
Effect of MethocelTM, Maltodextrin, Sodium Choloride, and pH on Foaming Properties and Foam-mat Drying of Aqueous Pandan (Pandanus amaryllifolius) Leaves Extract
Panida Rattanapitigorn, Masahiro Ogawa and Nithiya Rattanapanone*Published Date : 2019-08-23
DOI : 10.12982/cmujns.2016.00018
Journal Issues : Number3 ,September - December 2016
ABSTRACT
The pandan (Pandanus amaryllifolius) leaf is commonly used as a food coloring and flavoring agent. Aqueous pandan leaf extract has no foaming properties and deteriorates quickly. Finding the appropriate foaming agent and stabilizer during drying is a challenge. The objective of this research was to investigate the effect of Methocel™, maltodextrin, sodium chloride (NaCl), and pH on foaming properties and foam-mat drying of aqueous pandan leaf extract. Foaming properties of an aqueous extract from fresh pandan leaves were elucidated using Methocel™ K4M (1 and 1.5% w/w) as a foaming agent, maltodextrin (0, 5, 10 and 15% w/w) as a stabilizer, and NaCl (0, 3, and 5% w/w) as a foam improver. The foamed pandan leaf extract was dried using a batch-type cabinet dryer. The results showed that the appropriate concentrations of Methocel K4M™, maltodextrin, and NaCl were 1% or 1.5%, 15%, and 5% (w/w), respectively. Increasing the maltodextrin concentration in combination with NaCl significantly affected the foam density, foam overrun, and foam drainage volume of the pandan leaf extract (p<0.05). Adjusting the pH of the foam solution to 6.2-7.2 maintained the chlorophyll content during drying better than the control (p<0.05).
Keywords: Pandanus amaryllifolius, Foaming properties, Methocel™, Maltodextrin, Sodium chloride
INTRODUCTION
Pandan (Pandanus amaryllifolius) leaves have a unique scent, and are widely cultivated in Southeast Asia, including Thailand. The pandan leaf is used as a food coloring and fl avoring agent in many kinds of foods and desserts, including iced and herbal drinks (Yahya et al., 2010; Ping et al., 2014). The pandan leaf is also used in Thai alternative medicine for assisting relaxation and satisfying thirst. However, fresh pandan leaves deteriorate quickly; processing them into powder provides a more stable form for the market. Previous studies have spray dried pandan extract to produce a flavor encapsulation agent and colorant (Apintanapong and Noomhorm, 2003; Ratanasiriwat et al., 2013). However, the methods used in these studies were not economical. A more appropriate drying method is still needed; foam-mat drying offers one such alternative. Foam-mat drying is an advanced technique that uses a foaming agent, such as Methocel™ or egg white protein (Raikos et al., 2007), to introduce air bubbles into a liquid sample by whipping at high speed. The foam is then spread (1-5 mm thick) on a tray and dried by hot air. Foaming the liquid sample provides more surface area for evaporation, reducing the drying time.
In general, foam-mat drying is suitable for liquid foods with high sugar content, such as fruit juices (Chaves et al., 2013). However, for some kinds of liquid foods, foam-mat drying does not work well, because they possess no foaming properties or the foamed food is not stable during drying. Finding the appropriate ratio of foaming agent and stabilizer to stabilize foamed-food during drying is challenging. The FDA has approved Methocel™ K4M (hydroxypropyl methylcellulose, HPMC) food-grade products as generally recognized as safe (GRAS) (GRAS notification GRN 000213); they also comply with standards for direct food additives under 21 CFR172.874. Methocel™ is widely used in food and pharmaceutical products (Subramanian et al., 2003). Methocel™ K4M has an optimal hydration temperature of 29°C; soft gel forms at 70-90°C; and it is nonionic (Dow Chemical Company, 2002). Maltodextrin is generally used as a bulking agent and encapsulating material, because it is tasteless, dissolves well in water with low viscosity, and is commercially available in a variety of molecular weights (Takeiti et al., 2010; Caliskan and Dirim, 2013). Several researchers have reported that salt affects the foaming properties of protein-based foaming agents, especially whey protein isolate and egg white albumin (Raikos et al., 2007; Ercelebi and Ibanoglu, 2009; Schwenzfeier et al., 2013). However, few publications have studied the effect of NaCl on the foaming properties of polysaccharide-based foaming agents, especially Methocel™.
The objective of this study was to clarify the effect of Methocel™, maltodextrin, and NaCl concentrations on the foaming properties of aqueous extract of fresh pandan leaves and to investigate the impact of pH on the stability of chlorophyll of pandan leaf extract during drying.
MATERIALS AND METHODS
Pandan leaves
Fresh mature pandan leaves were purchased from a local market in Chiang Mai, Thailand. Methocel™ K4M (methoxyl 19.0-24.0% and hydroxypropoxyl 7.0-12.0%, food grade, Dow Chemical Company, USA) and maltodextrin (DE11.3) were purchased from Vicchi Enterprise, Ltd. (Bangkok, Thailand). NaCl was purchased from Merck (Germany).
Preparation of aqueous extract of pandan leaves
Whole pandan leaves were rinsed, chopped, and extracted with distilled water to provide a total soluble solid of 4.0°Brix in a blender for 1 min. The temperature of the aqueous extract was maintained at 23+2°C in an ice water bath. The extracts were passed through a cotton filter and the filtrate was kept at 4°C until used. The pH of freshly prepared aqueous extract of pandan leaves was 4.7. The aqueous extract was heated by microwave to 70°C for 25 sec to inactivate any microbes before adding the foaming agent.
Preparation of pandan foam solution
The foam solution of Methocel™ was prepared in concentrations of 1.0, 1.5, 2.0, 2.5, and 3.0% w/w for preliminary study of the foam ability of Methocel™ in aqueous extract of pandan leaves. The combination effect of Methocel™, maltodextrin, and NaCl on the foaming properties of aqueous extract from pandan leaves was studied. Methocel™ K4M was used as a foaming agent at concentrations of 1.0 and 1.5% w/w. Maltodextrin was used to stabilize the foam at concentrations of 0, 5, 10, and 15% w/w. NaCl at concentrations of 0, 3 and 5% w/w was used to investigate the combined effect with Methocel™ and maltodextrin on the foaming properties of an aqueous extract of pandan leaves. A foam solution of pandan was prepared by dispersing Methocel™, maltodextrin, and NaCl into a hot aqueous extract of pandan leaves, stirred for at least 10 min and kept overnight at 4°C to ensure a complete dispersion before using. The pH of the pandan foam solution was 5.6.
