
Binuclear Tetrahedral Dimeric Mg(II) Complexes of DibasicTridentate Schiff Base Ligands and Their Microbial Studies
Mohammad N. Uddin, Md. A. Salam, Didarul A. Chowdhury,and Md. Abu B. SiddiquePublished Date : 2019-08-24
DOI : 10.12982/cmujns.2014.0017
Journal Issues : Number 1, January-april 2014
ABSTRACT
Some Mg(II) complexes of dibasic tridentate Schiff base ligands derived from aldehydes and orthoaminophenol have been synthesized and characterized by some physico-chemical studies; elemental, spectral (IR, UV, NMR), magnetic and conductance analyses. Dimeric tetrahedral geometry for the prepared complexes has been proposed. The antibacterial activity of the prepared complexes was also studied against some gram positive and gram negative bacteria.
Keywords: Tridentate Schiff base, Orthoaminophenol, Mg(II) complexes, Antibacterial activity, Dimeric tetrahedral geometry
INTRODUCTION
A Schiff base named after Hugo Schiff, is a compound that contains azomethine group (>C=N-) connected to an aryl or alkyl group but not hydrogen. Schiff base ligands can be synthesized from an amine and a carbonyl compound by nucleophilic addition forming a hemi-aminal group followed by dehydration to generate an imine compound. Schiff base ligands are considered ‘privileged ligands’ because they are easily prepared by the condensation between aldehydes and amines. Schiff base ligands are able to coordinate with many metals to stabilize their various oxidation states. Schiff bases are generally monodentate, bidentate, tridentate, tetradentate etc. Ligands are capable of forming very stable complexes with transition metals. Most of the tridentate Schiff base ligands are formed by the condensation of β-diketones or o-hydroxyaldehydes or ketones with monoamines. These ligands with ONO donor atom set are well known to coordinate with various metal ions and this has attracted the interest of authors (Samanta, et al. 2007; Ghosh, et al. 2004; Salam, et al. 1997). Ligands with ONS donor atom set are also known to coordinate with metal ions (Chowdhury et al, 2010). Unsymmetrical Schiff bases functionalize as monobasic tridentate (NNO) ligands on complexation with some transition metal ions are reported (Jang, et al. 2005; Rabie, et al. 2008; Roberts, et al. 2010).
In contrast to the extensive converge of transition metal Schiff base complexes, there is a somewhat desultory presentation of magnesium complexes of these ligands. Sal-en complexes of magnesium have been first reported by Corazza et al. (Corazza, et al. 1988). Generally, Schiff bases, such as H2sal-en, containing phenolic groups can form complexes either by removal of the two phenolic protons to give a neutral metal complex, or by direct ligation as a mono- or non-deprotonated ligand. Zhang et al. studied the synthesis and DNA binding of Mg(II) complex of Schiff base derived from vanillin and L-tryptophan (Zhang, et al. 2011). The literature survey reveals that very little work has been done on the coordination chemistry of Mg(II). The present work has been an attempt to prepare and study some of the Mg(II) complexes using some dibasic tridentate Schiff bases. Antibacterial and antifungal activities of some of the prepared complexes were also study.
MATERIALS AND METHODS
Chemicals
2-hydroxy-1-naphthaldehyde, 2-hydroxyacetophenone and hydrazine hydrate were obtained from M/S. E. Merk (Germany). Salicylaldehyde, 2-aminophenol, ortho-phenylenediamine, ethyl benzoate, ethylenediamine, benzoylacetone, methanol, vanadin (IV) acetyleacetonate, chloroform, vanadium oxytrichloride (VOCl3), N-dimethylformamide (DMF), dimethylsulphoxide (DMSO) and carbon-tetrachloride were obtained from Aldrich Chemical Company Ltd. Ethyl alcohol was obtained from Carew and Company Ltd. Perchloric acid, nitric acid, sulphuric acid and disodiam salt of ethylenediaminetetraacitic acid were obtained from BDH Chemicals Ltd. All chemicals except solvents were used as received. Solvents were dried by standard methods and distilled under an inert atmosphere.
Analytical methods
The analysis of the metal content, Pb(II) of the prepared complexes were obtained by Atomic Absorption Spectrophotometer (Model Thermo Scientific ICE-3000) from B.C.S.I.R. Laboratories, Chittagong. Microanalyses on CHN CORDER MT-5, Yanaco, were performed in Prof. Shiumyozu’s Lab. in the Dept. of Applied Chemistry at Kyushu University, Japan. The Infrared spectra of the prepared complexes were obtained by FTIR spectrophotometer (Model- 8900, Shimadzu, Japan) using KBr as the matrix in the range 400-4000 cm-1 from B.C.S.I.R Laboratories, Dhaka. Polystyrene was used as the standard to calibrate the spectrophotometer. Electronic absorption spectra were run on Shimadzu UV-Visible Recording Spectrophotometer (Model-1800) using 1 cm cell in the research laboratory of the Department of Chemistry, University of Chittagong. Mass spectra were obtained from Shiumyozu’s Lab. in the Dept. of Applied Chemistry at Kyushu University, Japan, by Ultra-light Performance Mass Spectrometer (Model- JMS-HX 11 OA).
An electrothermal melting point apparatus was used for the determination of the melting or decomposition points of the complexes and the ligands. Using N, N-dimethylformamide (DMF) as the solvent the solutions of the complexes (of the order of 10-3 M) were used for conductivity measurements. Conductivity measurement was performed on a Philips Conductivity Meter (Model-HI 9255).
