
Studies on Some Dioxouranium(VI) Complexes with Dithiocarbamate Ligands
Mohammad Nasir Uddin*, D. A. Chowdhury and Mohammed Tazul IslamPublished Date : 2019-08-24
DOI : 10.12982/cmujns.2014.0015
Journal Issues : Number 1, January-april 2014
ABSTRACT
A number of dioxouranium(VI) complexes of some monobasic bidentate dithiocarbamate(dtc) ligands have been synthesized. Monobasic bidentate dithiocarbamate ligands were prepared by the reaction of 1:1 molar ratio of ethylenediamine, N,N-dimethylethylenediamine, N,N-diethylethylenediamine, 1,3-propanediamine, N,N-dibutyl-tri-methylenediamine, 1,6–hexanediamine with carbondisulphide. Physico-chemical analytical, e.g. elemental analysis, IR Spectral data suggest the formation of [UO2L2] type complexes. The molar conductance values obtained for a number of complexes indicate the non-electrolytic nature of the complexes. The magnetic and electronic spectral studies of the prepared complexes indicate the presence of the 5f 0 6d0 7s 0 electronic configuration characteristic of the 6+ oxidation state of uranium.
Keywords: Carbondisulphide, Dioxouranium(VI) complexes, Dithiocarbamate, Ligands, Octahedral geometry
INTRODUCTION
Transition elements of Group IV, V and VI are known to form mononuclear oxocations of the type MOn+ and MO2n+. The most thoroughly investigated, best characterized and most stable oxometal cations are the dioxouranium(VI), dioxomolybdenum(VI) and oxovanadium(IV) ions. Complexes of the uranyl ion, UO22+, are of interest since they show four, five, six or seven-coordinate, pentagonal-bipyramidal geometry (Gatto et al., 2004). Due to the spectral properties (absorption and luminescence) and excited-state electron-transfer properties ofthe UO22+ ion, dioxouranium(VI) complexes have possible applications in solar energy conversion systems (Signorni and Dockal, 1996).
The Schiff base complexes with many transition metal ions, focused on complexes of the d-block elements, have attracted considerable interest because of their growing importance as model molecules for biological systems such as oxygen carriers. Dioxouranium(VI) complexes of some aroylhydrazines (benzoylhydrazine, salicyloylhydrazine, nicotinoylhydrazine) and their Schiff bases withacetone have been characterized where ligand acted as bidentate using NO-donor set (Chowdhury et al., 2008). The structure of the chelates of dioxouranium(VI),UO22+, of the bidentate Schiff base ligands derived from o-hydroxyaldehyde or ketone, 2-hydroxy-1-naphthaldehyde and aniline was reported (Chowdhury et al., 2011). Several new dioxouranium(VI) complexes of Schiff bases [LH2], derived from salicylaldehyde, substituted salicylaldehydes, 2-hydroxy-1-naphthaldehyde and 2-aminothiophenol, have been prepared and characterized (Chowdhury et al., 2010).
Complexes of titanium(IV) containing mono-dithiocarbamate as a primary ligand derived from diamines have been reported where one NH2-site remained unreacted (Chowdhury and Uddin, 1998; Chowdhury et al., 2004). Some Dioxomolybdenum(VI) complexes of the type MoO2(dtc)2 have been prepared, where dtcH is the uninegatively charged bidentate ligand of diamine-monodithiocarbamate (Chowdhury et al., 2006). In continuation, the aim of this work is to study the behaviour, prepare and investigate the structure of the chelates dioxouranium(VI), UO22+ complexes of the bidentate ligand of diamine-monodithiocarbamate.
MATERIALS AND METHODS
Uranylnitrate hexahydrate, UO2(NO3)2.6H2O, was obtained from BDH Chemicals Ltd. Ethylenediamine, N,N-dimethylethylenediamine, N,N-diethylethylenediamine, 1,3-propanediamine, N,N-dibutyl-tri-methylenediamine, 1,6–hexanediamine, carbondisulphide, methanol, ethanol, chloroform, N,N-dimethylformamide were obtained from M/S. E. Merck, Germany.
