
Antioxidant and anticancer activities from leaf extracts of four Combretum species from northern Thailand
Wimaluk Nopsiri*, Sunee Chansakaow, Somporn Putiyanan, Surapol Natakankitkul and Dammrong SantiarwornPublished Date : 2019-08-25
DOI : 10.12982/CMUJNS.2014.0031
Journal Issues : Number 2,May-August-2014
ABSTRACT
Four Combretum species (Combretaceae) from northern Thailand (Combretum deciduum, Combretum griffithii, Combretum latifolium and Combretum quadrangulare) were tested for antioxidant and anticancer activities. Antioxidant activities were assessed by ABTS and DPPH radical scavenging capacity methods. Anticancer activity was tested against three cancerous human cell lines (KB, MCF7 and NCI-H187). All methanolic leaf extracts showed antioxidant activities with the ABTS and DPPH methods. The methanolic leaf extracts of C. deciduum inhibited KB-oral cavity and MCF7-breast cancer cell lines, C. latifolium inhibited MCF7-breast cancer cell line and C. quadrangulare inhibited KB-oral cavity and NCI-H187-small cell lung cancer cell lines. However, the methanolic leaf extracts of C. griffithii were inactive against all three cell lines. All methanolic leaf extracts exhibited non-cytotoxicity to Vero cell lines.
Keywords: Combretum deciduum, Combretum griffithii, Combretum latifolium, Combretum quadrangulare, Antioxidant activity, Anticancer activity
INTRODUCTION
The genus Combretum belongs to the family Combretaceae. This genus of trees, woody climbers and shrubs is distributed in the tropics, including southern Africa, Asia and America. The genus is well known in folk medicine for its medicinal value. In southern Africa, Combretum is used to treat abdominal disorders, backaches, bacterial infections, bilharzia, cancer, coughing, the urinary system, colds, conjunctivitis, constipation, diarrhea, dysentery, dysmenorrhea, earaches, fever, gastric ulcers, general weakness, gonorrhea, headaches, heart disease, hookworm, hypertension, jaundice, leprosy, nose bleeds, pneumonia, skin diseases, sore throats, swelling caused by mumps, syphilis, toothaches, malaria and diabetes (Clarke, 1878; Banskota et al., 2003; Eloff et al., 2008 and Lima et al., 2012).
Previous research on the genus includes the antioxidant activities of C. decandrum Roxb. (DC) and C. duarteanum Cambess. and the anticancer activities and cytotoxicity of C. duarteanum, C. collinum Fresen., C. apiculatum Sond. subsp apiculatum, C. fragrans F. Hoffm., C. micranthum G. Don, C. padoides Engl. & Diels, C. hereroense Schinz, C. psidioides Welw. and C. zeyheri Sond. (Lima et al., 2012).
According to the Thai Forest Bulletin, 19 species of the genus Combretum are found in Thailand (Nanakorn, 1986). Four Combretum species grow commonly in northern Thailand: C. deciduum Coll. & Hemsl., C. griffithii Heur. & M.A., C. latifolium Bl. and C. quadrangulare Kurz. The leaves, stem bark, root and seeds of C. quadrangulare have been used in Thailand and other countries as traditional medicine as antihepatitis, antipyretic, antidysenteric and anthelmintic agents (Banskota et al., 2003). Several studies in Thailand on the root and seeds of C. quadrangulare reported anthelmintic activity (Somanabandhu, 1984 and Euswas et al., 1988), antibacterial activity (Nantachit et al., 2006) and toxicity (Nakornchai et al., 1987; 1994). C. latifolium has not been reported on, but its stem and fruits have been used by rural people as an astringent and for dysentery, dysmenorrhea and nourishing the blood and body. Locals have used a water decoction of the stem of C. griffithii as a traditional medicine for hepatitis (Moosophon et al., 2011). The use of C. deciduum as a traditional medicine has not been reported, and no study on C. deciduum has been found.
Given the leaves can be harvested easily without killing the plants and several prior studies of Combretum species also used the leaves (Pettit et al., 1987; Banskota et al., 1998; 2003; McGaw et al., 2001; Fyhrquist et al., 2002; Inngjerdingen et al., 2004; Karou et al., 2005; Eldeen et al., 2007; Maregesi et al., 2007; Eloff et al., 2008; Gronhaug et al., 2008 and Coulidiati et al., 2009), this study has selected the leaves for extractions.
