
Producing Succinic Acid with Actinobacillus succinogenes: Optimizing the Composition of the Medium Using Plackett-Burman Design
Sukanya Phuengjayaem and Siriluk Teeradakorn*Published Date : 2019-08-23
DOI : 10.12982/cmujns.2016.00019
Journal Issues : Number3 ,September - December 2016
ABSTRACT
This study used Actinobacillus succinogenes DSMZ 22257 to produce succinic acid using sorghum straw hydrolysate (SSH) as a low-cost carbon source. In anaerobic fermentation, the maximum succinic acid concentration of 52.180 g/l, corresponding to a yield of 0.870 g/g glucose was obtained from 60 g/l of glucose and faster cells growth was also observed. When using 40 g/l of SSH as a carbon source, succinic acid of 16.671 g/l, corresponding to yield of 0.777 g/g substrate was achieved after 24 h of cultivation. Statistical method: Plackett-Burman Design (PBD) was applied for a preliminary optimization of succinic acid fermentation medium by A. succinogenes DSMZ 22257. The highest succinic acid of 15.746 g/l was obtained with fermentation medium contained 50.0 g/l of yeast extract, 5.0 g/l of urea, 5.0 g/l of CaCl2, 0.25 g/l of MnCl2, 2.50 g/l of Na2CO3 and 50 g/l of MgCO3. The results from PBD, yeast extract and MgCO3 were identified as the key medium components. The present study suggested that the renewable sorghum straw could be utilized as an alternative carbon source for succinic acid production. Further studies of the key medium components will be optimized using Response Surface Methodology to obtain optimum succinic acid production.
Keywords: Actinobacillus succinogenes, Hydrolysate, Sorghum straw, Succinic acid
INTRODUCTION
Succinic acid, a dicarboxylic acid with the molecular formula of C4H6O4, is an important platform chemical. It can be used as a precursor for many chemicals of industrial importance, including adipic acid; 1, 4-butanediol; tetrahydrofuran; N-methyl pyrrolidinone; 2-pyrrolidinone; succinate salts; and gamma-butyrolactone (Song and Lee, 2006; McKinlay et al., 2007). Succinic acid is used in the agricultural, food, and pharmaceutical industries, as well as in the synthesis of biodegradable polymers, such as polybutyrate succinate (PBS), polyamides, and various green solvents (Rudner et al., 2005). Succinic acid is produced commercially by catalytic hydrogenation of petrochemical-derived maleic acid or maleic anhydride. However, because of rising global demand for oil and the environmental impact of excessive fossil fuel use, using anaerobic bacteria to produce succinic acid from the fermentation of renewable biomass has become increasingly economically attractive.
Using renewable carbon resources to produce bio-based succinic acid has outstanding environmental benefits (McKinlay et al., 2007; Bechthold et al., 2008). Several bacteria have been shown to be candidates for producing bio-based succinic acids, including Anaerobiospirillum succiniciproducens, Actinobacillus succinogenes (Guettler et al., 1996, 1999; Liu et al., 2008), Mannheimia succiniciproducens, Prevotella ruminicola (Howlett et al. 1976), Escherichia coli AFP111 (Stols and Donnelly, 1997), and Corynebacterium glutamicum (Okino et al., 2005). However, whether bio-based fermentation can compete with petroleum-based production of succinic acid depends on cost, with cheap carbon sources, rather than glucose, offering potentially cost-effective options. Whey (Wan et al., 2008), wood hydrolysate (Hodge et al., 2009), and cane molasses (Liu et al., 2008) have already been reported as economic biomass feedstocks for producing bio-succinic acid.
Agricultural straw, one of the most abundant and renewable sources of lignocellulose biomass, is another potential feedstock for producing succinic acid. It is composed of 35-45% cellulose, 20-30% hemicellulose, and 8-15% lignin. Despite its low digestibility, agricultural straw is a good source of fermentable sugars. After pretreatment with dilute acid, alkali, or steam explosion, it can be enzymatically saccharified into fermentable sugars that are mainly a mixture of glucose and xylose. Therefore, straw hydrolysate offers an attractive low-cost feedstock for producing bio-based chemicals, such as ethanol and hydrogen (Saha et al., 1998).
In Thailand, sorghum straw is the most abundant and renewable lignocellulose biomass. It is a cane-like plant with a high biomass yield and sugar content, primarily composed of soluble (glucose and sucrose) and insoluble carbohydrates (cellulose and hemicellulose). Furthermore, it is drought tolerant and waterlog resistant, as well as offers salinity and alkalinity resistant properties (Li and Halbrendt, 2009). Despite its potential, the use of sorghum straw for the fermentation production of succinic acid has not been reported yet.
This present study investigated the potential of sorghum straw hydrolysate (SSH) as a carbon source for producing succinic acid using A. succinogenes, including the effects of the initial sugar concentration and nitrogen sources on cell growth and succinic acid production.