Preparation of some diamine-monodithiocarbamates
Diamine or their substituted diamine (150 mmol) in 50 cm3 methanol was introduced in a 250 cm3 conical flask, and the mixture was allowed to cool in a freezing mixture of ice and salt (0°C). To this, carbondisulphide (140 mmol; a bit less to protect formation of bis-derivative) was added drop-wise over a period of about half an hour with constant stirring. Immediately white oily precipitate was formed. The precipitate was allowed to stand for about five hours in the ice-salt bath and thereafter filtered off and washed with methanol and dried over calcium chloride in a vacuum desiccator.
Preparation of complexes, UO2L2
The ligand (2 mmol) was taken in a round flux. Ethanol (15 ml) and distilled water (10 ml) were added to this. The mixture was refluxed at 60°C in a paraffin bath and was stirred for a few minutes by a magnetic stirrer. Then uranylnitrate hexahydrate, UO2(NO3)2.6H2O (1 mmol) was added to this solution. The colour of the solution changed to orange-red. The mixture was refluxed at 60°C for half an hour with constant stirring when yellow precipitate separated out. The precipitate was filtered and washed with ethanol and finally dried under vacuum over CaCl2.
Physical measurements
Melting point of the ligands and complexes were determined by an electrothermal melting point apparatus. UV-absorption spectra were run on a Shimadzu UV-visible recording spectrophotometer (model-160). Infrared spectra were recorded on KBr pellets with Perkin-Elmer infrared spectrophotometer (Model-883). Magnetic moments were determined by the Gouy method. Conductivity measurements were performed on Philips conductivity meter (model-WPA CM-25) made by WPA, Saffron Walden, England. Metal analyses of the prepared complexes were done gravimetrically by oxine (Vogel A.I., 1961). Microanalysis was performed by the Central Drug Research Institute, Lucknow, India.
RESULTS AND DISCUSSION
UO2(NO3)2.6H2O reacts with the ligands in a 1:2 ratio following the reaction scheme as shown in Figure 1. As the ligand dissolved completely in general solvents, colour change of the reaction mixture shows the reaction is proceeding. Precipitates are formed upon cooling, which have been isolated and characterized by elemental analysis, IR and other conventional methods. All the complexes exhibit high melting points, indicating strong bonding between ligands upon complete deprotonation and neutral dioxouranium(VI) ion. All the complexes are stable atroom temperature. The yields of the purified dioxouranium(VI) complexes for this general procedure are in the range 70-85%. The complexes are insoluble in methanol, ethanol, acetone and chloroform, and fairly soluble in dimethylformamide. The analytical data support 1:2 metal-ligand stoichiometries. The ligands behave as monobasic bidentate coordinating via the SS atoms. Colour, yield and melting points of the prepared dithiocarbamate ligands are given in Table 1. Some analytical and physical data of complexes are included in Tables 2-4.
Table 1. Colour, yield and melting points of the prepared dithiocarbamate ligands.
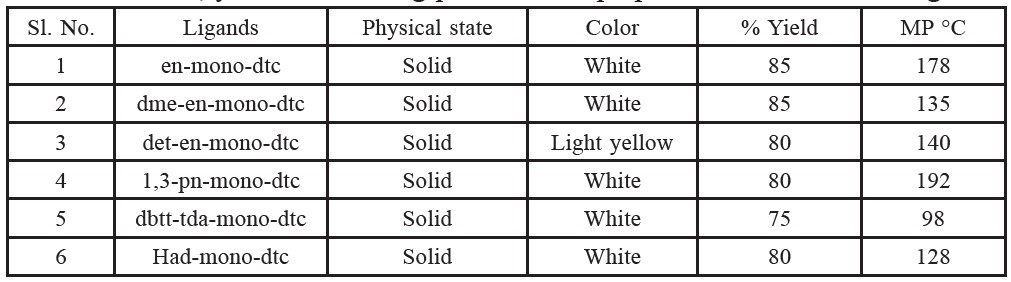
Table 2. Analytical and some physical data of the complexes.
