
Effect of Temperature on Brilliant Green Adsorption by Shrimp Shell: Equilibrium and Kinetics
Suchada Sawasdee and Prachart Watcharabundit*Published Date : 2019-08-23
DOI : 10.12982/cmujns.2016.0017
Journal Issues : Number3 ,September - December 2016
ABSTRACT
As the direct discharge of dying wastewater into the environment has adverse effects, there is a growing interest in using low-cost adsorbents or waste materials to adsorb dyes. In this study, the effect of temperature on the equilibrium and kinetic adsorption of brilliant green dye by shrimp shell (Macrobrachium rosenbergii) was studied using a batch process. The factors affecting the adsorption process, including contact time, initial dye concentration, and temperature, were investigated. The equilibrium data were analyzed by Langmuir, Freundlich, Temkin, and Dubinin-Raduskevich isotherm models. The Langmuir isotherm model fit the best, and the maximum adsorption capacity values were 8.13, 9.35, and 10.6 mg/g at 20, 30, and 40°C, respectively. The adsorption kinetic data corresponded to the pseudo-second order model at all temperatures. Thermodynamic parameters, such as ΔG, ΔH, and ΔS were calculated. Negative values of ΔG indicated that the overall adsorption was spontaneous. The characterization of surface adsorbent by FTIR confirmed that the shrimp shell can adsorb brilliant green dye and the proposed adsorption mechanisms were hydrogen bonding and n−π interaction. Experimental results showed that the adsorption capacity increased with temperature and the shrimp shell was an effective adsorbent for removing brilliant green dye.
Keywords: Temperature, Adsorption, Kinetic, Brilliant green, Shrimp shell
INTRODUCTION
Dyes can be classified as anionic, cationic, non−ionic, or zwitterionic. They are widely used in various industries, such as textile, paper, leather, rubber, plastics, and dyestuffs. The effluents containing dyes can cause drastic damage to the environment. In addition, most dye molecules are stable under various conditions of light, heat, and chemicals, and thus difficult to degrade (Han et al., 2012).Brilliant green (BG) is a cationic dye that is an odorless yellow-green to green powder used for various purposes, including as a biological stain and dermatological agent, in veterinary medicine, and as an additive to poultry feed to inhibit the propagation of mold, intestinal parasites, and fungus. It is also extensively used in textile dying and paper printing (Nandi et al., 2009; Mane and Babu, 2011). Brilliant green is an irritant, causing skin and eye burns, nausea, vomiting, diarrhea, and abdominal pain; it is classified as very toxic, with a probable lethal dose of 50-500 mg/kg in humans (Tavlieva et al., 2013).
Treatment of effluent containing synthetic dyestuffs is very difficult. While many techniques are used for dye removal, adsorption is an efficient option for removing dyes from water and wastewater (Daneshvar et al., 2014). Activated carbon is the most popular and widely used adsorbent for dye effluent. It is effective for removing organic matter because of its high adsorption capacity, large surface area, and microporous structure, but its use is usually limited due to high cost (Low et al., 2011) and activated carbons are difficult to regenerate. Using waste materials to treat dye effluent is the most popular option, because of their economic and eco-friendly traits, ready availability, and low cost (Chowdhury et al., 2011). Shrimp shell waste is composed of mainly calcium carbonate and chitin, along with some proteins. The functional groups of shrimp shell are hydroxyl (OH) and amine (NH), forming negatively charged adsorption sites that can be exchanged for cationic dye. Several reports used shell waste biosorbent to treat wastewater, such as biosorption of As by crab shell (Rahman et al., 2012), Acid Blue 25 by shrimp shell (Daneshvar et al., 2014), and Ag+ by crab shell (Jeon, 2014).
This study investigated the effect of temperature on the equilibrium and kinetics of brilliant green adsorption by shrimp shell as the biosorbent. The experiments were performed using a batch adsorption process using different contact times, initial dye concentrations, and temperatures (20-40°C). Equilibrium adsorption data were analyzed using Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherm models. The adsorption dynamic data were analyzed using pseudo-first order, pseudo-second order, and intraparticle diffusion kinetic models. We also determined the thermodynamic parameters of ΔG, ΔH, and ΔS. The results obtained from this study have been applied to batch design for the removal of brilliant green dye using shrimp shells.
MATERIALS AND METHODS
Preparation of adsorbent
The river shrimp shell (Macrobrachium rosenbergii) waste used as adsorbent in the present investigation was obtained from a restaurant in Lopburi City, Thailand. The collected shrimp shell was washed with tap water several times to remove all the dirt particles and dried in a hot air oven. The adsorbent was sieved to sizes of 50 to 100 mesh and stored in a desiccator until used.
Characterization of adsorbent
The surface functional group of the shrimp shell used in this study was analysed by FTIR (Perkin Elmer, model two) and its spectra were recorded from 4000- 50 cm-1.
Adsorbate
Brilliant green (C.I. 420140, molecular formula C27H34N2SO4, molecular weight = 482.65 g/mol; Merck, Germany) was used as the adsorbate in this study. The stock solution was prepared by dissolving 500 mg of brilliant green in one liter of distilled water. The working solutions (25, 50, 75, 100, 150, 200, 250, and 300 mg/L) were obtained by diluting with distilled water.