Determination of conductivity of an electrolytic solution involves measuring of the electrical resistance of that solution at a particular temperature, usually 25ºC. Magnetic susceptibility of some of the prepared complexes was determined using the Sherwood Scientific Magnetic Susceptibility Balance from Rajshahi University, Bangladesh.
Schiff base ligands of orthoaminophenol
Orthoaminophenol contains one hydroxyl group (-OH) and one amino group (-NH2) attached with benzene ring at the ortho positions. When an active carbonyl group reacts with –NH2 group of this compound, a Schiff base product containing the azomethine (>C=N–) functional group is obtained. Preparations of some tridentate ligands of orthoaminophenol1 are stated below:
Salicylaldehyde-orthoaminophenol, Sal-OAPH2 :
A mixture of salicylaldehyde and orthoaminophenol (25 mmol each) in ethanol (70 mL) was refluxed at 30ºC for one hour when an orange red crystalline solid appeared. This was filtered off, washed with and recrystallized from ethanol.
(b) 5-bromosalicyldehyde-para-methylorthoaminophenol,5BrSal-PMeOAPH2:
(c) 5-bromosalicyldehyde-para-chloro-orthoaminophenol, 5BrSal-PClOAPH2:
(d) 5-bromosalicylaldehyde-orthoaminophenol,5BrSal-OAPH2 :
(e) 5-chlorosalicylaldehyde-orthoaminophenol,5ClSal-OAPH2 :
(f) 2-hydroxy-1-naphthaldehyde- orthoaminophenol, HNP-OAPH2 :
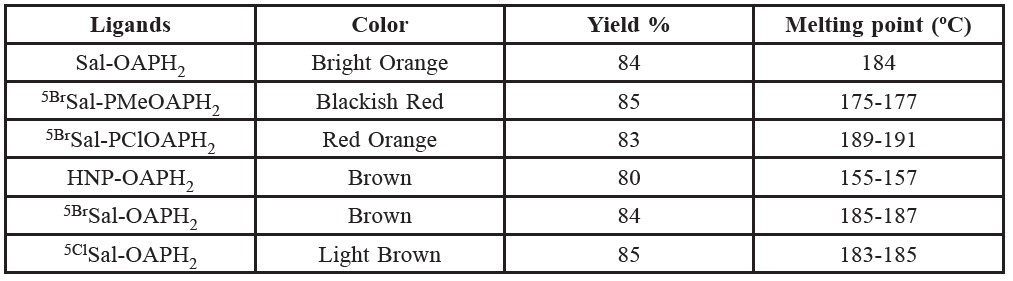
Table 1. Color, yield and melting points of the prepared ligands.
Preparation of magnesium(II) complexes
For the preparation of various complexes of the Schiff base ligands, the metal ion, Mg2+, was used in the form of magnesium chloride hexahydrate, MgCl2.6H2O. Pure and dry solvent was used in all the preparations. White colored magnesium chloride hexahydrate, MgCl2.6H2O (10 mmol) was dissolved in ethanol (30 mL) with gentle warming. In another beaker, the prepared Schiff base ligands of orthoaminophenol (10 mmol) were dissolved in the same solvent. The Mg(II) solution was then added to the ligand solution drop wise with continuous stirring. The mixture was refluxed for about two-three hours with continuous stirring by magnetic stirrer to complete the reaction. Then the reaction mixture was allowed to stand and the precipitate formed was filtered off. It was then washed with ethanol and dried over calcium chloride.
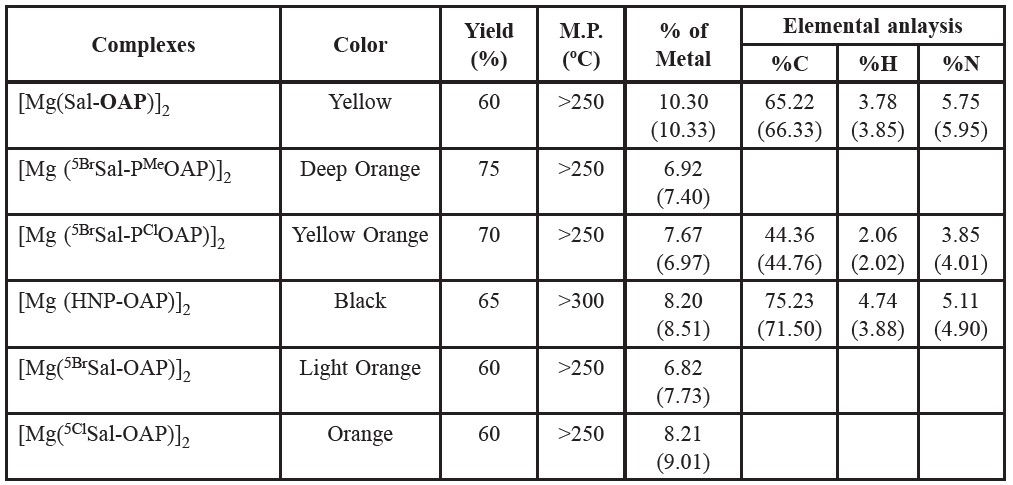
The following Magnesium (II) complexes of the prepared Schiff base ligands were prepared by following the same procedure as stated above. The prepared compounds seem to be dimeric in nature. Table -1 and table 2 shows the color, % of yield, melting points and % of metal contents of the prepared Schiff bases and complexes, respectively. Schemetic diagram for the preparation of a representative tridentate Schiff base ligand and its Mg(II) complex is given in figure 1.
