
Biotechnological Valorization of Cashew Apple: A Review
Trakul Prommajak,Noppol Leksawasdi and Nithiya Rattanapanone*Published Date : 2019-08-25
DOI : 10.12982/CMUJNS.2014.0029
Journal Issues : Number 2,May-August-2014
ABSTRACT
Cashew apple, the peduncle of cashew fruit, is an agricultural waste byproduct from harvesting cashew nuts. Cashew apple juice contains about 10% reducing sugar. Its bagasse contains about 20% of cellulose. The byproducts can be used as a substrate for several microbial fermentation processes. Wine and bioethanol were produced by Saccharomyces cerevisiae. Probiotic beverage and lactic acid were produced by Lactobacillus casei. Biosurfactants-rhamnolipids, emulsan and surfactin were synthesized by Pseudomonas aeruginosa, Acinetobacter calcoaceticus and Bacillus subtilis, respectively. Tannase and pectinase were produced during solid-state fermentation of Aspergillus spp. Prebiotic oligosaccharides were synthesized by the activity of dextransucrase produced by Leuconostoc spp. Cashew apple is a potential substrate for producing a variety of products, depending on the type of microorganisms used.
Keywords: Cashew apple, Ethanol, Biosurfactant, Beverage, Enzyme, Oligosaccharide
CASHEW APPLE
Cashew (Anacardium occidentale) is a tropical evergreen tree cultivated in a range of countries, including India, Vietnam, Brazil and Thailand (Clay, 2004). It is grown for the cashew nut industry. The peduncle, or cashew apple (Figure 1), is a waste byproduct of the cashew nut harvest. The cashew apple contains about 10 g of total sugar and 200 mg of ascorbic acid per 100 ml juice, as shown in Table 1 (Figueiredo et al., 2002; Attri, 2009). Most cashew apple is left in the field as agricultural waste (Figure 2). The weight of the leftover cashew apple is about 10 times of the harvested nuts (Attri, 2009). Global production of cashew nuts was 1.6 million tons in 2000, implying almost 16 million tons of cashew apples were underutilized.
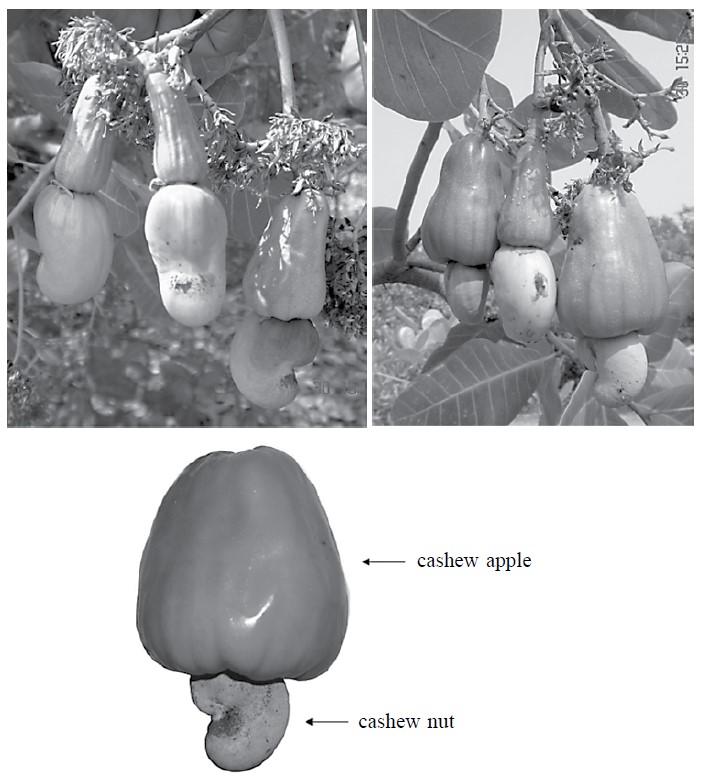
Figure 1. Cashew fruit, cashew apple and cashew nut.
Table 1. Chemical composition of cashew apple juice and bagasse.
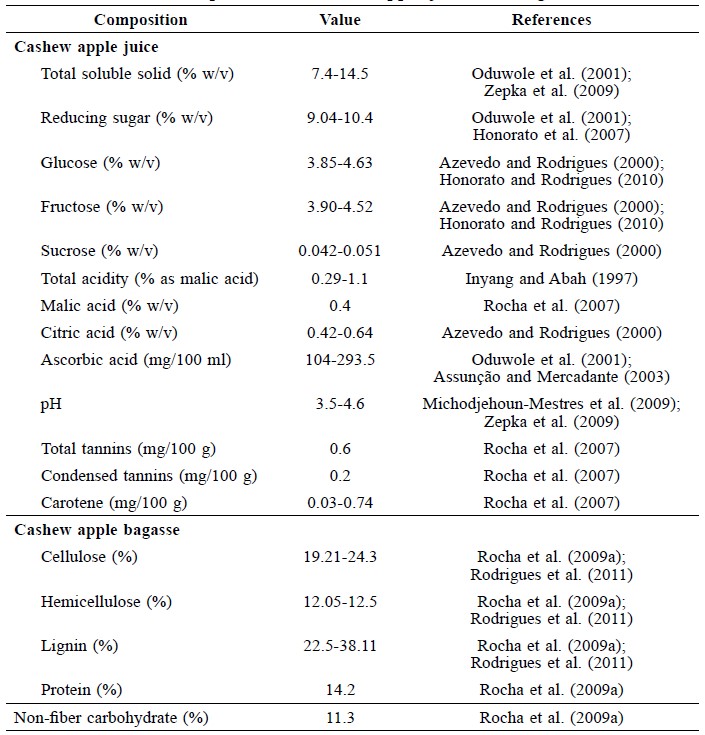

Figure 2. Cashew apple waste produced during harvesting of the cashew nut.
Cashew apples have the potential to be processed into juice, syrup, jam, ice cream, candy, chutney, pickle, and other products (Rabelo et al., 2009). Cashew apples can also be utilized through biotechnology, which depending on the substrates and microorganisms can yield a variety of products.
This review aims to summarize the current research regarding the potential of cashew apples to be fermented into different products, including: wine, bioethanol, enzymes, biosurfactants, probiotic beverages, lactic acid and oligosaccharides (Figure 3).
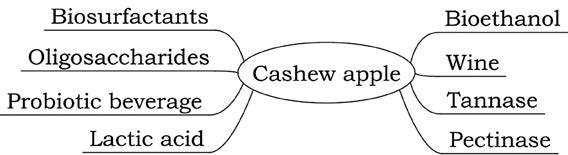
Figure 3. Potential products from fermentation of cashew apple.
PRE-FERMENTATION TREATMENT
The cashew apple can initially be decontaminated by washing in 100 ppm chlorine water before juice extraction (Muir-Beckford and Badrie, 2000).
Tannins are a group of phenolic compounds that can form strong complexes with proteins and other macromolecules. The cashew apple contains about 0.6 mg tannins/100 g juice (Rocha et al., 2007). The tannins can form complexes with salivary protein and glycoprotein, resulting in astringency (Fontoin et al., 2008). Ingested tannin could inhibit digestive enzymes and affect the utilization of nutrients (Chung et al., 1998a). However, tannins also have beneficial health effects, including: acceleration of blood clotting, reduction of blood pressure, treatment of burn wounds, modulation of immune response as well as antimicrobial and anticarcinogenic properties (Chung et al., 1998b; Chokotho and Hasselt, 2005). Removal of tannins from cashew apples can be accomplished by adding proteins (e.g., gelatin) or starch (e.g., cassava starch, rice gruel, sago), followed by filtration or siphoning (Jayalekshmy and John, 2004; Cormier, 2008). Among these tannin-precipitating agents, gelatin was the most commonly used. However, different levels of gelatin (ranging from 0.3 to 1.0% w/v) have been reported. The cost-effective amount of gelatin for precipitating tannins in cashew apples should be evaluated.