Viscosity measurements
The viscosity of the pandan foam solution was measured at 25±0.2°C, using a Brookfield LVD-II+ viscometer (Brookfield Engineering Laboratories Inc., Germany) with a spiral spindle adapter (#64). A sample solution (45 mL) was loaded into a 50 mL plastic tube (3 cm diameter) and the spindle was inserted down into the solution. The readings were collected at a rotational speed of 100 rpm for 20 seconds.
Foam formation
Foams were generated by whipping 100 mL of pandan foam solutions in a bowl-lift stand mixer equipped with a wire whip (Kitchen Aid Heavy Duty, St. Joseph’s, Mich., U.S.A.) for 15 min at a speed setting of 10 (planetary rpm of 310).
Foam properties
The foaming properties (density, overrun, and drainage volume) of the pandan foam solutions were determined by modified method of Phillips et al. (1987) and Raharitsifa et al. (2006). Each 100 mL dispersion of foam solution was whipped for 15 min before measuring the foaming properties. All measurements were done in triplicate.
Foam overrun. The foam overrun was calculated and presented as foaming capacity by the following equation:
Foam overrun (%) = [W(d) - W(f)] /W (f) x 100
where W(d) is the weight (g) of the unwhipped dispersion and W(f) is the weight (g) of the whipped foam.
Foam density. Foam density was calculated by weighing a known volume of foam divided by the weight of liquid in the same volume and expressed in the unit of g.mL–1.
Foam drainage volume. Foam drainage volume (FDV) was determined and presented as foam stability. The FDV was measured using a 100 mL graduated cylinder; a 100 mL Buchner funnel was filled to the top with each foam sample. The volume in mL of drained liquid-foam was read directly from the graduated cylinder after 10 min. Large FDV values indicated that the foam stability was poor.
Stability of chlorophyll during foam-mat drying For the variation of pH experiments, foam solutions of pandan were also prepared at pH 5.6, 6.2, 6.7, and 7.2 and kept overnight at 4°C before use. Foams were generated by whipping 100 mL of pandan foam solutions for 15 min at a speed setting of 10 (planetary rpm of 310). Pandan foam was dried in a laboratory-type, hot-air oven (Memmert Model UNE 400, Germany) at 70°C for 6 hrs; the foam-mat thickness was 5 mm. The temperature and the period of drying were set at the same condition from the preliminary study to make sure that all samples were dried completely and maintained in the same drying condition.
Chlorophyll content
Chlorophyll content was determined using a spectrophotometer by modified AOAC method 940.03 and 942.04 (AOAC, 2000) as follows: one gram of pandan powder was accurately weighed, then extracted with 85% acetone or ether, if necessary. The extracted solution was filtered. The residue was washed and the filtrate was diluted with 85% acetone to a final volume of 100 mL. The acetone and H2O was separated using a separator. Then the acetone solution was transferred to a 100 mL volumetric flask, diluted to volume, and the absorbance values were measured at 660 and 642.5 nm using a UV/visible diode array spectrophotometer (Agilent Technologies, model 8453 G1103A, Santa Clara, CA, USA). The total chlorophyll content, chlorophyll a, and chlorophyll b were calculated by the following equations:
Total chlorophyll content (mg/L) = 7.12 A660 + 16.8 A642.5
Chlorophyll a = 9.93 A660 – 0.777 A642.5
Chlorophyll b = 17.6 A642.5 – 2.81 A660.0
Color measurement
Color of the foam-mat dried pandan powder was measured with a Konica Minolta Chroma Meter (Model CR400, Japan) using the Hunter scale for L*, a*, b*, chroma, and hue values. L* value is in the range of 0 to 100 (black to white), a* value is in the range of -60 to +60 (greenness to redness), and b* value is in the range of -60 to +60 (blueness to yellowness). Chroma presented the intensity of color as compared to gray (0-90). The hue value used a scale of 0°= red, 90°=yellow-green, 180°=cyans, and 270°=blue-magenta (McGuire, 1992).
Water activity
Water activity was determined using a water activity meter (AQUA Lab, CX3TE, Decagen, USA).
Scanning electron microscope (SEM)
Morphology and surface appearance of foam-mat pandan powder was determined using a scanning electron microscope (JEOL, JSM-5910LV, Japan). The samples were attached with two-sided adhesive tape to specimen stubs and then coated with a gold-palladium mixture in a sputter coater. Magnification was set at 50%.
Statistical analysis
All experiments were carried out in at least two replications using freshly prepared samples. Results were reported as average values with standard deviation. Experimental results were subjected to one- and two-way analysis of variance (ANOVA). The analysis of variance (ANOVA) and a Pearson correlation test, at a confidence level of 95%, and the Duncan’s new multiple range test (as a mean difference test) were performed to ascertain the significance of the average values.
RESULTS
Foam properties of Methocel™
Microwave treatment of the aqueous extract of pandan leaves significantly decreased the total plate count of Escheria coli and Staphylococcus aureus to safe levels for food (data not shown). The foaming properties of Methocel™ in aqueous pandan extracts are shown in Figure 1. Increasing the Methocel™ concentration resulted in a high-viscosity, thick, foam solution with high foaming stability. Foam overrun of Methocel™ at concentrations of 1.5-3.0% w/w was in
the range of 100-123%, with no significant difference (P>0.05). This result showed that Methocel™ alone was not a suitable foaming agent for aqueous pandan leaf extract.
