
Combined Effect of Calcium Salt Treatments and Chitosan Coating on Quality and Shelf Life of Carved Fruits and Vegetables
Jomkhwun Suwannarak , Putkrong Phanumong and Nithiya Rattanapanone*Published Date : 2019-08-24
DOI : 10.12982/CMUJNS.2015.0088
Journal Issues : Number 3, September - December 2015
ABSTRACT
This study analyzed the combined effect of three types of calcium salts (CaCl2, Ca lactate, and Ca propionate) and chitosan coating on the quality and shelf life of carved fruits and vegetables (pumpkin, cantaloupe, carrot, Chinese radish, and Japanese cucumber). The best concentration of each salt was compared with chitosan (0.25 or 0.5%) coating combined with 0.5% CaCl2. The quality and shelf life of the five carved fruits/vegetables in rose-shape were investigated during storage in a clamshell container at 5±1°C. The study simulated the use of the carved samples as decorative food by transferring them daily between storage and room/display temperature (5 and 25°C). The combination of 0.5% CaCl2 + chitosan (0.25% or 0.5%) was the most effective for carved (rose shape) pumpkin, cantaloupe, and carrot. Chitosan coating helped delay dehydration and maintained the color of the carved samples better than the calcium salt alone. The shelf life of the carved (rose and carnation shapes) pumpkin and carrot was 6 and 12 days, respectively. The shelf life of the carved cantaloupe as rose and carnation shapes was 9 and 6 days, respectively; the shelf life of the carved Chinese radish (rose shape) and carved Japanese cucumber (lotus shape) was 9 and 6 days, respectively.
Keywords: Calcium salts, Carved fruit and vegetable, Quality and shelf life, Chitosan coating
INTRODUCTION
Fruit and vegetable carving has become popular across the globe and can be found in restaurants, hotels, catering halls, exhibitions, and cruise ships (Siam Carving Academy, 2011). The carvings decorate plates of food, enhancing their beauty and edibility (Suwannaruk, 2004). Fruit and vegetable carving is a delicate and highly skilled art. Given the effort to create, the carvings are often used more than once, alternating display with storage, with textural changes the main cause of quality loss. No published research has studied the effect of calcium salts on the texture of carved fruits and vegetables or explored the ability of edible coating to enhance shelf life. The objective of this study was to determine the efficacies of three types of calcium salt treatments at different concentrations to improve firmness and the use of chitosan coating to extend the shelf life of five carved fruits and vegetables.
MATERIALS AND METHODS
Plants materials
Mature, commercial cultivars of pumpkin (Cucurbita moschata Decne.) cv. Kangkok, cantaloupe (Cucumis melo L. var. cantaloupensis), carrot (Daucus carota subsp. sativus), Chinese radish (Raphanus sativus Linn.), and Japanese cucumber (Cucumis sativas) were purchased from a local market in Muang District, Chiang Mai, Thailand in August, 2013. Fruits and vegetables were transferred to the laboratory, washed with tap water, and air-dried.
Sample preparation
Pumpkin, cantaloupe, carrot, and Chinese radish were carved into rose and carnation shapes and Japanese cucumber was carved into lotus shape according to the method of Suwannarak et al. (2014a) (see Table 2, column 2 for initial appearance).
Experiment 1. Effect of calcium salts on texture
This experiment used a completely randomized design (CRD) methodology. The effects of three calcium salts [CaCl2 (Union Science, Thailand), Ca lactate (Sigma-Aldrich, USA), and Ca propionate (Sigma-Aldrich, USA)] on the texture of pumpkin, cantaloupe, carrot, Chinese radish and Japanese cucumber slices were compared. The samples were cut into appropriately thin slices (1.5mm) to measure firmness (Fig. 1). Three concentration levels of CaCl2 (0.5, 1.0, and 1.5%), Ca lactate (1.0, 1.5, and 2%), and Ca propionate (1.0, 1.5, and 2%) were used. Pumpkin and cantaloupe were separated into eight sections; each section was cut into a trapezoid shape, peeled, and sliced approximately 1.5 mm thick. Carrot and Chinese radish were peeled, sliced crosswise approximately 1.5 mm thick, and quartered (Fig 1). Japanese cucumber was sliced crosswise into 8 cm long pieces; these were then cut into three pieces, with each piece cut off from the skin into a flat shape 1.5 mm thick (Fig 1).
The samples were dipped in the various calcium salt solutions for 2 min. After draining, the samples were measured for firmness using a texture analyzer (TA-XTi / 50, Surrey, UK) by measuring the penetration force using a cylindrical probe (P/2 for carrot and P/6 for the others) with pre-test speed of 1 mm per second, test speed of 2 mm per second, and post-test speed of 10 mm per second; the probe was set to penetrate 5 mm. Values were reported as Newton (N). For pumpkin, cantaloupe, and Japanese cucumber, three samples were used per treatment, with penetration force measured at three different points on each sample. For carrot and Chinese radish, ten samples were used per treatment.
