
Comparison of the Fatty Acid Profiles of the Meat of Crossbreds with 75% Charolais Blood Proportion and Thai Indigenous Upland Cattle
Niraporn Chaiwang , Sanchai Jaturasitha* , Korawan Sringam , Michael Wicke and Michael KreuzerPublished Date : 2019-08-24
DOI : 10.12982/CMUJNS.2015.0082
Journal Issues : Number 2, May - August 2015
ABSTRACT
The objective of this study was to compare the fatty acid composition of the Longissimu sdorsi muscle of Charolais crossbreds (75%; with 25% Thai native cattle blood) and Thai indigenous Upland cattle (n=8 each). The animals were fed ad libitum grass and supplemented with concentrate at 1.5%/day of body weight. The cattle were on average four years old at slaughter. As a result, the crossbred beef had higher proportions of C16:0 and C18:0 of total lipids than the Upland cattle (P<0.05). At the same time, C14:1 and C18:2 cis-9, trans-11 (conjugated linoleic acid) was lower than in Upland cattle (P<0.05). Overall, this meant higher saturated fatty acid proportions in the crossbreds compared with the Upland cattle, while the opposite was true for the sums of monounsaturated fatty acids and polyunsaturated fatty acids, and the ratio of polyunsaturated to saturated fatty acids (P<0.05). This meant that the beef from the Upland cattle was better nutritionally for humans than the crossbreds.
Keywords: Fatty acid composition, Beef quality, Charolais crossbred, Thai Indigenous Upland cattle
INTRODUCTION
The fatty acid composition of meat is important, as it contributes to the nutritional value and affects various aspects of meat quality, including shelf life and flavor (Wood et al., 2003). Moreover, the fatty acid composition of fat in food has received increased attention, due to its potential impact on human health. Beef fat quality can be influenced by many factors, including genotype (breed) (Cuvelier et al., 2006). Genotype is one of the main factors affecting the fatty acid profile and carcass composition, because fat deposition differs between breeds (Wood et al., 1999). For higher nutritional value, the ratios between saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA) in the meat and between fatty acids of the n-6 and n-3 series should be low. In general, a ratio of PUFA to SFA
(P:S) above 0.45 and a ratio of n-6:n-3 below 4.0 in the diet are assumed to be effective in terms of human health (Simopoulos, 2004; Williams, 2000). Therefore, the objective of this study was to investigate the fatty acid composition of two extremely different genotypes, Thai indigenous Upland cattle and crossbreds with Charolais.
MATERIAL AND METHODS
Fattening and slaughtering procedures
The samples originated from an experiment described in detail in Chaiwang et al. (2014). Briefly, sixteen steers – eight crossbreds of Charolais (75%) and generic Thai native cattle (25%) and eight Thai indigenous Upland cattle breed (further on called Upland cattle) – were fed paragrass (Brachiariamutica) ad libitum from weaning at seven months of age. All animals were reared under the same condition and environment. They were reared on the research station in individual pens and supplemented with concentrate at a level of 1.5% of body weight/day. The concentrate was composed of corn, tapioca chips, rice bran, soybean meal, urea, dicalcium phosphate, salt and sulfur. The age of the cattle was on average four years when they were slaughtered at the Huay Kaew Slaughterhouse, Chiang Mai, Thailand, following procedures outlined by Jaturasitha (2007). At slaughter, the Charolais crossbreds had an average live weight of 649 kg and the Thai indigenous Upland cattle weighed 120 kg. All experimental procedures were carried out following the animal welfare standards of the Animal Care and Use Committee of the Thai Livestock Department based on the guidelines of the Federation of Animal Science Societies (1999). The Longissimus dorsi muscle (LD) between the 11th and 12th rib was removed and the fat taken for fatty acid analysis of its lipids.