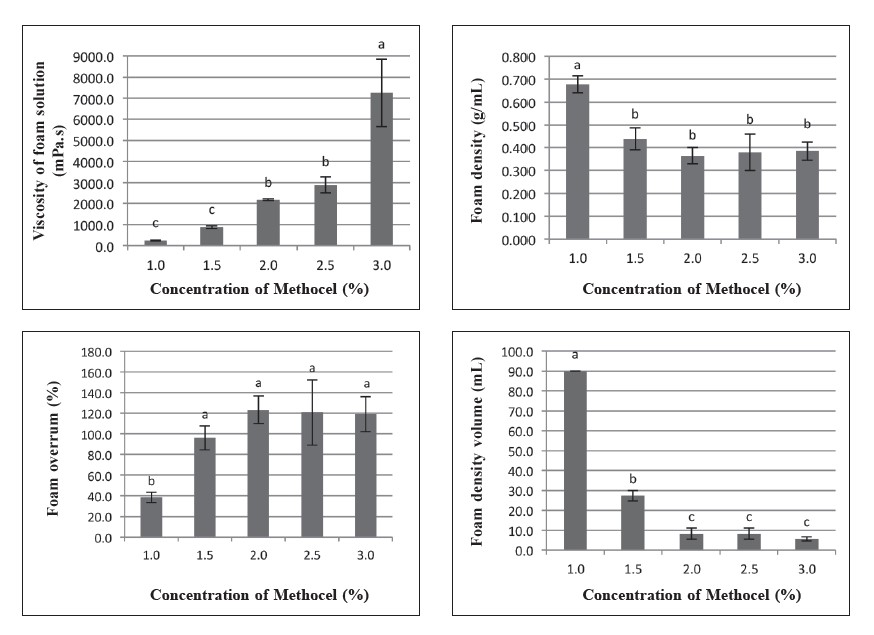
Figure 1. Viscosity of foam solution and foaming properties of Methocel™ K4M. Note: Results are means of triplicate determinations. Error bars are standard deviations. Different letters (a, b, c) differ significantly (P < 0.05) for the same treatment.
Foam properties of Methocel™, maltodextrin, and NaCl
Analysis of variance of the differences in the viscosity and foam properties (density, overrun, and drainage volume) of aqueous pandan leaf extract as a function of the various Methocel™ K4M, maltodextrin, and NaCl treatments are shown in Table 1. The treatments were significantly different (p<0.05). Adding NaCl enhanced the viscosity of foam solutions and foaming stability at low concentrations of maltodextrin (Figure 2 and Figure 5). Conversely, the viscosity sharply decreased as the NaCl concentration increased up to 5% in combination with the maltodextrin concentration of 15%, as shown in Figure 2, and the samples exhibited significantly highest overrun (Figure 4). The foamed pandan leaf extracts using Methocel™ (1 and 1.5%) as a foaming agent with maltodextrin (15%) andNaCl (5%) as stabilizing agent showed the appropriate foaming properties. The results clearly indicated that the different foaming properties were affected contrarily by concentrations of MethocelTM, maltodextrin, and NaCl.
A Pearson correlation test was applied to define the relationship between foaming properties of the pandan foam solutions; correlation data are shown in Table 2. Significant relations were not observed between the viscosity of foam solution and foam density or foam drainage volume. However, the foam overrun negatively correlated with the viscosity of foam solution (p<0.01). The foam density showed a significant correlation with foam drainage volume (r = 0.81) and foam overrun (r = -0.89). From the correlation coefficients, the foam density and the foam drainage volume decreased, while foam overrun increased.
Table 1. Analysis of variance of foam properties of aqueous pandan leaf extract as a function of Methocel™ K4M, maltodextrin, and NaCl concentrations.
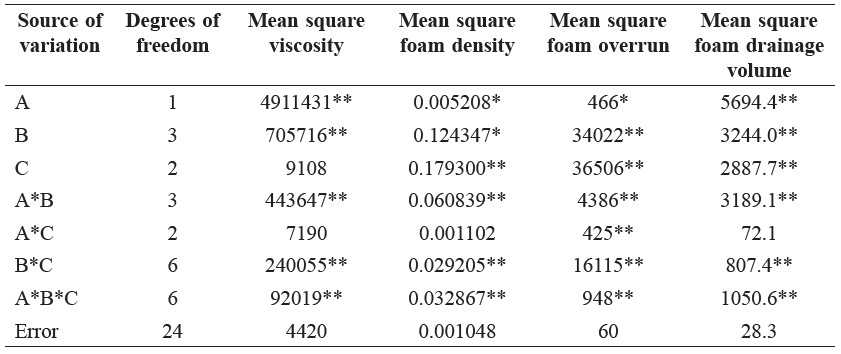
Note: A = Methocel™ K4M concentrations, B = Maltodextrin concentrations, and C = NaCl concentrations. *Significance level at p< 0.05. **Significance level at p< 0.01.
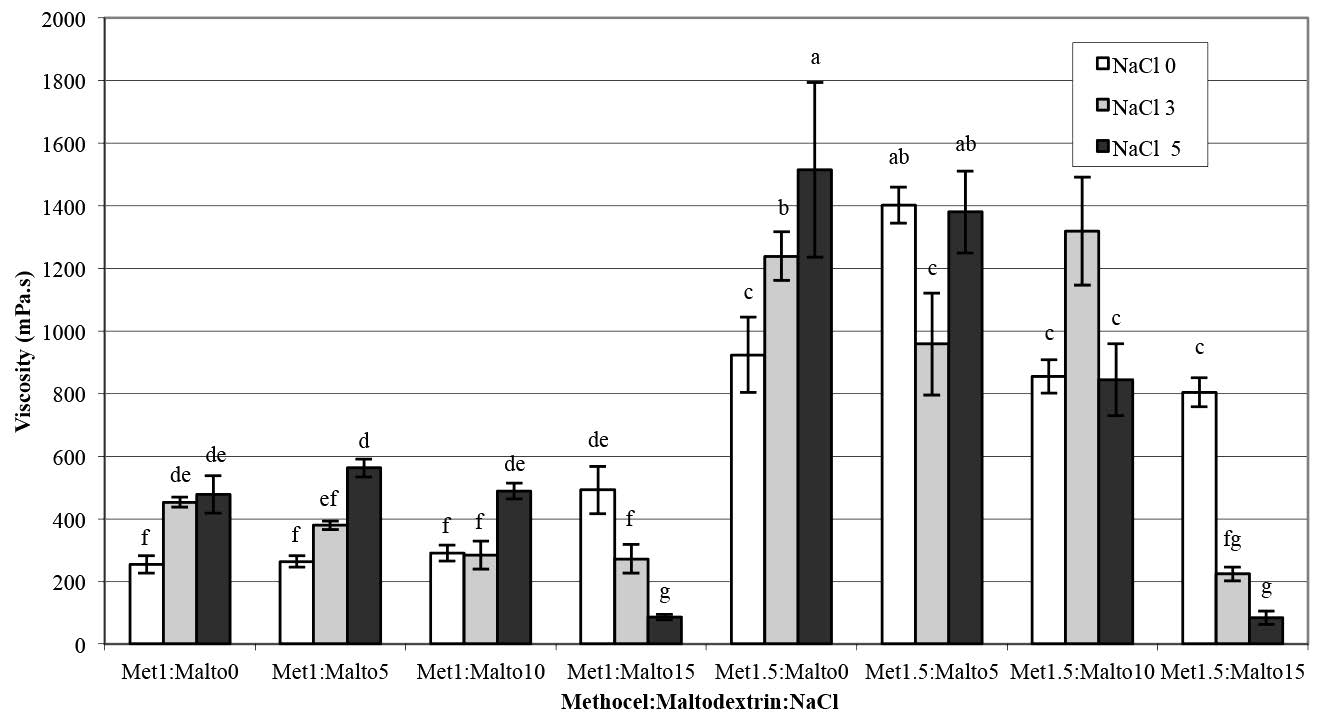
Figure 2. Viscosity of aqueous pandan leaf extract as a function of Methocel™ K4M, maltodextrin, and NaCl concentrations.