Pectinase can be added to increase the extraction yield and clarification of fruit juice (Gummadi et al., 2007). Pectinase is a group of enzymes, composed of pectin lyase, pectinesterase and polygalacturonase. However, pectin degradation caused by pectinesterase during fermentation releases methanol into the products. For example, application of pectinase (Rapidase ADEX-D at 100 g/ton) in apple juice increased methanol content in apple spirit from 51.9 mg/100 ml (no pectinase treatment) to 398.7 mg/100 ml, higher than the United States FDA limit for fruit spirits at 280 mg/100 ml (Zhang et al., 2011). Increasing of methanol content could be prevented by the use of pectin lyase instead of mixed pectinase containing pentinesterase (Wu et al., 2007).
Due to its high mineral content, adding minerals to cashew apple juice may not be necessary for production of dextransucrase, which is used for the synthesis of dextran from sucrose (Rabelo et al., 2009; Honorato and Rodrigues, 2010). Must has been nourished before wine fermentation by adding Becoplex (consisting of 10 mg vitamin B1, 3 mg B2, 1 mg B6 and 50 mg vitamin C), which served as coenzymes for the microorganism. The B-complex vitamins were essential for lactic acid bacteria, because the microorganism cannot synthesize them. Diammonium phosphate, a widely used assimilable nitrogen for wine yeast, can be added at 2.2% (Muir-Beckford and Badrie, 2000; Ribéreau-Gayon et al., 2006).
Depending on the microorganisms used in fermentation, the pH of the medium may be adjusted to the optimum pH of the microorganisms. In cashew wine production, the pH of must was adjusted down from pH 4.7-5.1 to pH 3.5 with citric acid (Muir-Beckford and Badrie, 2000). The pH of fermentation media were adjusted to 7.0 for production of biosurfactants from cashew apple juice by Pseudomonas aeruginosa and Acinetobacter calcoaceticus (Rocha et al., 2006; Rocha et al., 2007).
Elimination of wild microorganisms before wine fermentation can be accomplished by adding 50 ppm sodium metabisulfite (Muir-Beckford and Badrie, 2000). However, sodium bisulfate may cause off-flavor in wine and was also banned in the United States due to health concerns about the sodium content (Rivard, 2009). Potassium metabisulfite, with lower sulfur dioxide content, can be used instead (Sanchez, 2008). Filtration of the juice through 0.45 μm filter or exposing to ultraviolet radiation for 1 h can also be used (Rocha et al., 2006; Rocha et al., 2007). However, turbidity and juice color may interfere with exposure to ultraviolet light. Therefore, any ultraviolet process should be followed by filtration through 0.2 μm membrane for sterilization purposes (Udeh, 2004).
WINE AND BIOETHANOL
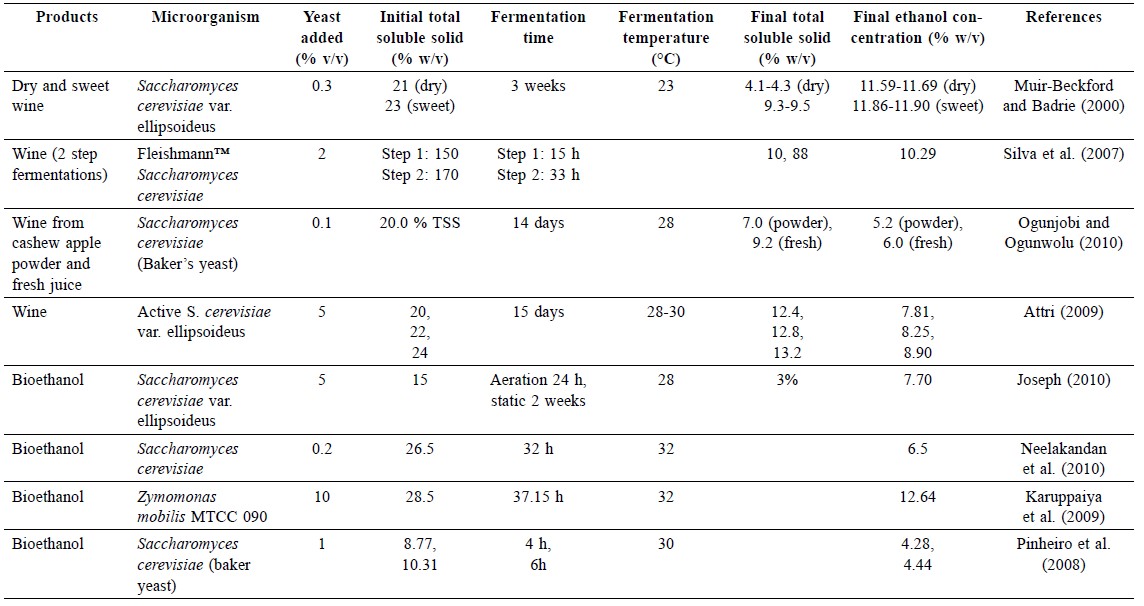
Cashew apple juice contained about 10% (w/v) of total sugar. Production of bioethanol from this level of sugar resulted in about 4.4% (w/v) of final ethanol concentration (Pinheiro et al., 2008). However, for production of cashew apple wine, initial sugar content was usually adjusted to above 20% (w/v) by adding sucrose to obtain a higher final ethanol concentration. The size of yeast inocula ranged between 0.1 to 12% (v/v) (Sudheer Kumar et al., 2009; Ogunjobi and Ogunwolu, 2010). However, an inoculum size of 105 cells/ml was desirable for wine making, because it provided high concentration of esters, lactones and free monoterpenes, while higher alcohols and medium chain fatty acids were less than other inoculum sizes (Carrau et al., 2010). Fermentation usually took place under ambient temperature for at least two weeks under static conditions. However, fermentation time depended on the fermentation temperature. For example, wine fermentation at 15°C required 500 h to reach dryness (less than 2 g sugar/l), while fermentation at 28°C required only 184 h (Molina et al., 2007). Final ethanol concentration ranged between 5 to 12% (Muir-Beckford and Badrie, 2000; Silva et al., 2007; Attri, 2009; Ogunjobi and Ogunwolu, 2010). Figure 4 shows the process for producing cashew apple wine. Fermentation conditions, initial sugar concentration and final ethanol concentration of cashew apple wine and ethanol are shown in Table 2.
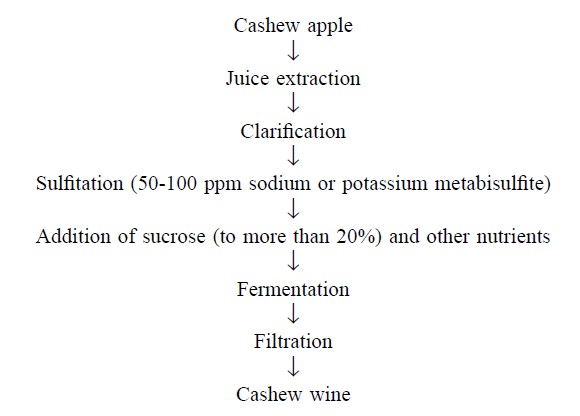
Figure 4. Processing diagram of cashew apple wine.
Osme GC-olfactory analysis revealed that the sweet, fruity and cashew-like aroma of cashew apple wine was contributed by ester compounds, mainly methyl 3-methyl butyrate, ethyl 3-methyl butyrate, methyl butyrate, ethyl butyrate, trans-ethyl crotonate and methyl 3-methyl pentanoate. The sweaty odor of 2-methyl butanoic acid was a primary reason for the unpleasant characteristic of the wine (Garruti et al., 2006b). Fermenting the wine at 18°C produced higher concentrations of fruity and sweet flavor compounds and lower concentrations of undesirable compounds when compared with fermentation at 30°C (Garruti et al., 2006a).
Cashew apple wine could also be produced from dried cashew apple. Because cashew apple is highly perishable and not available throughout the year, preservation of cashew apple can be accomplished by drying and grinding into cashew apple powder. This powder can be mixed with water at 75 g/L to prepare must for wine fermentation, which had initial total soluble solids of 20.0%. Alcohol content of wine from cashew apple powder was 7.0% v/v, lower than wine from fresh cashew apple juice (9.2% v/v). Although wine from cashew apple powder was light brown in color, its sensory scores were comparable to wine from fresh cashew apple juice and higher than commercial kola wine, cocoa wine and tea wine (Ogunjobi and Ogunwolu, 2010).