Fatty acid analysis
The lipids extracted from the meat samples were removed from the interior of each muscle, trimmed of intermuscular and subcutaneous fat, and ground using a
blender (Moulinex; model DPA1). Fifteen grams of each sample were homogenized for 2 min with 90 ml of chloroform-methanol (2:1) (Nissel AM-8 Homogenizer, Nihonseikikaisha, Ltd., Japan) (Folch et al., 1957). Fatty acid methyl esters were prepared according to Morrison and Smith (1964). Gas chromatographic analysis was accomplished with model GC-14B of Shimadzu (Kyoto, Japan) equipped with a 0.25 mm × 100 m × 0.25 μm wall-coated fused wax capillary column. The carrier gas was nitrogen. Oven temperature programming was increased from 50 to 220°C at a rate of 10°C/min, held for 35 min, then increased from 200 to 230°C at a rate of 5°C/min, and finally held at 230°C for 20 min. The injector volume was 1 μl and the flame ionisation detector (FID) temperature was 250°C. Chromatograms were processed using the Millenium 2010 Chromatography Manager (Millipore Corp., Milford, Massachusetts, USA). Identification was accomplished by comparing the retention time of peaks from samples with those of FAME standard mixtures (Supelco® 37 Component FAME Mix). Quantification of FAME was based on the internal standard technique, using margaric acid (17:0) and CLA as the internal standard and using the corrected response factor of each fatty acid (ES ISO 5508, 1990) to convert relative peak areas into weight percentages. Fatty acids were expressed in gravimetric contents (mg/100 g meat).
Statistical analysis
In total, 128 samples from sixteen animals were analyzed. Data were subjected to analysis of variance. The differences between crossbreds and Upland cattle were statistically analyzed by independent Student’s t-test (2-tailed). Treatment differences were considered significant at P<0.05. All calculations were performed with SAS version 6.12 (SAS, 1997).
RESULTS
Genotype affected (P<0.05) the proportions of C14:1, C16:0, C18:0, and C18:2 cis-9, trans-11 (conjugated linoleic acid; CLA) in total lipids (Table 1). Beef from the crossbreds had higher (P<0.05) proportions of C16:0 and C18:0 than Thai indigenous Upland cattle. In turn, Charolais crossbred presented lower (P<0.05) proportions of C14:1 and CLA than Thai indigenous Upland cattle. The genotypes also differed (P<0.05) in proportions of saturated fatty acids (SFA), monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA) and the ratios PUFA:SFA. When comparing the crossbreds with the Upland cattle, the proportion of SFA was higher by 6.7 percentage units and that of MUFA and PUFA lower by 4.0 and 2.7 percentage units, respectively.
Table 1. Fatty acid profiles (% of total fatty acids identified) of the Longissimus dorsi muscle found in the two genotypes.
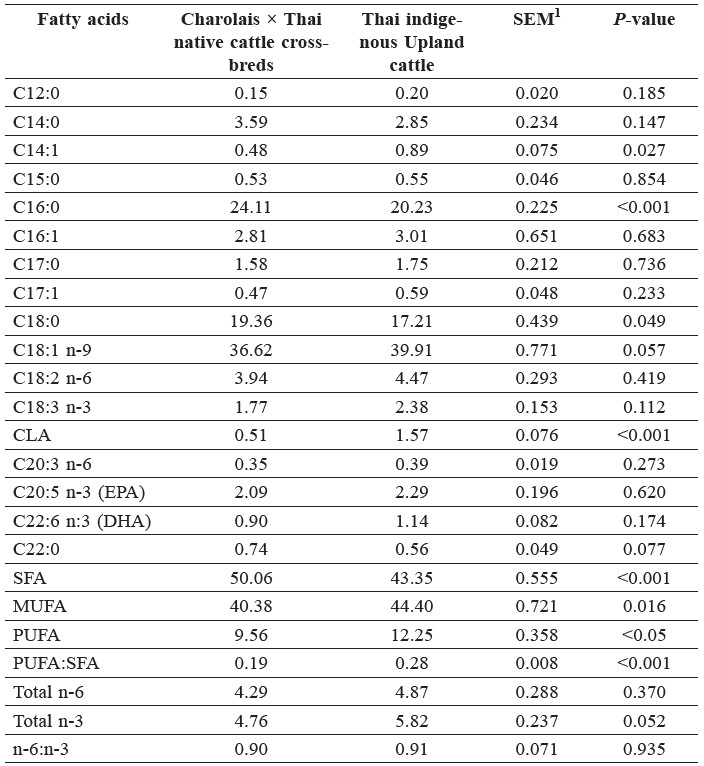
Note: 1Standard error of the means. CLA = conjugated linoleic acid (18:2 cis-9, trans-11); SFA = saturated fatty acids; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid, n-3 = omega-3 fatty acids; n-6 = omega-6 fatty acids. (n=128).
DISCUSSION
Even though in the present experiment, animals of both genotypes received the same amounts of concentrate per unit of body weight, certain diet effects could not be excluded due to the unknown forage intake. It could, therefore, have been that the crossbreds ingested proportionately more concentrate than the Upland cattle, where the concentrate likely contained proportionately more C16:0 and C18:0 than forage (Garcia et al., 2008). In addition, an influence of the level of fat deposition in the muscle cannot be totally excluded, as the intramuscular fat contents found in the crossbreds were almost five times higher than those in the Thai indigenous cattle (4.60 vs. 0.97%; Chaiwang et al., 2015).