No report has tested the antioxidant activity of the leaves of C. deciduum, C. griffithii, C. latifolium and C. quadrangulare. Only one of the four, C. griffithii, has been tested for anticancer activity. The purpose of this study, therefore, was to investigate these four species, focusing on validating their antioxidant and anticancer activities using leaf extracts from specimens growing in northern Thailand.
MATERIALS AND METHODS
Plant material
The plants were collected from convenient areas of several provinces in northern Thailand. C. latifolium specimens were collected in Wang Nua District, Lampang Province in December 2009. C. quadrangulare specimens were collected in Doi Saket District, Chiang Mai Province in June 2009. C. deciduum and C. griffithii specimens were collected in Mae Rim District, Chiang Mai Province in July 2010. The specimens were identified at the Chiang Mai University Herbarium, Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand. The voucher specimens were deposited in the Herbaria at the Faculty of Pharmacy and the Department of Biology, Faculty of Sciences, Chiang Mai University.
Plant extraction
The dried, powdered leaves of Combretum deciduum 264.35 g, C. griffithii 302.91 g, C. latifolium 611.29 g and C. quadrangulare 930.00 g were macerated in methanol for approximately 24 hours at room temperature and filtered. The marc was macerated again using the same procedure twice. The filtrates were then pooled and concentrated under reduced pressure until dry. In the same methods, n-hexane and dichloromethane extracts were prepared.
Antioxidant activity tests
The antioxidant activity of the crude plant extracts obtained from the above procedure was determined by the ABTS and DPPH methods.
ABTS method
The antioxidant activity of the crude extracts was investigated using the ABTS radical cation (ABTS•+) scavenging method following the methodology of Roberta et al. (1999) and compared with Trolox standards (concentration range 0.5-2.5 mM). For the ABTS method, 20 μl of crude plant extracts (0.1 g/ml) were mixed with 2.0 ml of diluted ABTS solution (A734nm = 0.700 ± 0.020) and the absorbance was determined at 734 nm after 5 minutes incubation at room temperature. A solvent blank was run in each assay. All determinations were carried out at least three times, and in triplicate. Inhibition of free radical by ABTS•+ in percent (I%) was calculated by the following equation:
I (%) = [ (Ablank – Asample) / Ablank ] × 100
Where Ablank is the absorbance of the control reaction and Asample is the absorbance of the test compound. The percentage inhibition of the absorbance at 734 nm was calculated and plotted as a function of the concentration of antioxidants and of Trolox and vitamin C for standard reference data.
DPPH method
A stock solution (5.0×10-4 mol/l) of DPPH was prepared by dissolving 10.0 mg in 50 ml 95% ethanol. This solution was stored at 4°C away from light, and was stable after a week. The DPPH working solution containing 1.0×10-4 mol/l was prepared by pipetting 50 ml of the stock solution into a 200 ml volumetric flask and diluting with 95% ethanol to volume. This working solution was prepared fresh daily and protected from light (Thongchai et al., 2009). The test sample (20 μl) was added to 180 μl of ethanolic DPPH solution in a 96-well microtiter plate. The reaction mixture was incubated at 37°C for 30 minutes, and then the absorbance of each well was measured at 540 nm. The DPPH solution was used as negative control. Trolox, vitamin C and quercetin were used as reference standards. For 50% inhibitory concentration (IC50) evaluation of the crude extracts, a graph showing concentration versus % DPPH reduction was plotted. The IC50 was calculated from the calibration curve and activity was expressed as the percentage DPPH scavenging relative to the control using the following equation:
DPPH scavenging activity (%) = [ (Acontrol - Asample) / Acontrol ] × 100
Where Acontrol is the absorbance of the control reaction and Asample is the absorbance of the test compound.