MATERIALS AND METHODS
Materials and microorganisms
Lignocellulosic material. The sorghum straw used in this study was obtained from the Suphanburi Field Crop Research Center, Suphanburi, Thailand. Sorghum straw consisted of 44.51% cellulose, 38.62% hemicellulose, 6.18% lignin and 10.69% ash. The chopped sorghum straw was dried in an oven at 70°C to a constant weight. Thirty grams of chopped sorghum straw was suspended in 300 ml of 3% aqueous solution of H2SO4 at 120°C for 10 minutes (Poonsrisawat et al., 2013). After the pretreatment step, the sorghum straw hydrolyzates (SSH) were neutralized with 40% NaOH, centrifuged, and filtered through 0.45 μm filter papers before analysis of the total reducing sugars by DNS method and monomeric sugars (glucose, xylose, galactose, arabinose and mannose) by HPLC.
Microorganism. A. succinogenes DSMZ 22257 was obtained from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cultures. The bacteria strain was maintained on TSA (Tryptice Soya Agar) agar slants and contained 17 g/l of pancreatic digest of casein, 3 g/l of soy peptone, 2 g/l of glucose, 5 g/l of NaCl, 2.5 g/l of KH2PO4, and 15 g/l of agar.
Medium for succinic acid fermentation
Succinic acid production was investigated by anaerobic fermentation in a medium that consisted of 30.0 g/l of yeast extract, 2.0 g/l of urea, 2 g/l of MgCl2.6H2O, 1.5 g/l of CaCl2, 0.07 g/l of MnCl2, 4.4 g/l of Na2HPO4, 3.3 g/l of NaH2PO4, and 30 g/l of MgCO3, with the pH adjusted to 7 (Li et al., 2011). First, glucose was separately sterilized at 115°C for 20 min and added to the medium to maintain the initial concentration of 60.0 g/l. Next, biotin 0.3 μg/l and thiamin 0.2 μg/l were prepared by sterile membrane filtration (0.22 μm nylon, Millipore Express, Ireland) and added to the medium. The seed culture medium contained 17 g/l of pancreatic digest of casein, 3 g/l of soy peptone, 2 g/l of glucose, 5 g/l of NaCl, and 2.5 g/l of KH2PO4. Ten percent of seed inoculum was added and cultivated at 37°C with 200 rpm for 24 h.
Effect of the sugar concentration on succinic acid production by A. succinogenes
The fermentative process was performed using different carbon sources (0-60 g/l of glucose and SSH) under anaerobic conditions at 37°C with 200 rpm for 24 h.
Optimizing the composition of the medium for succinic acid production using Plackett-Burman Design (PBD)
The first optimization approach using Plackett & Burman Design (PBD) with 12 experiments and 6 variables (yeast extract, urea, CaCl2, MnCl2, NaCO3, and MgCO3) was carried out. The experiment was designed with two levels, namely, minimum and maximum, coded as “-1” and “+1”, respectively, as shown in Table 1.
To determine the most important variables, a standardized Pareto chart was employed. Analysis of the measured response variables enabled one to obtain standardized Pareto charts to make predictions that were then compared against the actual plot. The standardized Pareto chart consisted of bars with a length proportional to the absolute value of the estimated effects, divided by the standard error. The bar was displayed in order of the size of the effects, with the largest effects on top. The chart included a vertical line at the critical t-value for α of 0.05. Confidence levels greater than 95% (p < 0.05) were considered significant. Effects for which the bars were smaller than the critical t-value were considered not significant and did not affect the response variables. The effect may be positive or negative.
The statistical software package “Minitab 17” was used to analyze the experimental data.
Table 1. Experimental design using Plackett–Burman methodology for succinic acid production by A. succinogenes.
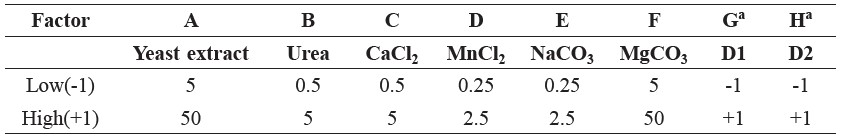
Note: aDummy variable. *Cultivation condition. Anaerobe at 37°C, 200 rpm for 24 h.
Analytical methods
Cell concentration. The insoluble MgCO3 in the sample was removed by adding 0.2 M of HCl (Zheng et al., 2009). Then the cell concentration was measured as the absorbance at wavelength 660 nm using a spectrophotometer (UV160, Shimadzu Corporation, Japan).
Reducing sugars. The reducing sugars in the sorghum straw hydrolysate (SSH) were measured by DNS (3, 5-dinitrosalicylic acid colorimetric) method applied from Miller (1959), with D-glucose as the standard. The mixtures containing 50 μl of sample and 150 μl of DNS reagent were heated in a boiling water bath for 10 minutes and were cooled immediately in an ice bath. Afterwards, 1 ml of distilled water was added and the absorbance at wavelength 540 nm was measured using a spectrophotometer.