Note: Results are means of two replieations determinations. Error bars are standard deviations. Different letters (a, b, c) differ significantly (P < 0.05) for the same treatment.
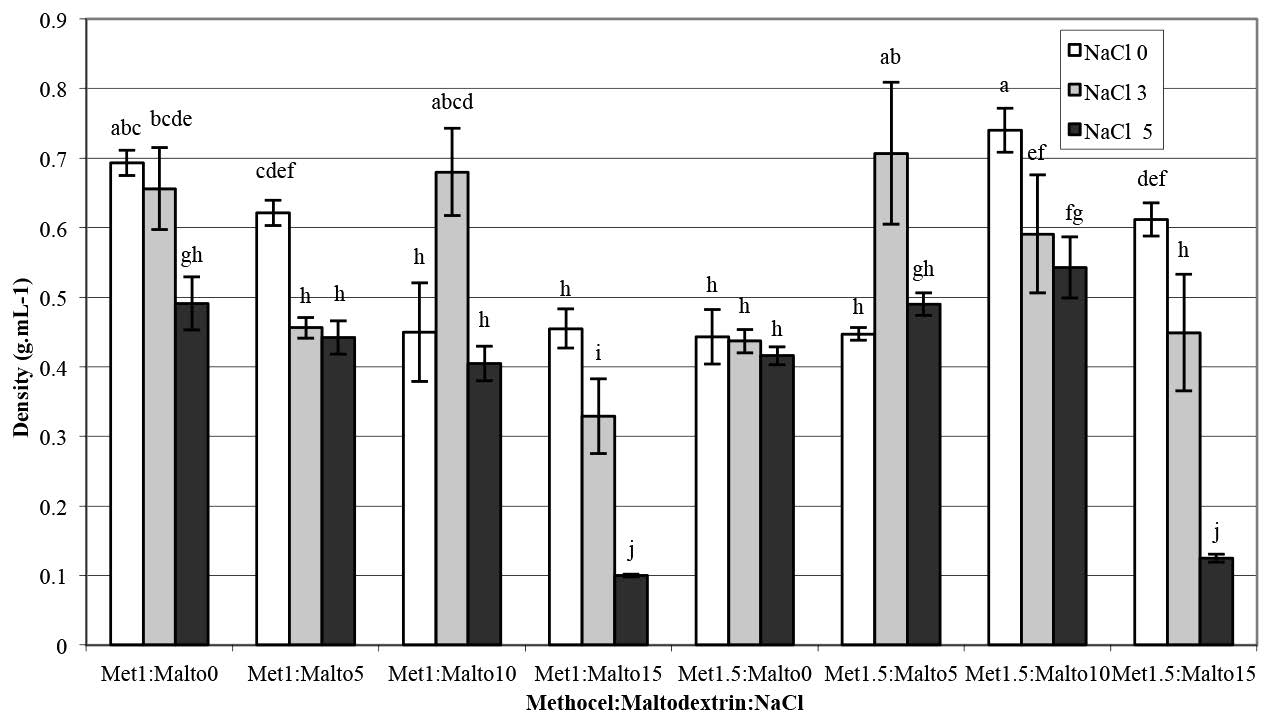
Figure 3. Foam density of aqueous pandan leaf extract as a function of Methocel™ K4M, maltodextrin, and NaCl concentrations.
Note: Results are means of two replications determinations. Error bars are standard deviations. Different letters (a, b, c) differ significantly (P < 0.05) for the same treatment.
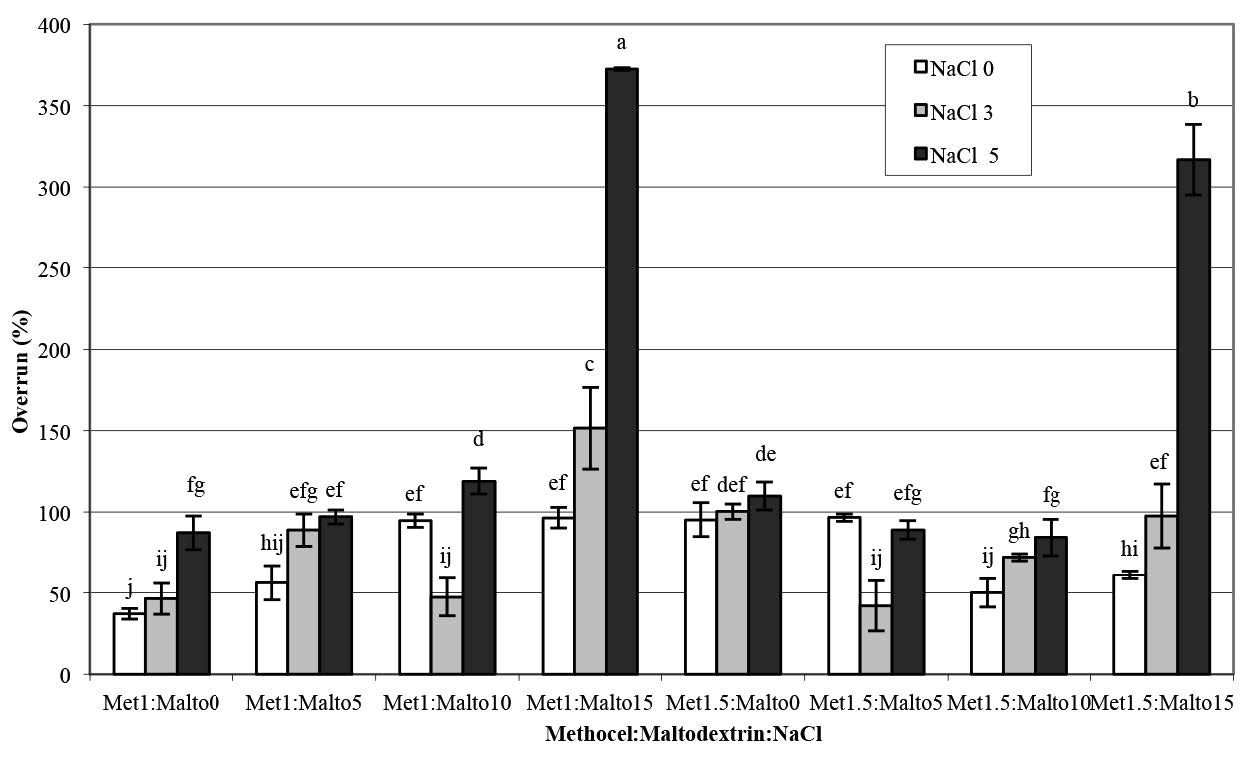
Figure 4. Foam overrun of aqueous pandan leaf extract as a function of Methocel™ K4M, maltodextrin, and NaCl concentrations.