Anticancer activity tests
Anticancer method. The anticancer activity of the crude extracts was tested using three cancerous human-cell lines: KB cell line (epidermoid carcinoma of the oral cavity, ATCC CCL-17), MCF7 cell line (breast adenocarcinoma, ATCC HTB-22) and NCI-H187 (small cell lung carcinoma, ATCC CRL-5804). This test was determined by resazurin microplate assay (REMA) following a modified method using fluorescent dye for mammalian cell cytotoxicity according to O’Brien et al. (2000). Ellipticine, Doxorubicin and Tamoxifen were used as positive controls. 0.5% DMSO and sterile water were used as negative controls. In brief, cells at a logarithmic growth phase were harvested and diluted to 2.2×104 cells/ml for KB and 3.3×104 cells/ml for MCF7 and NCI-H187 in fresh medium. Successively, 5 μl of the methanolic extract was diluted in 5% DMSO and 45 μl of the cell suspension was added to 384-well plates and incubated at 37°C in 5% CO2 incubator. After the incubation period (3 days for KB and MCF7; 5 days for NCI-H187), 12.5 μl of 62.5 μg/ml resazurin solution was added to each well and the plates were then incubated at 37°C for 4 hours. Fluorescence signal was measured using a SpectraMax M5 multi-detection microplate reader (Molecular Devices, USA) at the excitation and emission wavelengths of 530 nm and 590 nm. The inhibitory concentration (IC50) represented the concentration that caused a 50% reduction in cancer cell line growth.
Cytotoxicity method. The cytotoxicity method conformed to published standard methods (BS-EN30993-5 and ISO10993-5) using Vero cell lines (African green monkey kidney; ATCC Cat. No. CCL-81) by the MTT cytotoxicity method. The cells were exposed to the sample for 24 hours over the concentration ranges of 1000-7.8 μg/ml. The results were shown by percent survival of cells at each concentration compared to control and IC50 values. The method was a modified version of conventional direct and indirect contact tests. The MTT method (Plumb et al., 1989) is a tetrazolium-dye based colorimetric microtitration assay. Metabolism-competent cells are able to metabolize the tetrazolium (yellow) to formazan (blue); this color change is measured spectrophotometrically with a microplate reader. It is assumed that metabolically deficient cells will not survive, thus the MTT method is also an indirect measurement of cell viability. The cells were seeded in a 96-well plate at a density of 3,000 cells/well, and incubated for 48 hours. The samples at various concentrations were added to the cells and incubated for 24 hours. The test samples were removed from the cell cultures and the cells were reincubated for a further 24 hours in fresh medium and then tested by the MTT method. Briefly, 50 μl of MTT in phosphate buffer saline (PBS) at 5 mg/ml was added to the medium in each well and the cells were incubated for 4 hours. Medium and MTT were then aspirated from the wells and formazan solubilized with 200 μl of DMSO and 25 μl of Sorensen’s Glycine buffer, pH 10.5. The optical density was read with a microplate reader (Molecular Devices) at a wavelength of 570 nm. The average of four wells was used to determine the mean of each point. The data were analyzed with the SoftMax Program (Molecular Devices) to determine the IC50 for each toxin sample. A dose-response curve was derived from eight concentrations in the test range using four wells per concentration. Results of toxic compounds are expressed as the concentration of sample required to kill 50% (IC50) of the cells compared to the controls.
RESULTS
Among the three extracts, the methanolic extracts of the four Combretum species showed the most potent antioxidant activity using three conventional standards (Trolox, vitamin C and quercetin) (Table 1 and 2). These methanolic extracts were subsequently investigated for anticancer activity (Table 3).
Antioxidant activity
The antioxidant activities of the extracts are shown in Table 1 and 2. Methanolic extracts had the most potent antioxidant activity, followed by n-hexane extracts and dichloromethane extracts.
For the ABTS method (Table 1), the highest antioxidant activity was obtained with methanolic extracts (9.85-22.61 mM Trolox/mg of extracts and 2.15-4.96 mM vitamin C/mg of extracts), followed by dichloromethane extracts (5.75-9.82. mM Trolox/mg of extracts and 1.25-2.14 mM vitamin C/mg of extracts) and n-hexane extracts (3.87-8.74. mM Trolox/mg of extracts and 0.83-1.90 mM vitamin C/mg of extracts).
Table 1. Antioxidant capacities of extracts from leaves of four Combretum species.