Fermentation products. Fermentation products (succinic, acetic, and formic acids) were analyzed using high performance liquid chromatography (HPLC). The supernatants were filtered through a cellulose acetate membrane filter (pore size 0.45 μm). The injected volume was 20 μl. The HPLC system was equipped
with a cation-exclusion column (Aminex HPX-87H; 300 mm 7.8 mm; Bio-Rad Chemical) and a refractive index detector (Shimadzu Model RID-6A). The mobile phase consisted of a 5 mM H2SO4 solution at a flow rate of 0.6 ml/min and the column was operated at 55°C.
RESULTS
Effect of the glucose concentration on succinic acid production by A. succinogenes
The results showed fermentation cultivation using glucose as a carbon source with various concentrations in the range 0-60 g/l. The time course of cell growth and residual reducing sugars are shown in Figures 1(a) and (b), respectively.
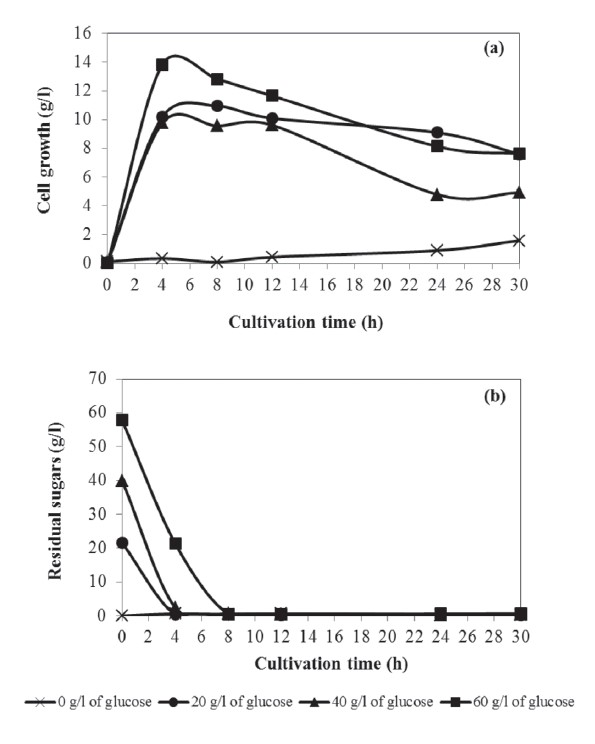
Figure 1. Time course of cell growth (a) and residual reducing sugars (b) in the succinic acid fermentation by A. succinogenes using glucose as a carbon source.
The cell growth of A. succinogenes increased rapidly and reached a maximum cell growth of 12.81 g/l after 4 h of cultivation (60 g/l of glucose as a carbon source). The lag phase of cell growth was not observed in this case. Figure 2 showed that increasing the initial glucose concentration from 0 to 60 g/l maximized the succinic acid concentration (52.180 g/l), corresponding to a yield of 0.870 g/g glucose (60 g/l of glucose). The residual reducing sugars diminished after 8 h of cultivation.
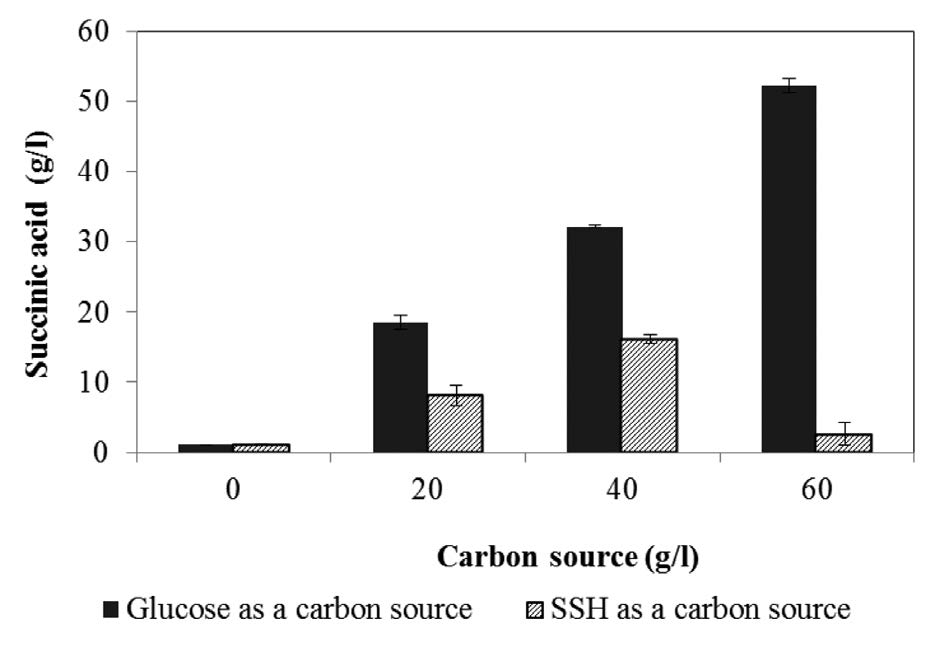
Figure 2. Succinic acid production by A. succinogenes using glucose or SSH as a carbon source.
