2008). Moreover, the low temperature also retarded metabolism and respiration in the cells, thereby slowing the chemical changes that affect the quality of ripe mango. The temperature at the core of the mango flesh was -16 to -18°C during frozen storage. Cryogenic freezing with liquid nitrogen created ice crystallization on the surface of the flesh. This reduces the exposure of the fruit to oxygen and the oxidation of phenolic compounds (Piskarev and Bornovalova, 1969; Khadatkar et al., 2004).
The freezing process destroyed some microorganisms to levels lower than the standard maximum limit for frozen fruit (less than 106 colonies/gram) (FAO/WHO, 1981; Gilbert et al., 2000). Freezing the fruit inhibited the growth of microorganisms. During the freezing of food, water inside the food is frozen rapidly. Decreasing the amount of free water inside the fruit would increase the concentration of solution. This inhibited the growth of microorganisms, becausethe higher solute concentration outside the cells caused the to shrink. The lack of moisture available when the water freezes to ice prevented bacteria from multiplying. In addition, cold storage preservation at temperatures below -10°C killed some microbes (Torreggiani and Maestrelli, 2006).
CONCLUSION
The immersion of mango flesh cv. ‘Maha-Chanok’ into a solution of 1% (w/v) citric acid and 1% (w/v) calcium chloride or dipping litchi flesh cv. ‘Hong Huay’ in a 0.5% (w/v) calcium chloride solution improved the fruit firmness. The treatments were also the most effective at inhibiting both PPO and POD activity. Cryogenic freezing with liquid nitrogen also minimized the changes in fruit quality during frozen storage at -24ºC for six months.
ACKNOWLEDGEMENTS
The funding support of the Thailand Research Fund (TRF-MAG) and Postharvest Technology Institute, Chiang Mai University are greatly appreciated.
REFERENCES
Agnelli, M.E., and R.H. Mascheroni. 2002. Quality evaluation of foodstuffs frozen in cryomechanical freezer. Journal of Engineering 52: 257-263. DOI: 10.1016/S0260-8774(01)00113-3
AOAC. 2000. Official Methods of Analysis of the Association of Official Analytical Chemists, Inc., Washington, D.C.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248-254.
Delgado, A.E., and D.W. Sun. 2000. Heat and mass transfer for predicting freezing processes, a review Journal of Food Engineering 47: 157-174.
FAO/WHO. 1981. Microbiological criteria for foods summary of recommendations of FAO/WHO expert consultation and working group. Veterinary Public Health 83: 1-32.
Flurkey, W.H., and J.J. Jen. 1978. Peroxidase and polyphenol oxidase activities in developing peaches. Journal of Food Science 43: 1826-1828.
Garcia, E., and D.M. Barrett. 2002. Preservative treatments for fresh-cut fruit and vegetable. In Fresh-cut Fruits and Vegetables, Science, Technology and Market. Ed O. Lamikanra. pp. 267-304. CRC Press Inc., Boca Raton, Florida.
Gilbert, R.J., J. de Louvois, T. Donovan, C. Little, K. Nye, C.D. Ribeiro, J. Richards, D. Robert, and F.J. Botton. 2000. Public health laboratory service guidelines for the microbiological quality of some ready-to-eat foods sampled at the point of sale. Communicable Disease and Publicable Health 3: 163-167.
Goncalves, N.B., V.D. de Carvalho, and J.R. de A. 2000. Effect of calcium chloride and hot water treatment on enzyme activity and content of phenolic compounds in pineapples. Pesquisa Agropecuaria Brasileira 35: 2075-2081.
Jiang, Y.M., and Y.B. Li. 2003. Effects of low temperature acclimation on browning of litchi fruit in relation to shelf life. Journal of Horticultural Science and Biotechnology 78: 437-440.
Khadatkar, R.M., S. Kumar, and S.C. Pattanayak. 2004. Cryofreezing and cryofreezer. Cryogenics 44: 661-678. DOI: 10.1016/j.cryogenics.2004.03.008
Kidmose, U., and H.J. Martens. 1999. Changes in texture, microstructure and nutritional quality of carrot slices during blanching and freezing. Journal of the Science of Food and Agriculture 79: 1747-1753. DOI: 10.1002/(SICI)1097-0010(199909)79:12<1747::AID-JSFA429>3.0.CO;2-B
Kondratowicz, J., and P. Matusevicius. 2002. Use of low temperatures for food preservation. Veterinarija Ir Zootechnika 17: 88-92.
Lee, C.Y., and N.L. Smith. 1979. Blanching effect on polyphenoloxidase activity in table beets. Journal of Food Science 44: 82-86.