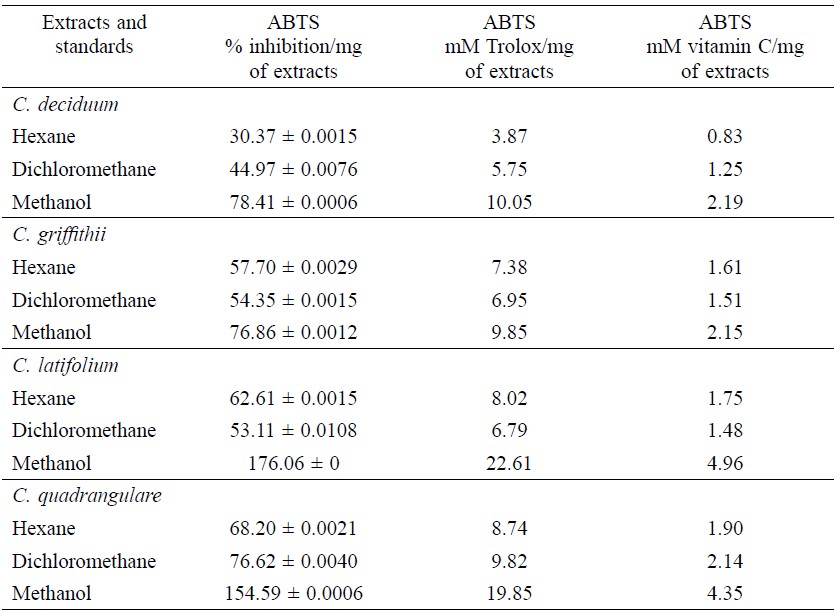
Note: NT = not tested. Values are given as mean ± S.D. of triplicate experiments. Values represent the significantly different results (p ≤ 0.05).
For the DPPH method (Table 2), the IC50 values of the n-hexane extracts were 0.52-6.53 μg/ml, the dichloromethane extracts were 1.52-5.93 μg/ml and the methanolic extracts were 0.13-0.81 μg/ml. The three standards showed antioxidant activity with the DPPH method with IC50 values of 0.06, 0.07 and 0.05 μg/ml, respectively.
Table 2. DPPH radical scavenging activity of extracts from leaves of four Combretum species.
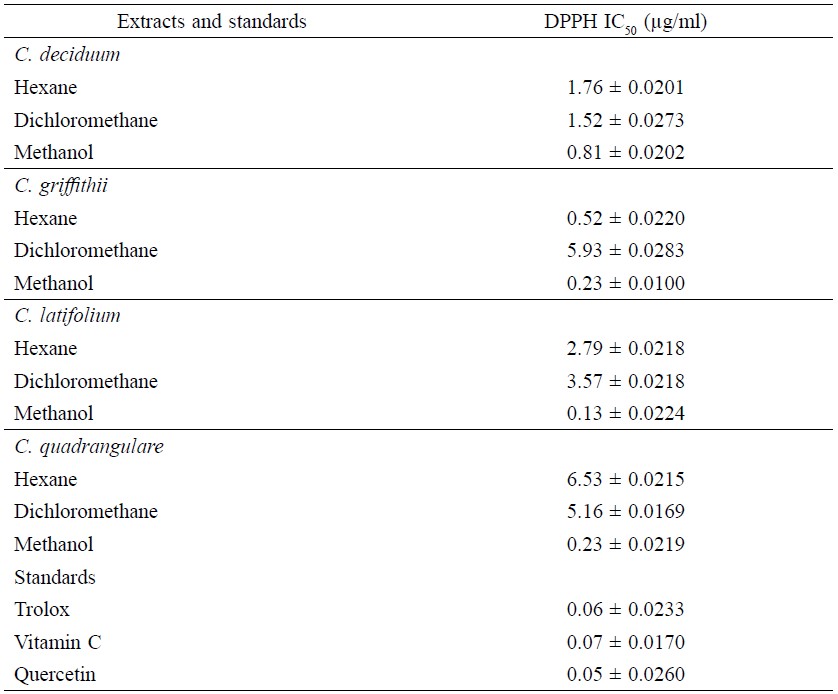
Note: NT = not tested. Values are given as mean ± S.D. of triplicate experiments. Values represent the significantly different results (p ≤ 0.05).
