
Effect of Fruit Size and Processing Time on Vacuum Impregnation Parameters of Cantaloupe and Apple
Aphirak Phianmongkol*, Hathaitip Rongkom and Tri Indrarini WirjantoroPublished Date : 2019-08-24
DOI : 10.12982/CMUJNS.2015.0075
Journal Issues : Number 2, May - August 2015
ABSTRACT
This study evaluated the effect of two fruit sizes (3.5×2.5×1.0 and 1.2×1.2×0.8 cm3) and processing time (impregnation and relaxation times; both for 10 and 20 min), on some vacuum impregnation parameters of cantaloupe and apple. Fruit size and processing time significantly affected the mass fraction of fruit occupied by impregnation liquid (X value) and the effective porosity (εe) of apples more than cantaloupe. High surface area of fruit and long processing times allowed for significant liquid penetration into the fruit. This study has shown that the effectiveness of vacuum impregnation is a surface-controlled phenomenon.
Keywords: Vacuum impregnation, Impregnation processing time, Fruit size, Cantaloupe, Apple
INTRODUCTION
Vacuum impregnation (VI) is a process that applies reduced pressure to a solid-liquid system, followed by restoration to atmospheric pressure. Vacuum pressure causes the gases inside tissue to expand and flow out of the extracellular spaces. When the pressure is restored, the residual gas is compressed and the external liquid flows into the product pores (Andrés et al., 2001; Krasaekoopt and Suthanwong, 2008). This process can be used to develop novel food products, especially fortified food products. The effects of vacuum impregnation on the properties of porous food materials, including fruits and vegetables, have been studied (Mújica-Paz et al., 2003a; Zhao and Xie, 2004). Some reports investigated using vacuum impregnation with different liquids for mineral fortification in fruits and vegetables (Fito et al., 2001; Gras et al., 2003; Zhao and Xie, 2004).
The quality of the final products after vacuum impregnation treatments was affected by several factors, such as the vacuum and relaxation times of the solid matrix (Derossi et al., 2010), mechanical properties of the materials, transport rate of hydrodynamic mechanism, food structure and size and shape of the sample (Zhao and Xie, 2004). The effect of vacuum impregnation on fruit was reported to be complicated, depending on types of the samples (Mujica-Paz et al., 2003b). However, the effect of fruit size, especially in cubic form, on vacuum impregnation parameters of fruit has not been reported. Although the vacuum impregnation properties of several tropical fruits have been studied, few have looked at cantaloupe.
This present study analyzed the effect of fruit size and processing time (both for impregnation and relaxation times) on some vacuum impregnation parameters of cantaloupe and apple. This understanding might lead to appropriate vacuum impregnation conditions for particular fruits and processes, including fortifying dehydrated fruit.
MATERIALS AND METHODS
Sample preparation for vacuum impregnation treatments
Fresh apple (Malussylvestris Mill var. Granny smith) and cantaloupe (Cucumismelo L. var. cantalupensis) were purchased from a local market in Chiang Mai, Thailand. The edible portions of apple and cantaloupe were cut into pieces 1.2×1.2×0.8 and 3.5×2.5×1.2 cm3. Sucrose solution was used as an impregnation solution and prepared by adding commercial sucrose (MitrPhol Sugar, Thailand) in distilled water until its water activity (aw) reached the value of the corresponding fruit pieces (Mujica-Paz et al., 2003b). During vacuum impregnation, fruit pieces were submerged in the solution at a ratio of fruit to impregnation liquid of 1:5 (w/w).
Vacuum impregnation treatments
The vacuum impregnation with sucrose solution was performed at 25°C in a vacuum oven (Binder VD23, Germany). The sample was treated under vacuum pressure of 50 mbar for a period of time (vacuum impregnation time), then the pressure was adjusted to atmospheric pressure for a preset time (relaxation time). Samples were then collected for analysis. Both the vacuum impregnation and relaxation times were varied at 10 and 20 min. Each experimental combination was run in triplicate. The amount of liquid incorporated into the fruit slices, expressed as X value, was obtained from Eq. (1) (Mújica-Paz et al., 2003a) and the volumetric deformation of the sample (γ) was calculated from Eq. (2) (Paes et al., 2008).