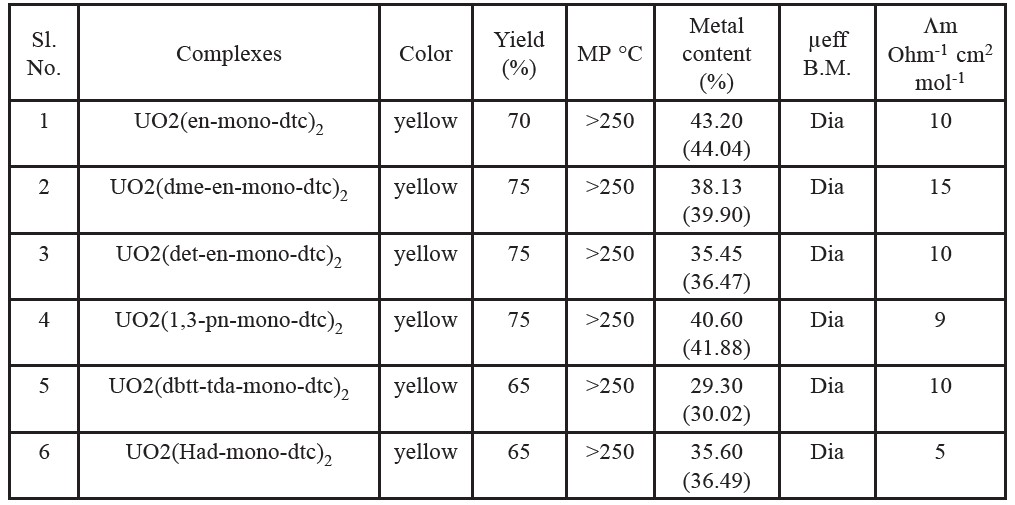
Note: M.P. = melting point, dia = diamagnetic. * Values in parentheses indicate calculated values.
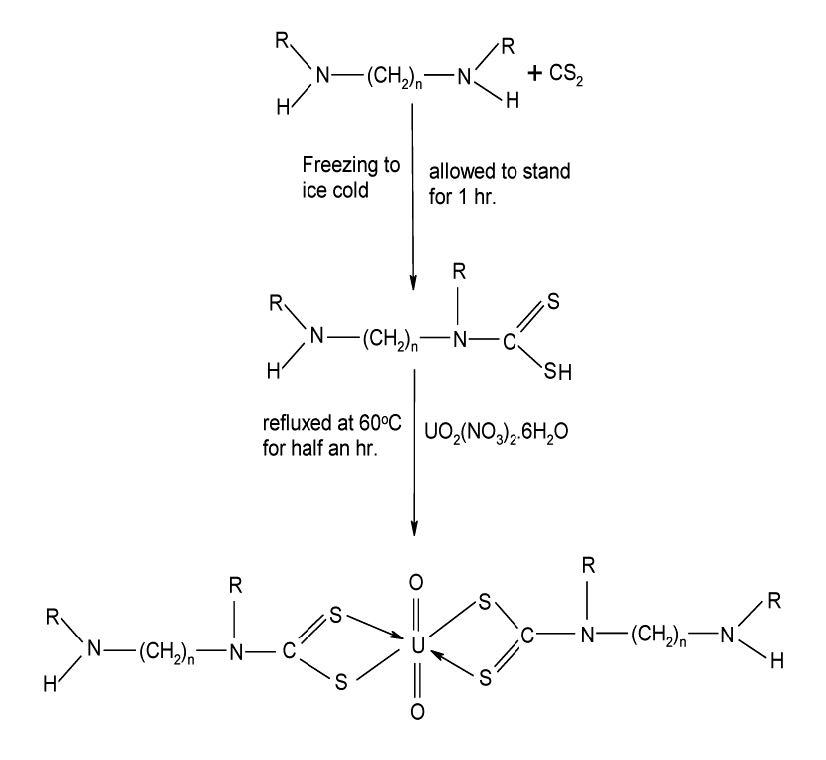
Figure 1. The proposed reaction scheme and structure of uranyl complex.