Batch adsorption
Adsorption experiments were performed using 50 ml of brilliant green dye solution (natural pH = 4.94 without any further adjustment) in 250 ml Erlenmeyer flasks; 1.0 g of adsorbent was added to each flask. Various adsorption conditions –contact time (1, 2, 3, 4, 5, 10, 15, 30, 45, 60, 90, 120, and 180 min) and initial dye concentrations (25, 50, 75, 100, 150, 200, 250, and 300 mg/L) – were investigated under an isothermal shaker (20-40°C) at an agitation speed of 250 rpm. Then, the suspended matter in each sample was filtered and supernatant was measured for dye concentration by a double beam UV-Vis spectrophotometer (Analytik Jena, Specord 210 plus) at 625 nm. The adsorption capacity (qt) was calculated as follows:
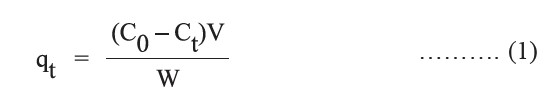
where C0 (mg/L) is initial concentration of adsorbent, Ct (mg/L) is the concentration at time t, qt (mg/g) is the amount adsorbed at time t, V(L) is the volume of the solution, and W(g) is the mass of adsorbent.
Adsorption isotherm
Four equilibrium isotherm models – Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich (D-R) – were used to describe experimental adsorption data. The Langmuir isotherm model is based on the assumption of monolayer coverage on a structurally homogeneous adsorbent, where all the adsorption sites are identical and energetically equivalent. The Langmuir isotherm in a linear form is represented as follows:
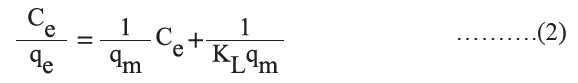
where Ce (mg/l) is the equilibrium concentration, qe (mg/g) is the amount adsorbed at equilibrium, KL is the Langmuir constant, and qmax (mg/g) is the maximum adsorption capacity.
The essential characteristics of a Langmuir isotherm can be expressed in terms of a dimensionless separation factor or equilibrium parameter (RL), which is defined by

The Freundlich isotherm model is an empirical expression that encompasses the heterogeneity of the surface and the exponential distribution of sites and energies. The Freundlich isotherm in a linear form is represented as follows:
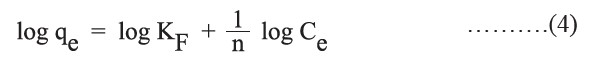
where KF (l/g) is the adsorption capacity and 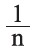 is the adsorption intensity.
is the adsorption intensity.
The Temkin isotherm assumes that the heat of adsorption of all molecules in a layer decreases linearly with the surface coverage of the adsorbent due to sorbate-adsorbate interactions. The Temkin isotherm in a linear form is represented as follows:
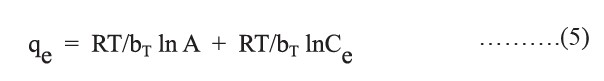
where RT/bT is B, A is Temkin isotherm constants (L/g) corresponding to the maximum binding energy, bT is Temkin constant related to heat of sorption (J/mol), R is the gas constant (8.314 J/mol.K), and T is the absolute temperature (K).
The Dubinin-Radushkevich (D-R) isotherm is used to determine whether the adsorption is chemical or physical in nature, and it is used to calculate sorption energy (Vadivelan and Vasanth, 2005; Mahmoud, 2015). The Dubinin-Radushkevich isotherm in a linear form is represented as follows:
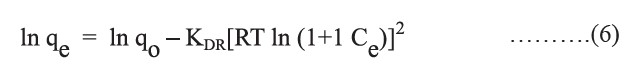
By plotting ln qe versus [RT ln (1+1 Ce)]2, a straight line is obtained, where KDR (mol2/kJ2) is calculated from the slope and the adsorption capacity [qo (mg/g)] is calculated from the intercept. The constant KDR represents the mean adsorption energy (E, kJ/mol) of the adsorbate; the equation is as follows:

If the value of E = 8-16 kJ/mol, then the adsorption process flows by chemical ion-exchange; if E < 8 kJ/mol, the adsorption process is physical in nature; and if the value > 16 kJ/mol, the adsorption process is chemisorption in nature (Youssef et al., 2008).
Adsorption kinetics
The kinetic models were applied mainly by pseudo-first order, pseudo-second order, and intraparticle diffusion.
The pseudo first-order kinetic in a linear form is written as follows:
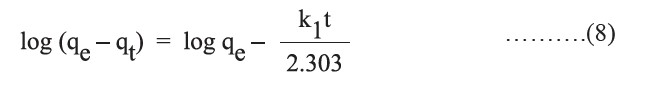
where k1 (min−1) is the rate constant of pseudo first-order adsorption, qe (mg/g) is the amount adsorbed at equilibrium, and qt (mg/g) is the amount adsorbed at time t (min). Values of k1 and qe can be calculated from the slope and intercept of the plot of log (qe −qt) versus t.
The pseudo second-order kinetic in a linear form is written as follows:

where k2 (g.mg−1.min−1) is the rate constant of pseudo second-order adsorption. Values of k2 and qe can be calculated from the intercept and slope of the plot 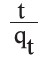 of versus t.
of versus t.
The intraparticle diffusion model shows that the adsorption process occurs in three steps, as follows (Nethaji et al., 2013; Mahmoud, 2015):
• Mass transfer across the external boundary layer film of liquid surrounding the outside of the particle.
• Adsorption at a site on the surface (internal or external), with the energy depending on the binding process (physical or chemical); this step is often assumed
to be extremely rapid.
• Diffusion of the adsorbate molecules to an adsorption site either by a pore diffusion process through the liquid filled pores or by a solid surface diffusion mechanism.
The intraparticle diffusion model is expressed as:

where qt is the amount of adsorbate retained at time t, Kid is the intraparticle diffusion rate constant (mg/g min1/2), and C is the intercept. The plot of qt versus the square root of time (t1/2) and Kid can be calculated from the slope of the straight line.