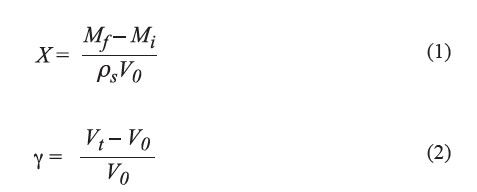
where Mf was the final mass of the fruit (kg), Mi was the initial mass of the fruit (kg), V0 was the initial volume of the fruit (m3), Vt was the final volume of samples (m3) and ρs was the density of the sucrose solution (kg/m3).
Effective porosity (εe) of the sample was determined using Eq. (3).

where r was a compression ratio (atmospheric/vacuum pressure) (Andrés et al., 2001).
The water loss (WL) and solid gain (SG) of the samples were calculated using Eq. 4 and 5 of Paes et al. (2008), respectively.
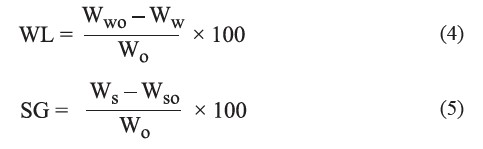
where wwo was the initial weight of water in the sample (kg), ww was the weight of water in the sample at the end of the treatment (kg), wo was the initial weight of the sample (kg), ws was the weight of dry solids at the end of the treatment (kg) and wso was the initial weight of dry solids in the sample (kg).
Statistical analysis
The experiment was set up using a Complete Randomized Design. Analysis of variance (one way ANOVA) was performed on the experimental results to determine the effect of treatments on the vacuum impregnation parameters. Mean differences were determined by Duncan’s New Multiple Range. All statistical analysis was performed using SPSS Statistic Base version 17.0 for Windows, serial number 5068035 (SPSS Inc., Chicago, USA).
RESULTS
The volumetric fraction of fruit occupied by impregnation liquid was affected by fruit type. The incorporated liquid in apple was much higher than that of cantaloupe. The impregnation times also played a role, especially in apple (Figure 1). Smaller sample sizes and longer processing times resulted in higher volumes of incorporated liquid.
Corresponding to the X value, the effect of the studied parameters on εe was clearly seen in apple (Figure 2). Long processing time provided higher space for liquid penetration. The fruit size also affected the εe in the same trend as the processing time.
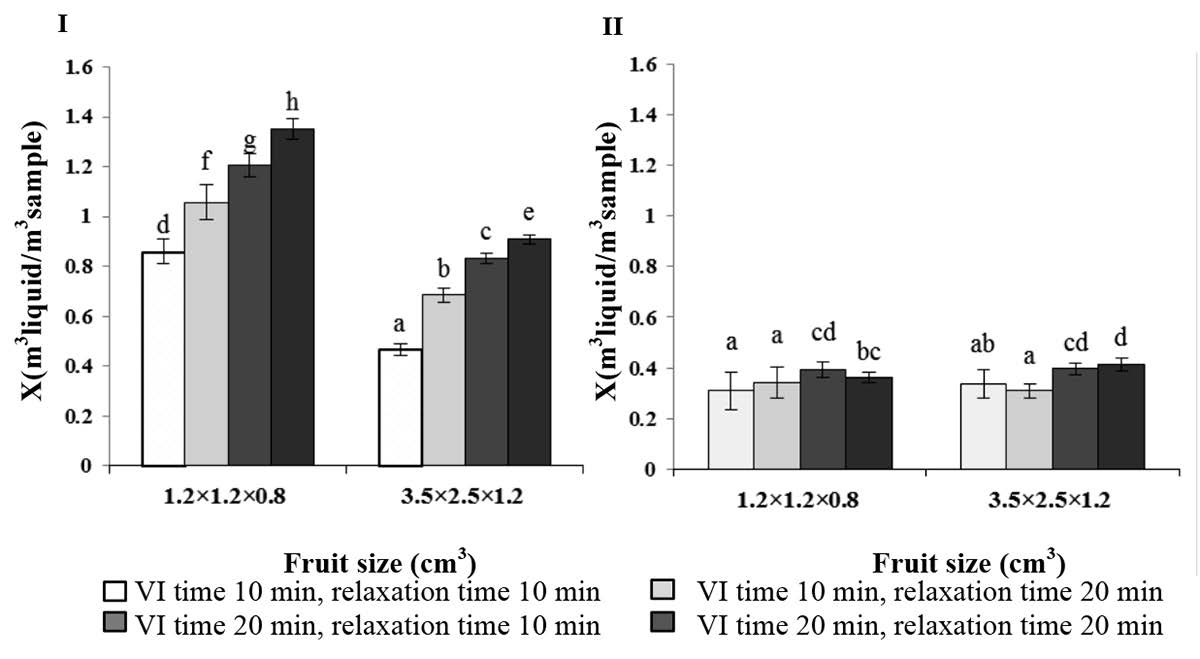
Figure 1. Effect of fruit size and impregnation time on mass fraction of fruits occupied by impregnation liquid (X value) of apple (left) and cantaloupe (right) after vacuum impregnation.