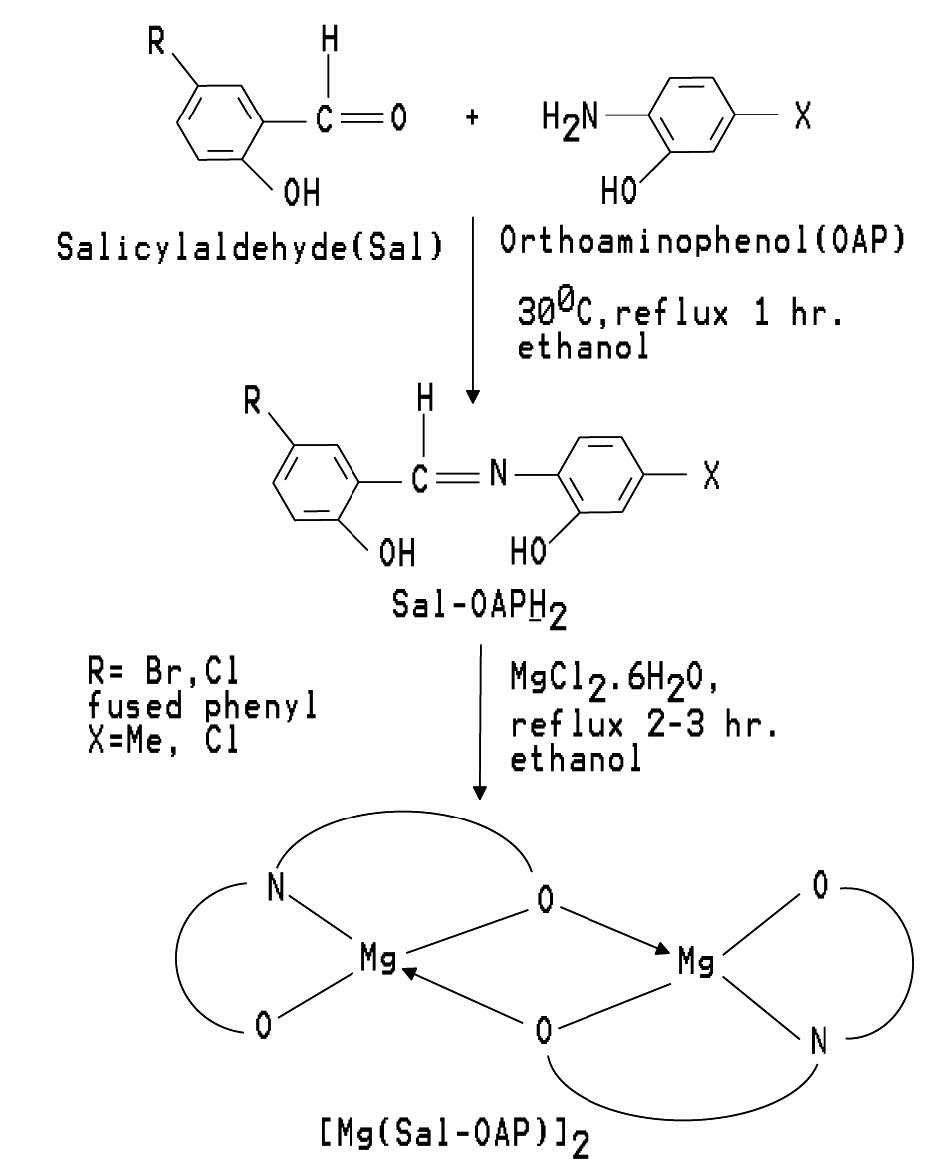
Figure 1. Schemetic diagram for the preparation of a representative tridentate Schiff base ligand and its Mg(II) complex.
Table 2. Color, % of yield, melting points and % of metal contents of the prepared Schiff base complexes.
Evaluation of chemicals against fungi
Preparation of the PDA (Potato Dextrose Agar) medium. PDA (Potato Dextrose Agar) medium was used throughout the study. The compositions of PDA medium are as Potato 200 gm, Dextrose 20 gm, Agar 15gm, Distilled water 1000 cm3. 200 gm of sliced potato was boiled in 500 cm3 distilled water and the extract was decanted after proper boiling. The extract was taken in a 1000 cm3 beaker and the solution was made up to the mark with distilled water and 20 gm dextrose was added in the solution, then 15 gm of agar powder was added in the solution and they were mixed thoroughly with a glass rod. After 10 minutes of boiling, the medium was transferred to a 250 cm3 conical flask. Then the medium was autoclaved at 121°C and 15 psi pressure for 15 minutes.
Preparation and maintenance of cultures. Glass petri plates were sterilized and the molted sterilized PDA medium was poured at the rate of 10 cm3 per petri plate. After solidification of the medium, the small portions of mycelium of each fungus were placed carefully at the centre of each PDA plate with the help of sterilized needles. Thus each fungus was transferred to a number of PDA plates.
Test tube slants of PDA medium were prepared for the maintenance of cultures. Small portions of mycelia of the collected pathogens were transferred to the test tubes separately from old cultures with the help of sterilized needles. A number of test tubes were freshly prepared for each fungal pathogen. The inoculated slants were incubated at room temperature under laboratory conditions.