Experiment 2. Effect of calcium treatments and chitosan coating on shelf life during simulated decorative use
The samples were stored in a clamshell at 5±1°C for 21 hours (simulating refrigerated storage) and then at 25±1°C for 3 hours (simulating decorative display). This cycle was repeated, simulating typical use – refrigerated in preparation for a decorative display on a daily basis, until no longer presentable. Shelf life was evaluated based on appearance. The experiment used a completely randomized design (CRD) with three rose-shaped samples of each fruit and vegetable per treatment. Five chemical treatments were used:
a) 0.5% CaCl2 + 0.25% chitosan (Bannawach Bio-line Co., Ltd., Thailand) in 1% citric acid (Union Science, Thailand)
b) 0.5% CaCl2 + 0.5% chitosan in 1% citric acid (Union Science, Thailand)
c) 0.5% CaCl2 + 80 mg/L PAA (PAA; Thai Peroxide Co., Ltd., Thailand)
d) 1.5% Ca lactate + 80 mg/L PAA
e) 2% Ca propionate + 80 mg/L PAA
The carved samples were dipped into CaCl2 solution for 2 min, followed by coating with 0.25 or 0.5% chitosan for 2 min, and then air-dried. For calcium treatments, the carved samples were dipped into calcium salt solutions for 2 min, followed by dipping in 80 mg/L PAA for 3 min as a sanitizer (Suwannarak et al., 2014b). After draining and air-drying, the carved samples were kept in polystyrene clamshell containers and stored at 5±1°C and 85+5% RH in a temperature and relative humidity controller (Sanyo, Taiwan). The controls were dipped in water or 1% citric acid solution. The carved samples were moved from a 5±1°C incubator into a 25±1°C incubator with open lid for 3 h to simulate daily use as decorative food on a plate at ambient temperature. After 3 h, the carved samples were dipped again in the same chemical solution and kept at 5±1°C. The samples were photographed and appearance quality compared daily. The point at which the carved samples first appeared unacceptable for display was considered the shelf life limit.
Experiment 3. Quality and shelf life during storage in clamshell container at 5±1°C (using the best chemical treatment only)
The experiment used a completely randomized design (CRD), with three carved samples per treatment. The samples were treated with the best chemical treatment determined in Experiment 2. The samples treated with calcium salts and chitosan solutions were dipped for 2 min; the samples treated with water were dipped for 3 min. After dipping, all samples were air dried for 15 min and stored in polystyrene clamshell containers at 5±1°C and 85±5% RH in a temperature and relative humidity controller (Sanyo, Taiwan). Samples were analyzed at two-day intervals for weight loss, color, microbial population, and appearance.
Weight loss. Weights were determined on a digital scale (Model PB 1502-5, Mettler-Toledu, Swizerland). Three carved samples were weighed (including clamshell box) at two-day intervals; weight loss was expressed as the percentage loss of the original weight.
Color measurement. Different carved samples were used for color measurement. The color of the samples was measured with a colorimeter (ColorQuest XE, HunterLab, USA) and expressed as L*, chroma (C*), and hue angle (H°) values. Pumpkin, cantaloupe, carrot, and Chinese radish were peeled and cut into a square shape (1.5 x 1.5 cm; 0.5 cm thick). Japanese cucumber was cut along the length into three sections, then the seeds were removed and the skin peeled, before cutting the flesh into a square shape (same dimensions). All samples were dipped in the same calcium salt solutions that were used for treating the carved samples, with fifteen pieces per treatment.
Microbial populations. Total bacteria count was evaluated by pour-plate method using one carved flower sample per replicate. The sample was transferred to a sterilized bag containing 100 ml of 0.1% peptone water (Merck, Germany) and hand-rubbed for 2 min. Then the samples were diluted serially by a factor of ten in peptone water. The dish was incubated at 35°C for 48 hr and the values were reported as log CFU/piece [Bacteriological Analysis Manual (BAM), 2001]. The shelf life limit of the samples was reached when the microbe counts exceeded the safe standard for ready-to-eat fruits and vegetables (6 log CFU/piece, Department of Medical Science, 2010), regardless of appearance.
Appearance. The appearance (color and texture) of the carved fruits and vegetables were evaluated visually at two-day intervals. The samples were also photographed.
Statistical analysis
There were three replications per treatment. Data were analyzed using SPSS program (V.16; IBM, Ontario, Canada) for analysis of variance at P ≤0.05. Duncan’s multiple range test was used to compare mean values to determine the differences between treatments.
RESULTS
Effect of three calcium salts on texture
The types and concentrations of calcium salts affected the firmness of pumpkin, cantaloupe, carrot, Chinese radish, and Japanese cucumber slices (Figure 1 a-e). Various concentrations of CaCl2, 2% Ca lactate and 2% Ca propionate were the best at retaining the original firmness of the carved samples (p ≤0.05) (Figure 1).
