
Production of Collagen Hydrolysate with Antioxidant Activity from Pharaoh Cuttlefish Skin
Sophawan Bousopha, Sitthipong Nalinanon* and Chodsana SriketPublished Date : 2019-08-23
DOI : 10.12982/cmujns.2016.00012
Journal Issues : Number2 ,May - August 2016
ABSTRACT
The aim of this study was to produce collagen hydrolysate with antioxidant activity. Pepsin soluble collagen (PSC) isolated from the skin of Pharaoh cuttlefish (Sepia pharaonis) was used as a substrate for hydrolysis at 25°C and pH 7.5 by using collagenase from Clostridium histolyticum. The result showed that rapid hydrolysis was observed within the first 90 min with a degree of hydrolysis (DH) approaching 35%. Thereafter, a slower rate of hydrolysis was generally observed. An increase in DH was generally observed with increasing enzyme concentration. When log10 (collagenase concentration) versus DH (%) was plotted, a linear relationship was found with a coefficient of determination (R2) of 0.9863. Electrophoretic study of collagen hydrolysates with 10% DH, 20% DH, and 30% DH revealed that their major peptides had molecular weights (Mr) ranging from 6.5 to 10.3 kDa; and 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2,2-diphenyl-1-picryl hydrazyl (DPPH) radical scavenging activities. The ferric reducing activity power (FRAP) of collagen hydrolysates with 20% DH and 30% DH were similar (p>0.05). Chelating activity of collagen hydrolysates with 20% DH was negligible and for 10% DH and 30% DH it was not detectable. Given these findings, Pharaoh cuttlefish skin, a byproduct of cuttlefish processing, is a promising source material for production of collagen hydrolysate with antioxidant activities.
Keywords: Pharaoh cuttlefish, Collagen hydrolysate, Collagenase, Antioxidant activity
INTRODUCTION
The cephalopod (fresh, chilled, frozen, dried, or otherwise processed), including cuttlefish, is one of Thailand’s most important fishery exports, with the majority sent to Japan, the European Union, and the United States. In 2013, cuttlefish exports were 13,648 metric tons at a value of THB 2,375 million (Fisheries Foreign Affair Division, 2015). When processing cuttlefish, byproducts such as viscera, skin, and pen (or bones) are generated in large quantity; these are mostly sold for producing animal feed and fertilizer with low value.
Cuttlefish skin contains a large amount of collagen. Nagai et al. (2001) reported that collagen from the skin of cuttlefish (Sepia lycidas) could be isolated with a total yield of 37% (dry-weight basis) by using acid and subsequent extraction. Collagen can be converted to gelatin by heat treatment and used as a substrate for hydrolysate production. Alemán et al. (2011) extracted gelatin from inner and outer giant squid (Dosidicus gigas), then hydrolyzed it using commercial proteases – the ABTS radical scavenging capacity of gelatin increased approximately 3-fold with protamex, neutrase, and NS37005 hydrolysates; 7- to 10-fold with trypsin, savinase, esperase, and alcalase hydrolysates. However, no information on the production and properties of collagen hydrolysate from Pharaoh cuttlefish (Sepia pharaonis) skin has been reported. The objective of this study was to produce collagen hydrolysates with antioxidant activity from Pharaoh cuttlefish skin.
MATERIAL AND METHODS
Chemicals
All chemicals were of analytical grade. Pepsin from porcine gastric mucosa (EC 3.4.23.1) (750 units/mg dry matter), collagenase from Clostridium histolyticum (EC 3.4.24.3) (≥ 125 CDU/mg solid), 2,4,6-trinitrobenzenesulfonic acid solution (TNBS), L-leucine, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt hydrate (Ferrozine), (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and Iron (II) chloride tetrahydrate were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Coomassie brilliant blue G-250 and N,N,N′,N′-tetramethyl ethylene diamine (TEMED) were procured from Bio-Rad Laboratories (Hercules, CA, USA). Iron (III) chloride anhydrous and Folin-Ciocalteu’s reagent were obtained from CARLO ERBA Reagents S.A.S. (Val-de-Reuil, France).