Efficacy of the chemicals
Efficacy of the chemicals was tested by “poison food technique” (Nene and Thapilyal, 2002). The petri plates of 70-90 mm in diameter were used throughout this experiment. 0.0 1 cm3 of test complexes with definite concentration (2%) was taken in sterilized glass petri plates and then molted sterilized PDA medium was poured at the rate of 10 cm3 per petri plate. Proper control was maintained separately with sterilized PDA medium without complex but in the presence of solvent. After solidification of the medium, the fungal inoculums (5 mm mycelia block) were placed at the center of each petri plate. All the plates were inoculated at room temperature on the laboratory desk for five days. The linear growth of fungal colony was measured in two directions at right angle to each other after five days of incubation. The percentage inhibition of mycelia growth of test fungi was calculated as follows:
I = [C-TIC] x 100
Where, I =Percentage of inhibition; C = Diameter of fungal colony in control; T = Diameter of fungal colony in treatment.
Evaluation of chemicals against bacteria
For the detection of antibacterial activities and sensitivity spectrum analysis, the disc diffusion method as followed by Chowdhury et al. (Chowdhury, et al. 2003) was followed. Nutrient Agar (NA) was used as basal medium for culture of test bacteria and N, N-dimethylformamide (DMF) was used as the solvent to prepare the desired solution (1%) of the compounds initially.
Nutrient Agar (NA) medium was prepared using the composition Beef extract (3 gm), Peptone (5 gm), NaCl (0.5 gm), Agar (15 gm) and Distilled water (1000 mL). 1000 mL of distilled water was taken in a beaker and 15 gm of agar powder, 3 gm of beef extract, 5 gm of peptone and 0.5 gm of NaCl were added slowly in and they were mixed thoroughly with a glass rod and then heated to boiling for 10 minutes. After 10 minutes of boiling, the medium was transferred in 250 mL conical flasks at the rate of 200 mL per flask. The conical flasks were closed with the cotton plugs and autoclaved at 121°C and 15 psi pressure for 15 minutes and then culturing of different micro-organisms was performed.
Sensitivity spectrum analysis: Paper discs of 5 mm diameter were soaked with 10 μL of 2% solution of the test complexes. 0.2 mL of the suspension of test organism was taken in sterilized glass Petri plates of 100 cm diameter and then the molted and cooled (45°C) NA medium was poured at the rate of 10 mL per Petri plate and shaked gently. Then the discs with test complexes were placed on the seeded agar plate. A control plate was also maintained in each case with solvent. The plates were kept firstly for 24 hours at low temperature (4°C) and the test complex diffused from the disc to the surrounding medium by this time. The plates were then incubated at 35±2°C for growth of test organisms and were observed at 24 hours interval for two days. The activity was determined by measuring the diameter of the zone of inhibition in mm.
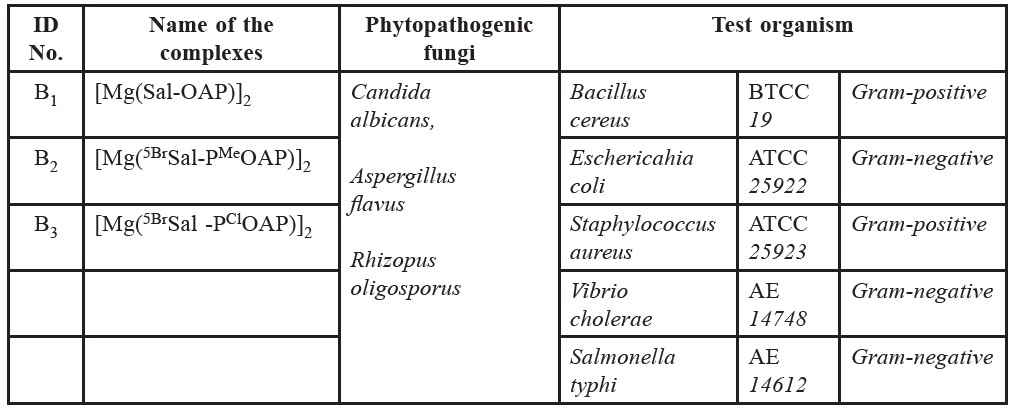
Table 3. Identification no, name of the compounds and test organisms.
Test organisms
In the present study, to screen the antibacterial activities of different complexes, a variety of bacterial strains were used as test organism. Bacterial strains were collected from the microbiology laboratory of the Department of Microbiology, University of Chittagong. The names of the collected pathogens, the names and Identification no. of test chemicals are listed in table 3.
RESULTS AND DISCUSSION
Primarily, the complexes of magnesium(II) were characterized qualitatively by the following procedure:
1. A little amount of the prepared complex was taken in a test tube. Then concentrated nitric acid (HNO3) was added to the sample and gently warmed on a water bath for the decomposition.
2. Excess acid was then neutralized with dilute ammonia solution to make the solution neutral.
3. Then a small amount of NH4Cl [to prevent precipitation of Mg(OH)2)] and few drops of dilute ammonia solution were added to the mixture drop wise with continuous shaking.
