
Influence of Solvents on Characteristics of Nanoparticles Prepared by Pulsed Laser Ablation on Iron Target
Htain Lin Aye, Supab Choopun* and Torranin ChairuangsriPublished Date : 2019-08-24
DOI : 10.12982/cmujns.2014.0018
Journal Issues : Number 1, January-april 2014
ABSTRACT
An iron target submerged in three different solvents (distilled water, absolute ethanol and acetone) was laser ablated to study the influence of solvents on the size and composition of the nanoparticles produced. A Nd:YAG laser ablation machine was operated at wavelength 1064 nm, pulse power 90 W, pulse frequency 30 kHz and pulse duration 3 ns. Nanoparticles were characterized by UV-Vis spectroscopy and transmission electron microscopy (TEM). TEM revealed that spherical nanoparticles, mainly FeO, were observed in all solvents. Fe2O3 was found only in distilled water. Well-dispersed nanoparticles with mono-modal distribution and a mean particle size of ca. 20 nm were produced only in the acetone. It is believed that higher dipole moment of the solvents should lead to an inhibition of growth or aggregation of nanoparticles, and hence the smallest nanoparticles were observed with the acetone.
Keywords: Laser ablation, Iron, Nanoparticles, Acetone, Ethanol, Water
INTRODUCTION
Nanoparticles, in the form of nanopowders or colloids, can be synthesized by laser ablating a solid target that lies in a gaseous or a liquid environment (Arul et al., 1998). The size, composition and crystal structure of the produced nanoparticles are influenced by parameters of laser ablation in liquids, including types of liquid media. It has been known that micrometer size of round and multi-twinned crystalline particles of copper and copper oxide can be produced in water, acetone and decane from metal or metal oxide targets by Nd:YAG laser (532-1064 nm wavelength) (Tilaki et al., 2007a; Amikura et al., 2008). Thraeja and co-workers (2007) reported that adding of isopropanol into water has an effect on the formation of zinc oxide nanoparticles by pulsed Nd:YAG laser (355 nm wavelength) on a zinc metal target. Tsuji and co-workers (2005) reported that different solvent species (water and hexane) led to a difference in atomic composition of cobalt nanoparticles. Optical properties and composition changes of produced noble metal nanoparticles in different liquids have been characterized in previous studies (Andrei et al., 2003; Pyatenko et al., 2004; Tarasenko et al., 2006; Tilaki et al., 2006; Tilaki et al., 2007b).
Iron and iron oxide nanoparticles have attracted an interest in environmental applications, such as reduction of dioxin (Arbain et al., 2011) and biosensors (Kaushik et al., 2008) because of large surface-to-volume ratio, high surface reaction activity, and other beneficial properties. In this work, pulsed laser ablation on an iron target in different solvent media was studied and the produced nanoparticles were characterized by UV-Vis spectroscopy and transmission electron microscopy (TEM). Influence of solvent media (distilled water, absolute ethanol and acetone) on type, morphology, size and size distribution of nanoparticles was discussed.
MATERIALS AND METHODS
The target (144 mm2) was cut from a 1 mm thick iron sheet (99.5% Fe, ADVENT Research Materials Ltd, Oxford, England). The target was washed by absolute ethanol and distilled water in a sonicator for several minutes to eliminate surface contaminants. Distilled water, 99% absolute ethanol (Merck KGaA, Germany) and AR grade acetone (Merck KGaA, Germany) were used as solvents. The iron target was placed at the bottom of a Petri-disc filled with the solvent to the desired height of 3 mm (about 10 ml). A Nd:YAG laser ablation machine (1064 nm wavelength, Kevron Marker Laser) was operated at the power of 90 W and 30 kHz for 7 min. Nanoparticles as colloids were characterized immediately by UV-Vis spectroscopy (CARY-50). Type, size and size distribution of the nanoparticles were analysed using a JEOL-2010 TEM/STEM microscope operated at 200 kV. The apparent size of the nanoparticles were measured from TEM micrographs based on 100 particles using Image J software.