Thermodynamics of adsorption
The Gibbs’ free energy change indicates the degree of spontaneity of a process and the higher negative value indicates a more energetically favorable adsorption. The Gibbs’ free energy change (ΔG), enthalpy change (ΔH), and entropy change (ΔS) in the adsorption process can be expressed as follows:

where K = qe/Ce is the equilibrium constant, R is the gas constant (8.314 J/ mol K), and T is the absolute temperature (K).
RESULTS
To investigate the surface characteristics of shrimp shell, FTIR spectra were performed in the range of 450 to 4000 cm–1. Figure 1(a) and Figure 1(b) represent the FTIR spectra of shrimp shell before and after adsorption, respectively. In Figure 1(a), the wide, strong, and broad band at around 3271 cm-1 is due to O−H and N−H stretching. The peak at 2929 cm-1 is due to N–H and O–H stretching. The strong band observed at 1626 cm-1 corresponds to N−H stretching. The peak around 1538 cm–1 is due to N−H stretching of the amino groups. The band at 1068 cm-1 is assigned to C−N bending. The strong band at 1230.7 cm–1 is due to C=O stretching. The bands at 951.75 cm–1 and 896.96 cm–1 are due to the β−D−glucose unit of polysaccharide (Kamala et al., 2013). The peak positions of the major bands in the spectrum of shrimp shell saturated with brilliant green (Figure 1(b)) are shifted from the same positions as in the spectrum of raw shrimp shell. In addition, there are new and stronger peaks at 1152.7 cm–1 due to C=O stretching, 1308.8 cm-1 due to C−N vibration, and 1008.2 cm–1 due to C−O stretching.
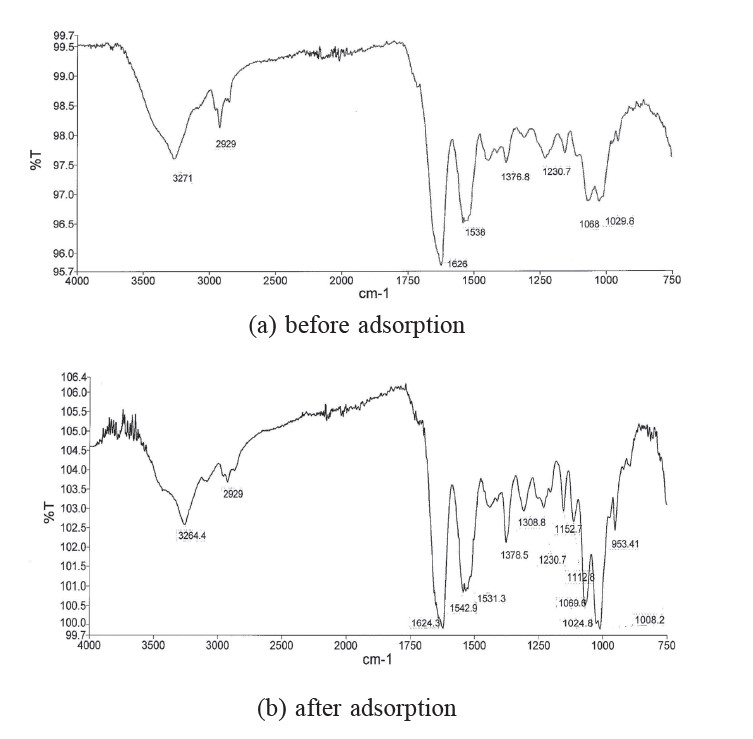
Figure 1. FTIR spectra of shrimp shell before and after adsorption of brilliant green.
Effect of contact time at different temperatures
The plots of the adsorption capacity of brilliant green dye onto shrimp shell versus the adsorption time at three temperatures (20-40°C) for 50 mg/L of brilliant green dye solution are shown in Figure 2. The results showed that the adsorption capacity increased with contact time and temperature. The adsorption capacity values at 5 min were 2.02, 2.14, and 2.22 mg/L at 20, 30, and 40°C, respectively. As shown in Figure 2, the rapid adsorption was observed during the first 5 min and, thereafter, the adsorption became less efficient and reached the equilibrium at 30 min for all temperatures. The equilibrium time was independent of temperature. Based on the results, 30 min was taken as the equilibrium time of adsorption for further studies.
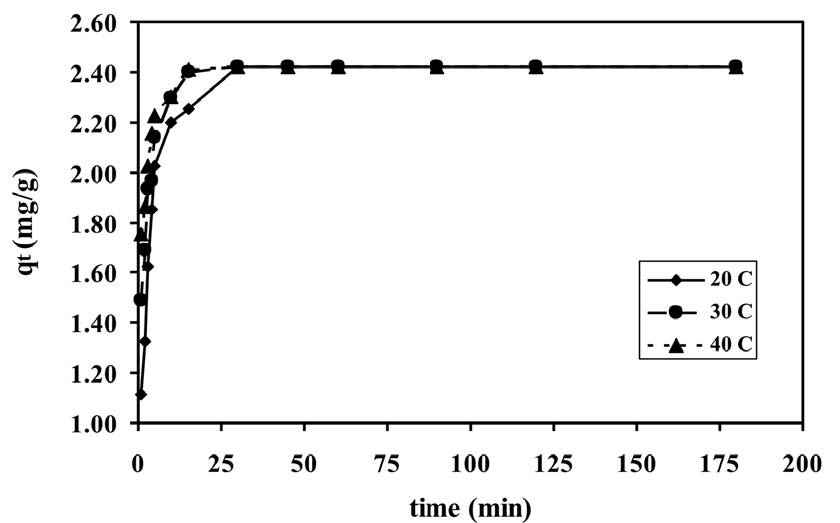
Figure 2. Effect of contact time for brilliant green adsorption onto shrimp shell at different temperatures (natural pH, shrimp shell dose = 1.0 g, brilliant green concentration = 50 mg/L).