4. Finally a few drops of Na2HPO4 was added to the mixture and a white crystalline precipitate of magnesium ammonium phosphate, Mg(NH4)PO4 was obtained by rubbing the test tube beneath the surface of the liquid with a glass rod. The possible reactions are as follows:
Mg2+ + HPO42- + NH3 → Mg(NH4) PO4, White crystalline Precipitate
The white precipitate thus obtained indicated the presence of magnesium(II) ion and hence formation of the desired magnesium complexes.
Infra-red spectra
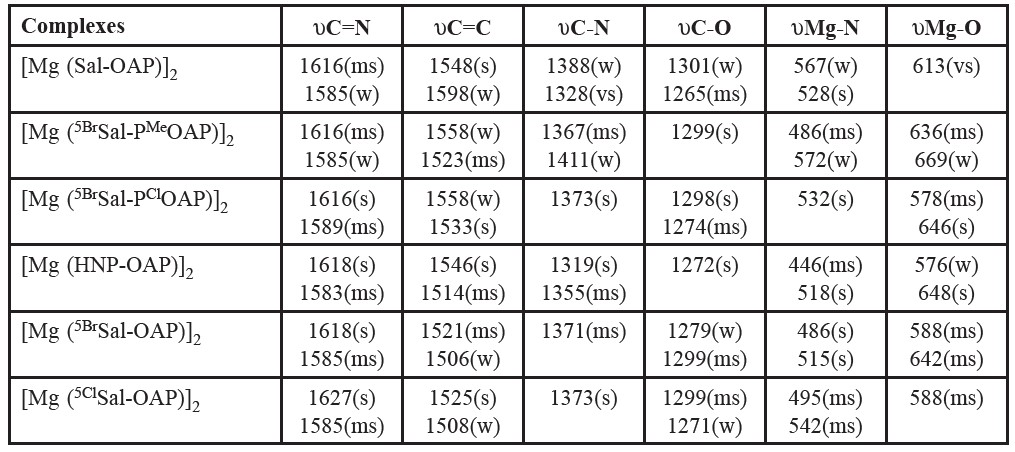
The tentative assignments of the characteristic infrared peaks of various complexes have been made empirically by comparison of the spectra of the corresponding ligands. Of the present compounds the infrared band assignments, that were made tentatively, are given in table 4. It is a general observation that the spectral behavior of the free ligands changes by chelation but the interpretation of the band shifts depends on the attribution of the observed frequencies to the various bands, C-N, C-O etc.
The present Schiff base ligands contain the υC=N modes. The peak between 1620 and 1580 cm-1 have been assigned to υC=N by Aliyu and Ado (Aliyu and Ado, 2010) and Johari et al. (Johari, et al. 2009). The bands appearing between 1520 and 1600 cm-1 have been assigned to aromatic υC=C. The υC=N modes are often mixed with higher frequency υC=C (near 1600 cm-1) and are seen as a strong band. The bands appearing at 1315-1400 cm-1 (specially the highest frequency ones near 1400 cm-1) have been assigned to υC-N mode. Aliyu and Ado (Aliyu and Ado, 2010) have assigned a band near 1400 cm-1 to υC-N for M(II) (M=Mn, Ni, Cu) Schiff base complexes. The υC-O (Phenolic) stretching frequency of ligand seen at around 1380 cm-1 gets shifted to a lower frequency region in the complexes in the range of 1372- 1326 cm-1, and this is indicative of bonding through the phenolic oxygen (Mounika, et al. 2010). On the basis of these studies, the band observed for the present complexes in the regions of 1580-1670, 1520-1600, 1360-1401 and 1260-1300 cm-1 have been assigned as due to υC=N, aromatic υC=C, υC-N and υC-O, respectively. The presence of υC=N and υC-O modes in the ligand and their negative shift on complexation confirm that the ligands act as N- donor and O- donor bases. El-ajaily et al. (El-Ajaily, et al. 2007; El-ajaily and Abdlseed, 2009) and Raman et al. (Raman, et al. 2001) have prepared and investigated new Schiff base complexes of Cr(II), Cu(II), Ni(II), Mn(II), Zn(II), Pb(II), TiO(IV) and VO(IV) and observed new bands at 575-671 cm-1 and 444-540 cm-1 in the spectrum which were attributed to υM-O and υM-N vibrations respectively. The presence of new bands in this region was an advantage for attempts of the present assignments. The bands appearing in the region 575-671 cm-1 have been assigned as υM-O stretching modes and the bands appearing below this υM-O (around 444-540 cm-1) frequency have been assigned to υM-N vibrations.
Table 4. Infrared spectral bands of some Schiff base complexes of magnesium(II).
1H NMR spectra
The 1H NMR spectrum of the ligand in CDCl3 shows the respective signals. The phenyl multiplet is seen at 6.5–7.3 δ and the azomethine proton is seen at 7.6 δ (singlet). The peak at 10.8 δ is attributed to phenolic OH group present in the ligand which is being absent in respective complex.
Electronic spectra
The electronic spectral bands for the prepared complexes are given in table 5. Being a d0 system, the present magnesium(II) complexes show no d-d transition in the visible region, and are colored only through their intense charge transfer absorptions tailing in from the ultraviolet. Besides, the other bands are due to intra-ligand transitions. The peaks observed below 350 nm are tentatively assumed as due to π →π*, n→π* and n→σ* transitions. The peaks observed above 350 nm are assumed to be due to charge transfer.