RESULTS
Type, morphology and size distribution of the produced nanoparticles were shown by bright-field (BF) TEM images and selected area electron diffraction (SADP) as ring patterns in Figure 1. Almost of the nanoparticles were spherical in shape. Broad size distribution was observed in the case of distilled water (Figure 1(a)). Two groups of particles were observed: large ones with a size on the order of hundred nanometers and finer ones of ten nanometers. Corresponding to the SADP of nanoparticles in distilled water as shown in Figure 1(b), the nanoparticles were mainly FeO of the fcc lattice type with a lattice parameter of a = 4.326 Å according to JCPDS file No. 89-0687. As a faint ring can be indexed as {1013}Fe2O3, some particles can be Fe2O3 with rhombohedral lattice type and lattice parameters of a = 5.038 Å and c = 13.772 Å, using hexagonal unit cell according to JCPDS file No. 89-0687.
Figure 1(c) shows spherical nanoparticles in absolute ethanol. It is likely that they were welded together as ligaments. Well-dispersed nanoparticles without agglomeration or welded ligament were observed in the case of acetone as shown in Figure 1(e). Corresponding SADPs of the nanoparticles in both cases, as shown in Figures 1(d) and 1(f), also indicated that the nanoparticles were mainly FeO.
Size distributions of the nanoparticles were measured from TEM micrographs and are given in Figure 2. The results were fitted as Gaussian-fitting curves. In the
case of nanoparticles in distilled water, the size distribution is tri-modal with three mean sizes as ca. 18, 68 and 121 nm, whereas for absolute ethanol and acetone, it is mono-modal with the mean size of ca. 20 nm and 19 nm, respectively.
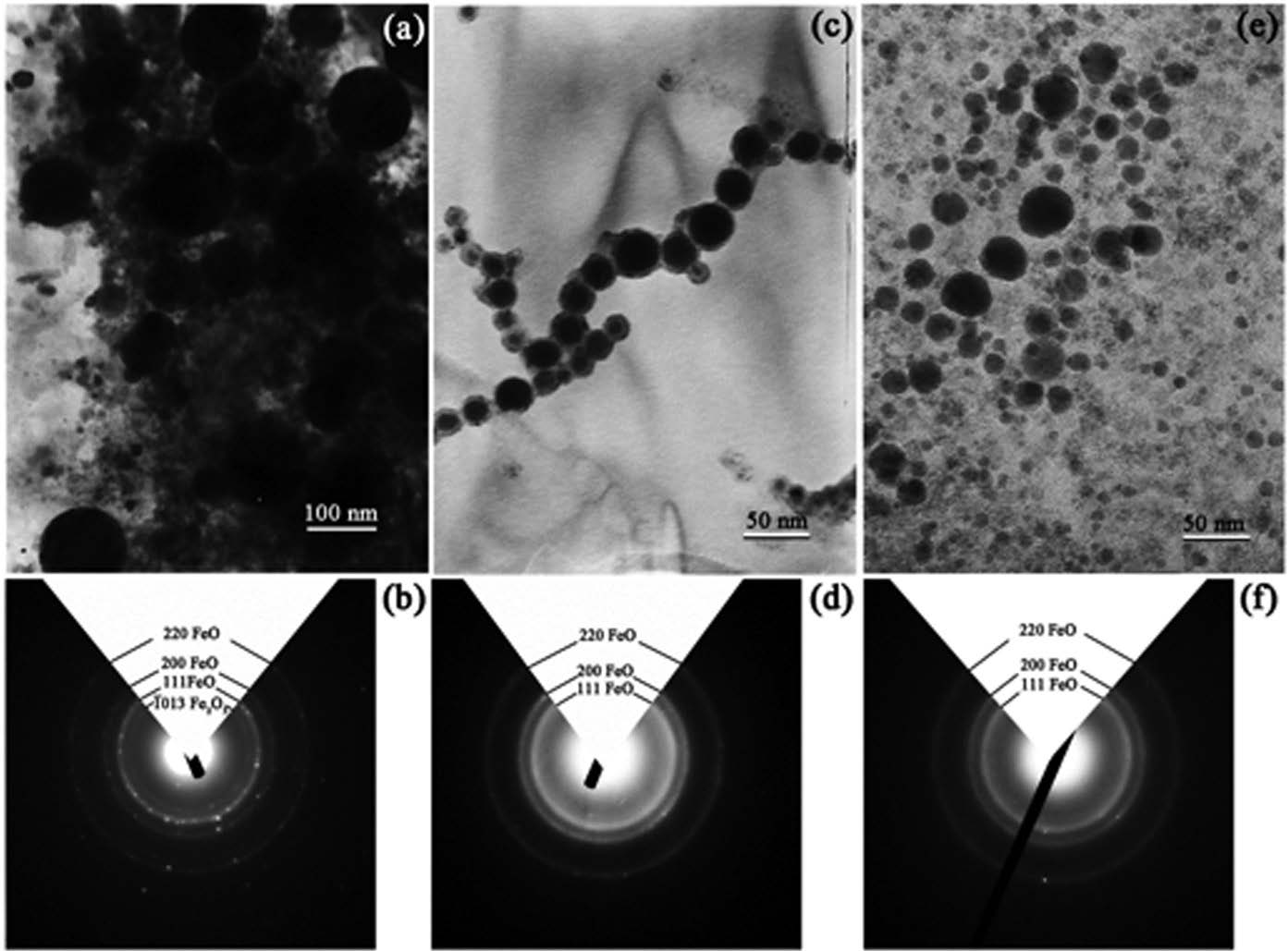
Figure 1. BF-TEM micrographs of the nanoparticles prepared in (a) distilled water, (c) absolute ethanol and (e) acetone, with the corresponding SADPs in (b), (d) and (f), respectively.
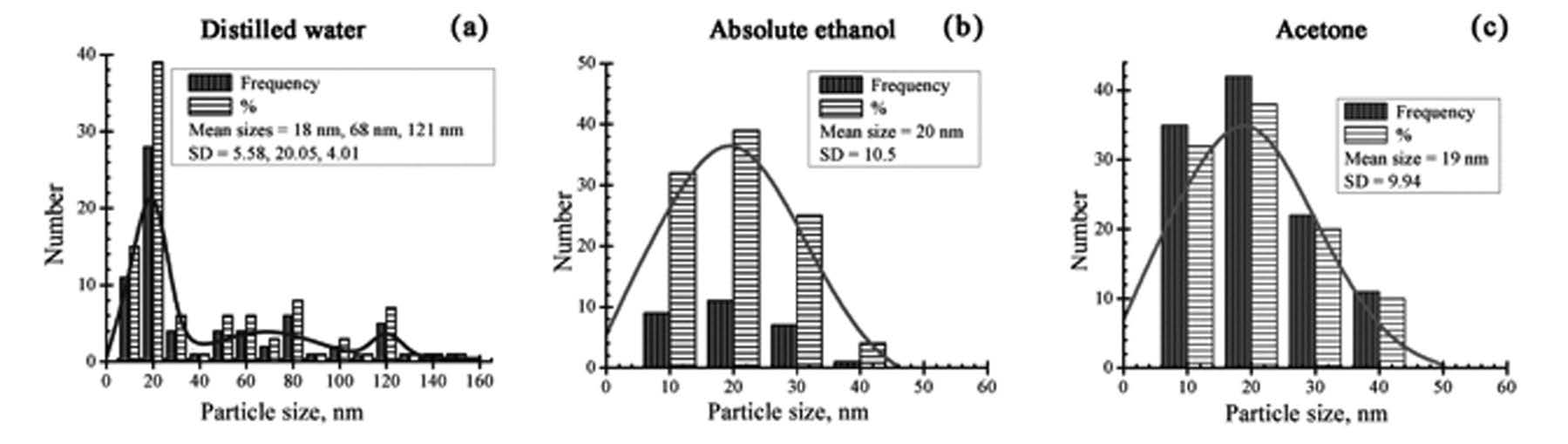
Figure 2. Size distribution and Gaussian-fitting curves of nanoparticles prepared in: (a) disilled water, (b) absolute ethanol and (c) acetone.
Table 1. Summary of characteristics of nanoparticles prepared by laser ablation in different solvent media.