Note: Results are means of two replications determinations. Error bars are standard deviations. Different letters (a, b, c) differ significantly (P < 0.05) for the same treatment.
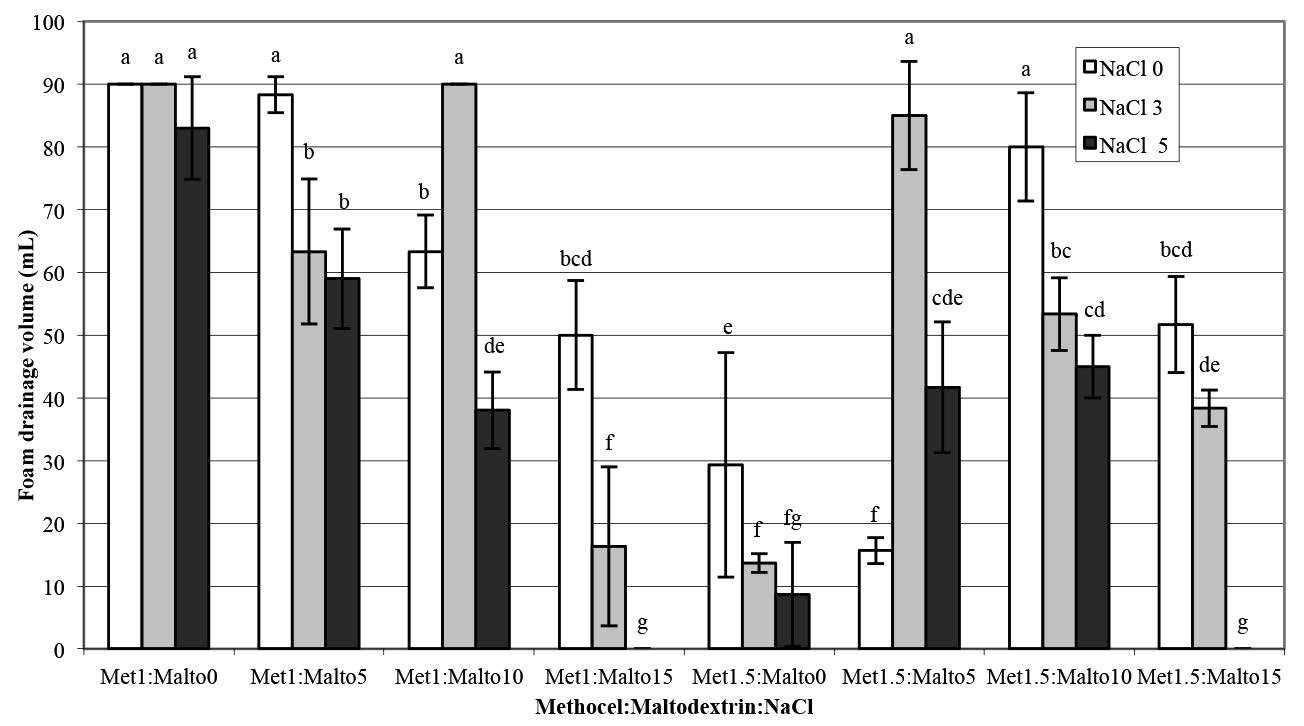
Figure 5. Foam drainage volume of aqueous pandan leaf extract as a function of Methocel™ K4M, maltodextrin, and NaCl concentrations.
Note: Results are means of two replications determinations. Error bars are standard deviations. Different letters (a, b, c) differ significantly (P < 0.05) for the same treatment.
Table 2. Pearson correlation coefficients of foam properties of aqueous pandan leaf extract as a function of Methocel™ K4M, maltodextrin, and NaCl concentrations.
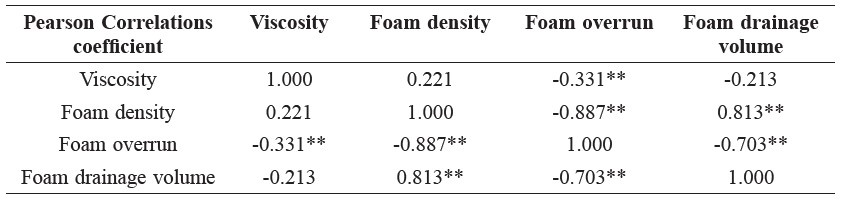
Note: ** Correlation is significant at the 0.01 level (2-tailed).
Stability of chlorophyll during foam-mat drying
The retention of total chlorophyll content, chlorophyll a, and chlorophyll b at all four pH values at a drying temperature of 70°C with and without pH control are shown in Figure 6. Adjusting the pH prevented denaturing of the chlorophyll of the pandan extracts during drying. The total chlorophyll content, chlorophyll a, and chlorophyll b of foam-mat dried pandan powder after adjusting the pH to 6.2-7.2 was in the range of 780-930, 500-610, and 250-340 mg/L, respectively.