Infrared study of the prepared dithiocarbamate complexes of Uranium (VI) The infrared studies of metal complexes help to demonstrate the presence and examine the effect of coordination of the ligands and assume structural properties of the complexes. For all the diamine-mono-dithiocarbamate ligands and their complexes, the main stretching modes are for –N-NH/NH2, C-N, C-S, C=S and S-H. The presence of M-S thiolato and M-S thioketo and the disappearance of vS-H vibrations confirms coordination of SS donors of the ligand to the metal ion and the monobasic nature of ligands. For the UO2 complexes, the O=U=O provide structural information about the coordination environment of the uranyl group. For the present project, the infrared spectra of the metal complexes provided information about their formation in respect to the presence and coordination of the diamine-mono-dithiocarbamate ligands in the form of bidentate L, with one of the NH/NH2 parts of the diamines being free. The absence of any S-H vibrations and the presence of vN-H mode confirm that the coordinated ligand contains a free NH/NH2 part of the diamine, thus enabling the ligand to act as a monobasic bidentate one. Comparison of the metal-complex spectra with that for the free ligands was most useful in rationalizing the infrared results and assigning of the various stretching modes. Of our presently prepared ligands and their complexes, the infrared spectral band assignments, made tentatively, are shown in Table 3and Table 4, respectively. The infrared spectra of the present compounds taken in the range 400-4000 cm-1, helped indicate regions of absorptions due to the above-mentioned vibrations.
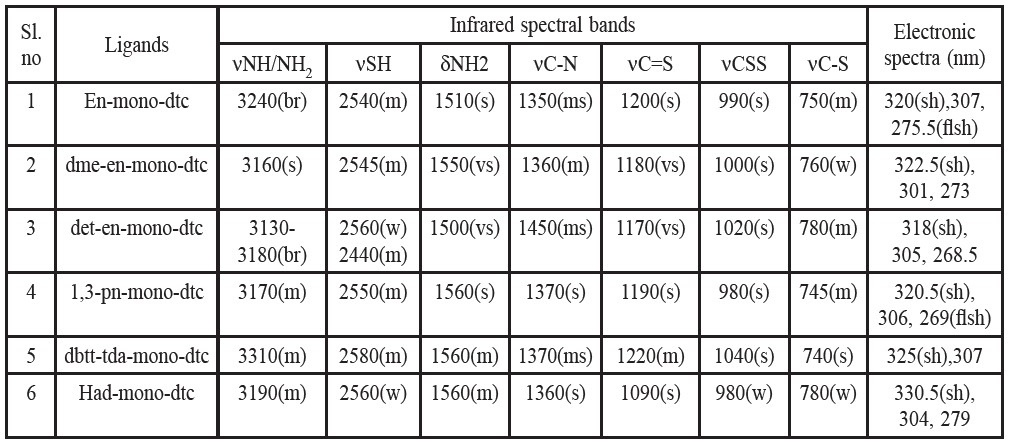
Table 3. Infrared spectral bands for the prepared ligands.
The high oxidation state 6+ of uranium is stabilized by formation of the dioxocation, UO22+. A number of authors (Gatto et al., 2004; Signorni and Dockal, 1996; Chowdhury et al., 2006, 2008, 2010, 2011) have assigned bands in the region 800-1000 cm-1 for different types of monomeric dioxourmium (VI) complexes as due to the asymmetric vibration of O=U=O group. The observation of the bands for the prepared complexes in the region 870-990 cm-1 given in Table 4 argues the presence of O=U=O group, which indicates the presence of uranium (VI) in the form of stable dioxocation UO22+. The bands attributed to the asymmetric stretching vibrations of O=U=O group indicates that linearity of the O=U=O group is retained in the complexes.
Table 4. Infrared spectral and electronic spectral bands for the prepared complexes.