Ruminants do not deposit tissue fatty acid in proportion to dietary lipid composition, as do swine or poultry, because of the biohydrogenating activity of rumen micoorganisms that first hydrolyze the triglycerides and then hydrogenate a large part of the dietary unsaturated fatty acid. Thus, ruminant tissue has a higher proportion of saturated fatty acids, and a lower proportion of polyunsaturated fatty acids than monogastics. This ruminal conversion of dietary unsaturated fatty acid is normally not complete (Byers and Schelling, 1993). Breed was found to affect the composition of saturated fatty acids. The content of C14:0 and C16:0 was significantly higher in Czech Fleckvieh bulls, while C18:0 and C20:0 content was higher in Montbeliarde bulls. This showed that each breed has a different potential to synthesize fatty acids under the same production conditions (Zapletal et al., 2009). The same is true for CLA, which is typically higher in the body fat of ruminants fed high-forage diets compared with those fed concentrate-based diets (French et al., 2000). The major dietary sources of CLA for humans are beef and dairy products. CLA is of interest because of its anticarcinogenic and antiatherogenic properties and its ability to reduce body fat, while enhancing lean body formation (Tsuboyama-Kasaoka et al., 2000).
In the present crossbred beef, the PUFA to SFA ratio was lower by about one third compared with the Upland cattle beef. Among the groups of fatty acids, SFA and MUFA always accumulate faster than PUFA do (Scollan et al., 2006). Consistent with this, Dinh et al. (2010) showed by correlation analysis that the SFA and MUFA proportions were more closely correlated with muscle fatness (r = 0.996 and 0.988, respectively) than was the PUFA proportion (r = 0.723). Choi et al. (2000) reported significant differences in specific PUFA between the muscles of dairy and traditional beef breeds, whereas the proportions of other fatty acids were not significantly different. On the contrary, Muchenje et al. (2009) reported no (P>0.05) breed effects on the proportions of most fatty acids. Some meats naturally have a PUFA to SFA ratio of around 0.1. Meat has been implicated in causing the imbalanced fatty acid intake of today’s consumers. Thus, the recommended ratio should be increased to higher than 0.4 (Raes et al, 2004). The PUFA to SFA ratio in the present study was lower than that recommended, with 0.19 and 0.28 for the crossbreds and the Upland cattle, respectively. A low ratio of n-6:n-3 is desirable for beef consumers’ health (Department of Health, 1994), because of its relationship with coronary heart diseases (American Heart Association, 2011). However, there were no breed differences in this ratio.
CONCLUSION
It is concluded that Upland cattle beef was slightly more favorable for consumers with particular health concerns or chronic heart diseases, because of the improved health benefit of its fatty acid composition, particularly that SFA was lower and EPA, DHA, PUFA and total n-3 were higher compared with crossbreds with high Charolais blood proportion.
ACKOWLEDGEMENT
The authors would like to express their gratitude to the National Research University under The Commission of Higher Education, Ministry of Education, Royal Thai Government for funding this research.
REFERENCES
American Heart Association. 2011. Heart and stroke encyclopedia. Dietary Guidelines for Healthy American Adults. Cholesterol. Fat.[Online]. Available: http://www.americanheart.org (May 13, 2011).
Byers, F.M., and G.T. Schelling. 1993. Lipids metabolism in ruminant nutrition. Pages 298–312 In: The Ruminant Animal: Digestive, Physiology, and Nutrition. D. C. Church, ed. Waveland Press Inc., Long Grove, IL.
Chaiwang, N., S. Jaturasitha, K. Sringram, M. Wicke, and M. Kreuzer. 2014. Comparison of the meat quality of Thai indigenous Upland cattle and F2-crossbreds with 75% Charolais blood proportion. Journal of Applied Animal Research, DOI: 10.1080/09712119.2014.963087, online first
Choi, N.J., M. Enser, J.D. Wood, and N.D. Scollan. 2000. Effect of breed on the deposition in beef muscle and adipose tissue of dietary n-3 polyunsaturated fatty acids. Journal of Animal Science 71: 509-519.
Cuvelier, C., A. Clinquart, J.F. Hocquette, J.F. Cabaraux, I. Dufrasne, L. Istasse, and J.L. Hornick. 2006. Comparison of composition and quality traits of meat from young fi nishing bulls from Belgian Blue, Limousin and Aberdeen Angus breeds. Meat Science 74: 522-531.