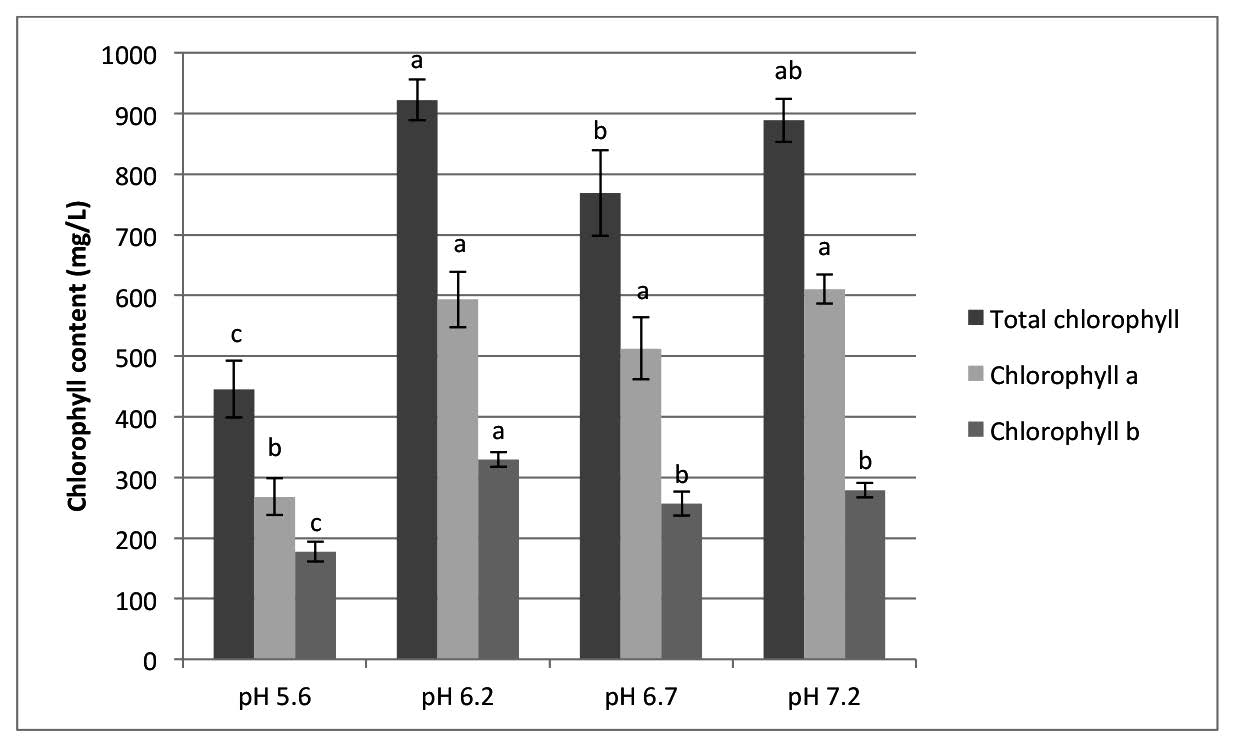
Figure 6. Chlorophyll content of foam mat-dried pandan powder as affected by pH.
Note: Results are means of triplicate determinations. Error bars are standard deviations. Different letters (a, b, c) differ significantly (P < 0.05) for the same treatment.
Color evaluation
The hue value of foam-mat dried pandan powder was in the range of 100-130°, or green color (Figure 7). The water activity of foam-mat dried pandan powder was similar for all treatments. Greenness (a* value) and hue value of foam-mat dried pandan color increased when pH increased (p<0.5). Yellowness (b* value) of the samples decreased at higher pH (p<0.5) and no significant difference was found between pH values of 6.7 and 7.2. The higher pH adjustment increased the greenness of foam-mat dried pandan powder.
Scanning electron microscope (SEM)
Morphology and surface appearance of foam-mated pandan powder was determined using SEM as shown in Figure 8. The microstructure of foam-mated pandan powder had a porous and small flake-like structure.
DISCUSSION
Methocel™ is an efficient foaming agent used in a wide variety of processed foods and pharmaceuticals (Dow Chemical Company, 2002). The ability of a foam solution to hold air bubbles in producing the foam structure is called foamability, while foam stability is expressed as the stability of foam over time after foam generation (Bureiko et al., 2014). From this experiment, as shown in Figure 1, Methocel™ alone was not a suitable foaming agent for aqueous pandan leaf extract. The addition of NaCl enhanced the viscosity of foam solutions and foaming stability at low concentrations of maltodextrin (Figure 2 and Figure 5).
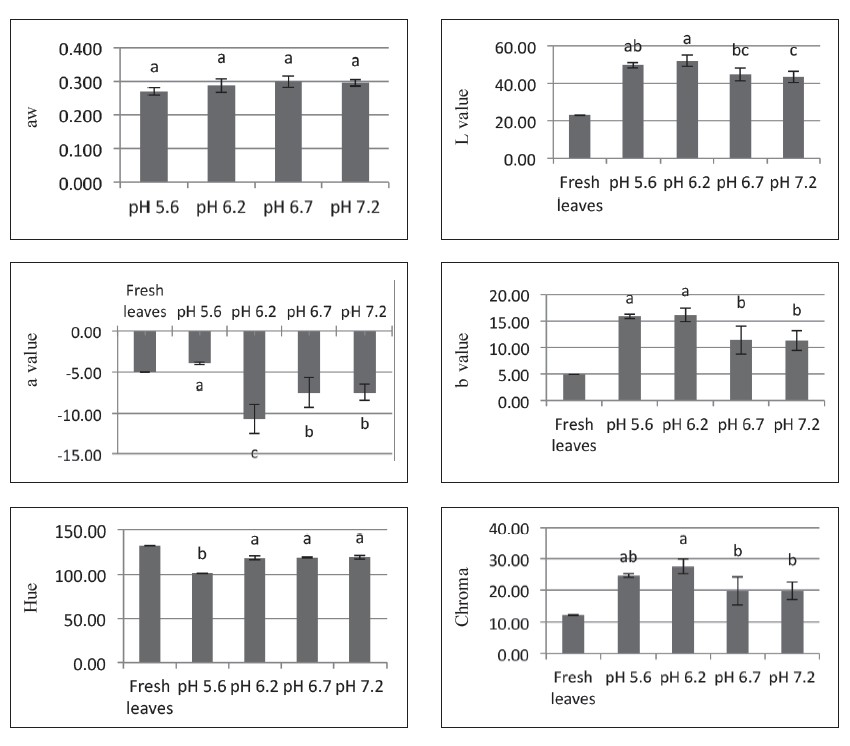
Figure 7. Physicochemical properties of foam-mat dried pandan powder as affected by pH.
Note: Results are means of triplicate determinations. Error bars are standard deviations. Different letters (a, b, c) differ significantly (P < 0.05) for the same treatment.

Figure 8. SEM image of foam-mat, hot-air dried, pandan powder (50x).