Cashew juice extraction leaves bagasse of about 20% of the total fruit weight. This bagasse contains 19-24% cellulose, 12% hemicelluloses and 22-38% lignin on a dry-weight basis (Rocha et al., 2009a; Rodrigues et al., 2011). Cellulose is a polymer of glucose units linked by β-glycosidic bond that can be hydrolyzed by β-glycosidase. Glucose obtained from enzymatic hydrolysis can be used for ethanol production. However, the cellulose molecules are naturally packed in a crystalline structure and associated with hemicelluloses and lignin. As a result, the cellulose molecules are inaccessible for enzymatic hydrolysis. Thus, pretreatment is required for removal of lignin to improve enzymatic saccharification (Laxman and Lachke, 2009).
Table 2. Processing conditions and final ethanol concentration of cashew apple wine and bioethanol.
Many pretreatment methods were introduced to improve enzymatic hydrolysis of cellulose. Steam explosion is widely used in the industry. Among chemical treatments, alkaline treatment is the most successful. Pretreatment of cashew apple bagasse in alkaline solution was shown to be effective for increase the availability of cellulose for enzymatic hydrolysis. Pretreatment of cashew apple bagasse by autoclaving (121°C, 15 min) in 0.8 M sulfuric acid followed by autoclaving in 4% sodium hydroxide solution for 30 min was more effective than using the acid solution alone. The autoclave was vented within 10 min of the cycle end. Cellulase released 52.4 g/L of glucose from a mixture containing 16% w/v of alkaline treated bagasse. After fermentation for 6 h, 20 g/L of ethanol was obtained (Rocha et al., 2009a).
Cashew apple bagasse contains about 12% hemicelluloses. Xylose is the most abundant monomer unit of the hemicelluloses. However, native strains of Saccharomyces cerevisiae cannot utilize xylose. But some native strains of Pichia, Candida and Kluyveromyces, as well as genetically modified S. cerevisiae strain, can convert xylose to ethanol (Rocha et al., 2011).
ENZYMES
Tannase
Tannase, or tannin acyl hydrolase (EC 3.1.1.20), is an enzyme that catalyzes the hydrolysis reaction of hydrolysable tannin and gallic acid esters. The products of the reaction are gallic acid and glucose, which can be utilized by microorganisms for energy metabolism (Rodrigues et al., 2008). Tannase is widely produced by the fungi in the genus of Aspergillus and Penicillium. Some yeast and bacteria also have tannase producing capability. Tannase has been used for production of gallic acid – a substrate for the manufacturing of propyl gallate and trimethoprim. Tannase has also been used for clarification of wine and fruit juices to prevent haze formation and sedimentation (Belur and Mugeraya, 2011).
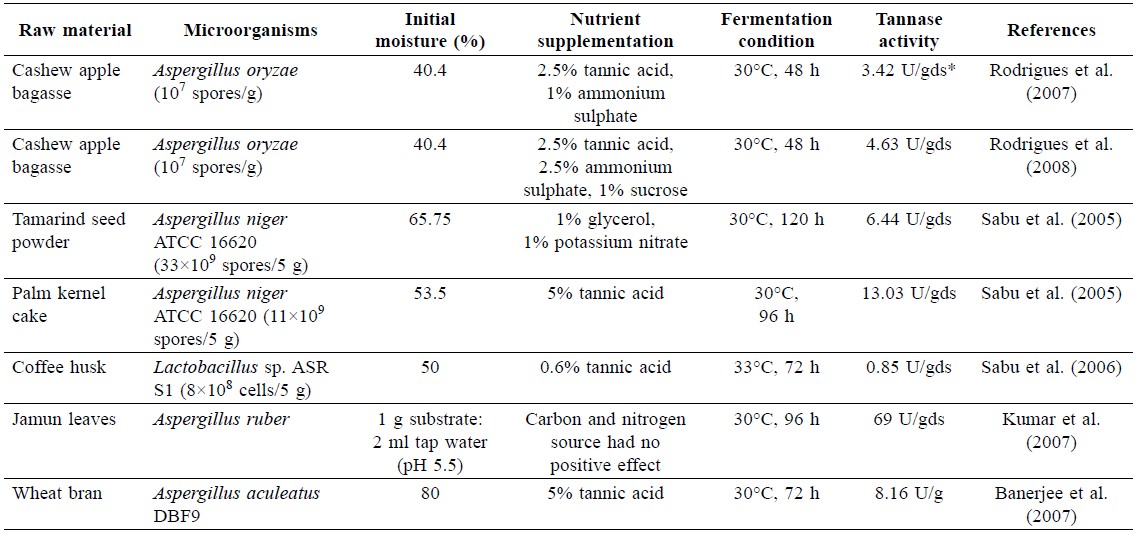
Tannase production from cashew apple bagasse can be acheived by solid-state fermentation of Aspergillus oryzae. The optimal moisture content for producing tannase was about 40%. Higher or lower moisture content decreased the enzyme production rate. Microbial production of tannase required an inducer-tannin. Due to the presence of tannin in cashew apple (0.64 mg/100 g cashew apple pulp), tannase activity was detectable after inoculation of the fungi (Campos et al., 2002). One unit of tannase activity was the amount of enzyme that catalyzed the production of 1 μmol of gallic acid/min under assay condition. However, addition of tannic acid at 2.5% w/w increased tannase activity more than fourfold. Supplementation with higher concentrations of tannic acid caused growth inhibition, resulting in less enzyme synthesis. Organic nitrogen sources such as peptone and yeast extract had no effect on enzyme synthesis due to complex formation between tannin and protein. In contrast, an inorganic counterpart, e.g. ammonium sulphate, increased enzyme production. Supplementation with ammonium sulphate at 2.5% was suitable for better productivity of tannase. Tannase activity and productivity reached its maximum (3.42 U/gds and 0.128 U/gds•h, respectively) at fermentation times between 24 to 48 h, before decreasing thereafter (Rodrigues et al., 2007).
Inoculum size also played an important role in tannase production, like other products produced by solid-state fermentation. Increasing size of inocula helped improve enzyme production. A temperature range between 30-35°C was suitable for tannase production by A. oryzae. Moreover, tannase activity was also increased by supplementation with sucrose and starch, but not glucose (Rodrigues et al., 2008). However, tannase produced from cashew apple bagasse was lower than tamarind seed, wheat bran or jamun leaves (Table 3).
Pectinase
Pectin or pectic substances are complex polysaccharides containing galacturonic acid as a basic monomer. The carboxyl groups of some galacturonic acids are methylesterified, with the degree of methoxylation used to determine the quality of pectin. Pectinases are a group of enzymes that catalyze the reaction for degrading pectic substances. Pectinase are divided into three groups: (1) protopectinases that degrade insoluble protopectin to polymerized soluble pectin; (2) esterases that act on the ester linkage and depolymerase that acts on the main polymer chain and (3) depolymerases that hydrolyse glycosidic bonds between galacturonic acid moieties and play a major role in pectin breakdown during fruit ripening (Jayani et al., 2005). Pectinases have many uses in the food industry, including clarification of fruit juice, extraction of juice and oil and treatment of wastewater (Gummadi et al., 2007).
Pectin esterase can be prepared by solid-state fermentation of fruit waste containing pectin, e.g. cashew apple, banana, pineapple and grape, by Aspergillus sp. The cashew apple bagasse was dried to a moisture content of 8 to 10% (w/w) and inoculated with A. foetidus at 2x107 spore/g for 6 days. A combination of urea and ammonium sulphate (1.5% and 5% of waste mass, respectively) was a suitable nitrogen source for growth of the fungi in cashew apple. The highest activity of pectin esterase in cashew apple waste (0.29 U/mg) was obtained by a fermentation temperature of 40°C for 8 days. However, the enzyme activity was lower than that prepared from grape waste (0.35 U/mg), but higher than a mixture of orange bagasse and wheat bran (0.071 U/mg) (Silva et al., 2005; Venkatesh et al., 2009).
Many factors influenced polygalacturonase production by Aspergillus niger CCT0916 in cashew apple bagasse. Moisture content positively effected polygalacturonase and pectinolytic activities (study range was between 30 to 50% wb). Ammonium sulphate (range from 0.5 to 1.5%) negatively effected enzyme production (Alcântara et al., 2010). In another study, treatment using an ammonium sulphate concentration of 1.5% resulted in the highest polygalacturonase activity, although this treatment also included other factors, including spore concentration of 106 spores/g medium, temperature of 35°C and fermentation period of 29 h (Alcântara and da Silva, 2011).