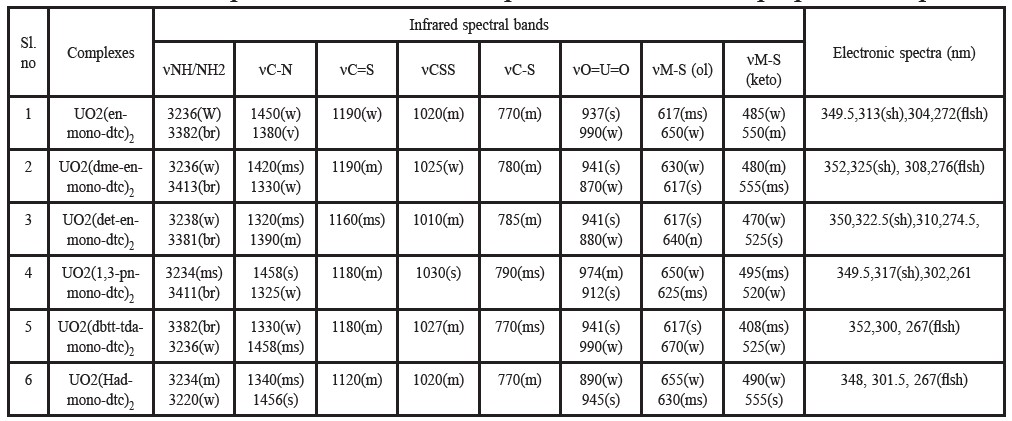
The bands in the region 3150-3300 cm-1 (Trifunovic et al., 2002) are assigned as due to the N-H stretching mode in the free ligands. In the ligand itself, vibrational stretching of the -NH and -NH2 groups give rise to a multiple absorption band in this region. The N-H absorption is usually broad unless spectrum is scanned in dilute solutions. Samples in the solid form show N-H stretching at lower wave number due to greater degree of hydrogen bonding. The N-H def. vibrations for amino group in free ligands appear as strong bands at 1500-1560 cm-1 (Sharma, 1989). For most complexes, these move to higher frequencies as a result of coordination. Primary amines show two sharp bands; secondary amines give only one band, while tertiary amines do not absorb in the said N-H str. region (Sharma, 1989). The infrared spectra of the free ligand exhibit medium intensity bands at 2550 cm-1 and 3150-3300 cm-1, which have been assigned to vS-H (Chowdhury et al., 2006; Trifunovic et al., 2002) and vNH/NH2 respectively. The vS-H disappears in the IR spectra of the complexes while no significant change is noticed in vNH/NH2 modes, indicating that the bonding is taking place through the sulphur atom only and the amino group of ligand is not involved in complexation.
15N and I8O isotope substitutions have been used by Percy et al. (1976) to identify the N and O sensitive interactions in N-alkyl and N-arylsalicylaldemine and salicylaldeneglycinate complexes. Authors (Suardi et al., 1997; Chowdhury et al., 2006) showed vC-N frequency in the region in their studies of iridium and molybdenum dithiocarbamate complexes, respectively. On the basis of these studies, the band in the region ~ 1350-1450 cm-1 of the present dithiocarbamate ligand have been assigned as due to vC-N that shifted to higher region 1320-1458 cm-1.
It has been suggested (Zhang et al., 2003; Jung and Sohn, 1988; Kaludjerovic et al., 2002; Husarek et al., 2003; Pastorek et al., 2002) that when both the sulphur atoms of the ligand are involved in bonding, the vasyCSS mode (at ~ 1000 cm-1)does not split, whereas, splitting of the band takes place when unidentate sulphur bonding is involved. The IR spectra of the ligands exhibit a strong band around 980-1040 cm-1 due to vasyCSS mode. The appearance of only a single band in the range ~ 930 ±50 cm-1, in the prepared complexes indicates that both the sulphur atoms of the ligand take part in bonding. The band at -1200 cm-1 (assigned to the vC=S) shifts by about 20 cm-1 to a lower wave number in the case of the prepared complex indicates the involvement of thioketone sulphur in coordination. The absence of any band around 2550 cm-1 in the complex indicates deprotonation of the thiol group, S-H. The vC-S at -760 cm-1 shifts by about ~(10-20) cm-1 to a higher energy, coordinated through the sulphur atom. The vC-S shifts to higher energy due to the maintenance of a ring current arising for electron derealization in a chelate ring.
The assignments of vM-S (thioketo) and vM-S (thiophenolic) bands (Table 4) in the present study are tentative and these assignments have been made on the basis of the previous studies (Trifunovic et al., 2002; Jung and Sohn, 1988). Salam et al. (1990) observed the vM-S (thioketo) band at -400 cm-1 and vM-S (thiolato) band at -620 cm-1. The bands appearing in the region 617-670 cm-1 have been assigned as vM-S (thiol) stretching modes and the bands appearing in the region 408-555 cm-1 have been assigned as vM-S (thioketo) vibrations.