Effect of initial dye concentration at different temperatures
The adsorption capacity versus the initial brilliant green dye concentration of 25-300 mg/L at three temperatures (20-40°C) are shown in Figure 3. The results showed that the adsorption capacity increased with the increase in the initial dye concentration and temperature. At a brilliant green dye concentration of 25-50 mg/L, the difference in equilibrium capacity was negligible at the three temperatures. However, at the concentration over 75 mg/L, the result showed a significant increase in adsorption as the temperature increased. As shown in Figure 3, at the fixed temperature, the equilibrium adsorption capacity increased with the increase in brilliant green dye concentration.
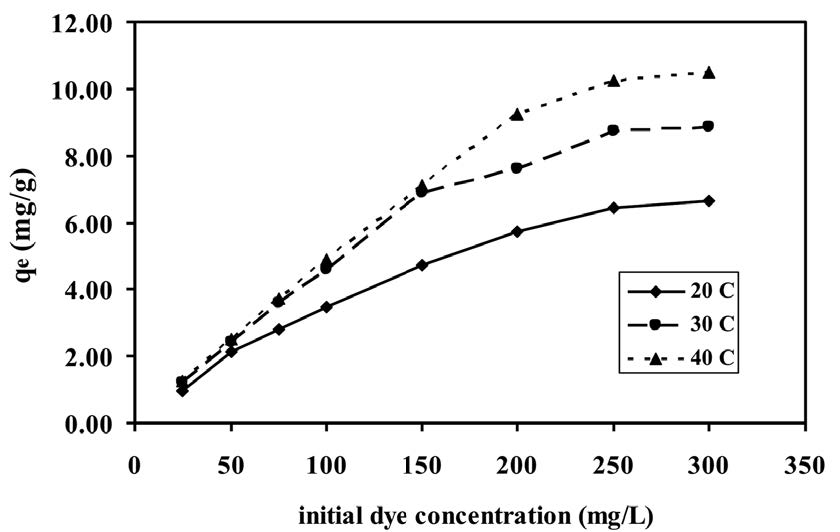
Figure 3. Effect of initial brilliant green dye concentration at different temperatures (natural pH, shrimp shell dose = 1.0 g, contact time = 30 min).
Adsorption isotherm
Equilibrium adsorption isotherm is very useful for the analysis and design of adsorption systems. The equilibrium adsorption experiments were carried out by batch process for different initial dye concentrations (25 to 300 mg/L) at 20, 30, and 40°C. The adsorption experimental data were plotted for linear isotherm models (not shown) and isotherm constants were calculated as shown in Table 1.
Table 1. Isotherm parameters of brilliant green dye adsorption by shrimp shell at different temperatures (natural pH, shrimp shell dose = 1.0 g, contact time = 30 min).
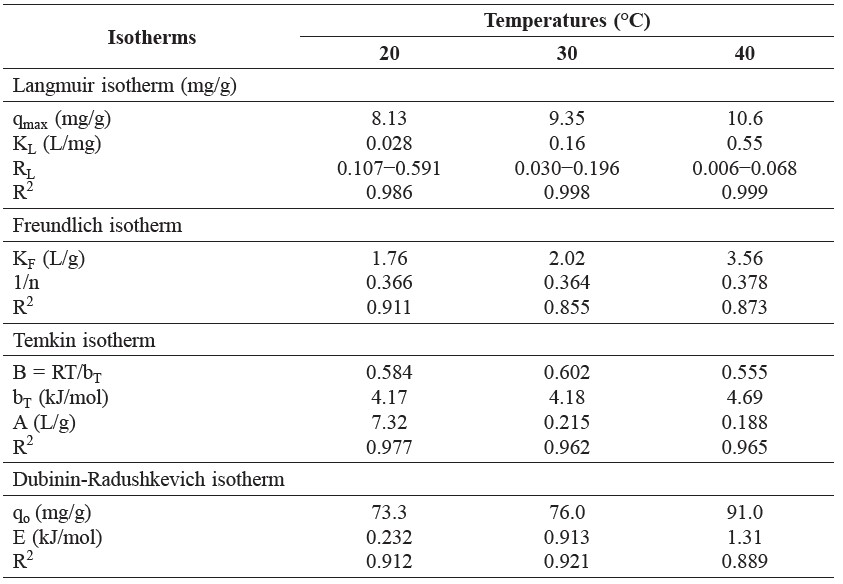
The equilibrium adsorption data were analyzed by Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich isotherm models. The correlation coefficients (R2) showed that the obtained equilibrium data for three temperatures were better fitted to the Langmuir isotherm model than Freundlich, Temkin, and Dubinin-Radushkevich isotherm models (Table 1). The maximum adsorption values calculated from the Langmuir isotherm model were 8.13, 9.35, and 10.6 mg/g at 20, 30, and 40°C, respectively. The essential characteristic of the dimensionless separation factor (RL) values calculated as Equation (3) were in the range of 0.107-0.591, 0.030-0.196, and 0.006-0.068 at 20, 30, and 40°C, respectively. The values of 0 < RL < 1 indicated that the adsorption of brilliant green was favorable at all temperatures.