Effect of the SSH concentration on succinic acid production by A. succinogenes
The fermentation cultivation used SSH as a carbon source with various concentrations in the range 0-60 g/l (equivalent to glucose concentration). The maximum succinic acid concentration of 16.671 g/l from 40 g/l of SSH is shown in Figure 2, corresponding to a yield of 0.777 g/g substrate at 24h of cultivation. The time course of cell growth and residual reducing sugars are shown in Figure 3(a) and (b), respectively. The cell growth of A. succinogenes increased with the increasing of the initial SSH concentration from 0 to 40 g/l. The lag of cell growth was observed for 4 h. Noticeably, at the initial SSH concentration 60 g/l, the residual sugars were slowly consumed, resulting in a small amount of cell growth and succinic acid concentration.
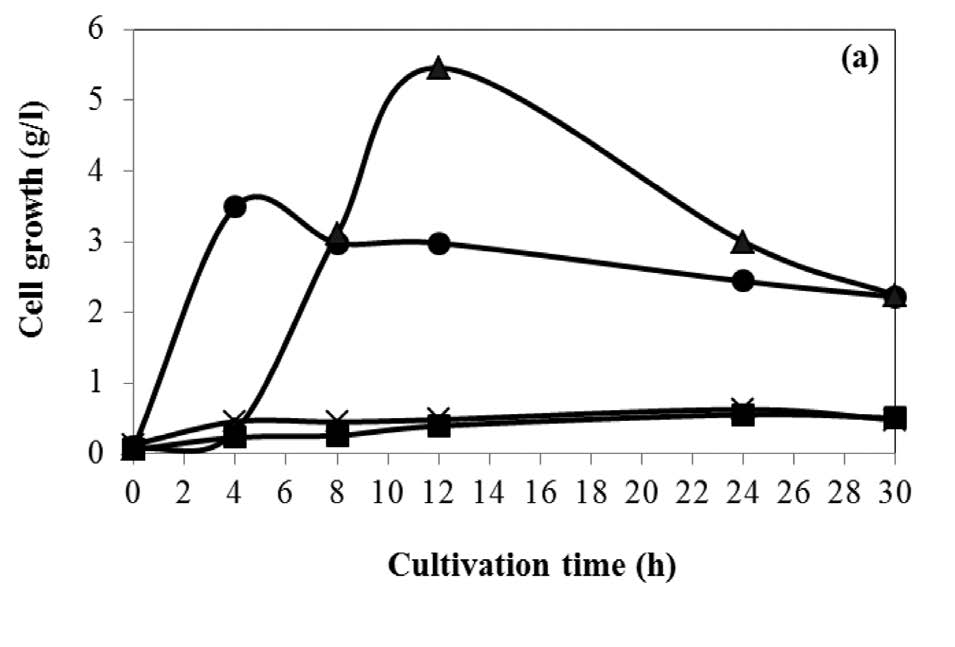
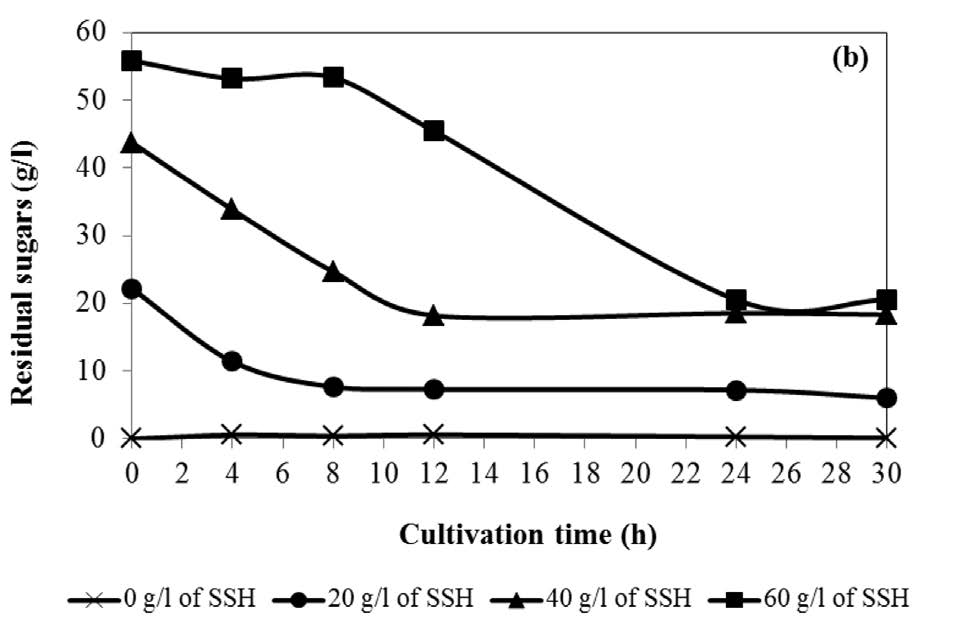
Figure 3. Time course of cell growth (a) and residual sugars (b) in succinic acid fermentation by A. succinogenes from SSH as a carbon source.