Pharaoh cuttlefish skin sample preparation
Pharaoh cuttlefish (Sepia pharaonis) skin was obtained dockside in Samut Sakhon, Thailand, kept in ice with sample/ice ratio of 1:2 (w/w), and transported to the Faculty of Agro-Industry, King Mongkut’s Institute of Technology Ladkrabang within 2 h. Upon arrival, the skin was washed with cold tap water (0-4°C), drained, and cut into small pieces (0.5 x 0.5 cm2) using a scissor. Then, the samples were kept at -20°C in polyethylene bags and used within 2 months.
Preparation of pepsin soluble collagen
Pepsin soluble collagen (PSC) from the skin of Pharaoh cuttlefish was prepared according to the method of Nalinanon et al. (2007), with some modifications. All procedures were performed at 4°C. Briefly, the cuttlefish skin was pretreated by stirring in 0.1 M NaOH at a ratio of 1:10 (w/v) to remove non-collagen proteins and washed with water until a neutral or faintly basic pH was obtained. Then the fat was removed by extracting with 10% (v/v) butyl alcohol at a ratio of 1:10 (w/v) and the skin was washed with cold water. To isolate pepsin soluble collagen, the defatted skin was then soaked in 0.5 M acetic acid at a ratio of 1:30 (w/v) in the presence of pepsin (10 g/100 g of defatted skin) for 72 h with gentle stirring, using an overhead stirrer model RW 20 digital (IKA-Werke GmbH & Co. KG, Staufen, Germany). Thereafter, the mixture was centrifuged at 15,000xg for 1 h using a refrigerated centrifuge model Legend Mach 1.6R (Thermo Fisher Scientific, PA, USA). The supernatant was salted-out by addition of NaCl to a final concentration of 2.6 M in 0.05 M Tris-HCl (pH 7.5) and allowed to stand for 1 h to inactivate pepsin. The resultant precipitate was obtained by centrifugation at 15,000xg for 1 h. The pellet was dissolved in 0.5 M acetic acid and dialyzed against 0.1 M acetic acid in a dialysis bag (MW cut-off 14 kDa). Subsequently, the solution was dialyzed with distilled water until a neutral pH of washed water was obtained. The resulting dialysate was lyophilized using a SCANVAC FD8 Coolsafe freeze-dryer (LaboGene™, Lynge, Denmark); this is what is referred to in this paper as ‘pepsin soluble collagen (PSC)’.
Effects of collagenase on PSC hydrolysis
Preparation of PSC substrate. The PSC (3 g) was suspended in 97 mL of distilled water (pH 7.5). The mixture was subjected to heat at 60°C for 15 min before filtering through filter paper (Whatman no.4). The filtrate was used as a substrate for collagenase hydrolysis to prepare collagen hydrolysate.
Effect of hydrolysis time. The PSC substrate (30 mg/mL) was subjected to enzymatic hydrolysis by collagenase from Clostridium histolyticum with an enzyme-substrate ratio of 1 unit/mg PSC. The collagenase was prepared by dissolving in buffer containing 50 mM Tricine with 10 mM CaCl2 and 400 mM NaCl at pH 7.5. The reaction was run at pH 7.5 and 25°C for 0, 15, 30, 45, 60, 75, 90, and 150 min. The α-amino content was determined by the 2,4,6-trinitrobenzene sulfonic acid (TNBS) method as described by Adler-Nissen (1979). The degree of hydrolysis (DH) was determined following the method of Benjakul and Morrissey (1997). A graph between time (min) and DH (%) was plotted to determine the pattern and optimum reaction time of collagenase on PSC hydrolysis.
Effect of collagenase concentration. Five levels of enzyme concentration (0.1, 0.2, 0.4, 0.6, and 1.2 unit/mg PSC) were used to hydrolyze PSC substrate (30 mg/mL) at 25°C for 90 min. The α-amino content and DH of the hydrolysates were determined as described previously. A linear relationship was plotted between the log10 (collagenase concentration) and DH (%).