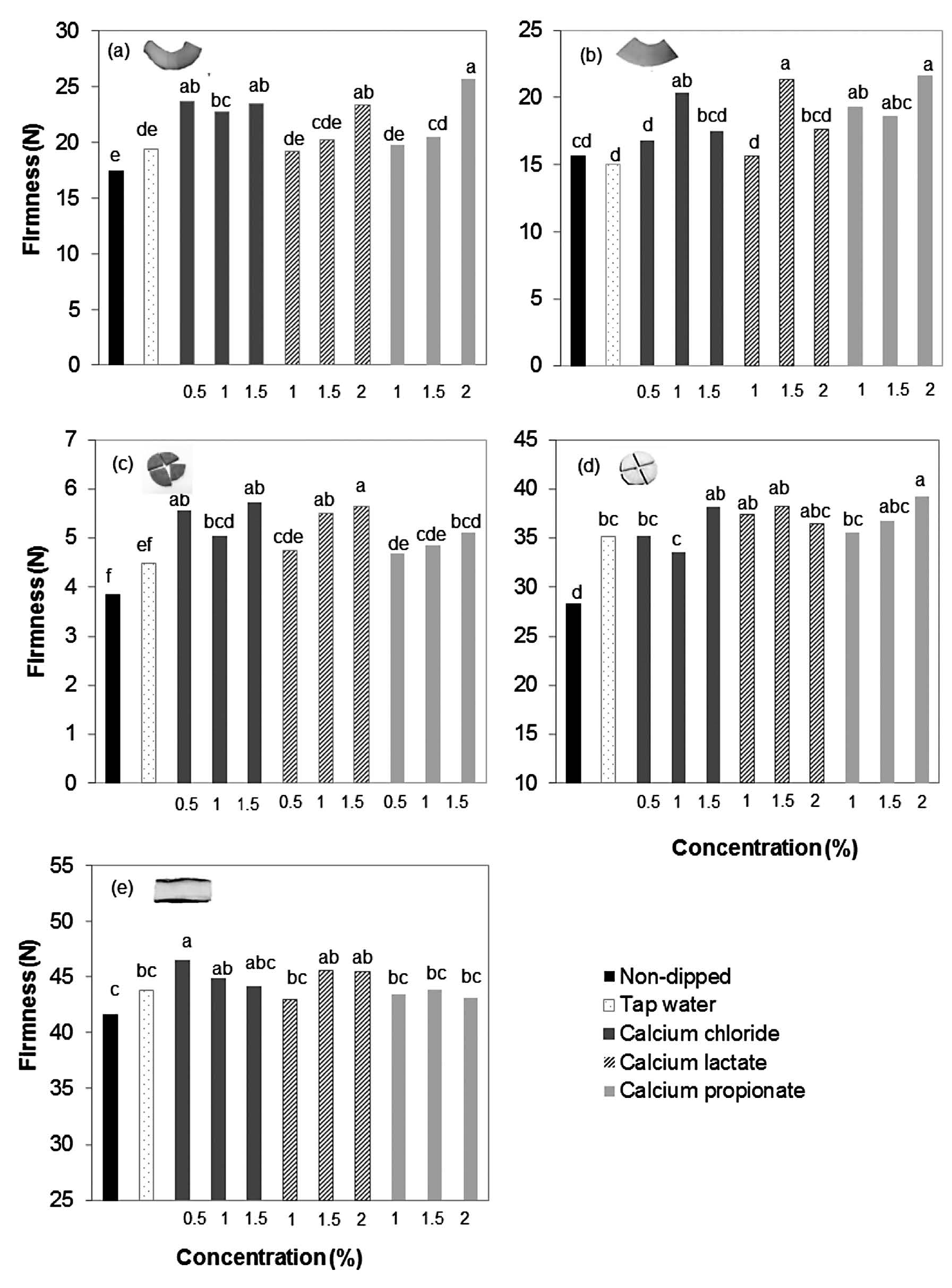
Figure 1. Firmness (N) of pumpkin (a), cantaloupe (b), carrot (c), Chinese radish (d), and Japanese cucumber (e) slices after dipping in CaCl2, Ca lactate and Ca propionate at the concentrations of 0.5-2.0%, compared with non-dipped and dipped in tap water as control. Different letters shows statistically significant differences between values (P ≤0.05).
Effect of calcium treatments and chitosan coating on shelf life of carved samples during simulated decorative use
The appearance of carved-rose samples dipped in the best treatment (Table 1) at the beginning and end of storage are shown in Table 2. For pumpkin, no salt treatment prevented color fading during storage, particularly after day 5 (data not shown). After day 7, all treatments turned white and unacceptable, except the two treatments with chitosan coating, which remained acceptable up to 12 days of storage. Similar results were observed in carrot and cantaloupe.
Tap water was the best treatment for carved Chinese radish and Japanese cucumber. None of the calcium treatments were useable after 7 days. In sharp contrast to pumpkin, carrot, and cantaloupe, the chitosan coating shortened usability even more, to a maximum of 5 days.
Table 1. The best chemical treatments for dipping of five carved fruits and vegetables.
Table 2. Appearance of carved rose and carved lotus of five fruits and vegetables dipped in the five best treatment solutions after simulating decorative use.