Optimizing the composition of the medium for succinic acid production using Plackett-Burman Design (PBD)
As shown in Table 2, PBD for 12 trials at two different concentrations was undertaken to evaluate the significance of six medium components. The Pareto chart of the standardized effects is shown in Figure 4. The parity plot between the actual and predicted values of succinic acid production by A. succinogenes is shown in Figure 5.
Table 2. Experimental design using PBD and the result for the optimization of succinic acid production by A. succinogenes DSMZ 22257.
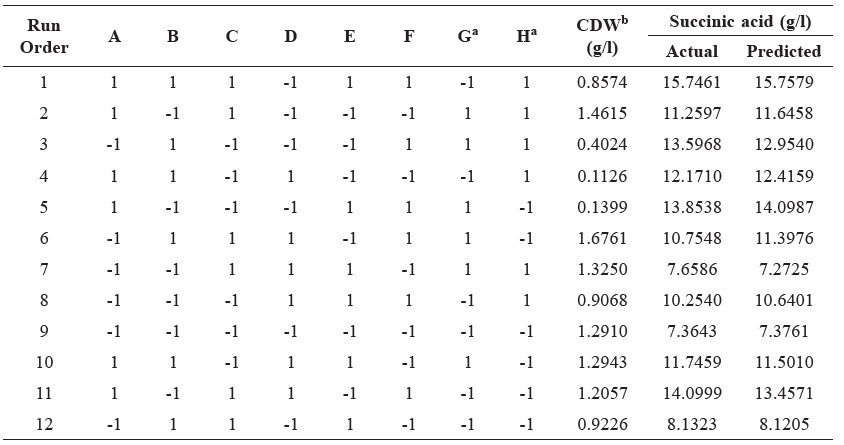
Note: aDummy variable. bCDW means cell dry weight.
From Figure 4, yeast extract and MgCO3 were significant (p < 0.05) for biosynthesis of succinic acid. From the PBD analysis using Minitab Program (version 17), a first-order regression equation was determined as shown in Equation 1:
Y = 11.386 + 1.760A + 0.638B - 0.111C - 0.272D - 0.155E + 1.664F + 0.092G + 0.395H (Eq.1)
In this equation, Y was the succinic acid production. A, B, C, D, E, and F were the values of yeast extract, urea, CaCl2, MnCl2, Na2CO3, and MgCO3, respectively, while G and H were dummy variables.
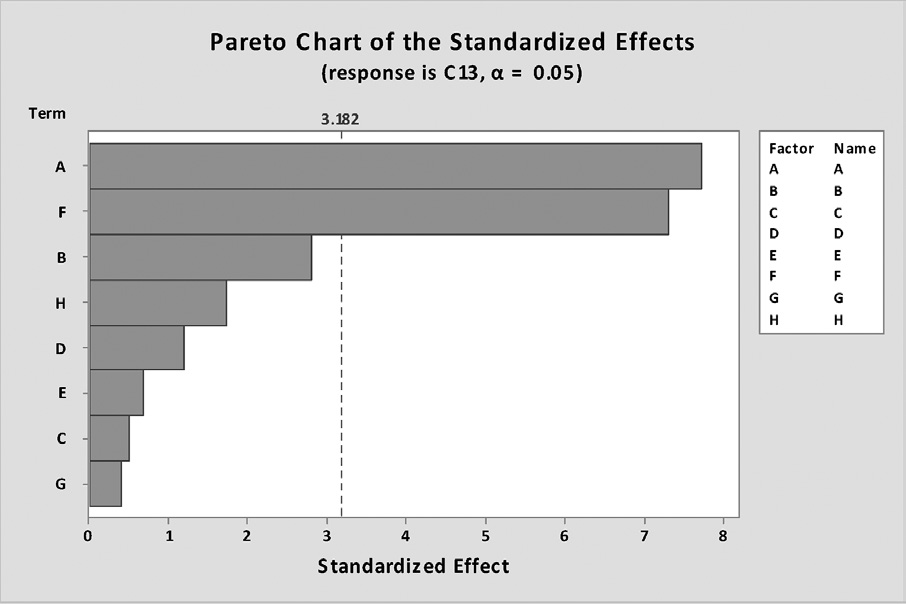
Figure 4. Pareto chart of the standardized effects on succinic acid production. The chart included a vertical line (i.e., standardized effect = 3.182) at the critical t-value for α of 0.05. The bars were displayed in order of the size of the effects and the standardized effect of each term was shown on the top of its corresponding bar.