Department of Health. 1994. Nutritional Aspects of Cardiovascular Disease. Report on Health and Social Subject No. 46. Her Majesty’s Stationary Office, London
Dinh, T.T.N., J.R. Jr. Blanton, D.G. Riley, C.C. Jr. Chase, S.W. Coleman, W.A. Phillips, J.C. Brooks, M.F. Miller, and L.D. Thompson. 2010. Pure breeds of cattle intramuscular fat and fatty acid composition of longissimus muscle from divergent pure breeds of cattle. Journal of Animal Science 88: 756-766.
Federation of Animal Science Societies. 1999. Guidelines for the care and use of agricultural animals in agricultural research and teaching. 1st rev. ed. Federation of Animal Science Societies., Savoy, IL.
Folch, J., M. Lee, and G.H.S. Stanley. 1957. A simple method for the isolation and purification of total lipid from animal tissue. Journal of Biological Chemistry 226: 497-509.
French, P., E.G. O’Riordan, F.J. Monahan, P.J. Caffrey, M. Vidal, and M.T. Mooney. 2000. Meat quality of steers finished on autumn grass, grass silage or concentrate-based diets. Meat Science56: 173-180.
García, P.T., N.A. Pensel, A.M. Sancho, N.J. Latimori, A.M. Kloster, M.A. Amigone, and J.J. Casal. 2008. Beef lipids in relation to animal breed and nutrition in Argentina. Meat Science79: 500-508.
Jaturasitha, S. 2007. Meat Management. Chiang Mai, Thailand: Mingmuang Press. 170 p.
Morrison, W.R., and L.M. Smith. 1964. Preparation of fatty acid methyl esters and dimethyl acetals from lipids with boron trifluoride-methanol. Journal of Lipid Research 5: 600-608.
Muchenje, V., A. Hugo, K. Dzama, M. Chimonyo, P.E. Strydom, and J.G. Raats. 2009. Cholesterol levels and fatty acid profiles of beef from three cattle breeds raised on natural pasture. Journal of Food Composition Analysis 22: 354-358.
Raes, K., D. De Smet, and D. Demeyer. 2004. Effect of dietary fatty acids on incorporation of long chain polyunsaturated fatty acids and conjugated linoleic acid in lamb, beef and pork meat: A review. Animal Feed Science Technology 113: 199-221.
SAS Institute. 1997. SAS Systems for Windows, release 6.12, version 8.2. Cary, NC: SAS Institute Inc.
Scollan, N., J.F. Hocquette, K. Nuernberg, D. Dannenberger, I. Richardson, and A. Moloney. 2006. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Science74: 17-33.
Simopoulos, A.P. 2004. Omega-6/Omega-3 essential fatty acid ratio and chronic diseases. Food Reviews International 20: 77-90.
Tsuboyama-Kasaoka, N., M.Takahashi, K. Tanemura, H.J. Kim, T. Tange, H.M. Okuyama, S. Kasai, Ikemoto, and O. Ezaki. 2000. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes 49: 1534-1542
Williams, C.M. 2000. Dietary fatty acids and human health. Annual Zootechnie 49: 165-180.
Wood, J.D., R.I. Richardson, G.R. Nute, A.V. Fisher, M.M. Campo, E. Kasapidou, P.R. Sheard, and M. Enser. 2003. Effects of fatty acids on meat quality: a review. Meat Science66: 21-32.
Wood, J.D., M. Enser, A.V. Fisher, G.R. Nute, R.I. Richardson,and P.R Shear. 1999. Manipulating meat quality and composition. Proceedings of the Nutrition Society 58: 363-370.
Zapletal, D., G. Chladek, and J. Šubrt. 2009. Breed variation in the chemical and fatty acid compositions of the longissimus dorsi muscle in Czech Fleckvieh and Montbeliarde cattle. Livestock Science 123: 28-33.
Niraporn Chaiwang1, Sanchai Jaturasitha1*, Korawan Sringam2, Michael Wicke3 and Michael Kreuzer4
1 Department of Animal and Aquatic Science, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand
2 Central Laboratory, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand
3 Department of Animal Breeding and Husbandry, Georg-August University of Göttingen, Albrecht-Thaer-Weg 3, 37075 Göttingen, Germany
4 ETH Zurich Institute of Agricultural Sciences, Universitaetstrasse 2, 8092 Zurich, Switzerland
*Corresponding author. E-mail: ja.sanchai@gmail.com
Total Article Views