Magnetic measurement
From the experimental values, (χM) -266.08 × 10-6 of magnetic measurements of the prepared complex, [Mg(5BrSal-PMeOAP)]2 revealed that magnesium (II) complexes of the tridentate Schiff bases are diamagnetic in nature and thus indicating the d0 electronic configuration of magnesium(II) in such complexes.
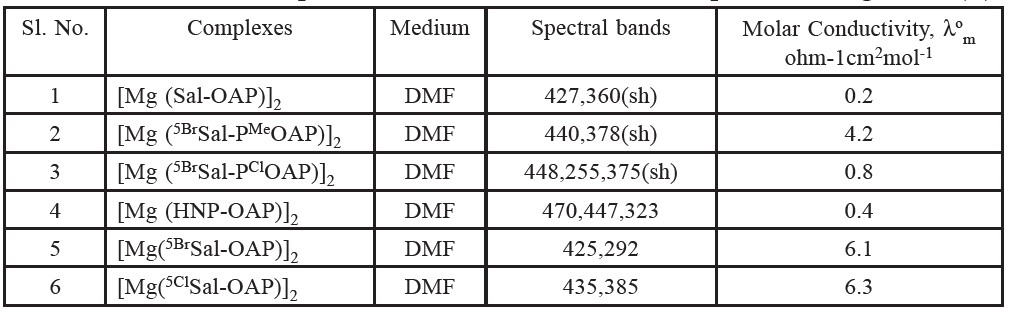
Molar conductance
The molar conductance values of the presently prepared complexes are shown in table 5. (Solution of complexes is ca. 10-3 M). The low conductance values of the prepared complexes in DMF solutions indicate their non-electrolytic nature (Raman, et al. 2001). This suggests +2 oxidation state of magnesium which is satisfied by the dinegatively charged tridentate ligand as expected. The molar conductance values of the prepared oxovanadium(V) complexes in DMF Solutions indicate their non-electrolytic nature. However, the observed conductance values might be due to coordination of the solvent molecule in the solution state.
Table 5. The electronic spectral data for the Schiff base complexes of magnesium(II).
Effect of chemicals on the bacterial growth
In the present work, Magnesium (II) complexes were selected for antibacterial activity against five human pathogenic bacteria. The inhibition zones of test organisms for different complexes are presented in figure 2. The results of the inhibition zone of the selected bacteria due to the effect of compounds are presented in graphical figure 3.
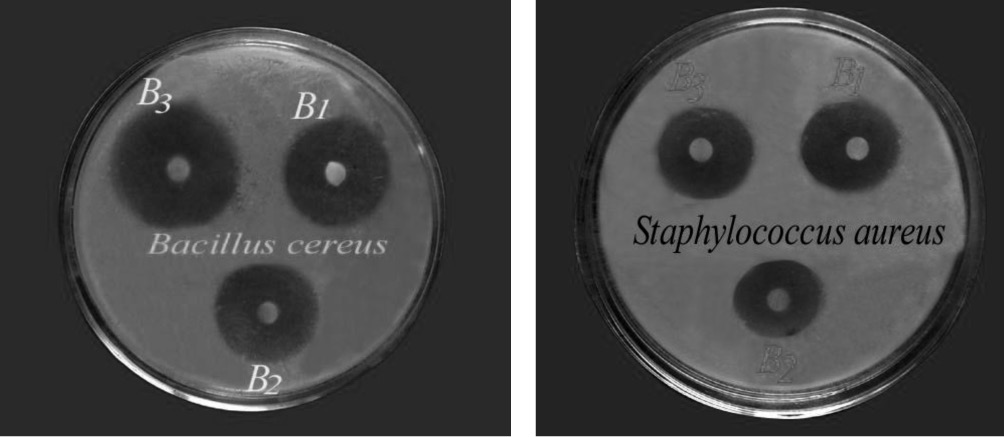
Figure 2. Zone of inhibition against Bacillus cereus and Staphylococcus aureus by complexes B1, B2, B3.
It is found that compound B1, B2, B3 are more effective against Bacillus cereus, Eschericahia coli and, Staphylococcus aureus than B7, B9 and B14. Compound B7, B9 and B14 are quite effective against Bacillus cereus, Eschericahia coli and, Staphylococcus aureus. Compound B3 is more effective against Vibrio cholerae and compound B1 is quite effective against Vibrio cholerae. On the other hand compound B2, B7, B9 and B14 were not effective against Vibrio cholerae. Compound B3 is quite effective against Salmonella typhi while Compound B1, B2, B7, B9 and B14 were not effective against Salmonella typhi.
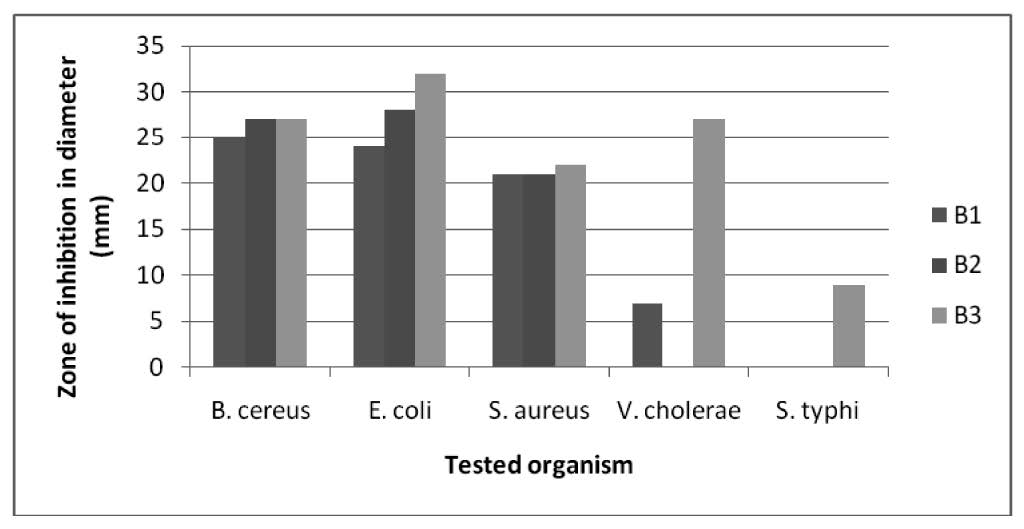
Figure 3. Zone of inhibition by complexes B1, B2, B3 against tested organisms.