Production of collagen hydrolysate
Collagen hydrolysates with different DHs were produced according to the methods of Kittiphattanabawon et al. (2012) and Li et al. (2007), with some modifications. The hydrolysis reaction was started by adding collagenase from Clostridium histolyticum to PSC substrate at the amount calculated from a linear relationship between log10 (collagenase concentration) and % DH to obtain 10% DH, 20% DH, and 30% DH. The reaction was carried out at 25°C at the optimum reaction time (90 min). The enzyme was inactivated by heating for 15 min at 95°C. The mixture was then centrifuged at 5,000xg at room temperature for 10 min. The supernatant was collected, lyophilized, kept in a polyethylene bag, and stored at -20°C.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed according to the method of Laemmli (1970) using a Dual Mini Slab Kit model AE-640 (ATTO, Tokyo, Japan). Protein sample (15 μg) was loaded onto a polyacrylamide gel made of 4% stacking gel and running gel (18% for hydrolysates and 7.5% for PSC). Gel was stained in 0.05% w/v Coomassie brilliant blue G-250 and destrained in 30% methanol and 10% acetic acid. Mr of protein bands was estimated by using protein standard (Bio-Rad Laboratories, Inc., Hercules, CA).
Determination of antioxidant activity
Antioxidant activities of the collagen hydrolysates (10% DH, 20% DH, and 30% DH) at a concentration of 2 mg/mL were determined with different assays.
ABTS radical scavenging activity. ABTS radical scavenging activity was determined as described by Binsan et al. (2008). A standard curve of Trolox ranging from 100 to 600 μM was prepared. The absorbance was measured at 734 nm using V-1200 MAPADA spectrophotometer (Shanghai Mapada Instruments Co., Ltd., Shanghai, China). The activity was expressed as μmol Trolox equivalents (TE)/g sample.
DPPH radical scavenging activity. DPPH radical scavenging activity was determined as described by Binsan et al. (2008). A standard curve was prepared using Trolox in the range of 0-50 μM. The absorbance was measured at 517 nm using a V-1200 MAPADA spectrophotometer. The activity was expressed as μmol Trolox equivalents (TE)/g sample.
Ferric reducing antioxidant power (FRAP). FRAP was determined according to the method of Benzie and Strain (1996). The standard curve was prepared using Trolox with concentration ranging from 0 to 500 μM. Absorbance values were measured at 593 nm using a V-1200 MAPADA spectrophotometer. The activity was expressed as μmol Trolox equivalents (TE)/g sample.
Chelating activity on ferrous ion. Ferrous ion chelating activity was measured by the method of Boyer and McCleary (1987). The absorbance was read at 562 nm using a V-1200 MAPADA spectrophotometer. Chelating activity was expressed as a percentage and calculated as follows:
Chelating activity (%) = [(B-A)/B] x100
where A was the absorbance at 562 nm of the sample and B was the absorbance at 562 nm of the blank. The blanks were run by replacing the sample with deionized water.
Statistical analysis
All experiments were performed in triplicate. The data were subjected to analysis of variance (ANOVA) using the SPSS® computer program (SPSS Statistical Software, Inc., Chicago, IL, USA). Duncan’s multiple range tests were used with a level of significance of p ≤ 0.05.
RESULTS
Effect of hydrolysis time on DH of collagen hydrolysates
The DH of collagen hydrolysate using collagenase increased as the reaction time increased (p ≤ 0.05) (Figure 1). DH increased rapidly in the first 90 min (reaching 35%), after which the rate slowed and leveled off. The relatively high initial rate indicated that the maximum cleavage of peptide occurred within 90 min of hydrolysis; this was chosen as the optimum reaction time for further experiments.