Electronic spectra
The electronic spectra of the prepared ligands and complexes were recorded on nujul mull and observed bands are given in Table 3 and Table 4, respectively. The electronic spectra of the ligand (LH) in ethanol shows absorption bands at 276-325 nm. The absorption bands of the complexes are shifted to longer wavelength compared to those of the ligand. A moderately intensive band observed in the range of 300–350 nm is attributable to the n - π* transitions of the complexes. However, the typical band of UO22+ expected around 350 nm seems to be overlapped by fairly strong ligand-to-metal charge-transfer bands. These charge-transfer transitions probably occur from the n- π* orbitals of the ligands to the f-orbitals of uranium (Bansse et al., 1995). The colour of the complexes is due to charge transfer absorption tailing in from the ultraviolet.
Conductivity measurement
The molar conductance values as shown in Table 2 measured in DMF (10-3 M) solution of the complexes gave a value of 5-15 ohm-1 cm2 mol-1. The low conductance of the complexes [UO2L2] indicates their non-electrolytic nature as tentative for the complexes (Husarek et al., 2003). The low molar conductance values of the complexes are supporting neutral [UO2L2] formulation of the compounds.
Magnetic measurement
The magnetic susceptibility of the complexes were found to be negative indicating the complexes to be diamagnetic as expected fo, 5f°6d°7s°, U(VI) complexes possessing the 6+ oxidation state. The magnetic measurements of the dioxouranium (VI) complexes are independent of field strength and temperature and the ground states of dioxouranium(VI) compounds contain no unpaired electrons. This is consistent with diamagnetic behaviour expected for the U(VI) electronic spectra of complexes. The absence of any band above 500 nm in UVvisible region indicates the absence of d-d transition confirming the 6d°-system of UO22+ moiety. The fact that the complexes with UO2(NO3)2.6H2O involving 1:2 UO22+ to ligand ratio give solid complexes (Table 1) have been isolated.
CONCLUSION
The dithiocarbamate (dtcH) possess a planar configuration with respect to the position of the donor atoms/ions. As a general conclusion, the prepared Schiff bases behave as a monobasic bidentate ON donor ligands in 1:2 complexes. Uranyl nitrate reacts with LH, in water to yield a yellow complex of the composition [UO2L2] for neutral cationic complexes. Therefore, on the basis of metal analysis and physical measurement data, an octahedral geometry (Trifunovic et al., 2002) has been proposed for the [UO2L2] type complexes. The proposed structure of uranyl complexes has been given in reaction scheme in Figure 1. Magnetic properties clearly illustrate that the ligands under study do not introduce sufficiently severe steric hindrance as to preclude the formation of complexes. In addition, steric features and arrangement in space can also favourably influence the stabilization of 1:2 complexes. However, it is difficult to suggest the exact geometry for each compound without crystal structural evidence.
REFERENCES
Bansse W., E. Ludwig, U. Schilde, E. Uhlemann, and F. Weller. 1995. Ligand exchange reactions of bis(acetylacetonato) dioxo-molybdenum(VI) and molybdenum hexacarbonyl. J. Inorg. Biochem. 59(2): 730-730. 10.1016/0162-0134(95)97817-A
Chowdhury D. A., and M. N. Uddin. 1998. Some Mixed-Ligand Complexes of Titanium(IV) Containing Mono-Dithiocarbamates as the Primary Ligands. Bull.of Pure and Appl. Sci. 17C(2): 57-60.
Chowdhury D. A., M. N. Uddin, and N. Lucky. 2004. Some Mixed-Ligand Titanium(IV) Complexes Containing Mono-Dithiocarbamate as the Primary and Dibasic Bidentates as the Secondary Ligands. The Chit. Univ. J. of Sci. 28(2): 1-5.
Chowdhury D.A., M.N. Uddin, and A. K.M. L. Rahman. 2006. Synthesis and Characterization of Dioxo-molybdenum (VI) Complexes of Some Dithiocarbamates, Chiang Mai J. Sci. 33(3): 357-362.
Chowdhury D. A., M. N. Uddin, and M.A.H. Sarker. 2008. Synthesis and characterization of dioxo-uranium(VI) complexes of some aroylhydrazines and their Schiff bases with acetone. Chiang Mai J. Sci. 35(3): 483-494.