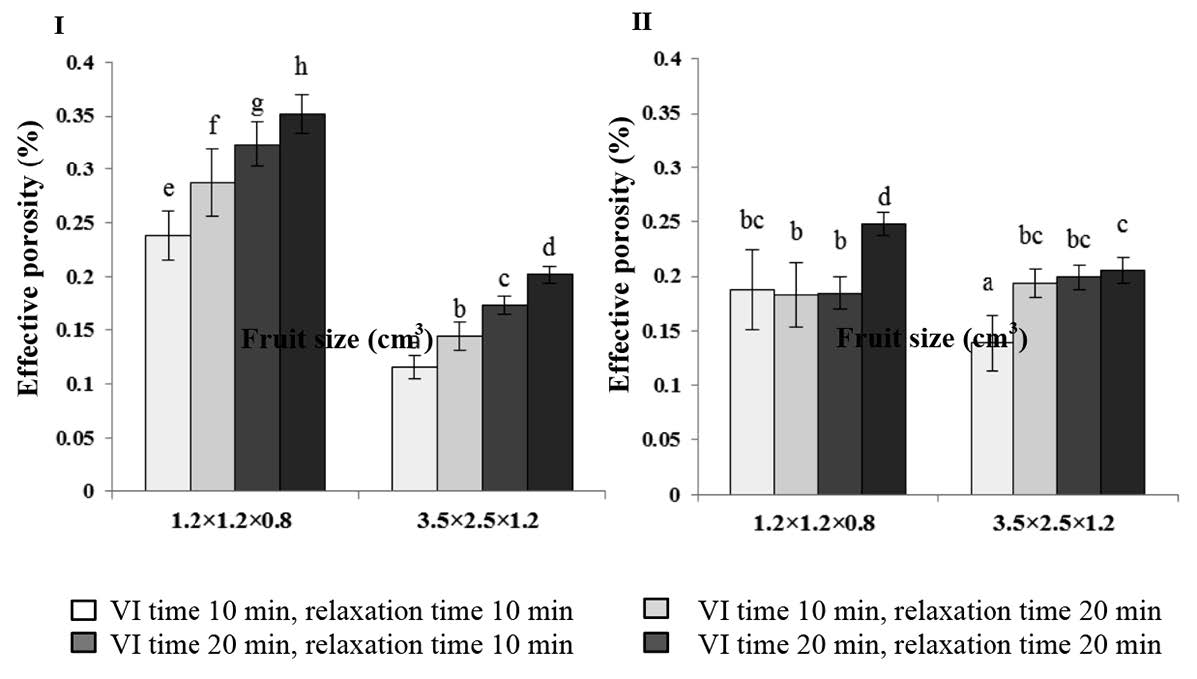
Figure 2. Effect of fruit size and impregnation time on the effective porosity (εe) of apple (left) and cantaloupe (right) after vacuum impregnation.