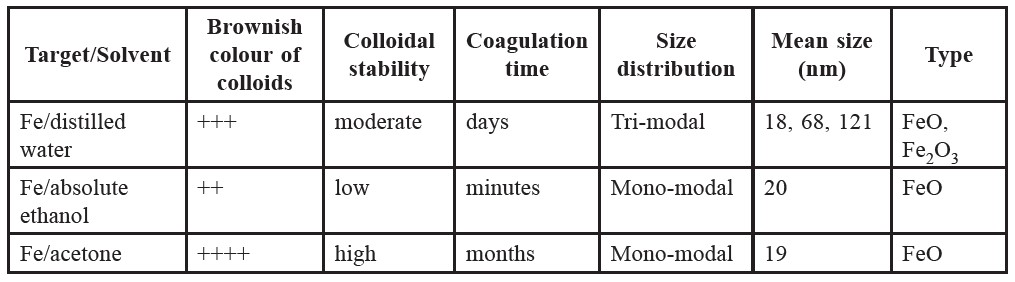
Table 1 summarizes the characteristics of the nanoparticles produced in this study. The colloids are brownish. Coagulation time of the yielded colloids is in an order of minutes, days and months for the cases of absolute ethanol, distilled water and acetone, respectively. This indicates that the colloidal stability of the nanoparticles is the highest in acetone, but the least in absolute ethanol.
DISCUSSION
The particle formation mechanism in liquid was explained by Yang (2007). A dense cloud of plume including iron atoms can accumulate in the laser spot of the target during ablation. The plume should be composed of a number of small species, which later aggregate to form embryonic nanoparticles. In some cases, some of the particles became larger by aggregation with other small species through a growth mechanism resulting from the attractive van-der-Waals force of the electrical double layers (Tilaki et al., 2006). From the results in this study, it is expected that the particle size and size distribution may be influenced by the dipole moment of the solvent media. Higher dipole moment of the surrounding molecules should lead to stronger bonds between these molecules, prohibiting the further growth mechanism in liquid. The smallest nanoparticles were thus observed in the case of acetone.
The UV-Vis absorption spectra of the yielded colloids containing nanoparticles exhibited very weak plasmon resonance as shown in Figure 3. The absorption spectra gradually decreased with increasing wavelength towards the visible region and the maximum absorption peak did not appear in all cases. The possible explanation for this is that nanoparticles are mainly iron oxide (as has been confirmed by TEM), which cannot exhibit plasmon resonance as proposed by Amikura and co-workers (2008).
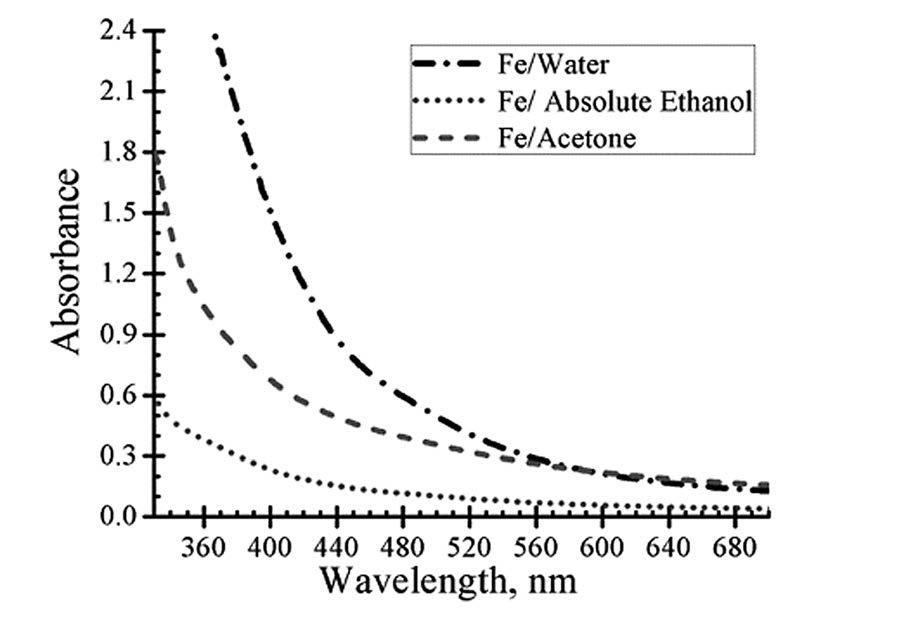
Figure 3. Absorption spectra of colloidal nanoparticles in distilled water, absolute ethanol and acetone.