Chowdhury D.A., M.N. Uddin, and F. Hoque. 2010. Dioxouranium(VI) Complexes of Some Bivalent Tridentate Schiff-base Ligands Containing ONS Donor Set, Chiang Mai J. Sci. 37(3): 443-450.
Chowdhury D. A., M. N. Uddin, and F. Hoque. 2011. Dioxouranium(VI) complexes of some monovalent bidentate Schiff base ligands derived from aniline. Chiang Mai Uni. J. Sci. 10(1): 261-268.
Gatto C.C., E.S. Lang, A. Kupfer, A. Hagenbach, D. Wille, and U. Abram. 2004. Dioxouranium complexes with acetylpyridine benzoylhydrazones and related ligands. Z. Anorg. Allg. Chem. 630(8-9): 735-741. 10.1016/S1387-7003(03)00165-5
Husarek J., B. Cvek, R. Pastorek, Z. Sindelar, and M. Pavlicek. 2003. Nickel(II) Cyclohexylethyl and Di(Pentyl)Dithiocarbamate Complexes with 1,1,1- Tris (Diphenylphosphinomethyl) Ethane in the Coordination Sphere. Chemica. 42: 7-11.
Jung O., and Y. S. Sohn. 1988. Coordination Chemistry of Organotin(IV) Dithiocarbamate Complexes, Bull. Korean Chem. Soc. 9(6): 365-368.
Kaludjerovic G. N., V. M. Djinovic, S. R. Trifunovic, I. M. Hodzic, and T. J. Sabo. 2002. Synthesis and Characterization of Tris[Butyl-(1-Methyl-3-Phenyl-Propyl)- Dithiocarbamato]- Cobalt(III) Seskvitoluene, J. Serb. Chem. Soc. 67(2): 123-126.
Pastorek R., J. Kamenicek, M. Pavlicek, J. Husarek, and Z. Sindelar. 2002. Nickel(II) Dithiocarbamates with Polydentate P-Ligands in the Coordination Sphere. Chemica. 41: 35-41.
Percy G. C., and J. S. Stenton. 1976. IR spectra of isotopically labelled anhydrous cobalt(II) complexes of N-salicylideneglycinates, J. Inorg. Nucl. Chem. 38(7): 1255-1258.
Salam M. A., Moniruddin, and A. J. Begum. 1990. Chitt. Univ. Studies, part-II, Science, 14 (2):
Sharma Y. R. 1989. "Elementary Organic Spectroscopy", S. Chand and Company Ltd, P(120-122), 2nd edn-, Rep. 1996.
Signorni O., and E.R. Dockal. 1996. Synthesis and characterization of aquo[N,N -ethylenebis(3-ethoxysalicylideneaminato)] dioxouranium(IV). Polyhedron. 15(2): 245-255.
Suardi G., B. P. Cleary, S. B. Duckett, C. Sleigh, M. Rau, E. W. Reed, J. A. B. Lohman, and R. Eisenberg. 1997. Luminescent Iridium(I) Diethyldithiocarbamate Reactivity Including Stereo selictive Hydrogen Oxidative Addition, J. Am. Chem. Soc. 119: 7716-7725. 10.1021/ja970208t
Trifunovic S. R, Z. Markovic, D. Sladic, K. Andjelkovic, T. Sabo, and D. Minic. 2002. The Synthesis and Characterization of Nickel(II) and Copper(II) Complexes with the Polydentate Dialkyl Dithiocarbamic Acid Ligand 3-Dithiocarboxy-3-Aza- 5-Aminopentanoate. J. Serb. Chem. Soc. 67(2): 115-122. 10.3390/80500411
Vogel A.I. 1961. Quantitative Inorganic Analyses. London.
Zhang W., Y. Zhong, M. Tan, N. Tang, and K. Yu. 2003. Synthesis and Structure of Bis(Dibutyldithiocarbamate)Zinc(II): Zn2[(n-Bu)2NCSS]4. Molecules. 8: 411-417.
Mohammad Nasir Uddin*, D. A. Chowdhury and Mohammed Tazul Islam
Department of Chemistry, University of Chittagong, Chittagong-4331, Bangladesh
*Corresponding author. E-mail: nasiru_cu@yahoo.com
Total Article Views