Various solvents can extract the enzyme from the fermentation medium. Distilled water was better than calcium chloride solution for extracting pectin esterase (Venkatesh et al., 2009). For polygalacturonase, 200 mM acetate buffer pH 4.5 was used (Alcântara et al., 2010). Water and acetate buffer were not compared for cashew apple. However, for extracting polygalacturonase fermented from wheat bran, water was better than acetate buffer for extracting the enzyme produced by Aspergiilus carbonarius (Singh et al., 1999). In another study, acetate buffer was better than water for extracting the enzyme produced by Aspergillus niger. These contradictory results may be due to extraction time and temperature, which significantly affected enzyme activity (Castilho et al., 2000). Nevertheless, adding sodium sulphate to either water or acetate buffer increased enzyme recovery (Singh et al., 1999).
Table 3. Tannase produced from cashew apple bagasse compared with other substrates.
BIOSURFACTANTS
Surfactants are surface-active compounds that can decrease superfacial and interfacial tension between solids, liquids and gases (Rocha et al., 2009b). Currently, most surfactants are chemically synthesized, resulting in toxic and non-biodegradable compounds. Biosurfactants produced by various microorganisms offer more environmentally friendly alternatives. Examples of biosurfactants are shown in Figure 5. Biosurfactants can be used in food, pharmaceutical and environmental applications as emulsifying, foaming, detergency, wetting, dispersing and solubilizing agents (Rocha et al., 2006). However, barriers to their use include high cost and low yield. Lower cost substrates and simpler substrates that reduce purification steps could help counter this. The yield problem could be overcome by process optimization (Makkar et al., 2011). Cashew apple, an agro-industrial waste, is a potential substrate for producing biosurfactants.
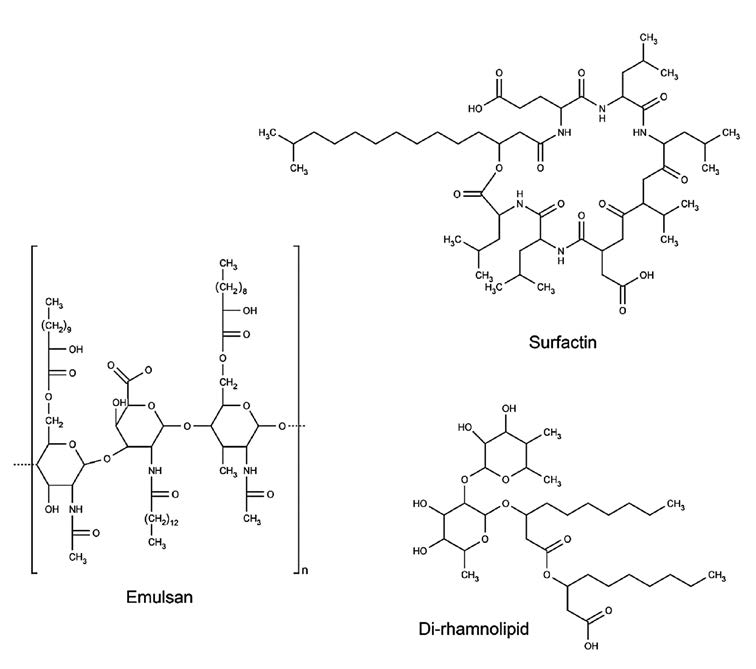
Figure 5. Examples of biosurfactants (adapted from: Banat et al., 2010).
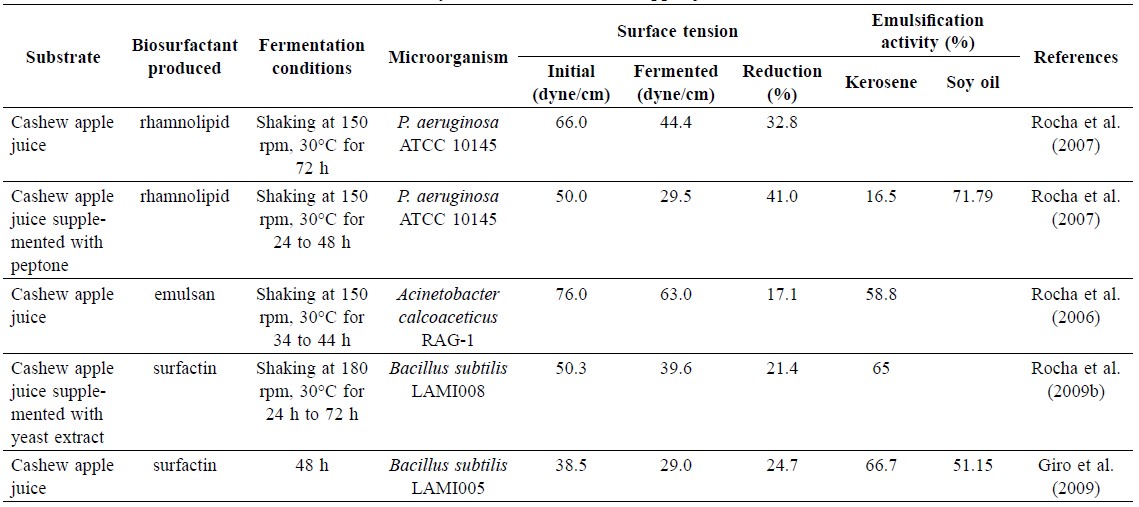
The biosurfactant rhamnolipid from Pseudomonas species has been studied extensively (Banat et al., 2010). Rhamnolipid is a glycolipid containing rhamnose and 3-hydroxy fatty acid. Biosurfactant can be produced from cashew apple juice by P. aeruginosa ATCC 10145. The highest reduction of surface tension (50.0 to 29.5 dyne/cm, or 41.0%) was obtained when cashew apple juice was supplemented with 5 g/L peptone. Suitable biosurfactants should reduce the surface tension of the medium to less than 30 dyne/cm. The highest surfactant production was 3.86 g/L, after fermentation at 30°C for 48 h. Emulsification activity was determined by mixing cell-free supernatant and hydrocarbon; then the emulsion height after 24 h was measured and calculated as a percentage of the total solution height. The emulsion activity of the biosurfactant was the highest with soy oil (71.79%) and the lowest with kerosene (16.50%). Although cashew apple juice contains glucose and fructose in equal amounts, only glucose was used by P. aeruginosa ATCC 10145 while the fructose concentration remained constant. Rhamnolipid could be purified by solvent extraction using chloroform/methanol in a 2:1 ratio (Rocha et al., 2007).
Emulsan is a lipopolysaccharide biosurfactant comprised of a sugar backbone linked with fatty acids (Castro et al., 2008). Many microorganisms are capable of producing bioemulsan, but Acinetobacter calcoaceticus has been widely studied. Bioemulsan is used in the food, agriculture, bioremeditation, detergent and cosmetic industries (Rosenberg and Ron, 1997). Cashew apple juice could be used as a substrate for production of emulsan by A. calcoaceticus RAG-1. The medium showed emulsifying activity with kerosene of 58.8% after 34 h of fermentation, while the surface tension was decreased about 17% (Rocha et al., 2006). Thus, bioemulsan has higher emulsifying activity, but lower surface activity than that of rhamnolipid. Generally, high-molecular-weight polymers, such as emulsan, are effective in emulsion stabilization and ineffective in surface tension reduction (Banat et al., 2010).
Surfactin is a cyclic lipopeptide biosurfactant produced by Bacillus subtilis. Surface activity of surfactin is higher than that of sodium lauryl sulfate. Surfactin has potential applications in the healthcare and environmental sectors. Surfactin can be used as a blood-clotting inhibitor (Sen, 2010). Surfactin exhibits antibiotic properties. It has a non-specific antibacterial property, which can disrupt the cell membranes of both Gram-positive and Gram-negative bacteria. A study of 20 multidrug-resistant bacteria showed that all strains, especially Enterococcus faecalis, were sensitive to surfactin (Fernandes et al., 2007). Antiviral properties of surfactin include inactivation of herpes and retroviruses. Surfactin possess antitumor and antiproliferative activities against cancer cell lines (Seydlová and Svobodová, 2008).