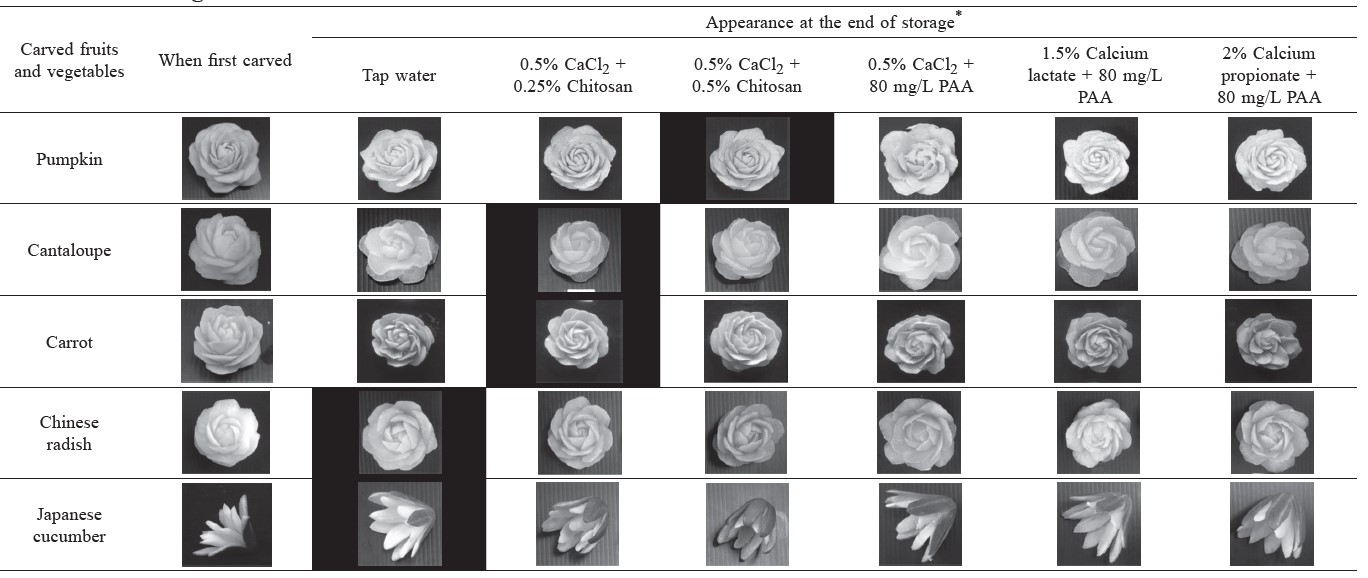
Note: The pictures with black color background were the best treatment for each fruit and vegetable. *Appearance at the end of storage: 6 days for carved-rose cantaloupe, 7 days for carved-rose pumpkin and Chinese radish and 8 days for carved-rose carrot and carved-lotus Japanese cucumber.
Quality and shelf life during storage in clamshell container at 5±1°C (using the best chemical treatment only)
Color. Initially, pumpkin cubes were a strong/deep yellow color (L* value 75.1, C* 58.7 and H° 92.4), carrot a strong/deep orange color (L* value 61.0, C* 58.2 and H° 56.7), and cantaloupe a light orange color (L* value 66.9, C* 25.5 and H° 71.5). After treating all samples with the best chemical treatment (0.5% CaCl2 + 0.25 or 0.5% chitosan), the colors faded, with lightness, chroma, and hue angle all worsening significantly (Table 3). They all remained within acceptable appearance, as can be seen in the photographs (Table 5). The color of Chinese radish and Japanese cucumber turned to brown after 12 and 9 days, respectively, and the L* value decreased continuously during storage (Table 3).
Weight loss. Regardless of carving style, the weight loss during storage for 12 days was minimal (0.01-2.39%) and showed significant increase with longer storage days (Table 4). The carved fruits and vegetables showed some surface drying and hardening (Table 5). All carved samples remained usable from a weight-loss perspective. Carving pumpkin and carrot into rose shape had a higher weight loss compared to carnation, in contrast to that found in carved cantaloupe and Chinese radish.
Total bacteria count. The initial total bacteria count of all carved fruits and vegetables was 2.1-4.3 log CFU/piece. Carved carrots had a lower initial total bacteria count than the other carved fruits and vegetables. The carnation shape had higher initial total bacteria counts than the rose shape, as its detailed petals were harder to cover with sanitizer. During storage at 5±1°C, total bacteria count of all carved fruits and vegetables gradually increased until the end of storage (data not shown), reaching 5.1-9.3 log CFU/piece after 9 days of storage. Due to microbe counts, the shelf life of carved pumpkin (rose and carnation shapes) and Japanese cucumber (lotus shape) did not exceed 6 days at 5±1°C, even though the appearance remained good. Chitosan showed effective antimicrobial property in carrots (rose and carnation shapes), with total bacteria count lower than 6 log CFU/piece throughout the 12 days of storage. Chinese radish (rose and carnation shapes) lasted 9 days before exceeding the microbe limit. Rose-shaped cantaloupe also lasted 9 days, but carnation-shaped cantaloupe only lasted 6 days.
Appearance. The initial versus final appearance of the three carved shapes of the five fruits and vegetables samples are shown in Table 5.
For pumpkin (dipped in 0.5% CaCl2 for 2 min followed by 0.5% chitosan coating), the rose and carnation carvings, initially bright yellow, started fading after 3 days, and worsened by day 6 of storage at 5±1°C. The carved rose faded and softened faster than the carnation. Microorganisms could be seen growing on the surface after 12 days of storage.
For cantaloupe (dipped in 0.5% CaCl2 followed by 0.25% chitosan coating), the rose and carnation carvings were initially light orange, with the carnation more translucent, perhaps due to the more detailed carving pattern, resulting more severe tissue damage. The rose shape had a better appearance throughout 12 days of storage.
For carrot (dipped in 0.5% CaCl2 followed by 0.25% chitosan coating), the rose and carnation carvings were initially orange-red. After 3 days of storage at 5±1°C, the carved shape faded in correlation with decreasing C* and H° values, while the carved carnation exhibited a white blush on the petals, which was caused by a thin layer of dehydrated carrot. For both shapes during day 6 to 12 of storage, the color faded only slightly, while no texture softening was observed.
For Chinese radish (dipped in tap water), the rose and carnation carvings turned brown by day 3, and exhibited continuously increased browning throughout the 12 days of storage, in line with the decreasing L* value.
For Japanese cucumber (dipped in tap water), the inner petal color of the carved lotus changed to solid white after 3 days and became slightly darker after 6 days of storage, in line with the decreasing L* value.
Table 3. Changes in color (L*, C*, H°) of pumpkin, carrot, cantaloupe, Chinese radish, and Japanese cucumber cubes during storage in clamshell container at 5±1°C for 12 days.