Anticancer Activity
The methanolic extracts of all four Combretum species exhibited significant anticancer activity against KB, MCF7 and NCI-H187 cell lines with IC50 (Table 3). The methanolic extract of C. deciduum inhibited KB and MCF7 cell lines with an IC50 value of 34.34 μg/ml and 28.84 μg/ml, respectively. The methanolic extract of C. latifolium inhibited MCF7 cell line with an IC50 value of 26.63 μg/ml. Methanolic extract of C. quadrangulare inhibited KB and NCI-H187 cell lines with IC50 values of 26.76 μg/ml and 46.88 μg/ml, respectively. However, methanolic extract of C. griffithii was inactive against all three cell lines. Ellipticine, Doxorubicin and Tamoxifen were used as standard compounds. All extracts were non-cytotoxic against Vero cell lines.
Table 3. Anticancer activity of methanolic extracts from leaves of four Combretum species.
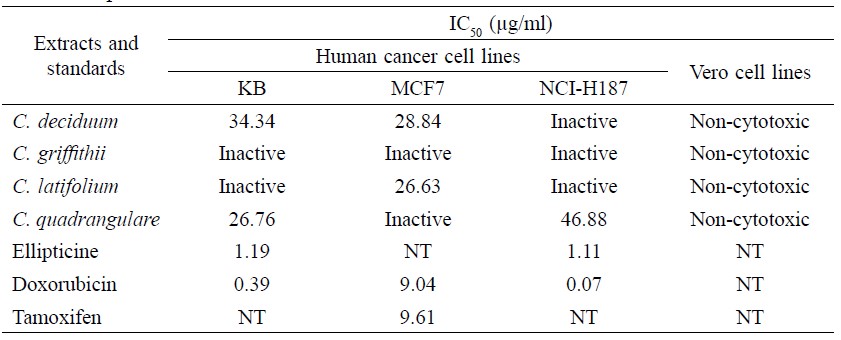
Note: 0.5% DMSO was used as negative control. IC50 > 50 μg/ml = inactive. NT = not tested.
DISCUSSION
The leaf extracts of four Combretum species showed antioxidant and anticancer activities, which suggests the presence of antioxidant and anticancer compounds in the leaves. The antioxidant activity of these four species is newly reported here. Anticancer activity on KB, MCF-7 and NCI-H187 cell lines of C. deciduum and C. latifolium are initially reported here.
Methanolic extracts of the four species displayed the most potent antioxidant activity against both ABTS•+ and DPPH• radicals. Methanolic extracts of C. latifolium leaves showed the highest antioxidant activity, suggesting that this extract is a rich source of antioxidants. The antioxidant activity of C. quadrangular, C. griffithii and C. deciduum followed. To study antioxidant activity, it is recommended to use at least two methods, as we did here. Our results showed that extracts with different polar compounds exhibit different antioxidant activities.
As traditional medicinal plants associated with anticancer uses might be potential sources of potent natural antioxidant, we also investigated these extracts for anticancer activity. The methanolic extracts from the leaves of C. deciduum, C. latifolium and C. quadrangulare exhibited anticancer activity against cancer cell lines (KB, MCF7 NCI-H187). However, the methanolic extract from the leaves of C. griffithii was inactive against all three cell lines. All methanolic leaf extracts were non-cytotoxic against Vero cell lines. It is generally accepted that free radicals react with biological molecules, leading to the possible development of cancer. Considerable laboratory evidence from chemical, cell culture and animal studies indicates that antioxidants may slow or possibly prevent cancer by stabilizing biological molecules and preventing damage to cells. The study confirmed that the methanolic extracts are the most potent in terms of their antioxidant, suggesting that the polar compounds residing in these extracts may be responsible. However, the anticancer activity of the plants could not be associated with these polar extracts, which have shown strong radical scavenging activity. These results suggest that free radical scavenging activity may not be the only mechanism preventing development of cancer.
Lima et al. (2012) reviewed the bioactivities of the genus Combretum, in which the authors stated that ethanolic leaf extract of C. decandrum showed antioxidant activity with IC50 value of 0.75 g/kg by ferrous ion oxidation-xylenol orange method in rats. And ethanolic leaf extracts of C. duarteanum possess a strong antioxidant potential by using thiobarbituric acid reactive species (TBARS), hydroxyl radical–scavenging and scavenging activity of nitric oxide assays. In the review, Lima et al. (2012) also stated that the ethanolic extracts of the leaves, root and stem of C. duarteanum showed anticancer activity against KB cells. Methanolic and ethanolic extracts of dried air parts of C. collinum exhibited anticancer activity against squamous carcinoma KB with IC50 value of 20.00 μg/ml and methanolic extracts of leaves and root exhibited anticancer activity against MCF7 breast cancer with IC50 value of 25.00 μg/ml. Methanolic extracts of leaves and root of C. apiculatum subsp apiculatum, C. fragrans, C. micranthum; methanolic extracts of stem bark and root of C. padoides; methanolic extracts of stem bark of C. hereroense and C. psidioides; and methanolic extracts of root and fruits of C. zeyheri inhibited MCF7 breast cancer cells with IC50 value of 25.00 μg/ml.