The Freundlich isotherm model is an empirical model that describes adsorption with heterogeneous surface. As shown in Table 1, the value for 1/n below 1 indicated a normal Langmuir isotherm model and reflected the favorable adsorption (Kaur et al., 2013).
In Table 1, the Temkin constant, AT, can be considered a sort of adsorption potential for dye adsorption and the calculated values were 7.32, 0.215, and 0.188 L/g at 20, 30, and 40°C, respectively. The bT values, related to the heat of adsorption, were 4.17, 4.18, and 4.69 kJ/mol, suggesting a strong interaction between dye molecules and adsorbent (Daneshvar et al., 2014).
The Dubinin-Radushkevich isotherm is an empirical model that predicts the nature of adsorption processes as physical or chemical by calculating sorption energy. As shown in Table 1, the adsorption capacity values were 73.3, 76.0, and 91.0 mg/g at 20, 30 and 40°C, respectively. The adsorption capacity increases with higher temperature. The mean adsorption energy (E) was 0.232, 0.913, and 1.31 kJ/mol at 20, 30, and 40°C, respectively.
Adsorption kinetics
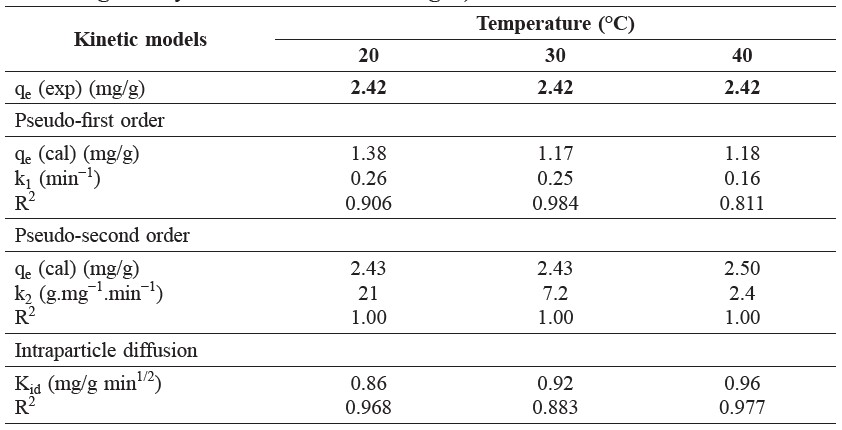
From the results of effect of contact time to adsorption, the kinetic data of the initial dye concentration of 50 mg/L were analyzed by plots of pseudo-first kinetic, pseudo-second kinetic, and intraparticle diffusion models (not shown) at different temperatures (20, 30, and 40°C); the parameters were calculated from the slopes and intercepts as shown in Table 2. The correlation coefficients of straight lines of kinetic adsorption models were 0.906, 0.984, and 0.811 for pseudo-first order and 1.00, 1.00, and 1.00 for pseudo-second kinetic model, respectively. Therefore, the kinetic data were better fitted with the pseudo-second kinetic model at different temperatures. As seen from Table 2, the values of Kid for the intraparticle diffusion were 0.86, 0.92, and 0.96 mg/g min1/2 at 20, 30, and 40°C, respectively.
Table 2. Kinetic parameters of brilliant green dye adsorption by shrimp shell at different temperatures (natural pH, shrimp shell dose = 1.0 g, brilliant green dye concentration = 50 mg/L).
Thermodynamic parameters
The equilibrium adsorption of brilliant green by shrimp shell was studied at different temperatures (20-40°C). The equilibrium adsorption constant (K=qe/Ce) obtained at each temperature was used for determining free energy of adsorption (ΔG). At the initial brilliant green dye concentration of 50 mg/L, the ΔG values were 3.10, -1.00, and -6.82 kJ/mol at 20, 30, and 40°C, respectively. The plot (not shown) between ΔG against temperature (T, °K) was a straight line. Then, ΔH and ΔS were determined from the intercept and slope of the straight line, respectively. ΔG values became more negative with increasing temperature, supporting that adsorption was favored with increasing temperature. The negative value of ΔG indicated the feasibility of the process and the spontaneous nature of the adsorption. ΔH and ΔS were 123 and 0.410 kJ/mol, respectively.
DISCUSSION
This research studied the effect of temperature on adsorption of brilliant green dye by river shrimp shell (Macrobrachium rosenbergii). FTIR spectra were obtained before and after adsorption of dye onto shrimp shell. The spectra peaks of shrimp shell saturated with brilliant green dye were shifted. The presence of hydroxyl and amine groups in the shrimp shell surface can attract the brilliant green dye molecule. The proposed adsorption mechanisms are hydrogen bonding (- - - -) and n−π interaction (…….), as shown in Figure 4 (Chowdhury et al., 2011; Heibati et al., 2014).