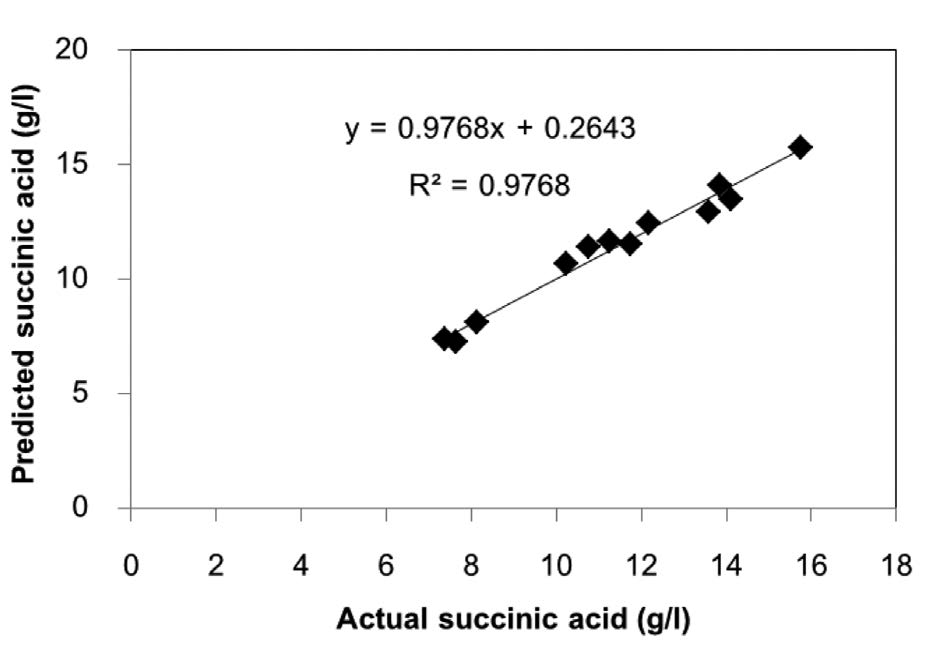
Figure 5. Parity plot between the actual and predicted values of succinic acid production by A. succinogenes.
The goodness of the regression was checked by the coefficient of determination R2; the model explained 97.68% of the total variation. It was reasonable to use the regression model to analyze the trend in this response. The succinic acid production was affected by the coefficients of yeast extract (1.760) and MgCO3 (1.664). The positive coefficient indicated that the high concentration of yeast extract helped succinic acid production.
Similarly, the positive result of MgCO3 indicated that the high concentration of MgCO3 helped succinic acid production. The optimal medium composition was 50.0 g/l of yeast extract, 5.0 g/l of urea, 5.0 g/l of CaCl2, 0.25 g/l of MnCl2, 2.50 g/l of Na2CO3, and 50 g/l of MgCO3, which maximized the succinic acid yield (15.746 g/l). The productivity, specific productivity, and CDW were 0.656 g/l/h, 15.746 g/l and 0.8574 g/l, respectively.
DISCUSSION
The fermentation result using glucose as a carbon source indicated that cell growth of A. succinogenes and succinic acid concentration increased with increasing glucose concentration. Lee et al. (1999) reported that cell growth and metabolites production from A. succiniciproducens were affected by the initial glucose concentration. A longer lag phase was accompanied by lower biomass and succinic acid concentration (at a high initial glucose concentration of 80 g/l). Liu et al. (2008) also reported that succinic acid production by A. succinogenes CGMCC1593 was inhibited by a high initial glucose concentration. When the initial glucose concentration increased from 50 g/l to 75 g/l, the maximum specific growth rate dropped from 0.77 h-1 to 0.58 h-1, which corresponded to a decline in the rate of succinic acid production from 1.3 g/l/h to 1.0 g/l/h. However, Lin et al. (2008) reported that A. succinogenes tolerated up to 143 g/l of glucose with the maximum specific growth rate of 0.50 h-1, and cell growth was completely inhibited at a glucose concentration over 158 g/l. A significant decrease in succinic acid yield and a prolonged lag phase were observed with glucose concentration above 100 g/l. Among the end-products investigated, formate was found to have the greatest inhibitory effect on succinic acid fermentation. Moreover, Guettler et al. (1996) reported that A. succinogenes could grow in aqueous media containing over 150 g/l of glucose. Indeed, Liu et al. (2008) also reported that A. succinogenes could tolerate up to 160 g/l initial glucose concentration, but with a reduction of yield and productivity of succinic acid. The tolerance of a microorganism to glucose concentration might be due to differences in growth media, such as the presence of a nitrogen source, MgCO3 or a metal solution. These factors will be investigated in the future.