The fruit size and processing time significantly affected (p<0.05) both the water loss and solid gain of impregnated cantaloupe and apple (Table 1). In general, smaller fruit pieces and longer processing time increased absorption of the liquid into the fruit pieces. The water loss increased with longer vacuum impregnation and relaxation times. However, an increase in solid gain with the processing times was mainly observed in cantaloupe.
Table 1. Water loss and solid gain of vacuum impregnated cantaloupe and apple pieces.
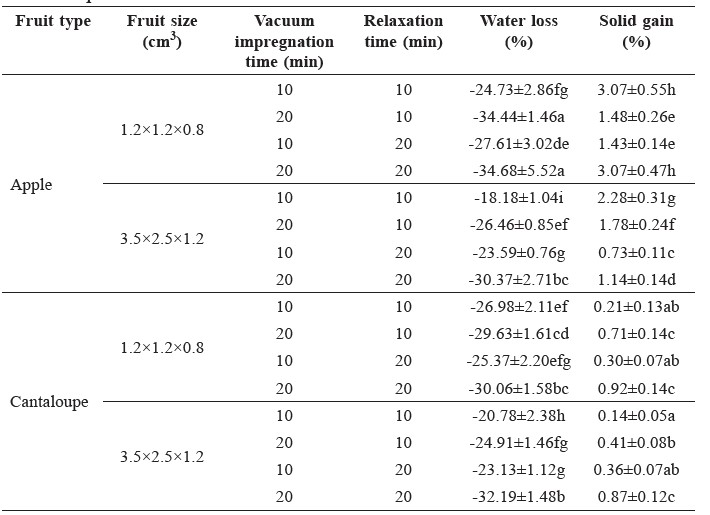
Note: *The value presented is mean + standard deviation. Different letters within a column are significantly different (p<0.05).
DISCUSSION
The volumetric fraction of apple occupied by the impregnation liquid was higher than that of cantaloupe, indicating the difference in their tissue microstructure (Mújica-Paz et al., 2003a). The low impregnation level of cantaloupe could be affected by its fruit tissue structure that might involve an overlapping
of different transport mechanisms during vacuum impregnation (Derossi et al., 2010). The insignificant effect of the processing time for cantaloupe in this study had also been reported for eggplant, carrot and mushroom (Gras et al., 2003). However, in general, longer processing time provided longer mass transportation time, which resulted in a higher volume of the external liquid being incorporated. The higher surface area of the small-sized apple samples also significantly (p<0.05) contributed to the transport mechanism as compared with the larger sample size.
The effective porosity, or εe, was described as the volume of the sample that could be occupied by the external liquid in the product tissue (Zhao and Xie, 2004). Longer processing time provided higher space for liquid penetration, which was probably due to attainable mechanical equilibrium (Cháfer et al., 2003). Krasaekoopt and Suthanwong (2008) reported a similar finding with the present study for guava and papaya.
All of the impregnated fruits had negative water loss values, indicating that the fruit samples absorbed the liquid during the impregnation process (Mújica-Paz et al., 2003b). The vacuum impregnation time had a much stronger effect on the water loss value than the relaxation time. Generally, water loss increased with longer vacuum impregnation and relaxation times. However, an increase in solid gain with longer vacuum impregnation and relaxation times was mainly observed in cantaloupe. This finding was similar to a previous report for mango (Khan et al., 2008). Martinez-Valencia et al. (2008) also reported increased water loss and solid gain of melon with increased immersion times, although the increase in water loss was higher than solid gain. At 10 min relaxation time, the solid gain of apple reduced at the longer vacuum impregnation time. The finding was affected by the fact that during reduced-pressure, native solution leached from the fruit intercellular spaces (Carciofi et al., 2012; Jacob and Paliyath, 2012). The loss of this native solution might not be fully replaced by solids in the impregnation solution both by the infused amount of the solution, which was due to a short relaxation time, and/or the low molecular weight of the solids in the external solution.
CONCLUSION
This present study demonstrated that fruit type, fruit size and impregnation processing time (both vacuum impregnation and relaxation times) played an important role in the mass transport phenomena during vacuum impregnation. Proportionally large surface area samples and long processing times provided better impregnation in terms of some vacuum impregnation parameters – mass fraction of fruit occupied by impregnation liquid, effective porosity, water loss and solid gain.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the Division of Food Science and Technology, Faculty of Agro-Industry, Chiang Mai University, Thailand for providing equipment and laboratory facilities.