Effect of chemicals on mycelia growth of the fungi
Candida albicans, Aspergillus flavus and Rhizopus oligosporus were selected for mycelia growth tests. The growth inhibition of Candida albicans and Aspergillus flavus due to the treatment of B3 are mentioned in figure 4. The results of the percentage inhibitions of mycelia growth of the selected phytopathogenic fungi due to the effect of compounds are presented graphically in figure 5.
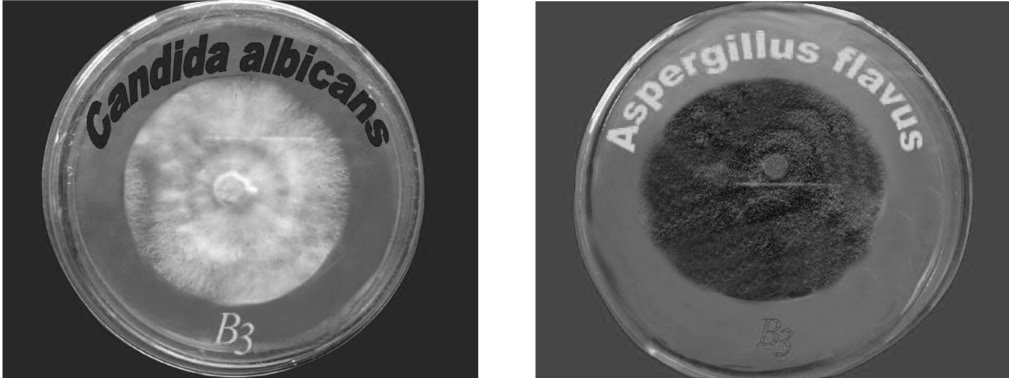
Figure 4. % inhibition against Candida albicans and Aspergillus flavus by B3 complexe.
The inhibitions of the mycelia growth of the chemicals were like B1(66%), B2(17%), B3(12%), B7(25%), B9(20%) and B14(39%). The screening data presented in figure 8 suggest that B1(25%), B2(21%), B3(17%), B7(38%), B9(15%) and B14(34%) were toxic to the Aspergillus flavus. The screening data presented in figure 5 suggest that B1(11%), B2(14%), B3(9%) and B14(35%) were toxic to the Rhizopus oligosporus. On the other hand, B7(3%) and B9(6%) were comparatively less toxic to the Rhizopus oligosporus.
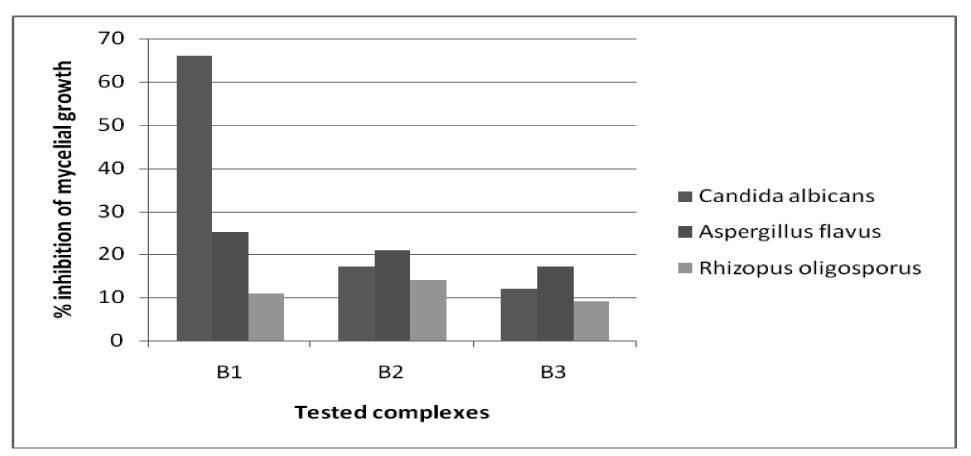
Figure 5. % inhibition against tested fungi by B1, B2, B3 complexes.
CONCLUSION
The Schiff bases were derived by the condensation of o-hydroxyaldehydes or ketones with amines. The prepared Mg(II) complexes with such ligands have been found to be of the type [MgL]2 (where, LH2 = dibasic tridentate ligands). The analytical data indicate that the complexes have 1:1 (metal: ligand) stoichiometry. Conductivity measurement indicates their non-electrolytic nature. From IR data, elemental analysis and other analytical and physicochemical information, the magnesium(II) complexes seem to be dimeric in nature. The Schiff base ligands possess a planar configuration with respect to the position of the donor atoms or ions. The prepared four-coordinated (dimeric) Mg(II) complexes of the type [MgL]2 are expected to be tetrahedral in structure. However, it is difficult to suggest the exact structure of any of the prepared compounds without any crystal structural evidences. Very good antimicrobial activity of the selected complexes was found against tested organisms.