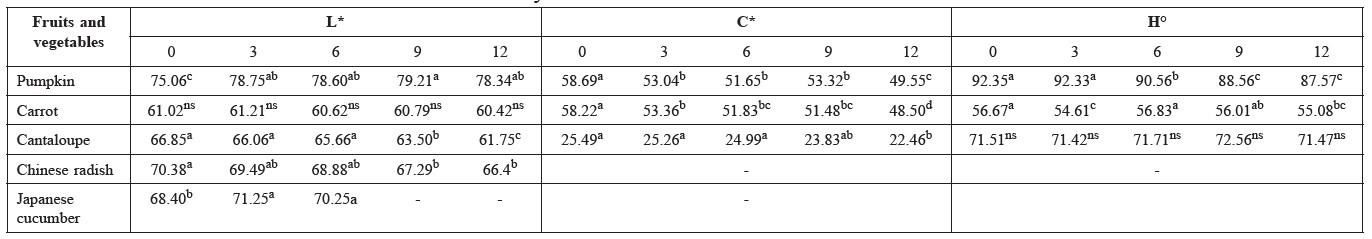
Note: Data expressed as mean of n=15. Means followed by values in each row with distinct lower case letters represent significantly different results (p ≤ 0.05). ns = non significant. Where L* values are not shown, the samples had been spoiled by microorganisms.
Table 4. Changes in weight loss (%) and total bacteria count (log CFU/g) of five carved fruits and vegetables during storage in clamshell container at 5±1°C for 12 days.
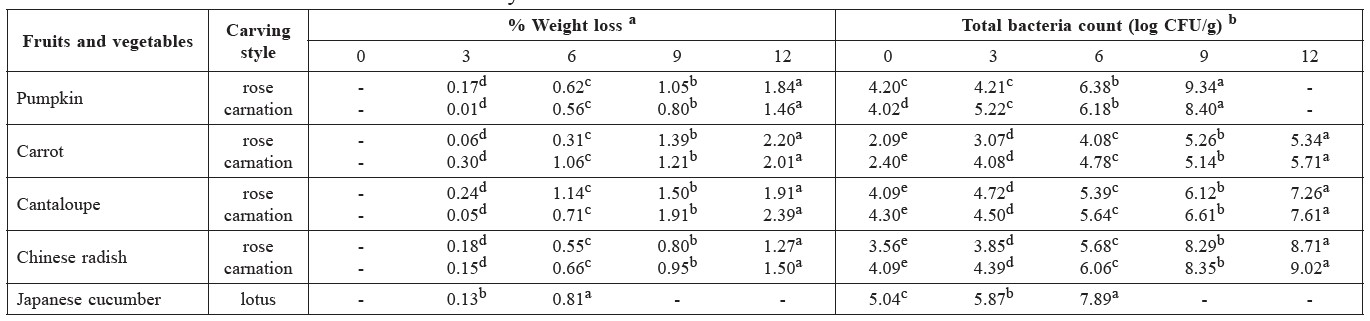
Note: a Weight loss data expressed as mean of n=3. Means followed by values in each row with distinct lower case letters represent significantly different results (p ≤ 0.05). b Total bacteria count data expressed as mean of n=6. Means followed by values in each row with distinct lower case letters represent significantly different results (p ≤ 0.05). Where data is not shown, the samples had been spoiled by microorganisms.
Table 5. Appearance quality of pumpkin, cantaloupe, carrot, Chinese radish, and Japanese cucumber carved into rose, carnation, and lotus shapes initially compared with the end of storage at 5±1°C in clamshell container.
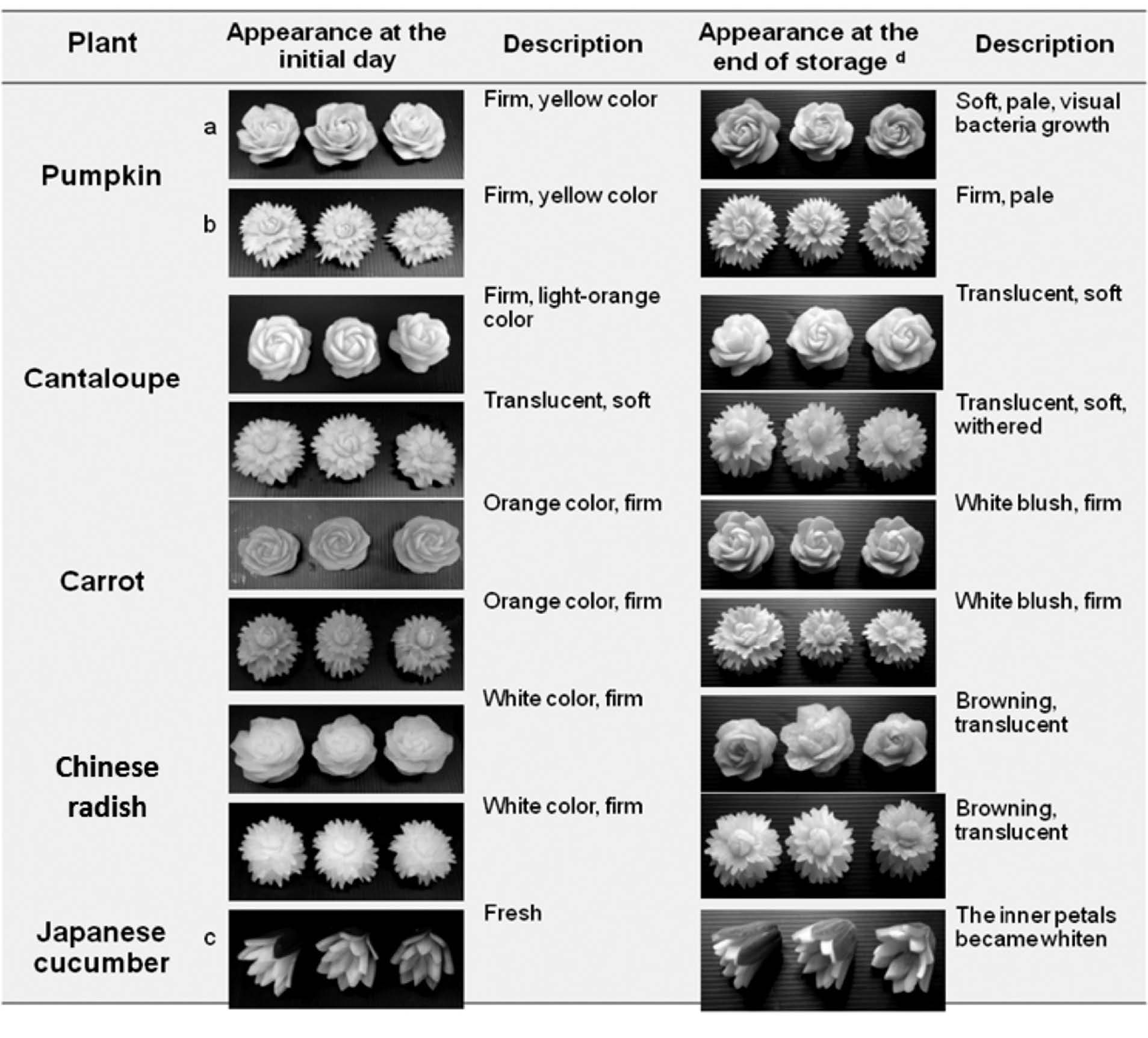
Note: acarved rose, bcarved carnation, and ccarved lotus. dAppearance at the end of storage: 9 days for pumpkin, 12 days for cantaloupe, 12 days for carrot, 12 days for Chinese radish, and 6 days for Japanese cucumber.
DISCUSSION
Effect of concentrations and types of calcium salts on texture of pumpkin, cantaloupe, carrot, Chinese radish, and Japanese cucumber slices
Application of 0.5-2% CaCl2, Ca lactate, and Ca propionate to improve the texture of pumpkin, cantaloupe, carrot, Chinese radish, and Japanese cucumber slices showed a good firming effect at different concentrations for each salt. The best concentration of CaCl2, Ca lactate, and Ca propionate for improving the firmness of the five fruits and vegetables were 0.5, 1.5, and 2% for 2 min, respectively. CaCl2 is advantageous, as it easily dissolves at room temperature; the other two salts have low solubility (Kubantseva et al., 2004). Thus, CaCl2 at low concentration (0.5%) was more effective than the other two salts. The effectiveness of calcium salts to improve the firmness in fresh-cut fruit and vegetables depended not only on the type of salt, but also the pectin content of the plant tissue. Pectin content has been reported for carrot and cantaloupe – 2.04 and 0.88 g/100g, respectively (Baker, 1997; Mahattanatawee et al., 2006). Amodio et al. (2010) reported that 2.5% Ca lactate for 1 min under controlled atmosphere delayed firmness loss in fresh-cut pumpkin. Rico et al. (2007) reported that 1.5% Ca lactate