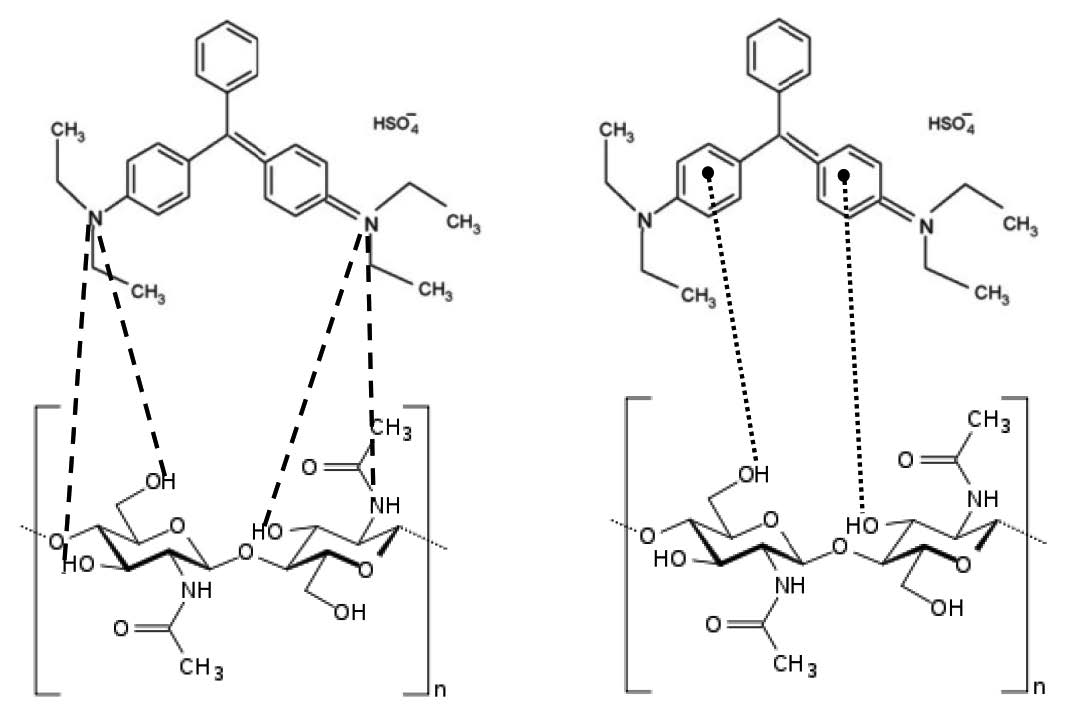
Figure 4. The interaction of hydrogen bonding and n−π between brilliant green dye and shrimp shell.
The experimental results showed that the adsorption capacity of brilliant green dye onto shrimp shell increased rapidly with contact time, reaching equilibrium at about 30 min. Errais et al. (2012) made a similar observation in a study of anionic RR120 dye adsorption onto raw clay. The rapid adsorption involves a surface reaction process (Alkan et al., 2007) and might be due to physical adsorption or ion exchange on the biosorbent surface (Daneshvar et al., 2014). Initially, the rate of adsorption was rapid due to the adsorption of dye molecules onto the exterior surface. Then, the dye molecules moved into pores, a relatively slow process. The initial rapid rates of adsorption may be due to a large number of vacant sites at the beginning, with fewer sites available as treatment time increased (Al-Khatib et al., 2012).
The equilibrium adsorption results showed that the adsorption capacity increased with increasing concentration of brilliant green dye. This may due to the increase in the driving force of the concentration gradient for mass transfer with the increase in initial dye concentration (Auta and Hameed, 2012).
The equilibrium adsorption capacity increased with the increase of temperature, because of the increased interaction between the brilliant green dye molecules and the shrimp shell surface at high temperature. At concentrations of 25-300 mg/L, the adsorption capacity of brilliant green dye onto shrimp shell increased with increasing temperature, from 1.15-7.87 mg/g at 20°C to 1.20-8.88 at 30°C to 1.20-9.49 at 40°C, indicating that the dye adsorption on the adsorbent was favored at higher temperature. An increase in temperature results in an increased mobility of the adsorbate and a decrease in the retarding forces acting on the diffusion adsorbates (Lakshmi et al., 2009).
The equilibrium adsorption data were analyzed by four isotherm models – Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich. The Langmuir isotherm model fit best, with better correlation coefficients (R2) than the Freundlich, Temkin, and Dubinin-Radushkevich isotherm model at all temperatures. The Langmuir isotherm indicated monolayer coverage onto a homogeneous surface adsorbent; the maximum monolayer adsorption capacity values (qmax) were 8.13, 9.35, and 10.6 mg/g at 20, 30, and 40°C, respectively. The Langmuir isotherm model fitted the experimental data very well. This confirmed the homogeneous distribution of active sites on the shrimp shell surface. Similar observations have been reported in the literature for monolayer adsorption of brilliant green dye following the Langmuir isotherm model (Nandi et al., 2009; Kismir and Aroguz, 2011; Dahri et al., 2015). In addition, the effect of Langmuir isotherm shape was investigated by a dimensionless separation factor (RL); this could be used to predict whether the adsorption system favorable or unfavorable. In this investigation, the RL values were less than one, indicating that the overall adsorption was favorable. The Freundlich isotherm showed that 1/n values were in the range 0.364-0.367. With these values < 1, the Freundlich isotherm further demonstrated favorable adsorption of brilliant green onto shrimp shell. The Temkin isotherm showed that bT values were 4.17, 4.18, and 4.69 kJ/mol at 20, 30 and 40°C, respectively. Therefore, bT increased with increasing temperature, indicating endothermic adsorption. The magnitude of E calculated by the Dubinin-Radushkevich isotherm equation were 0.232, 0.913, and 1.31 kJ/mol, E < 8 kJ/mol, indicating that the adsorption was of a physical nature (Youssef et al., 2008).
The experimental data of adsorption capacity varying with contact time were also applied to three kinetic models – pseudo-first order, pseudo-second order, and intraparticle diffusion models. Adsorption kinetic studies were performed for initial brilliant green dye concentrations (25-300 mg/L) at three temperatures. The correlation coefficients (R2) for the pseudo-second order kinetic model were higher than the pseudo-first order, indicated that the dye adsorption process followed the pseudo-second order model. The pseudo-second order model is based on the assumption that the reaction is chemisorption involving valence force or exchange of electron between adsorbent and adsorbate (Ibrahim et al., 2006). Similar observations have been reported in the literature for monolayer adsorption of dye following a pseudo-second order model (Nandi et al., 2009; Kobiraj et al., 2012).