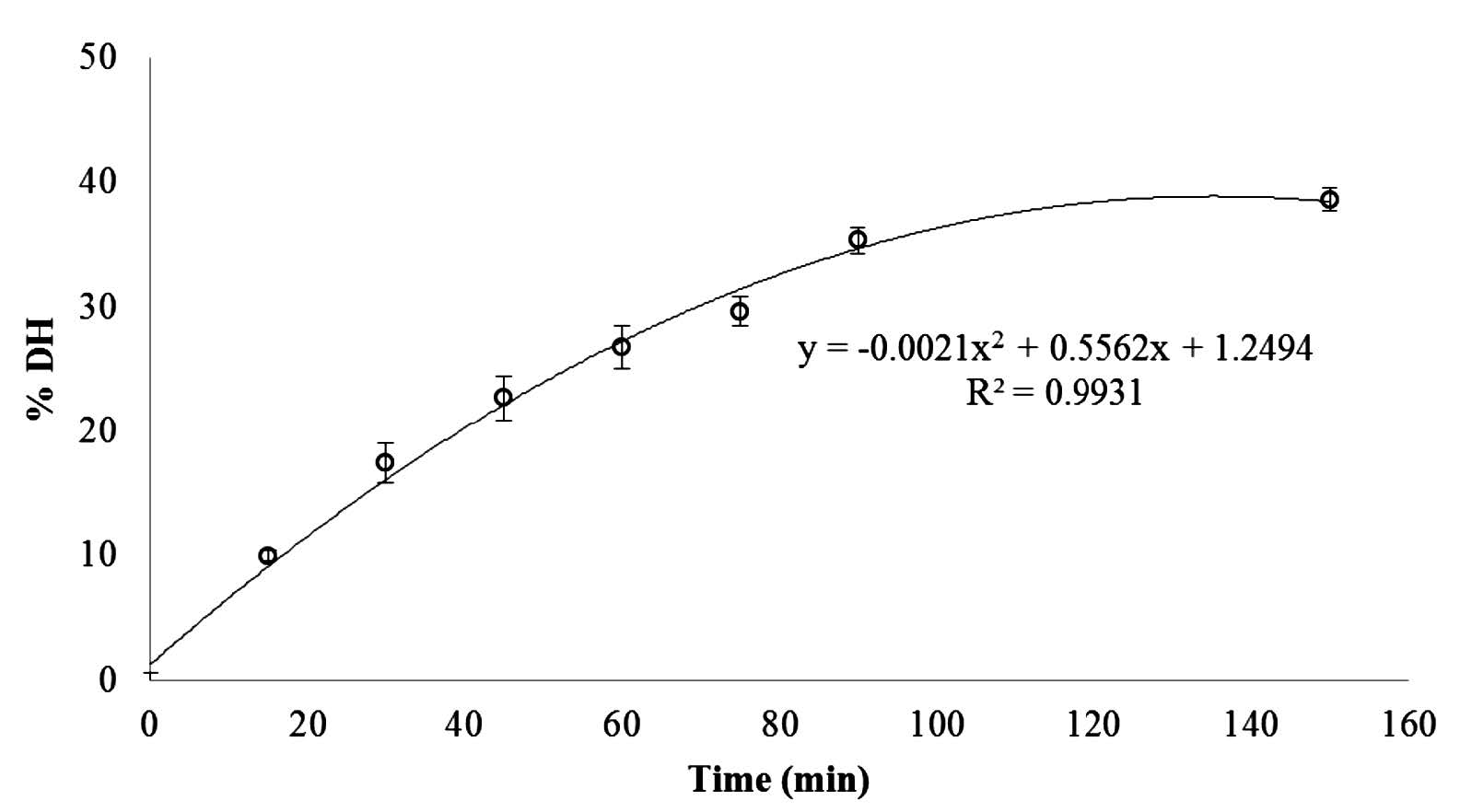
Figure 1. Degree of hydrolysis (DH) of pepsin soluble collagen (PSC) from the skin of Pharaoh cuttlefish (Sepia pharaonis) hydrolyzed by collagenase from Clostridium histolyticum (1 unit/mg PSC) for various times (0-150 min). The reaction was run at pH 7.5 and 25°C.
Effect of enzyme concentration on DH of collagen hydrolysates
PSC was hydrolyzed for 90 min with different concentrations of collagenase from Clostridium histolyticum. An increase in DH was generally observed with increasing enzyme concentration (Figure 2). When log10 (collagenase concentration) versus DH (%) was plotted, a linear relationship was found with a coefficient of determination (R2) of 0.9863. From this relationship, the exact concentrations of collagenase required to hydrolyze PSC to obtain the required 10% DH, 20% DH, and 30% DH under the same hydrolytic conditions were calculated – 159.39, 408.74, and 1048.21 unit/g collagen, respectively.