Conversely, the viscosity sharply decreased with an increasing NaCl concentration up to 5%, in combination with a 15%-maltodextrin concentration. NaCl is a strong electrolyte, and it may lower the gel point of Methocel™, because the electrolytes have a greater affinity for water and dehydrate the cellulosic polymer (Liu et al., 2008). The decrease of viscosity of 5% NaCl in the presence of the optimum concentration of maltodextrin (15%) in this system (Figure 2) was presumably due to the salting out effect of Methocel™, in which maltodextrin and NaCl might interact with water, expelling it from the structural MethocelTM gel network. Hence, the viscosity decreased, resulting in breaking down the gel structure of Methocel™ into small fractions. In this experiment, the small fractional gel structure of Methocel™ in the pandan foam solution was clearly observed visually. Liu et al. (2008) and Joshi (2011) reported that NaCl interacted with the gelling properties of HPMC; NaCl decreased the gelation temperature of HPMC, when determined using a micro-differential scanning calorimeter, confirming the salting-out phenomenon. A negatively charged chloride ion affects a water molecule’s structure via interactions between the hydroxyl groups and the water molecules and inter-chain hydrogen bonding of different HPMC chains. These are involved in the sol-gel transition of HPMC. The strength of inter-chain hydrogen bonding depended on the temperature and salt concentration. In addition, the strength of the water cages can constrain the hydrophobic association of HPMC chains (Liu et al., 2008). Ford (2014) explained that HPMC molecules fully swell in an aqueous solution, and polymer-polymer interactions are largely restricted to entanglements at low temperatures. When increasing the temperature, the viscosity of the HPMC solution initially decreased, then increased sharply as a sequence of the generation of a three-dimensional insoluble gel network via hydrophobic associations. The thermal gelation temperature of HPMC is influenced by the degree of substitution and existence of ionic molecules, which may ‘salt out’ the polymer.
The Pearson correlation test (Table 2) was used to explain the relationship between the foaming properties of the pandan foam solution. The correlation between foam overrun and viscosity of the foam solution was significantly negative (p<0.01). This result was caused by a high concentration (5%) of NaCl with maltodextrin at 15% led to low viscosity (Figure 2). Visual observation confirmed the rupture of the gel structure of the foam solution in this experiment. The foam density showed a significant correlation with foam drainage volume (r = 0.81) and foam overrun (r = -0.89). From the correlation coefficient, the foam density and foam drainage volume decreased, while foam overrun increased. Previous studies on the interaction between Methocel™ and NaCl on foaming properties are limited to a single report on egg white protein, in which Raikos et al. (2007) found that NaCl enhanced the foaming ability and overrun of egg white proteins, similar to our findings. As Raikos explained, NaCl counter ions may decrease the electrostatic repulsion of the layer charge on the protein molecule, which increases the adsorption between the air-water interfaces. Xu et al. (2009) also reported that the surface tension of sodium dodecyl sulfate (SDS), an anionic surfactant, reduced as the NaCl concentration increased above 0.25%. Adding NaCl at an SDS concentration lower than the critical micelle concentration resulted in decreased air bubble size. The authors suggested that adding NaCl promoted foam generation and its stability by providing an appropriate structure for the adsorption monolayer and interfacial film.
The foamed pandan extracts using Methocel™ (1 and 1.5%) as a foaming agent with maltodextrin (15%) and NaCl (5%) as stabilizing agents showed the lowest foam drainage volume. This observation indicated that it was the most stable foam (Figure 5). The stability of the foam of the aqueous pandan leaf extract may relate to the layer ions of NaCl counter ions interacting with the Methocel™ gel fraction after salting out. This stability was observed when concentrations of maltodextrin and NaCl were increased up to 15% and 5%, respectively. Hoey et al. (2016) reported that the thermodynamic incompatibility between HPMC and potato maltodextrin (dextrose equivalent less than 3) assisted the hydrophobic association of HPMC at low temperatures to form a gel. In addition, HPMC can assist self-association of maltodextrin.
In this experiment, adjusting the pH to a range of 6.2-7.2 kept the chlorophyll of the pandan extract from denaturing during drying at 70°C for 6 hrs. The greenness (a* value) and hue value of foam-mat dried pandan color increased when the pH increased (Figure 6). The total chlorophyll content, chlorophyll a, and chlorophyll b of foam-mat dried pandan powder, after adjusting the pH to 6.2-7.2, was higher than the control (without pH adjustment) and was in the range of 780- 930, 500-610, and 250-340 mg/L, respectively. Ryan-Stoneham and Tong (2000) and Koca et al. (2006) showed that the degradation of chlorophyll to pheophytin was acid catalyzed and caused the color change from bright green color to an olive-brown color, which could be visually detected. Ping et al. (2014) reported the total chlorophyll content, chlorophyll a, and chlorophyll b of pandan leaves was affected by water quality and was in the range of 1,000-1,600, 650-1,100, and 300-400 mg/kg fresh weight, respectively. Ryan-Stoneham and Tong (2000) reported that the chlorophyll content in peas was more stable at higher pH when blanching them in hot water.
The porous and small flake-like structure of foam-mat dried pandan powder revealed by SEM image (Figure 8) demonstrated its high hygroscopic. Franco et al. (2016) reported that foam-mat dried yacon juice powder has a porous and irregular structure, similar to freeze-dried mango juice powder.
CONCLUSION
Foaming properties of Methocel™ K4M were significantly affected by the concentrations of maltodextrin and NaCl. Using 1, 1.5% Methocel™, 15% maltodextrin and 5% NaCl exhibited good foaming properties. The foam-mat dried pandan powder could be formulated for specific end-uses by adjusting foaming agents, foam stabilizer, and pH, showing potential as an additive in food products.
ACKNOWLEDGMENTS
The authors thank the Thailand Research Fund and Commission on Higher Education for their financial support via a TRF-CHE Research Grant for New Scholar, and also Assoc. Prof. Dr. Phuriwat Leesawat, Department of Pharmaceutical Science, Faculty of Pharmacy, Chiang Mai University, for his advice about the hydrocolloid principle.
REFERENCES
AOAC. 2000. Official Methods of Analysis, 17th ed., Official Methods of Analysis of the Association of Official Analytical Chemists, Inc., Washington, D.C.