When using sorghum straw hydrolysate as a carbon source, a lag phase of 4 h was observed, while no distinct lag phase was seen with glucose as a carbon source. These results indicated that the inhibitory effect of higher concentration of sugars from SSH on A. succinogenes was also observed in this study. Inhibitors, such as 5-hydroxy methyl fufural and fufural, might have affected cell growth and, subsequently, succinic acid production.
From this research, the yield of succinic acid concentration from SSH was 0.777 g/g substrate, close to the 0.870 g/g substrate from 60 g/l glucose, indicating that the sugars liberated from sorghum straw could be used as an alternative carbon source. Moreover, sorghum straw is cheap, abundant, and renewable. Therefore, agriculture biomass offers a more cost efficient process for producing succinic acid, and increases the value of the agriculture waste.
Plackett-Burman Design (PBD) was applied for a preliminary optimization of the succinic acid fermentation medium by A. succinogenes DSMZ 22257. Yeast extract and MgCO3 were screened to be the key factors for producing succinic acid. Yeast extract affected cell growth directly as a nutrient. It contained many trace substances, such as folic acid, pantothenic acid, biotin, and vitamins B1, B2, B6, and B12. This may be the reason why many kinds of vitamins could be omitted, while still efficiently producing succinic acid.
The pH value of the culture medium was one of the key factors controlling succinic acid production. MgCO3 was added to the fermentation medium to neutralize
the pH of the culture medium. Wang et al. (2012) reported the pattern of succinic acid production at a culture pH of 7.0 was similar to that obtained at 7.5. When the initial culture pH was adjusted to 6.5 or 8.0, they observed a significant decrease of succinic acid production was observed, with the highest succinic acid production of 48.2 g/l obtained at a culture pH of 7.5. Consequently, the effect of MgCO3 on optimizing the medium may be partly due to its influence on the pH. On the other hand, sufficient CO2 supplement in the fermentation broth has been shown to strongly influence the metabolic flux of carbon and the activities of phosphoenolpyruvate (PEP) carboxykinase, which are important steps in the biosynthesis of succinic acid (McKinlay and Vieille, 2008). As an important CO2 donor in A. succinogenes fermentation, MgCO3 could react with organic acids in the fermentation broth and cause an increase in the dissolved concentrations of HCO3−, CO32−, and CO2.
Zhu et al. (2012) reported that a higher amount of MgCO3 more effectively promoted the synthesis of succinic acid by A. succinogenes. Moreover, they obtained a maximum succinic acid production of 61.92 g/l at 159.22 mM dissolved CO2 concentration, which was supplied by 40 g/l MgCO3 with 100% CO2 gas. This means that the dissolved CO2 concentration was another factor affecting succinic acid synthesis. Indeed, during the fermentation process, insoluble MgCO3 caused turbid broth, spreading the cells uniformly in the broth and avoiding cell flocculation. Because of these properties, MgCO3 plays a key role in improving succinic acid production.
CONCLUSION
This study demonstrated that Packett-Burnman Design was effective at selecting and optimizing the significant factors for producing succinic acid by A. succinogenes DSMZ 22257. Yeast extract and MgCO3 were the key factors for succinic acid production. The optimal concentrations of these two key components, including sugar concentration, will be studied using response surface methodology (RSM) in the future.
ACKNOWLEDGEMENTS
The authors acknowledge with thanks the financial support from the Annual Government Statement of Expenditure (2015) (GRB_BSS_81_58_61_09), Thailand.
REFERENCES
Bechthold, I., K. Bretz, S. Kabasci, R. Kopitzky, and A. Springer. 2008. Succinic acid: a new platform chemical for bio-based polymers from renewable resources. Chemical Engineering and Technology. 31: 647-654.
Guettler, M.V., D. Rumler, and M.K. Jain. 1999. Actinobacillus succinogenes sp. nov., a novel succinic-acid producing strain from the bovine rumen. International Journal of Systematic Bacteriology. 49: 207-216. doi: 10.1099/00207713-49-1-207
Guettler, M.V., M.K. Jain, and B.K. Soni. 1996. Process for making succinic acid, microorganisms for use in the process and methods of obtaining the microorganisms, Michigan Biotechnology Institute, USA, US patent. 5: 504-004.
Hodge, D.B., C. Andersson, K.A. Berglund, and S.C. Park. 2009. Detoxification requirements for bioconversion of softwood dilute acid hydrolyzates to succinic acid. Enzyme Microbial Technology. 44: 309-316. doi: 10.1016/j.enzmictec.2008.11.007
Howlett, M.R., D.O. Mountfort, K.W. Turner, and A.M. Roberton. 1976. Metabolism and growth yields in Bacteroides ruminicola strain b14. Applied and Environmental Microbiology. 32(2): 274-283.
Lee P.C., W.G. Lee, S. Kwon, S.Y. Lee, and H.N. Chang. 1999. Succinic acid production by Anaerobiospirillum succiniciproducens: effects of the H2/CO2 supply and glucose concentration. Enzyme Microbial Technology. 24:549-54. doi: 10.1016/S0141-0229(98)00156-2
Li, S. Z., and C.C. Halbrendt. 2009. Ethanol production in (the) people’s republic of china: potential and technologies. Applied Energy. 86: S162-S169.