Our findings support the above research, having found similar antioxidant and anticancer properties for extracts from the genus Combretum. These results indicate that properties of Combretum species might be further explored in the search for new antioxidant and anticancer compounds. This work could be extended by testing other parts of the four species studied here, or expanding to additional species.
ACKNOWLEDGEMENTS
The authors wish to express their grateful thanks to the Faculty of Pharmacy and the Graduate School, Chiang Mai University for their financial support. We also thank J. F. Maxwell, CMU Herbarium, Department of Biology, Faculty of Sciences, Chiang Mai University, for identifying and curating the plant specimens.
REFERENCES
Banskota, A.H., Y. Tezuka, L.K. Phung, K.Q. Tran, I. Saiki, Y. Miwa, T. Taga, and S. Kadota. 1998. Cytotoxic cycloartane-type triterpenes from Combretum
quadrangulare. Bioorganic and Medicinal Chemistry Letters 8: 3519-3524. 10.1016/S0960-894X(98)00644-1
Banskota, A.H., Y. Tezuka, Q.L. Tran, and S. Kadota. 2003. Chemical constituents and biological activities of Vietnamese medicinal plants. Current Topics in Medicinal Chemistry 3: 227-248.
Clarke, C.B. 1878. Combretaceae, In Flora of British India Vol. p. 452-459. Brothers LTD, England.
Coulidiati, T.H., H. Millogo-Kone, A. Lamien-Meda, C.E. Lamien, M. Lompo, M. Kiendrebeogo, S. Bakasso, M. Yougbare-Ziebrou, J. Millogo-Rasolodimby, and O.G. Nacoulma. 2009. Antioxidant and antibacterial activities of Combretum nioroense Aubrev. ex Keay (Combretaceae). Pakistan Journal of Biological Sciences 12(3): 264-269. 10.3923/pjbs.2009.264.269
Eldeen, I.M.S., and J. van Staden. 2007. In vitro pharmacological investigation of extracts from some trees used in Sudanese traditional medicine. South African Journal of Botany 73: 435-440. 10.1016/j.sajb.2007.03.009
Eloff, J.N., D.R. Katerere, and L.J. McGaw. 2008. The biological activity and chemistry of the southern African Combretaceae. Journal of Ethnopharmacology 119: 686-699. 10.1016/j.jep.2008.07.051
Euswas, P., S. Srirod, P. Chunthanon, and T. Chompoochant. 1988. Studies on anthelmintic activity of Sakae naa (Combretum quadrangulare Kurz) seeds on roundworms of buffalo calves. Kasetsart University Journal 22: 201-206.
Fyhrquist, F., L. Mwasumbi, C.-A. Haeggström, H. Vuorela, R. Hiltunen, and P. Vuorela, 2002. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) in Tanzania. Journal of Ethnopharmacology 79: 169-177. 10.1016/S0378-8741(01)00375-0
Gronhaug, T.E., S. Glaeserud, M. Skogsrud, N. Ballo, S. Bah, D. Diallo, and B.S. Paulsen. 2008. Ethnopharmacological survey of six medicinal plants from Mali, West-Africa. Journal of Ethnobiology and Ethnomedicine 4(26): 1-11. 10.1186/1746-4269-4-26
Inngjerdingen, K., C.S. Nergard, D. Diallo, P.P. Mounkoro, and B.S. Paulsen. 2004. An ethnopharmacological survey of plants used for wound healing in Dogonland, Mali, West Africa. Journal of Ethnopharmacology 92: 233-244. 10.1016/j.jep.2004.02.021
Karou, D., M.H. Dicko, J. Simpore, and A.S. Traore. 2005. Antioxidant and antibacterial activities of polyphenols from ethomedicinal plants of Burkina Faso. African Journal of Biotechnology 4(8): 823-828.