In this study, the intraparticle diffusion rate constant (Kid) increased with increasing temperature. The observed increase in Kid values can be explained by the growing effect of the driving force, which resulted in reducing the diffusion of dye species in the boundary layer and enhancing the diffusion in the solid (Mall et al., 2006; Gamal et al., 2011). The linear portion of the plot (qt against t1/2) did not pass through the origin. This deviation from the origin may be due to the variation of mass transfer in the initial and final stages of adsorption. Furthermore, such deviation from origin indicates that the pore diffusion is not the only rate-controlling step (Mohanty et al., 2005).
Adsorption thermodynamic values of Gibbs’ free energy (ΔG) at an initial brilliant green dye concentration of 50 mg/L were 3.10, -1.00 and -6.83 kJ/mol at 20, 30, and 40°C, respectively. The negative value of ΔG indicated that the overall adsorption was favorable and spontaneous in nature. The Gibbs’ free energy changes for physical and chemical adsorption are usually in the range of 0.0 to 20 kJ/mol and 80 to 400 kJ/mol, respectively. Therefore, the adsorption of brilliant green onto shrimp shell can be considered as physisorption. Similar observations have been reported for the adsorption of brilliant green dye by rice husk ash (Mane et al., 2007) and Saklikent mud (Kismir and Aroguz, 2011). In this study, ΔH and ΔS were positive. The positive value of ΔH reflected that the adsorption was endothermic in nature, while the positive value of ΔS showed that an increase in randomness occurred at the interface during the adsorption process.
CONCLUSION
In this study, the investigation of brilliant green dye adsorption onto shrimp shell was carried out at 20, 30, and 40°C in batch process. The initial rate was rapid and reached equilibrium at 30 min. The Langmuir, Freundlich, Temkin, and Dubinin-Radushkevic isotherm models were used to fit the equilibrium data and estimated parameters; the overall data best fit the Langmuir isotherm model. The maximum adsorption capacity values were 8.13, 9.35, and 10.6 mg/g at 20, 30, and 40°C, respectively. The RL values showed that shrimp shell was favorable for the adsorption of brilliant green. Adsorption kinetics can be predicted by the pseudo-second order model. However, the adsorption may be controlled by external mass transfer followed by intraparticle diffusion mass transfer. Thermodynamic parameters showed that brilliant green adsorption onto shrimp shell was spontaneous, endothermic, and physical in nature.
ACKNOWLEDGEMENTS
The authors would like to thank Thepsatri Rajabhat University for financial support.
REFERENCES
Al−Khatib, L., F. Fraige, M. Al−Hwaiti, and O. Al−Khashman. 2012. Adsorption from aqueous solution onto natural and acid activated bentonite. American Journal of Environmental Science. 8(5): 510-522. doi: 10.3844/ajessp.2012.510.522
Alkan, M., O. Demirbas, and M. Dogan. 2007. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous and Mesoporous Materials. 101: 388-396.
Auta, M., and B.H. Hameed. 2012. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chemical Engineering Journal. 198: 219-227. doi: 10.1016/j.cej.2012.05.075
Chitin. 2016. Online http//Wikipedia/chitin.
Chowdhury, S., R. Mishra, P. Saha, and P. Kushwaha. 2011. Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination. 265: 159-168. doi: 10.1016/j.desal.2010.07.047
Dahri, M.K., L.B.L Lim, and C.C. Mei. 2015. Cempedak durian as a potential biosorbent for the removal of brilliant green dye from aqueous solution: equilibrium, thermodynamics and kinetics studies. Environ Monit Assess. 187: 546. doi: 10.1007/s10661-015-4768-z
Daneshvar, E., M.S. Sohrabi, M. Kousha, A. Bhatnagar, B. Aliakbarian, A. Converti, and A−C. Norrström. 2014. Shrimp shell as an efficient bioadsorbent for Acid Blue 25 dye removal from aqueous solution. Journal of the Taiwan Institute of Chemical Engineers. 45(6): 2926-2934. doi: 10.1016/j.jtice.2014.09.019
Errais, E., J. Duplay, M. Elhabiri, M. Khodja, R. Ocampo, R. Batenweck−Guyot, and F. Darragi. 2012. Anionic RR120 dye adsorption onto raw clay: Surface properties and adsorption mechanism. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 403: 69-78. doi:10.1016/j.colsurfa.2012.03.057
Gamal, A. M., S. A. Abo Farha, H. B. Sallam, G. E. A. Mahmoud, and L. F. M. Ismail. 2011. Kinetic Study and Equilibrium Isotherm Analysis of Reactive Dyes Adsorption onto Cotton Fiber. Nature and Science. 8(11): 96-110.
Han, X., X. Niu, and X. Ma. 2012. Adsorption characteristics of methylene blue on poplar leaf in batch mode: Equilibrium, kinetics and thermodynamics. Korean Journal of Chemical Engineering. 29(4): 494-502. doi: 10.1007/s11814-011-0211-5
Heibati, B., S. Rodriquez-Couto, A. Amrane, M. Rafatullah, A. Hawari, and M.A. Al-Ghouti. 2014. Uptake of Reactive Black 5 by pumice and walnut activated carbon: Chemistry and adsorption mechanisms. Journal of Industrial and Engineering Chemistry. 20: 2939-2947. doi: 10.1016/j.jiec.2013.10.063
Ibrahim, S.C., M. Hanafiah, and M.Z.A. Yahya. 2006. Removal of Cadmium from Aqueous Solutions by using Low−Cost by Adsorption onto Sugarcane Bagasse. American−Eurasian J.Agric & Environ. Sci. 1(3): 179-184.