Lu, S., Luo, Y., Turner, E., and Feng H. 2007. Efficacy of sodium chlorite as an inhibitor of enzymatic browning in apple slices. Food Chem 104: 824-829. DOI: 10.1016/j.foodchem.2006.12.050
Mahattanatawee, K., J.A. Manthey, G. Luzio, S.T. Talcott, K. Goodner, and E.A. Baldwin. 2006. Total antioxidant activity and fiber content of select Florida-grown tropical fruits. Journal of Agricultural and Food Chemistry 54: 7355-7363. DOI: 10.1021/jf060566s
Mahmud, T.M.M., A. Al Eryani-Raqeeb, S.R. Syed Omar, A.R. Mohamed Zaki, and A.R. Al Eryani. 2008. Effects of different concentrations and applications of calcium on storage life and physicochemical characteristics of papaya (Carica Papaya L.). American Journal of Agricultural and Biological Sciences 3: 526-533.
Marín, M.A., P. Cano, and C. Füster. 2005. Freezing preservation of four Spanish mango cultivars (Mangifera indica L.). Chemical and Biochemical Aspects 194: 566-569.
Miller, G.L. 1959. Use of dinitrosaIicyIic acid reagent for determination of reducing sugar. Analytical Chemistry 31: 426-428.
Narciso, J., and A. Plotto. 2005. A comparison of sanitation system for fresh-cut mango. Horticultural Technology 15: 837- 842.
Piskarev, A.I., and A.P. Bornovalova. 1969. Investigation on quality changes during cold storage of fish frozen in liquid nitrogen, Frozen foods: products freezing techniques. Bull. Int. Inst. Refrig. Annexe; Commissions IV and V. pp. 151-154.
Pukszta, T., and P. Palich. 2007. The effect of freezing conditions of strawberry storage on the level of thawing drip loss. Acta Agrophysica 9: 203-208.
Ramakrishnan, S., R.A. Wysk, and V.V. Prabhu. 2004. Prediction of process parameters for intelligent control of tunnel freezers using simulation. Journal of Food Engineering 65: 23-31. DOI: 10.1016/j.jfoodeng.2003.05.001
Ranganna, S. 1986. Handbook of Analysis and Quality Control for Fruit and Vegetable Products 2nd ed., Tata McGraw-Hill, New Delhi.
Saengnil, K., K. Lueangprasert, and J. Uthaibutra. 2006. Control of enzymatic browning of harvested ‘Hong Huay’ litchi fruit with hot water and oxalic acid dips. Science Asia 32: 345-350. DOI: 10.2306/scienceasia1513-1874.2006.32.345
Salcini, M.C., and R. Massantini. 2005. Minimally Processed Fruits: an Update on Browning Control. Department of Food Science and Technology, University of Tuscia, Viterbo, Italy. DOI: 10.2212/spr.2005.3.7
Singleton, U.L., and J. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-posphotungustic acid reagent. American Journal of Enology and Viticulture 16(3): 144-158.
Souza, B.S., T.J. O’ Hare, J.F. Durugan., and P.S. Souza. 2006. Impact of atmostphere, oeganic acid and calcium on quality of fresh-cut ‘Kensington’ mango. Postharvest. Biol. Technol 2: 161-167.
Sun, J., J. Shi, M. Zhao, S.J. Xue, J. Ren, and Y. Jiang. 2008. A comparative and analysis property of lychee polyphenoloxidase using endogenous and exogenous substrates. Food Chemistry. 108: 818-823.
Suttirak, W., and S. Manurakchinakorn. 2010. Potential application of ascorbic acid, citric acid and oxalic acid for browning inhibition in fresh-cut fruits and vegetables. Walailak Journal of Science and Technology 7: 5-14.
Suutarinen, J., K. Seiska, P. Moss, and K. Auto. 2000. The effects of calcium chloride and sucrose prefreezing treatments on the structure of strawberry tissues. Lebensm.-Wiss. u.-Technology 33: 89-102.
Torreggiani, D., and A. Maestrelli. 2006. Quality and safety of frozen fruits. In Handbook of Frozen Food Processing and Packaging. Ed. D.W. Sun. pp. 417-440. CRC Press, Boca Raton.
Julaluk Tangtua1, Noppol Leksawasdi2 and Nithiya Rattanapanone1,3*
1 Postharvest Technology Research Institute, Chiang Mai University, Chiang Mai 50200, Thailand
2 Division of Food Engineering, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
3 Division of Food Science and Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
*Corresponding author. E-mail: agfsi001@gmail.com
Total Article Views