Surfactin production from cashew apple juice by various strains of B. subtilis has been studied. B. subtilis LAMI008 was inoculated in clarified cashew apple juice supplemented with mineral medium and produce surfactin at a concentration of 3.5 g/L after 24 h of fermentation. Surface tension of the medium was reduced by 21%. The emulsification index with kerosene was 65% (Rocha et al., 2009b). B. subtilis LAMI005 produced surfactin in the same medium at 123 mg/L after 48 h of fermentation. Surface tension of the medium was decreased by 25%. The emulsification index was 67% and 51% with kerosene and soybean oil, respectively. Moreover, critical micelle concentration was 2.5-fold lower than a medium using glucose and fructose as carbon sources (Giro et al., 2009). Yeast extract was important for producing surfactin; no reduction in surface tension was observed without yeast extract in the medium (Rocha et al., 2008). A summary of biosurfactants produced from cashew apple juice is shown in Table 4.
Table 4. Surface tension and emulsification activity of fermented cashew apple juice.
PROBIOTIC BEVERAGE AND LACTIC ACID
Probiotics are microorganisms that survive ingestion in certain numbers and provide health benefits to the host beyond general nutrition (Prado et al., 2008). Probiotics have many health benefits, including stimulating the immune system, preventing pathogens, reducing gastrointestinal tract disease, preventing cancer and reducing food allergies (Swennen et al., 2006).
Traditionally, probiotics are presented in dairy products. However, probiotics are increasingly being offered in non-dairy products, which have many advantages over the dairy products, e.g. casein allergy, lactose intolerance and cholesterol content (Prado et al., 2008). Many fruits and vegetables have proven to be good media for probiotics, including pineapple, orange, mango, beet, cabbage and cashew apple juice (Yoon et al., 2005; Yoon et al., 2006; Pereira et al., 2011).
Probiotic foods should have minimal counts of 7 log CFU/mL. Probiotic beverages produced from cashew apple juice, using Lactobacillus casei NRRL B-442, had viable cell counts of more than 8 log CFU/mL throughout 42 days of storage. L. casei overcame spoilage microorganisms, although heat treatment of the medium was not used in this study. The optimum fermentation condition was 30°C, initial pH 6.4, inoculation at 7.48 log CFU/mL and fermentation for 16 h, based on viable cells count and a final pH level below 4.6, which inhibited pathogenic microorganisms. The first 28 days of storage showed increasing viability, making this period most suitable for consumption with maximum benefits. Even with viability loss after 28 days due to the pH falling below 4.0, cell viability was still higher than 8 CFU/ml for at least 42 days (Pereira et al., 2011).
Lactobacillus spp. can also be used for lactic acid production. Lactic acid can be produced by chemical and fermentation processes. The chemical process produces a racemic mixture of lactic acid. The D-lactic acid was not metabolized by humans. Absorption of large amount of D-isomer can cause encephalopathy and acidosis (Uribarri et al., 1998). Lactic acid obtained by fermentation contained about 90% L-lactic acid, an isomer used in the food industry (Guilherme et al., 2011).
A study of cashew apple juice (25 to 37.5 g/L reducing sugar obtained by dilution) fermentation with L. casei NRRL B-442 found that the reducing sugar concentration had a significant effect on lactic acid production through carbohydrate metabolism. High concentration of reducing sugar increased lactic acid productivity until the concentration reached 60 g/L. After this point, lactic acid production decreased due to substrate inhibition. Ammonium sulfate affected the biomass because nitrogen is needed for creation of cells wall. The optimal condition for lactic acid production was 6 g/L ammonium sulfate (12% w/w nitrogen/carbon ratio), pH 6.5 and 37°C – at which lactic acid yield and productivity were about 95% and 2.3 g/L·h, respectively. However, the condition yielding the highest productivity may not be the most economical, given it was obtained from a low initial concentration of reducing sugar and, therefore, the lactic acid produced in each batch was not high (Silveira et al., 2012; Guilherme et al., 2011).
PREBIOTIC OLIGOSACCHARIDES
Prebiotics are food ingredients that are not digested and absorbed in the upper part of the gastrointestinal tract, but rather enter the large intestine to become substrates for probiotic bacteria, e.g. lactobacilli and bifidobacteria. Among prebiotics, non-digestible oligosaccharides have received the most attention (Swennen et al., 2006).
Dextransucrase (EC 2.4.1.5) is a glycosyltranferase enzyme that synthesizes dextran from sucrose. If a carbohydrate other than sucrose was in the medium, the enzyme pathway was shifted from dextran synthesis to oligosaccharide synthesis. Glycosyl moiety is transferred from a donor molecule (sucrose) to an acceptor by α-1,6-glycosidic bond (Figure 6). Acceptor molecules can be mono-, di-, oligosaccharides and also the products of this enzyme. In the latter case, the acceptors become longer, producing oligosaccharides or polysaccharides. During transfer of the glycosyl unit, fructose is left as a by-product that can be used to monitor the process. Dextransucrase is produced by certain lactic acid bacteria, e.g. Leuconostoc mesenteroides (Demuth et al., 2000; Chagas et al., 2007; Rabelo et al., 2009).
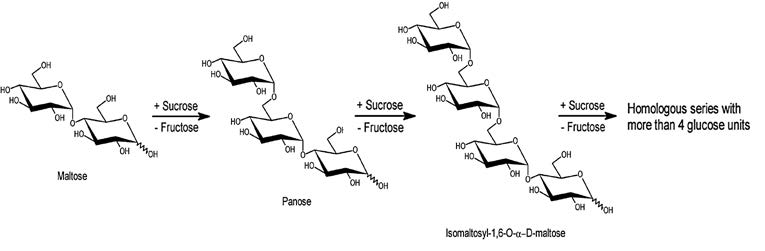
Figure 6. Dextransucrase acceptor reaction with maltose (adapted from: Rodrigues et al., 2005).
Oligosaccharides are produced by enzymatic method in two main steps: (i) production of dextransucrase and (ii) synthesis of oligosaccharides by the crude or purified dextransucrase obtained from the first step.
Cashew apple is a good substrate for dextransucrase production. L. mesenteroides NRRL B-512F was able to produce dextransucrase with high activity in a medium containing cashew apple juice (diluted to 5 g/L reducing sugar) and 5 g/L sucrose, without addition of other nutrients. Due to the fact that the primary sugars in cashew apple juice are glucose and fructose, adding sucrose is required to induce the enzyme. Dextransucrase activity in cashew apple was at least 3.5 times higher than synthetic medium. Juice supplementation with phosphate and yeast extract increased cell biomass (Chagas et al., 2007).
The stability of dextransucrase depended on the specific strain of microorganism. Dextransucrase from L. citreum B-742 had optimum activity at pH 6.5. This is also the optimum pH for Leuconostoc spp. The falling pH level throughout the fermentation process should be stopped when the pH of the medium reaches 5.5 because dextransucrase is denatured at a pH lower than 5.0 (Rabelo et al., 2009). The enzyme from L. mesenteroides B-512F had optimum stability at pH 5.2 (Rodrigues et al., 2005). Controlling the pH during fermentation at 6.5 results in decreased enzyme activity as dextransucrase activity from this microbe was not stable at this pH level (Chagas et al., 2007). The effect of controlling the pH level on the stability of dextransucrase from L. citreum B-742 has not yet been investigated.
Stability of dextransucrase in cashew apple juice (27.35 g/L of fructose, 22.47 g/L of glucose, 50 g/L of added sucrose, 20 g/L of yeast extract and 20 g/L of K2HPO4) was higher than that in synthetic medium (50 g/L of sucrose, 20 g/L of yeast extract, 20 g/L of K2HPO4 and minerals). Synthetic medium was used to investigate whether stability of the enzyme was caused by fermentation metabolites or by the cashew apple juice itself. Activity of dextransucrase from L. citreum B-742 and L. mesenteroides B-512F in synthetic crude fermented broth was completely lost after 20 h and 6 h, respectively. Thus, enzyme precipitation and stabilization should be performed immediately after fermentation. However, in cashew apple juice medium, the enzyme from L. citreum B-742 was stable for 48 h at 25°C and 20 h at 30°C. Maximum enzyme activity was obtained at 25°C after 20 h and 30°C at 3 h (Rabelo et al., 2011). The enzyme from L. mesenteroides B-512F was stable at least 30 h at pH 4.5 to 5.5. In addition, at pH 5.5, relative activity of the enzyme increased fivefold at the 30 h reaction time. Cashew apple juice from both fermented and non-fermented conditions maintained activity of dextransucrase. The partially purified enzyme was stable for 96 h at pH 5.5, 30°C in non-fermented cashew apple juice. However, the juice compositions responsible for stabilizing dextransucrase have not been studied (Honorato and Rodrigues, 2010).