Nanoparticles were fabricated successfully by pulsed laser ablation using an iron target immersed in different solvent media. Spherical nanoparticles were observed for all solvents. However, well-dispersed, mono-modal distribution of nanoparticles with the mean size of ca. 20 nm was obtained only in the case of acetone. In all cases, the produced nanoparticles are mainly FeO.
ACKNOWLEDGEMENTS
The authors would like to thank the Graduate School, Chiang Mai University and the Thailand International Development Cooperation Agency for their financial support. The authors would also like to thank the Electron Microscopy Research and Service Centre, Faculty of Science, Chiang Mai University, for use of its TEM facilities.
REFERENCES
Amikura K., T. Kimura, M. Hamada, N. Yokoyama, J. Miyazaki, and Y. Yamada. 2008. Copper oxide particles produced by laser ablation in water. J. Appl. Surf. Sci. 254: 6976-6982.
Andrei K.V., M. Michel, K. Christopher, and H.T.L. John. 2003. Fabrication and characterization of gold nanoparticles by femtosecond laser ablation in an aqueous solution of cyclodextrins. J. Phys. Chem. B 107: 4527-4531.
Arbain R., M. Othman, and S. Palaniandy. 2011. Preparation of iron oxide nanoparticles by mechanical milling. J. Minerals. Eng. 24:1-9.
Arul D.N., R.C. Paul, and K. Gedanken. 1998. Synthesis, characterization, and properties of metallic copper nanoparticles. J. Chem. Matter. 10: 1446-1452.
Kaushik A., R. Khan, PR. Solanki, P. Pandey, J. Alam, S. Ahmad, and B.D. Malhotra. 2008 Iron oxide nanoparticles–chitosan composite based glucose biosensor. J. Biosens. Bioelectro. 1-8.
Pyatenko A., K. Shimokawa, M. Yamaguchi, O. Nishimura, and M. Suzuki. 2004. Synthesis of silver nanoparticles by laser ablation in pure water. J. Appl. Phys. A 79: 803-806.
Tarasenko N.V., A.V. Butsen, E.A. Nevar, and N.A. Savastenko. 2006. Synthesis of nanosized particles during laser ablation of gold in water. J. Appl. Surf. Sci. 252: 4439-4444.
Thareja R.K., and S. Shukla. 2007. Synthesis and characterization of zinc oxide nanoparticles by laser ablation of zinc in liquid. J. Appl. Surf. Sci. 253: 8889-8895.
Tilaki R.M., A.I. Zad, and S.M. Mahdavi. 2007. Size, composition and optical properties of copper nanoparticles prepared by laser ablation in liquids. J. Appl. Phys. a; A 88: 415-419.
Tilaki R.M., A.I. Zad, and S.M. Mahdavi. 2006. Stability, size and optical properties of silver nanoparticles prepared by laser ablation in different carrier media. J. Appl. Phys. A 84: 215-219.
Tilaki R.M., A.I. Zad, and S.M. Mahdavi. 2007. The effect of liquid environment on size and aggregation of gold nanoparticles prepared by pulsed laser ablation. J. Nano. Res. b; 9: 853-860.
Tsuji T., M. Hashimoto, Y. Nishizawa, M. Kubokawa, and M. Tsuji. 2005. Laser ablation of cobalt and cobalt oxides in liquids: influence of solvent on composition of prepared nanoparticles. J. Appl. Surf. Sci. 243: 243-214.
Yang G.W. 2007. Laser ablation in liquids: applications in the synthesis of nanocrystals. J. Progr. Mater. Sci. 52: 648-698.
Htain Lin Aye1, Supab Choopun2* and Torranin Chairuangsri1
1 Department of Industrial Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
2 Department of Physics, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: supab99@gmail.com
Total Article Views