Lima, G.R.M., I.R.P. Sales, M.R.D.C. Filho, N.Z.T. Jesus, H.S. Falcão, J.M. Barbosa-Filho, A.G.S. Cabral, A.L. Souto, J.F. Tavares, and L.M. Batista. 2012. Bioactivities of genus Combretum (Combretaceae): A review. Molecules 17: 9142-9206. 10.3390/molecules17089142
Maregesi, S.M., O.D. Ngassapa, L. Pieters, and A.J. Vlietinck. 2007. Ethnopharmacological survey of the Bunda district, Tanzania: Plants used to treat infectious diseases. Journal of Ethnopharmacology 113: 457-470. 10.1016/j.jep.2007.07.006
McGaw, L.J., T. Rabe, S.G. Sparg, A.K. Jager, J.N. Eloff, and J. van Staden. 2001. An investigation on the biological activity of Combretum species. Journal of Ethnopharmacology 75: 45-50. 10.1016/S0378-8741(00)0045-0
Moosophon, P., S. Kanokmedhakul, and K. Kanokmedhakul. 2011. Diarylpropanes and an arylpropyl quinone from Combretum griffithii. Journal of Natural Products 74: 2216-2218. 10.1021/np200593d
Nakornchai, S., R. Temsiririrkkul, Y. Wongkrajang, and K. Atisook. 1994. Toxicity study of Combretum quadrangulare Kurz (part II). Mahidol University Journal of Pharmaceutical Science 21(4): 118-125.
Nakornchai, S., R. Temsiririrkkul, Y. Wongkrajang, P. Jongtaveesuk, and P. Junjamjang. 1987. Toxicity study of Combretum quadrangulare Kurz I. Mahidol University Journal of Pharmaceutical Science 14(4): 69-74.
Nanakorn, W. 1986. The genus Combretum (Combretaceae) in Thailand. Thai Forest Bulletin 16: 154-204.
Nantachit, K., D. Santiarvorn, and B. Khantawa. 2006. Antibacterial activity of the seeds of Combretum quadrangulare Kurz (Combretaceae). Chiang Mai University Journal 5: 333-339.
O’Brien, J., I. Wilson, T. Orton, and F. Pognan. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry 267: 5421-5426.
Pettit, G.R., G.M. Cragg, and S.B. Singh. 1987. Antineoplastic agent, 122. constituents of Combretum caffrum. Journal of Natural Products 50(3): 386-391.
Plumb, J.A., R. Milroy, and S.B. Kaye. 1989. Effect of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltretrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium base assay. Cancer Research 49: 4435-4440.
Premjet, D., S. Premjet, R.A. Ario Lelono, and S. Tachibana. 2010. Callus induction and determination of iridoid glycosides from Barleria prionitis Linn leaf explants. Australian Journal of Basic and Applied Sciences 4(9): 4461-4467.
Roberta, R., P. Nicoletta, P. Anna, P. Ananth, Y. Min, and R.-E. Catherine. 1999. Antioxidant activity applying an improved ABTS radical cation decororization assay. Free Radical Biology and Medicine 26: 1231-1237.
Somanabandhu, A. 1984. Thai crude drugs their preparations and specifications (Combretum quadrangulare Kurz, Sakae naa). Mahidol University Journal of Pharmaceutical Science 11(1): 1-7.
Thongchai, W., B. Liawruangrath, and S. Liawruangrath. 2009. Flow injection analysis of total curcuminoids in turmeric and total antioxidant capacity using 2,2-diphenyl-1-picrylhydrazyl assay. Food Chemistry 122: 494-499. 10.1016/j.foodchem.2008.05.083
Ueda, J., Y. Tezuka, A.H. Banskota, Q.L. Tran, Q.K. Tran, Y. Harimaya, I. Saiki, and S. Kadota. 2002. Antiproliferative activity of Vietnamese medicinal plants. Biological and Pharmaceutical Bulletin 25(96): 753-760. 10.1248/bpb.25.753
Wimaluk Nopsiri*, Sunee Chansakaow, Somporn Putiyanan, Surapol Natakankitkul and Dammrong Santiarworn
Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: wimsiri@hotmail.com, tksiri@yahoo.com
Total Article Views