The second step is an oligosaccharide synthesis. A study of oligosaccharide synthesis by crude enzyme from L. citreum B-742 used substrate media containing sucrose (25 to 75 g/L) and reducing sugar (62.5 to 125 g/L). Sucrose was an added disaccharide, while glucose and fructose were reducing sugars from concentrated cashew apple juice. It was found that oligosaccharide yield depended on the sugar composition of the medium. Both sucrose and reducing sugar had positive effects on oligosaccharide concentration. However, in terms of oligosaccharide yield, only reducing sugar had a positive effect and sucrose had no significant effect. The increment of acceptor concentration shifted the acceptor mechanism toward oligosaccharide synthesis instead of highly-polymerized dextran production. High concentration of sucrose and low concentration of reducing sugar enhanced dextran formation. The optimal medium condition for high oligosaccharide yield contained sucrose below 60 g/L and reducing sugar above 100 g/L. The reducing sugar substrate was almost totally consumed within 72 h (Rabelo et al., 2009).
Oligosaccharides could also be produced by direct inoculation of L. mesenteroides into cashew apple juice. Sucrose was added to the medium for dextransucrase induction. Fermentation was conducted while shaking at 30°C for 24 h., producing oligosaccharides with up to six degrees of polymerization, similar to the synthetic medium. Prebiotic effect of fermented cashew apple juice was tested using the probiotic Lactobacillus johnsonii NRRL B-2178. In vitro growth of L. johnsonii in fermented cashew apple juice was about three times higher than non-fermented juice. Although reducing sugar in fermented cashew apple juice was about five times lower than MRS broth containing fructose as the carbon source, the growth of L. johnsonii in both media was not significant (Vergara et al., 2010).
Levan, a fructose polymer synthesized by levansucrase (EC 2.4.1.10), is another polymer similar to dextran. This enzyme releases fructose from sucrose and adds it to the acceptor (Tanaka et al., 1979 and Yoo et al., 2004). Zymomonas mobilis has been widely studied for levan production (Bekers et al., 2001; de Paula et al., 2008; Ernandes and Garcia-Cruz, 2011). Levan production from cashew apple juice has not been studied.
Because cashew apple juice contains sucrose concentrations of less than 1 g/L (Azevedo and Rodrigues, 2000), and sucrose is a substrate for dextran and levan production, fortification of sucrose is required. Thus, production of dextran and levan from a mixture of high reducing sugar juice, such as cashew apple juice, and high sucrose juice, such as sugar cane, beet root and longan juice, should be considered.
CONCLUSION
From the single substrate cashew apple, many products can be prepared through the use of a variety of different microorganisms and processing conditions. Due to its moderate concentration of initial sugar, using cashew apple to produce ethanol and lactic acid may not be appropriate compared with other raw materials. However, cashew apple wine and probiotic beverage contained unique aroma, differentiating it from other juice products. Cashew apple offers a potential source for enzyme production due to the presence of substrates, e.g. lignocellulosic material, pectin and tannin. Screening microorganisms from rotting cashew apples should be investigated to identify microorganisms that can produce mixed enzymes. Biosurfactants produced from underutilized crops such as cashew apple offers an alternative to chemically-synthesized surfactants due to low cost and safety. However, product purification was still lacking in most products and an economic evaluation should be performed before commercialization.
REFERENCES
Alcântara, S.R., F.A.C. de Almeida, and F.L.H. da Silva. 2010. Pectinase production by solid state fermentation with cashew apple bagasse: water activity and influence of nitrogen source. Chemical Engineering Transactions 20: 121-126.
Alcântara, S.R., and F.L.H. da Silva. 2011. Influence of spores concentration, moisture, ammonium sulphate concentration and temperature on polygalacturonase production using cashew apple in the solid state fermentation process. Chemical Engineering Transactions 24: 949-954.
Assunção, R.B., and A.Z. Mercadante. 2003. Carotenoids and ascorbic acid from cashew apple (Anacardium occidentale L.): variety and geographic effects. Food Chemistry 81: 495-502. 10.1016/S0308-8146(02)00477-6
Attri, B.L. 2009. Effect of initial sugar concentration on the physico-chemical characteristics and sensory qualities of cashew apple wine. Natural Product Radiance 8: 374-379.
Azevedo, D.C.S., and A. Rodrigues. 2000. Obtainment of high-fructose solutions from cashew (Anacardium occidentale) apple juice by simulated moving-bed chromatography. Separation Science and Technology 35: 2561–2581.
Banat, I., A. Franzetti, I. Gandolfi, G. Bestetti, M. Martinotti, L. Fracchia, T. Smyth, and R. Marchant. 2010. Microbial biosurfactants production, applications and future potential. Applied Microbiology and Biotechnology 87: 427-444. 10.1007/S00253-010-2589-0
Banerjee, D., K. Mondal, and B. Pati. 2007. Tannase production by Aspergillus aculeatus DBF9 through solid-state fermentation. Acta Microbiologica et Immunologica Hungarica 54: 159-166. 10.1556/AMicr.54.2007.2.6
Bekers, M., J. Laukevics, A. Karsakevich, E. Ventina, E. Kaminska, D. Upite, I. Vı̄Na, R. Linde, and R. Scherbaka. 2001. Levan-ethanol biosynthesis using Zymomonas mobilis cells immobilized by attachment and entrapment. Process Biochemistry 36: 979-986. 10.1016/S0032-9592(01)00140-6
Belur, P.D. and G. Mugeraya. 2011. Microbial production of tannase: state of the art. Research Journal of Microbiology 6: 25-40. 10.3923/jm.2011.25.40
Campos, D.C.P., A.S. Santos, D.B. Wolkoff, V.M. Matta, L.M.C. Cabral, and S. Couri. 2002. Cashew apple juice stabilization by microfiltration. Desalination 148: 61-65. 10.1016/S0011-9164(02)00654-9
Carrau, F., K. Medina, L. Fariña, E. Boido, and E. Dellacassa. 2010. Effect of Saccharomyces cerevisiae inoculum size on wine fermentation aroma compounds and its relation with assimilable nitrogen content. International Journal of Food Microbiology 143: 81-85. 10.1016/j.ijfoodmicro.2010.07.024
Castilho, L.R., R.A. Medronho, and T.L.M. Alves. 2000. Production and extraction of pectinases obtained by solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresource Technology 71: 45-50. 10.1016/S0960-8524(99)00058-9
Castro, G.R., B. Panilaitis, and D.L. Kaplan. 2008. Emulsan, a tailorable biopolymer for controlled release. Bioresource Technology 99: 4566-4571. 10.1016/j.biortech.2007.06.059
Chagas, C., T. Honorato, G. Pinto, G. Maia, and S. Rodrigues. 2007. Dextransucrase production using cashew apple juice as substrate: effect of phosphate and yeast extract addition. Bioprocess and Biosystems Engineering 30: 207-215.
Chokotho, L., and E. Hasselt. 2005. The use of tannins in the local treatment of burn wounds - a pilot study. Malawi Medical Journal 17: 19-20.
Chung, K.T., C.I. Wei, and M.G. Johnson. 1998a. Are tannins a double-edged sword in biology and health? Trends in Food Science & Technology 9: 168-175. 10.1016/S0924-2244(98)00028-4
Chung, K.T., T.Y. Wong, C.I. Wei, Y.W. Huang, and Y. Lin. 1998b. Tannins and human health: a review. Critical Reviews in Food Science and Nutrition 38: 421-464.
Clay, J.W. 2004. World Agriculture, and the Environment: A Commodity-by-commodity Guide to Impacts and Practices. Island Press, Washington, D.C.
Cormier, R. 2008. Clarification of cashew apple juice and commercial applications. available online at: http://anacardium.info/IMG/pdf/Clarification_and_Microfiltration_of_Cashew_Apple_Juice.pdf (Accessed 23 May 2011).
de Paula, V.C., I.O. Pinheiro, C.E. Lopes, and G.C.M.T. Calazans. 2008. Microwave-assisted hydrolysis of Zymomonas mobilis levan envisaging oligofructan production. Bioresource Technology 99: 2466-2470.