combined with mild heat treatment improved firmness and increased pectin methyl esterase activity of fresh-cut carrot. For fresh-cut cantaloupe, 1.0 and 2.5% CaCl2 for 1 min showed better firmness than Ca lactate at the same concentration. However, CaCl2 at concentrations higher than 1% results in a bitter taste compared with Ca lactate. Dipping fresh-cut cantaloupe in 2.5% Ca lactate increased firmness ~25-33% over the just-cut sample (Luna-Guzman et al., 2000). With fresh-cut Amarillo melon, 0.46% Ca carbonate, 1.42% Ca lactate, 0.5% CaCl2, and 0.9% Ca propionate at 60°C retarded softening better than the control treatment (Aguayo et al., 2008). No one has reported on improving firmness with calcium salts in Chinese radish and Japanese cucumber.
Effect of calcium treatments and chitosan coating on shelf life of carved samples during simulated decorative use
The carved fruits and vegetables could be reused daily, if after presented for decorative display (ambient temperature of 25±1°C for 3 hours), they were refrigerated (5±1°C) until the next day. CaCl2 (0.5%) combined with chitosan coating (0.25 or 0.5%) was appropriate for the colorful fruits and vegetables
(pumpkin, carrot, and cantaloupe) helping retard color, texture, and dehydration changes better than only calcium salt treatments, extending shelf life up to 2, 5, and 6 days for pumpkin, cantaloupe, and carrot, respectively. Simões et al. (2009) reported similar findings with carrot sticks; chitosan coating preserved the overall visual quality and reduced surface whiteness during storage for 12 days at 4°C in modified atmosphere packaging. Chitosan is a natural biopolymer that shows antimicrobial, antioxidant, and emulsifying properties; it can be used as an edible coating on fresh-cut fruit and vegetables. Chitosan decreases the water vapor transmission rate by forming a barrier on the fruit or vegetable surface, slowing water loss and texture softening (Baldwin, 2007). Adding a texture enhancer (CaCl2) to chitosan coatings can minimize softening of carved fruits and vegetables during storage. In addition to Ca ions, some carbohydrates undergo conformation changes, giving the “egg box” model of gelation (Rojas-Graü et al., 2009). The crosslinking of carbohydrate polymers (alginate, gellan, and pectin) with 2% CaCl2 maintained fresh-cut melon firmness during 15 days storage at 4°C (Oms-Oliu et al., 2008). A similar result was observed with apple wedges coated with calcium-crosslinked alginate and gellan-based edible coating (Rojas-Graü et al., 2008). In another case, Montero-Calderón et al. (2008) observed a reduction of juice leakage of fresh-cut pineapple coated with alginate and CaCl2.