Apintanapong, M., and A. Noomhorm. 2003. The use of spray drying to microencapsulate 2-acetyl-1-pyrroline, a major flavour component of aromatic rice. International Journal of Food Science and Technology. 38: 95-102. doi: 10.1046/j.1365-2621.2003.00649.x
Bureiko,A., A. Trybala, N. Kovalchuk, and V. Starov. 2014. Current applications of foams formed from mixed surfactant-polymer solutions. Advances in Colloid and Interface Science. 222: 670-677. doi: 10.1016/cis.2014.10.001
Caliskan,G., and S. N. Dirim. 2013. The effects of the different drying conditions and the amounts of maltodextrin addition during spray drying of sumac extract. Food and Bioproducts Processing. 91: 539-548. doi: 10.1016/j.fbp.2013.06.004
Chaves, M.A., I.M.A. Barreto, R.C. Reis, and D.M. Kadam. 2013. Physicochemical and sensory properties of purple Brazilian cherry (Eugenia uniflora, L.) foams. International Journal of Food Science and Technology. 48: 1688-1697.
Dow Chemical Company. 2002. Methocel cellulose ethers: Technical handbook. Form No. 192-01062-0902 AMS. USA.
Ercelebi, E.A., and E. Ibanoglu. 2009. Effects of ionic strength on the foaming properties of whey protein isolate and egg white in the presence of polysaccharides. Journal of Food Processing and Preservation. 33: 513-526. doi: 10.1111/j.1745-4549.2008.00302.x
Ford, J.L. 2014. Design and evaluation of hydroxypropyl methylcellulose matrix tablets for oral controlled release: A historical perspective. In P. Timmins, S.R. Pygall, C.D., Melia (eds.), Hydrophilic matrix tablets for oral controlled release, AAPS Advances in the Pharmaceutical Sciences Series 16, American Association of Pharmaceutical Scientists. doi: 10.1007/978-1-4939-1519-4_2
Franco, T.S., C.A. Perussello, L.N. Ellendersen, and M.L. Masson. 2016. Effects of foam mat drying on physicochemical and microstructural properties of yacon juice powder. LWT - Food Science and Technology. 66, 503-513.
Hoey, A., J.T. Ryan, S.M. Fitzsimons, and E.R. Morris. 2016. Segregative interactions in single-phase mixtures of gelling (potato) maltodextrin with other hydrocolloids. In P.A., Williams and G.O., Philips (eds.). The proceeding of the 18th gums and stabilisers for the food industry: Hydrocolloid functionality for affordable and sustainable global food solutions. Royal Society of Chemistry.
Joshi, S.C. 2011. Sol-gel behavior of hydroxypropyl methylcellulose (hpmc) in ionic media including drug release. Materials, 4, 1861-1905. doi: 10.3390/ma4101861.
Koca, N., F. Karadeniz, and H.S. Burdurlu. 2006. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chemistry. 100: 609-615. doi: 10.1016/j.foodchem.2005.09.079
Liu, S.Q., S.C. Joshi, and Y.C. Lam. 2008. Effects of salts in the Hofmeister series and solvent isotopes on the gelation mechanisms for hydroxypropyl-methylcellulose hydrogels. Journal of Applied Polymer Science. 109(1): 363-372. doi: 10.1002/app.28079
McGuire, R.G. 1992. Reporting of objective color measurements. Horticultural Science. 27: 1254-1255.
Phillips, L.G., Z. Haque, and J.E. Kinsella. 1987. A method for the measurement of foamformation and stability. Journal of Food Science. 52(4): 1074-1077. doi: 10.1111/j.1365-2621.1987.tb14279.x
Ping, H., K. Prakash, and O. Bee-Lian. 2014. Remediation of nutrient-rich waters using the terrestrial plant, Pandanus amaryllifolius Roxb. Journal of Environmental Science. 26: 404-414. doi: 10.1016/S1001-0742(13)60426-X
Raharitsifa, N., D.B. Genovese, and C. Ratti. 2006. Characterization of apple juice foams for foam-mat drying prepared with egg white protein and methylcellulose. Journal of Food Science. 71(3):142-151.
Raikos, V., L. Campbell, and S.R. Euston. 2007. Effects of sucrose and sodium chloride on foaming properties of egg white proteins. Food Research International.
40: 347-355. doi: 10.1016/j.foodres.2006.10.008
Ratanasiriwat, P., W. Worawattanamateekul, and W.Klaypradit. 2013. Properties of encapsulated wasabi flavour and its application in canned food. International
Journal of Food Science and Technology. 48: 749-757. doi: 10.1111/ijfs.12023
Ryan-Stoneham, T., and C.H.Tong. 2000. Degradation kinetics of chlorophyll in peas as a function of pH. Journal of Food Science. 65(8): 1296-1302. doi: 10.1111/j.1365-2621.2000.tb10600.x
Schwenzfeier, A., F. Lech, P.A. Wierenga, M.H.M. Eppink, and H. Gruppen. 2013. Foam properties of algae soluble protein isolate: Effect of pH and ionic strength. Food Hydrocolloids. 33: 111-117.
Subramanian, R., K. Muthukumarappan, and S. Gunasekaran. 2003. Effect of methocel as a water binder on the linear viscoelastic properties of mozzarella cheese during early stages of maturation. Journal of Texture Studies. 34: 361-380. doi: 10.1111/j.1745-4603.2003.tb01069.x
Takeiti, C.Y., T.G. Kieckbusch, and F.P. Collares-Queiroz. 2010. Morphological and physicochemical characterization of commercial maltodextrins with different degrees of dextrose-equivalent. International Journal of Food Properties. 13: 411-425. doi: 10.1080/10942910802181024
Xu, Q., M. Nakajima, S. Ichikawa, N. Nakamura, P. Roy, H. Okadome, and T. Shiina. 2009. Effects of surfactant and electrolyte concentrations on bubble formation and stabilization. Journal of Colloid and Interface Science. 332: 208-214. doi: 10.1016/j.jcis.2008.12.044
Yahya, F., T. Lu, R.C.D. Santos, P.J. Fryer, and S. Bakalis. 2010. Supercritical carbon dioxide and solvent extraction of 2-acetyl-1-pyrroline from pandan leaf: The effect of pre-treatment. Journal of Supercritical Fluids. 55: 200-207. doi: 10.1016/j.supflu.2010.05.027
Panida Rattanapitigorn1, Masahiro Ogawa2 and Nithiya Rattanapanone1,3*
1 Division of Food Science and Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
2 Department of Applied Biological Science, Faculty of Agriculture, Kagawa University, Kagawa 761-0795, Japan
3 Postharvest Technology Research Institute, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: agfsi001@gmail.com
Total Article Views