Joen, C. 2014. Adsorption characteristics of waste crab shells for silverions in industrial wastewater. Korean Journal of Chemical Engineering. 31(3): 446-451. doi: 10.1007/s11814-013-0234-1
Lakshmi, U.R., V.C. Srivastava, I.D. Mall, and D.H. Lataye. 2009. Rice husk ash as an effective adsorbent: Evaluation of adsorptive characteristics for Indigo Carmine Dye. Journal of Environmental Management. 90: 710-720. doi: 10.1016/j.jenvman.2008.01.002
Low, L.W., T.T. Teng, F. M. Alkarkhi, Abbas, A. Ahmad, and N. Morad. 2011. Optimization of the Adsorption Conditions for the Decolorization and COD Reduction of Methylene Blue Aqueous Solution using Low−Cost Adsorbent. Water Air Soil Pollute. 214: 185-195. doi: 10.1007/s11270-010-0414-0
Kamala, K., P. Sivaperumal, and R. Rajaram. 2013. Extraction and Characterization of Water Soluble Chitosan from Parapeneopsis Stylifera Shrimp Shell Waste and Its Antibacterial Activity. International Journal of Scientific and Research Publications. 3(4): 1-8.
Kaur, S., S. Rani, and R.K. Mahajan. 2013. Adsorption Kinetics for the Removal of Hazardous Dye Congo Red by Biowaste Materials as Adsorbents. Journal of Chemistry: Online. ID 628582 pp.1–12. Online.http://www.hindawi.com/journals/jchem/2013/628528/ doi: 10.1155/2013/628582
Kismir, Y., and A. Z. Aroguz. 2011. Adsorption characteristics of the hazardous dye Brilliant Green on Saklikent mud. Chemical Engineering Journal. 172: 199-206. doi: 10.1016/j.cej.2011.05.090
Kobiraj, R., N. Gupta, A. K. Kushwaha, and M. C. Chattopadhyaya. 2012. Determination of equilibrium, kinetic and thermodynamic parameters for the adsorption of Brilliant Green dye from aqueous solutions onto eggshell powder. Indian Journal of Chemical Technology (IJCT). 19(1): 26-31.
Mahmoud, M.A. 2015. Kinetics and thermodynamics of aluminum oxide nanopowder as adsorbent for Fe (III) from aqueous solution. Beni−Suef University Journal of Basic and Applied Sciences. 4: 142-149. doi: 10.1016/j.bjbas.2015.05.008
Mall, I. D., V.C. Srivastava, and N.K. Agarwal. 2006. Removal of Orange–G and Methyl Violet dyes by adsorption onto bagasse fly ash−kinetic study and equilibrium isotherm analyses. Dyes and Pigments. 69: 210-223. doi: 10.1016/j.dyepig.2005.03.013
Mane, V. S., and P. V. V. Babu. 2011. Studies on the adsorption of Brilliant Green dye from aqueous solution onto low–cost NaOH treated saw dust. Desalination. 273(2-3): 321-329. doi: 10.1016/j.desal.2011.01.049
Mane, V.S., I. D. Mall, and V. C. Srivastava. 2007. Kinetic and equilibrium isotherm studies for the adsorptive removal of brilliant green dye from aqueous solution by rice husk ash. Journal of Environmental Management. 84: 390-400. doi: 10.1016/j.jenvman.2006.06.024
Mohanty, K., D. Das, and M.N. Biswas. 2005. Adsorption of phenol from aqueous solutions using activated carbons prepared from Tectona grandis sawdust by ZnCl2 activation. Chemical Engineering Journal. 115: 121-131. doi: 10.1016/j.cej.2005.09.016
Nandi, B. K., A. Goswami, and M. K. Purkait. 2009. Adsorption characteristics of brilliant green dye on kaolin. Journal of Hazardous Materials. 161: 387-395. doi: 10.1016/j.jhazmat.2008.03.110
Nethaji, S., A. Sivasamy, and A.B. Mandal. 2013. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int J Environ Sci Technol. 10: 231-242. doi: 10.1007/s13762-012-0112-0
Rahman, M. A., M. M. Hossain, A. Samad, and A. M. Shafiqul Alam. 2012. Removal of Arsenic from Ground Water with Shrimp Shell. Dhaka University Journal of Science. 60(2): 175-180.
Tavlieva, M. P., S. D. Genieva, V. G. Georgieva, and L. T. Vlaev. 2013. Kinetic study of brilliant green adsorption from aqueous solution onto white rice husk ash. Journal of Colloid and Interface Science. 409: 112-122. doi: 10.1016/j.jcis.2013.07.052
Vadivelan, V., and K. K. Vasanth. 2005. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interface Sci. 286: 90-100. doi: 10.1016/j.jcis.2005.01.007
Youssef, A. M., S. El−Khouly, and T.H. El−Nabarawy. 2008. Removal of Pb(2+) and Cd(II) from aqueous solution using activated carbons from pecan shells. Carbon Lett. 9: 8-13.
Suchada Sawasdee1 and Prachart Watcharabundit1*
1 Department of Chemistry, Faculty of Science and Technology, Thepsatri Rajabhat University, Lopburi 15000 Thailand
*Corresponding author. E-mail: prachartw@hotmail.com
Total Article Views