Dipping in tap water was a good treatment for white-colored plant tissues (Chinese radish and Japanese cucumber). The carved Japanese cucumber did not brown and lost less water when treated with tap water. Coating with 0.25 or 0.5% chitosan caused browning in our study, in contrast with Pushkala et al. (2013), who reported that powder coating shredded-radish with chitosan browned less during storage at 10°C for 10 days in macro-perforated LDPE resealable pouches. Browning is an enzymatic reaction resulting from the interaction of oxygen, phenolic compounds, and polyphenol oxidase (PPOs) (Sapers, 1993). Pen and Jiang (2003) found that chitosan coating reduced enzymatic browning in fresh-cut water Chinese chestnut during storage at 4±1°C. Similar results were observed in fresh-cut ‘Rose’ apple (Worakeeratikul et al., 2007) and fresh-cut lotus root (Xing et al., 2010) when used in combination with hydro-cooling and modified atmosphere packaging, respectively.
In Experiment 3, all of the fruit and vegetable samples, regardless of carving shape, lost weight during storage at 5±1°C in clamshell containers. With pumpkin and carrot, the rose shape lost more weight than the carnation shape; this implies carving into the rose causes more serious tissue damage and an increased surface area, resulting in higher dehydration and respiration rates (Suwannarak et al., 2014b). In contrast, with the Chinese radish and cantaloupe in our study, the carnation shape lost more weight than the rose shape.
Chitosan coating retained color better than the other chemical treatments, as shown in Experiment 2. White color effect or white blush is often found in fresh-cut carrot. Vargas et al. (2009) also observed a white blush in fresh-cut carrot coated with chitosan.
Chitosan also displayed antimicrobial properties, for which three mechanisms have been proposed: 1) ionic surface interaction, resulting in cell wall leakage, 2) inhibition of mRNA and protein synthesis via the penetration of chitosan into the nuclei of the microorganism, and 3) the formation of an external barrier, chelating metal and provoking suppression of essential nutrients for microbial growth (Goy et al., 2009).
CONCLUSION
For the fruits and vegetables in this study, the combination of 0.5% CaCl2 with chitosan coating (0.25 or 0.5%) was the most effective treatment for improving the quality and extending the shelf life of the carved samples with lower water content – pumpkin, cantaloupe, and carrott. Dipping in tap water was the best treatment for retaining white color and avoiding dehydration in the carved samples with higher water content – Chinese radish and Japanese cucumber.
ACKNOWLEDGEMENTS
Financial support was provided by National Research Universities, Office of the Higher Education Commission, and Faculty of Home Economics Technology, Rajamangala University of Technology Phra-Nakhon. The authors thank the Postharvest Technology Research Institute, Chiang Mai University for providing instruments.
REFERENCES
Aguayo, E., V. H. Escalona, and F. Artés. 2008. Effect of hot water treatment and various calcium salts on quality of fresh-cut ‘Amarillo’ melon. Postharvest Biology and Technology, 47 (3): 397-406. doi:10.1016/j.postharvbio.2007.08.001
Amodio, M. L., R. Rinaldi, and G. Colelli. 2010. Extending shelf life of freshcut pumpkin (Cucurbita maxima): effect of pre-treatments and storage conditions. Acta Horticulturae, 876: 333-340. doi: 10.17660/ActaHortic.2010.876.44
Bacteriological Analytical Manual [BAM]. [online]. Available: http://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm2006949. htm. [2012, September 27]
Baker, R.A. 1997. Reassessment of some fruit and vegetable pectin levels. Journal of Food Science, 62(2):225-229. doi: 10.1111/j.1365-2621.1997.tb03973.x
Baldwin, E. 2007. Surface treatments and edible coatings in food preservation. pp. 477-507. In M. S. Rahman (ed.) Handbook of Food Preservation. CRC Press, Boca Raton, FL.