Demuth, K., B. Demuth, H.J. Jördening, and K. Buchholz. 2000. Oligosaccharide synthesis with dextransucrase: Kinetics and reaction engineering p.123-135. In S. Bielecki, J. Tramper and J. Polak (eds.) Food Biotechnology. Elsevier Science, Amsterdam. 10.1016/S0921-0423(00)80059-3
Ernandes, F.M.P.G., and C.H. Garcia-Cruz. 2011. Nutritional requirements of Zymomonas mobilis CCT 4494 for levan production. Brazilian Archives of Biology and Technology 54: 589-600.
Fernandes, P.A.V., I.R.D. Arruda, A.F.A.B.D. Santos, A.A.D. Araújo, A.M.S. Maior, and E.A. Ximenes. 2007. Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Brazilian Journal of Microbiology 38: 704-709.
Figueiredo, R.W.D., F.M. Lajolo, R. Elesbo Alves, and H.A.C. Filgueiras. 2002. Physical-chemical changes in early dwarf cashew pseudofruits during development and maturation. Food Chemistry 77: 343-347. 10.1016/S0308-8146(01)00358-2
Fontoin, H., C. Saucier, P.L. Teissedre, and Y. Glories. 2008. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Quality and Preference 19: 286-291. 10.1016/j.foodqual.2007.08.004
Garruti, D.S., F.A.P. De Abreu, M.R.B. Franco, M.A.A.P. Da Silva, L.P.B. Wender, and P. Mikael Agerlin. 2006a. The influence of fermentation temperature and sulfur dioxide on the volatile composition and flavour profile of cashew wine. Developments in Food Science 43: 109-112. 10.1016/S0167-4501(06)80026-9
Garruti, D.S., M.R.B. Franco, M.A.A.P. Da Silva, N.S. Janzantti, and G.L. Alves. 2006b. Assessment of aroma impact compounds in a cashew apple-based alcoholic beverage by GC-MS and GC-olfactometry. LWT-Food Science and Technology 39: 373-378. 10.1016/j.lwt.2005.02.006
Giro, M.E.A., J.J.L. Martins, M.V.P. Rocha, V.M.M. Melo, and L.R.B. Gonçalves. 2009. Clarified cashew apple juice as alternative raw material for biosurfactant production by Bacillus subtilis in a batch bioreactor. Biotechnology Journal 4: 738-747. 10.1002/biot.200800296
Guilherme, A., M. Silveira, C. Fontes, S. Rodrigues, and F. Fernandes. 2011. Modeling and optimization of lactic acid production using cashew apple juice as substrate. Food and Bioprocess Technology 1-8. 10.1007/S11947-011-0670-Z
Gummadi, S.N., N. Manoj, and D.S. Kumar. 2007. Structural and biochemical properties of pectinases. p. 99–115. In J. Polaina and A. P. MacCabe (eds.) Industrial Enzymes. Springer, Dordrecht. 10.1007/1-4020-5377-0-7
Honorato, T.L., M.C. Rabelo, L.R.B. Gonçalves, G.A.S. Pinto, and S. Rodrigues. 2007. Fermentation of cashew apple juice to produce high added value products. World Journal of Microbiology and Biotechnology 23: 1409-1415. 10.1007/S11274-007-9381-Z
Honorato, T.L., and S. Rodrigues. 2010. Dextransucrase stability in cashew apple juice. Food and Bioprocess Technology 3: 105-110. 10.1007/S11947-008-0053-2
Inyang, U.E., and U.J. Abah. 1997. Chemical composition and organoleptic evaluation of juice from steamed cashew apple blended with orange juice. Plant Foods for Human Nutrition 50: 295-300.
Jayalekshmy, V.G., and P.S. John. 2004. ‘Sago’ - a natural product for cashew apple juice clarification. Journal of Tropical Agriculture 42: 67-68.
Jayani, R.S., S. Saxena, and R. Gupta. 2005. Microbial pectinolytic enzymes: A review. Process Biochemistry 40: 2931-2944. 10.1016/j.procbio.2005.03.026
Joseph, A.D.O. 2010. Comparative studies of wine produced by spontaneous and controlled fermentation of preserved cashew (Anacardium occidentale) juice. Research Journal of Biological Sciences 5: 460-464.
Karuppaiya, M., E. Sasikumar, T. Viruthagiri, and V. Vijayagopal. 2009. Optimization of process conditions using response surface methodology (RSM) for ethanol production from waste cashew apple juice by Zymomonas mobilis. Chemical Engineering Communications 196: 1425-1435. 10.1080/00986440902938972
Kumar, R., J. Sharma, and R. Singh. 2007. Production of tannase from Aspergillus ruber under solid-state fermentation using jamun (Syzygium cumini) leaves. Microbiological Research 162: 384-390. 10.1016/j.micres.2006.06.012
Makkar, R., S. Cameotra, and I. Banat. 2011. Advances in utilization of renewable substrates for biosurfactant production. AMB Express 1: 1-19. 10.1186/2191-0855-1-5
Michodjehoun-Mestres, L., J.M. Souquet, H. Fulcrand, C. Bouchut, M. Reynes, and J.M. Brillouet. 2009. Monomeric phenols of cashew apple (Anacardium occidentale L.). Food Chemistry 112: 851-857. 10.1016/j.foodchem.2008.06.056
Molina, A., J. Swiegers, C. Varela, I. Pretorius, and E. Agosin. 2007. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Applied Microbiology and Biotechnology 77: 675-687. 10.1007/S00253-007-1194-3
Muir-Beckford, M., and N. Badrie. 2000. Consumer acceptance of tropical wines from aloe vera (Aloe barbadensis) and cashew apples (Anacardium occidentale L.) in the British Virgin Islands. Foodservice Research International 12: 185-196.
Neelakandan, T., G. Usharani, and C. Sekar. 2010. Bioethanol production from cashew apple juice using Saccharomyces cerevisiae. International Journal of Current Research 11: 151-153.
Oduwole, O.O., T.O. Akinwale, and O. Olubamiwa. 2001. Economic evaluation of a locally fabricated extraction machine for a cottage cashew juice factory. The Journal of Food Technology in Africa 6: 18-20.
Ogunjobi, M.A.K., and S.O. Ogunwolu. 2010. Development and physicochemical evaluation of wine produced from cashew apple powder. Journal of Food Technology 8: 18-23.
Pereira, A.L.F., T.C. Maciel, and S. Rodrigues. 2011. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Research International 44: 1276-1283. 10.1016/j.foodres.2010.11.035
Pinheiro, Á., M. Rocha, G. Macedo, and L. Gonçalves. 2008. Evaluation of cashew apple juice for the production of fuel ethanol. Applied Biochemistry and Biotechnology 148: 227-234. 10.1007/S12010-007-8118-7
Prado, F.C., J.L. Parada, A. Pandey, and C.R. Soccol. 2008. Trends in non-dairy probiotic beverages. Food Research International 41: 111-123. 10.1016.j.foodres.2007.10.010
Rabelo, M.C., C.P.M.L. Fontes, and S. Rodrigues. 2009. Enzyme synthesis of oligosaccharides using cashew apple juice as substrate. Bioresource Technology 100: 5574-5580. 10.1016/j.biortech.2009.06.060
Rabelo, M., C. Fontes, and S. Rodrigues. 2011. Stability study of crude dextransucrase from Leuconostoc citreum NRRL B-742. Indian Journal of Microbiology 51: 164-170. 10.1007/S12088-011-0114-5
Ribéreau-Gayon, P., D. Dubourdieu, B. Donèche, and A. Lonvaud. 2006. Handbook of Enology. John Wiley & Sons, Chichester. 10.1002/0470010398
Rivard, D. 2009. The Ultimate Fruit Winemaker's Guide. Bacchus Enterprises, Ludhiana.