REFERENCES
Aliyu, H. N., and I., Ado. 2010. Studies of Mn (II) and Ni (II) complexes with Schiff base derived from 2-amino benzoic acid and salicylaldehyde. Bayero J. Pure Appl. Sci. 3(1): 245-249.
Chowdhury, D. A., M. N., Uddin, and N, Lucky. 2003. Antimicrobial activities of some dithiocarbamates and their Titanium(IV) complexes. Bull. Pure Appl. Sci. 22C(1): 39-45.
Chowdhury, D. A., M. N. Uddin, and F. Hoque. 2010. Dioxouranium(VI) Complexes of Some Bivalent Tridentate Schiff-base Ligands Containing ONS Donor Set. Chaing Mai J. Sci. 37(3): 443-450.
Corazza F., C. Floriani, A. Chiesi-Villa, C. Guastini, and S. Ciurli. 1988. Fiveco-ordinate magnesium complexes: synthesis and structure of quadridentate Schiff-base derivatives. J. Chem. Soc., Dalton Trans. 2341-2345.
El-Ajaily, M. M., F. A. Abdlseed, and S. B. Gweirif. 2007. Preparation, Characterization and Antibacterial Activity of Some Metal ion Complexes. E-Journal Chem. 4: 461-466.
El-ajaily, M.M., and F.A. Abdlseed. 2009. Preparation and spectroscopic investigation of a Schiff base metal complexes. Int. J. Pharm. Tech. Res.,1(4): 1097-1103.
Ghosh, T., C., Bandyopadhyay, S., Bhattacharya, and G., Mukherjee. 2004. Synthesis, Spectral and Electrochemical Studies of Mixed-Ligand Oxovanadium(IV) and Oxovanadium(V) Complexes Incorporating the Tridentate ONO Donor Schiff Base Derived from Acetylacetone and Benzoylhydrazine. Trans. Met. Chem. 29(4): 444-450. 10.1023/B:TMCH.0000027457.32094.aa
Jang, Y. J., U.K., Lee, and B. K., Koo. 2005. Synthesis and Crystal Structures of Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) Metal Complexes with NNO Functionalized Ligands. Bull. Korean Chem. Soc. 26(6): 925-929.
Johari, R., G., Kumar, D., Kumar, and S., Singh. 2009. Synthesis and antibacterial activity of M(II) Schiff-Base complex. J. Ind. Council Chem. 26(1): 23-27.
Mounika, K. and B., Anupama, J., Pragathi, and C., Gyanakumari. 2010. Synthesis, Characterization, Antifeeding and Insect Growth-Regulating Activities of Cr(III), Mn(II), Fe(III), Co(II), Ni(II) and Cu(II) Complexes with N-Acetylacetonyl-3-Aminocoumarin. J. Sci. Res. 2(3): 513-524.
Nene Y., and Thapilyal L. 2002. Poisoned food technique of fungicides in plant disease control (3rd eds). Oxford and IBH Publishing Company, New Delhi.
Rabie, U. M., A.S.A. Assran, and M.H.M. Abou-El-Wafa. 2008. Unsymmetrical Schiff bases functionalize as bibasic tetradentate (ONNO) and monobasic tridentate (NNO) ligands on complexation with some transition metal ions. J. Molecular Structure. 872: 113-122. 10.1016/j.molstruc.2007.02.023
Raman, N., Y. P. Raja, and A. Kulandaisary. 2001. Synthesis and characterization of Cu(II), Ni(II), Mn(II), Zn(II) and VO(IV) Schiff base derived from ophenylenediamine, and acetanilide. Ind. Aca. Sci. 113: 183-185.
Roberts, C. C., and J. M. Fritsch. 2010. Synthesis and crystal structures of magnesium complexes with NNO Schiff base ligands bearing quinolyl pendant donors. Polyhedron. 29(40): 1271-1278.
Salam, M. A., S. M. Jahangir, D. A. Chowdhury, and Z. A. Siddique. 1997. Dioxouranium (VI) complexes of some dibasic tridentate ONO donor ligand systems. Chitt. Univ. Studies, Part II. 21(1): 23-28.
Samanta, B., J., Chakraborty, D. K., Dey, V., Gramlich, and S., Mitra. 2007. Synthesis, characterisation and structural aspects of a new diorganotin(IV) complex with N_-(5-bromo-2-hydroxybenzylidene) benzoylhydrazone ligand. Struct Chem. 18: 287-293. 10.1007/S11224-006-9133-y
Zhang Y, X, Wang, and L Ding. 2011. Synthesis and DNA binding studies of Mg(II) complex of Schiff base derived from vanillin and L-tryptophan. Nucleosides Nucleotides Nucleic Acids. 30(1): 49-62. 10.1080/15257770.2010.543117
Mohammad N. Uddin, Md. A. Salam, Didarul A. Chowdhury, and Md. Abu B. Siddique
Department of Chemistry, University of Chittagong, Chittagong-4331, Bangladesh
*Corresponding author. E-mail: nasircu72@gmail.com
Total Article Views