REFERENCES
Andrés, A., D. Salvatori, A. Albors, A. Chiralt, and P. Fito. 2001. Vacuum impregnation viability of some fruits and vegetables. p. 53-60. In P. Fito, A. Chiralt, J.M. Barat, W.E.L. Spiess, and D. Behsnililan (eds.) Osmotic dehydration and vacuum impregnation: Applications in food industries. Technomic Publishing Company, Pennysylvania.
Association of Official Analytical Chemists. 2000. Official methods of analysis of AOAC International, 17th ed. AOAC International, Gaithersburg, USA.
Carciofi, B.A.M., M. Prat, and J.B. Laurindo. 2012. Dynamics of vacuum impregnation of apples: Experimental data and simulation results using a VOF model. Journal of Food Engineering 113: 337-343.
Cháfer, M., C. González-Martínez, A. Chiralt, and P. Fito. 2003. Microstructure and vacuum impregnation response of citrus peels. Food Research International 36(1): 35-41.
Derossi, A., T.D. Pilli, and C. Severini. 2010. Reduction in the pH of vegetables by vacuum impregnation: A study on pepper. Journal of Food Engineering 99: 9-15.
Fito, P., A. Chiralt, J.M. Barat, A. Andrés, J. Martínez-Monzó, and N. Martínez-Navarrete. 2001. Vacuum impregnation for development of new dehydrated products. Journal of Food Engineering 49: 297-302.
Gras, M.L., D. Vidal, N. Betoret, A. Chiralt, and P. Fito. 2003. Calcium fortification of vegetables by vacuum impregnation interactions with cellular matrix. Journal of Food Engineering 56: 279-284.
Jacob, J.K., and G. Paliyath. 2012. Infusion of fruits with nutraceuticals and health regulatory components for enhanced functionality. Food Research International 45: 93-102.
Khan, M.A.M., L. Ahrné, J.C. Oliveira, and F.A.R. Oliveira. 2008. Prediction of water and soluble solids concentration during osmotic dehydration of mango. Food and Bioproducts Processing 86(1): 7-13.
Krasaekoopt, W., and B. Suthanwong. 2008. Vacuum impregnation of probiotics in fruit pieces and their survival during refrigerated storage. Kasetsart Journal 42: 723-731.
Martínez-Valencia, B.B., M. Abud-Archila, M.A. Ruiz-Cabrera, A. Grajales-Lagunes, L. Dendooven, S.L. Ovando-Chacón, and F.A. Gutiérrez-Miceli. 2011. Pulsed vacuum osmotic dehydration kinetics of melon (Cucumismelo L.) var. cantaloupe. Africa Journal of Agricultural Research 6(15): 3588-3596.
Mújica-Paz, H., A. Valdez-Fragoso, A. López-Malo, E. Paloub, and J. Welti-Chanes. 2003a. Impregnation properties of some fruits at vacuum pressure. Journal of Food Engineering 56: 307-314.
Mújica-Paz, H., A. Valdez-Fragoso, A. López-Malo, E. Paloub, and J. Welti-Chanes. 2003b. Impregnation and osmotic dehydration of some fruits: Effect of the vacuum pressure and syrup concentration. Journal of Food Engineering 57: 305-314.
Paes, S.S., B.G. Stringari, and J.B. Laurindo. 2008. Effect of vacuum impregnation temperature on the mechanical properties and osmotic dehydration parameters of apples. International Journal of Brazilian Archive of Biology and Technology 51(4): 799-806.
Zhao, Y., and J. Xie. 2004. Practical applications of vacuum impregnation in fruit and vegetable processing. Trends in Food Science and Technology 15: 434-451.
Aphirak Phianmongkhol*, Hathaitip Rongkom and Tri Indrarini Wirjantoro
Division of Food Science and Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
*Corresponding author. E-mail: aphirak.p@cmu.ac.th
Total Article Views