Rocha, M.V., A.H. Oliveira, M.C. Souza, and L.R. Gonçalves. 2006. Natural cashew apple juice as fermentation medium for biosurfactant production by Acinetobacter calcoaceticus. World Journal of Microbiology and Biotechnology 22: 1295-1299. 10.1007/S11274-006-9175-8
Rocha, M.V., M.C. Souza, S.C. Benedicto, M.S. Bezerra, G.R. Macedo, G.A. Pinto, and L.R. Gonçalves. 2007. Production of biosurfactant by Pseudomonas aeruginosa grown on cashew apple juice. Applied Biochemistry and Biotechnology 136-140: 185-194. 10.1007/S12010-007-9050-6
Rocha, M.V.P., R.V. Gomes, V.M.M. Melo, and L.R.B. Gonçalves. 2008. Evaluation of different carbon sources to surfactin production by Bacillus subtilis LAMI007.
Rocha, M., T. Rodrigues, G. De Macedo, and L. Gonçalves. 2009a. Enzymatic hydrolysis and fermentation of pretreated cashew apple bagasse with alkali and diluted sulfuric acid for bioethanol production. Applied Biochemistry and Biotechnology 155: 104-114. 10.1007/S12010-008-8432-8
Rocha, M.V.P., R.V.G. Barreto, V.M.M. Melo, and L.R.B. Goncalves. 2009b. Evaluation of cashew apple juice for surfactin production by Bacillus subtilis LAMI008. Applied Biochemistry and Biotechnology 155: 366-378. 10.1007/S12010-080-8459-X
Rocha, M.V.P., T.H.S. Rodrigues, V.M.M. Melo, L.R.B. Gonçalves, and G.R. Macedo. 2011. Cashew apple bagasse as a source of sugars for ethanol production by Kluyveromyces marxianus CE025. Journal of Industrial Microbiology & Biotechnology 38: 1099-1107. 10.1007/S10295-010-0889-0
Rodrigues, S., L. Lona, and T. Franco. 2005. The effect of maltose on dextran yield and molecular weight distribution. Bioprocess and Biosystems Engineering 28: 9-14. 10.1007/S00449-005-002-7
Rodrigues, T., M. Dantas, G. Pinto, and L. Gonçalves. 2007. Tannase production by solid state fermentation of cashew apple bagasse. Applied Biochemistry and Biotechnology 137-140: 675-688. 10.1007/S12010-007-9088-5
Rodrigues, T., G. Pinto, and L. Gonçalves. 2008. Effects of inoculum concentration, temperature, and carbon sources on tannase production during solid state fermentation of cashew apple bagasse. Biotechnology and Bioprocess Engineering 13: 571-576. 10.1007/S12257-008-0014-7
Rodrigues, T., M. Rocha, G. De Macedo, and L. Gonçalves. 2011. Ethanol production from cashew apple bagasse: improvement of enzymatic hydrolysis by microwave-assisted alkali pretreatment. Applied Biochemistry and Biotechnology 164: 929-943. 10.1007/S12010-011-9185-3
Rosenberg, E., and E.Z. Ron. 1997. Bioemulsans: microbial polymeric emulsifiers. Current Opinion in Biotechnology 8: 313-316. 10.1016/S0958-1669(97)80009-2
Sabu, A., A. Pandey, M. Jaafar Daud, and G. Szakacs. 2005. Tamarind seed powder and palm kernel cake: two novel agro residues for the production of tannase under solid state fermentation by Aspergillus niger ATCC 16620. Bioresource Technology 96: 1223-1228. 10.1016/j.biortech.2004.11.002
Sabu, A., C. Augur, C. Swati, and A. Pandey. 2006. Tannase production by Lactobacillus sp. ASR-S1 under solid-state fermentation. Process Biochemistry 41: 575-580. 10.1016/j.procbio.2005.05.11
Sanchez, P.C. 2008. Philippine Fermented Foods: Principles and Technology. The University of the Philippines Press, Quezon.
Sen, R. 2010. Surfactin: Biosynthesis, Genetics and Potential Applications. p. 316-323. In R. Sen (ed.) Biosurfactants. Springer, New York. 10.1007/978-1-4419-5979-9-24
Seydlová, G., and J. Svobodová. 2008. Review of surfactin chemical properties and the potential biomedical applications. Central European Journal of Medicine 3(2): 123-133. 10.2478/S11536-008-0002-5
Silva, D., K. Tokuioshi, E. Da Silva Martins, R. Da Silva, and E. Gomes. 2005. Production of pectinase by solid-state fermentation with Penicillium viridicatum RFC3. Process Biochemistry 40: 2885-2889. 10.1016/j.procbio.2005.01.008
Silva, M.E., A.B.T. Neto, W.B. Silva, F.L.H. Silva, and R. Swarnakar. 2007. Cashew wine vinegar production: alcoholic and acetic fermentation. Brazilian Journal of Chemical Engineering 24: 163-169.
Silveira, M., C. Fontes, A. Guilherme, F. Fernandes, and S. Rodrigues. 2012. Cashew apple juice as substrate for lactic acid production. Food and Bioprocess Technology 5: 947-953. 10.1007/S11947-010-0382-9
Singh, S.A., M. Ramakrishna, and A.G. Appu Rao. 1999. Optimisation of downstream processing parameters for the recovery of pectinase from the fermented bran of Aspergillus carbonarius. Process Biochemistry 35: 411-417. 10.1016/S0032-9592(99)00089-8
Sudheer Kumar, Y., R.S. Prakasam, and O.V.S. Reddy. 2009. Optimisation of fermentation conditions for mango (Mangifera indica L.) wine production by employing response surface methodology. International Journal of Food Science & Technology 44: 2320-2327. 10.1111/j.1365-2621.2009.02076.X
Swennen, K., C.M. Courtin, and J.A. Delcour. 2006. Non-digestible Oligosaccharides with Prebiotic Properties. Critical Reviews in Food Science and Nutrition 46: 459-471. 10.1080/10408390500215746
Tanaka, T., S. Oi, and T. Yamamoto. 1979. Synthesis of levan by levansucrase. Journal of Biochemistry 85: 287-293.
Udeh, P.J. 2004. A Guide to Healthy Drinking Water: All You Need to Know About the Water You Drink. iUniverse, Lincoln.
Uribarri, J., M.S. Oh, and H.J. Carroll. 1998. D-lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine 77: 73-82. 10.1097/00005792-199803000-00001
Venkatesh, M., P.B. Pushpalatha, K.B. Sheela, and D. Girija. 2009. Microbial pectinase from tropical fruit wastes. Journal of Tropical Agriculture 47: 67-69.
Vergara, C.M.D.A.J.C., T.L. Honorato, G.A. Maia, and S. Rodrigues. 2010. Prebiotic effect of fermented cashew apple (Anacardium occidentale L) juice. LWT-Food Science and Technology 43: 141-145.
Wu, M.C., C.M. Jiang, P.H. Huang, M.Y. Wu, and Y.T. Wang. 2007. Separation and utilization of pectin lyase from commercial pectic enzyme via highly methoxylated cross-linked alcohol-insoluble solid chromatography for wine methanol reduction. Journal of Agricultural and Food Chemistry 55: 1557-1562. 10.1021/jf062880S
Yoo, S.H., E.J. Yoon, J. Cha, and H.G. Lee. 2004. Antitumor activity of levan polysaccharides from selected microorganisms. International Journal of Biological Macromolecules 34: 37-41. 10.1016/j.ijbiomac.2004.01.002
Yoon, K.Y., E.E. Woodams, and Y.D. Hang. 2005. Fermentation of beet juice by beneficial lactic acid bacteria. LWT - Food Science and Technology 38: 73-75.
Yoon, K.Y., E.E. Woodams, and Y.D. Hang. 2006. Production of probiotic cabbage juice by lactic acid bacteria. Bioresource Technology 97: 1427-1430. 10.1016/j.biortech.2005.06.018
Zepka, L.Q., C.D. Borsarelli, M.A.A.P. Da Silva, and A.Z. Mercadante. 2009. Thermal degradation kinetics of carotenoids in a cashew apple juice model and its impact on the system color. Journal of Agricultural and Food Chemistry 57: 7841-7845. 10.1021/jf900558a
Zhang, H., E.E. Woodams, and Y.D. Hang. 2011. Influence of pectinase treatment on fruit spirits from apple mash, juice and pomace. Process Biochemistry 46: 1909-1913.
Trakul Prommajak1, Noppol Leksawasdi2 and Nithiya Rattanapanone1,3*
1 Division of Food Science and Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
2 Division of Food Engineering, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
3 Postharvest Technology Research Institute, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author: E-mail: agfsi001@gmail.com
Total Article Views