Department of Medical Science. 2010. Microbiological quality criteria of foods and utensils; 2006 [cited 2010 September 27]. Avialable from : http://dmsc2.dmsc.moph.go.th/webroot/BQSF/File/VARITY/dmscguide1.pdf
Goy, R.C., D. Britto, and O. B G. Assis. 2009. A review of the antimicrobial activity of chitosan. Polímeros, 19 (3): 1-7. doi: 10.1590/S0104-14282009000300013
Kubantseva, N., R.W. Hartel, and P.A. Swearingen. 2004. Factors affecting solubility of calcium lactate in aqueous solutions. Journal of Dairy Science, 87(4):863-867. doi: 10.3168/jds.S0022-0302(04)73230-0
Luna-Guzmán, I., and D. M. Barrett. 2000. Comparison of calcium chloride and calcium lactate effectiveness in maintaining shelf stability and quality of fresh-cut cantaloupes. Postharvest Biology and Technology, 19 (1): 61-72. doi:10.1016/S0925-5214(00)00079-X
Mahattanatawee, K., J.A, Manthay, G. Luzio, S.T. Talcutt, K. Goodner, and E.A. Baldwin. 2006. Total antioxidant activity and fiber content of select Florida-grown tropical fruits. Journal of Agricultural and Food Chemistry, 54:7355-7363. doi: 10.1021/jf060566s
Montero-Calderón, M., M.A. Rojas-Graü and O. Martín-Belloso. 2008. Effect of packaging conditions on quality and shelf life of fresh-cut pineapple (Ananas comosus). Postharvest Biology and Technology, 50 (2-3): 182-189. doi:10.1016/j.postharvbio.2008.03.014
Oms-Oliu, G., R. Soliva-Fortuny and O. Martín-Belloso. 2008. Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT - Food Science and Technology, 41 (10): 1862-1870. doi:10.1016/j.lwt.2008.01.007
Pen, L. T., and Y. M. Jiang. 2003. Effects of chitosan coating on shelf life and quality of fresh-cut Chinese water chestnut. LWT-Food Science and Technology, 36 (3): 359-364. doi: 10.1016/S0023-6438(03)00024-0
Pushkala, R., P. K. Raghuram, and N. Srividya. 2013. Chitosan based powder coating technique to enhance phytochemicals and shelf life quality of radish shreds. Postharvest Biology and Technology, 86: 402-408. doi:10.1016/j.postharvbio.2013.07.025
Qia, H., W. H. A. Jianga, M. Tiana, and Y. Lib. 2011. Extending shelf life of fresh-cut ‘Fuji’ apples with chitosan-coatings. Innovating Food Science & Emerging Technologies, 12 (1): 62-66. doi:10.1016/j.ifset.2010.11.001
Rico, D., A. B. Martín-Diana, J. M. Frías, J. M. Barat, G. T. M. Henehan, and C. Barry-Ryan. 2007. Improvement in texture using calcium lactate and heat-shock treatments for stored ready-to-eat carrots. Journal of Food Engineering, 79 (4): 1196-1206. doi:10.1016/j.jfoodeng.2006.04.032
Rojas-Graü, M. A., M. S. Tapia, and O. Martín-Belloso. 2008. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT - Food Science and Technology, 41 (1): 139-147. doi:10.1016/j.lwt.2007.01.009
Rojas-Graü, M. A., G. Oms-Oliu, R. Soliva-Fortuny, and O. Martín-Belloso. 2009. The use of packaging techniques to maintain freshness in fresh-cut fruits and vegetables: a review. International Journal of Food Science and Technology. 44 (5): 875-889. doi: 10.1111/j.1365-2621.2009.01911.x
Sapers, G.M. 1993. Browning of foods: Control by sulfites, antioxidants and other means. Food Technology, 47: 75-84.
Siam Carving Academy. The history of fruit carving [online]. Available: http://www.siamcarvingacademy.com/the-historyof-fruit-carving. [2013, May 31]
Simões, A. D.N., J. A. Tudela, A. Allende, R. Puschmann, and M. I. Gil. 2009. Edible coatings containing chitosan and moderate modified atmospheres maintain quality and enhance phytochemicals of carrot sticks. Postharvest Biology and Technology, 51 (3): 364-370. doi:10.1016/j.postharvbio.2008.08.012
Suwannarak, J. 2004. Vegetable Fruit Carving and Banana-leaf Arts. Odean Store Publishing, Bangkok, p.112.
Suwannarak, J., P. Phanumong, and N. Rattanapanone. 2014a. Physiological changes of fruit and vegetable carving. Chiang Mai University Journal of Natural Sciences, 13 (1): 77-86. doi: 10.12982/cmujns.2014.0023
Suwannarak, J., P. Phanumong, and N. Rattanapanone. 2014b. The efficacy of peroxyacetic acid and sodium hypochlorite solutions in reducing microorganisms on surface of carved fruits and vegetables. RMUTP Research Journal 8, (2): 92-106 (in Thai).
Vargas, M., A. Chiralt, A. Albors, and C. González-Martínez. 2009. Effect of chitosan-based edible coatings applied by vacuum impregnation on quality preservation of fresh-cut carrot. Postharvest Biology and Technology, 51 (2): 263-271. doi:10.1016/j.postharvbio.2008.07.019
Worakeeratikul, W., V. Srilaong, A. Uthairatanakij, and P. Jitareerat. 2007. Effects of hydrocooling and chitosan coating on browning and physiological changes in fresh-cut rose apple. Acta Horticulturae, 746: 427-434. doi: 10.17660/ActaHortic.2007.746.52
Xinga, Y., X. Lia, Q. Xua, Y. Jiang, J. Yuna, and W. Lia. 2010. Effects of chitosanbased coating and modified atmosphere packaging (MAP) on browning and shelf life of fresh-cut lotus root (Nelumbo nucifera Gaerth). Innovating Food Science & Emerging Technologies, 11 (4): 684-689. doi:10.1016/j.ifset.2010.07.006
Jomkhwun Suwannarak1, Putkrong Phanumong2 and Nithiya Rattanapanone2,3*
1 Faculty of Home Economics Technology, Rajamangala University of Technology Phra-Nakhon, Bangkok 10300, Thailand
2 Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand 3 Postharvest Technology Research Institute, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: agfsi001@gmail.com
Total Article Views