Li, J., X.Y. Zheng, X.Y. Fang, S.W. Liu, K.Q. Chen, M. Jiang, P. Wei, and P.K. Ouyang. 2011. A complete industrial system for economical succinic acid production by Actinobacillus succinogenes. Bioresource Technology. 102: 6147-6152. doi: 10.1016/j.biortech.2011.02.093
Lin, S.K.C., C. Du, A. Koutinas, R. Wang, and C. Webb. 2008. Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochemical Engineering Journal. 41: 128-135. doi: 10.1016/j.bej.2008.03.013
Liu, Y.P., P. Zheng, Z.H. Sun, Y. Ni, J.J. Dong, and L.L. Zhu. 2008. Economical succinic acid production from cane molasses by Actinobacillus succinogenes. Bioresource Technology. 99: 1736-1742. doi: 10.1016/j.biortech.2007.03.044
McKinlay, J.B., C. Vieille, and J.G. Zeikus. 2007. Prospects for a bio-based succinate industry. Applied Microbiology and Biotechnology. 76: 727-740. doi: 10.1007/s00253-007-1057-y
McKinlay, J.B., and C. Vieille. 2008. 13C-metabolic flux analysis of Actinobacillus succinogenes fermentative metabolism at different NaHCO3 and H2 concentrations. Metabolic Engineering. 10: 55-68. doi: 10.1016/j.ymben.2007.08.004
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 31: 426-428.
Okino, S., M. Inui, and H. Yukawa. 2005. Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Applied Microbiology and Biotechnology. 68: 475-480. doi: 10.1007/s00253-005-1900-y
Plackett, R.L., and J.P. Burman. 1946. The design of optimum multifactorial experiments. Biometrika. 33: 305-325.
Poonsrisawat, A., S. Phuengjayaem, A. Petsom, and S. Teeradakorn. 2013. Conversion of sweet sorghum straw to sugars by dilute acid saccharification. Sugar Tech. 15: 322-327. doi: 10.1007/s12355-013-0235-8
Rudner, M.S., S. Jeremic, K.A. Petterson, D.R. Kent, K.A. Brown, M.D. Drake, et al. 2005. Intramolecular hydrogen bonding in disubstituted ethanes. A comparison of NH-R-O- and OH-R-O- hydrogen bonding through conformational analysis of 4-amino-4-oxobutanoate (succinamate) and monohydrogen 1, 4-butanoate (monohydrogen succinate) anions. The Journal of Physical Chemistry A. 109: 9076-9082. doi: 10.1021/jp052925c
Saha, B.C., B.S. Dienand, and R.J. Bothast. 1998. Fuel ethanol production from corn fiber:current status and technical prospects. Applied Biochemistry and Biotechnology. 70–72: 115-125. doi: 10.1007/BF02920129
Song, H., and S.Y. Lee. 2006. Production of succinic acid by bacterial fermentation. Enzyme and Microbial Technology. 39: 352-361. doi: 10.1016/j.enzmictec.2005.11.043
Stols, L., and M.I. Donnelly. 1997. Production of succinic acid through overexpression of NAD(+)-dependent malic enzyme in an Escherichia coli mutant. Applied and Environmental Microbiology. 63(7): 2695-2701.
Wan, C., Y. Li, A. Shahbazi, and S. Xiu. 2008. Succinic acid production from cheese whey using Actinobacillus succinogenes 130z. Applied Biochemistry and Biotechnology. 45: 111-119. doi: 10.1007/s12010-007-8031-0
Wang, C.C., L.W. Zhu, H.M. Li, and Y.J. Tang. 2012. Performance analyses of a neutralizing agent combination strategy for the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Bioprocess and Biosystems Engineering. 35: 659-664. doi: 10.1007/s00449-011-0644-6
Zheng, P., J.J. Dong, Z.H. Sun, Y. Ni, and L. Fang. 2009. Fermentative production of succinic acid from straw hydrolysate by Actinobacillus succinogenes. Bioresource Technology. 100: 2425-2429. doi: 10.1016/j.biortech.2008.11.043
Zhu, L.W., C.C. Wang, R.S. Liu, H.M. Li, D.J. Wan, and Y.J. Tang. 2012. Actinobacillus succinogenes ATCC 55618 fermentation medium optimization for the production of succinic acid by response surface methodology. Journal of Biomedicine and Biotechnology. 2012: 1-9. doi: 10.1155/2012/626137
Sukanya Phuengjayaem1 and Siriluk Teeradakorn2*
1 Program in Biotechnology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
2 The Institute of Biotechnology and Genetic Engineering, Chulalongkorn University, Bangkok 10330, Thailand
*Corresponding author. E-mail: siriluk.T@chula.ac.th
Total Article Views