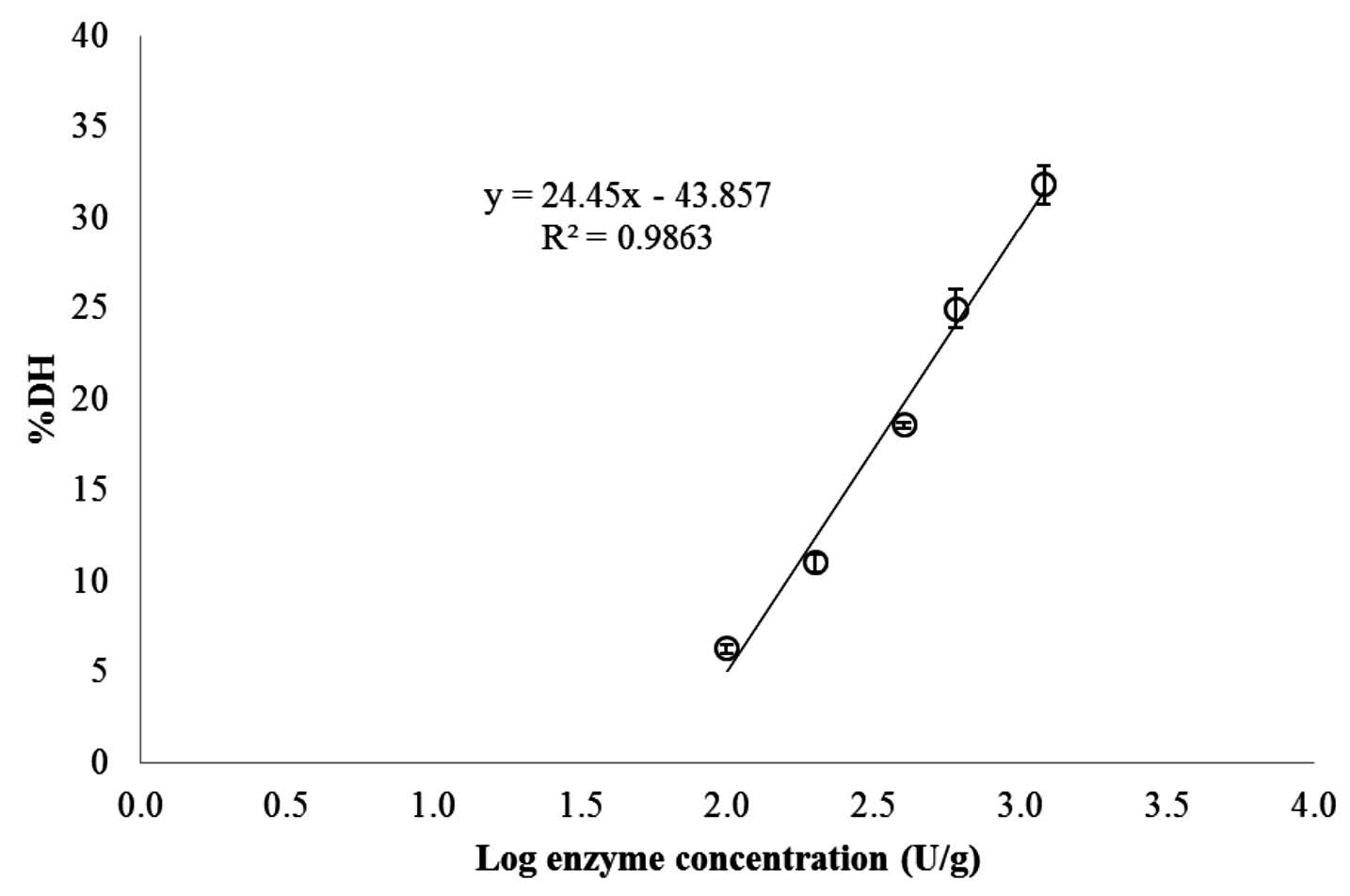
Figure 2. Relationship between degree of hydrolysis (DH) and log10 concentration of collagenase from Clostridium histolyticum for hydrolysis of pepsin soluble collagen (PSC) from the skin of Pharaoh cuttlefish (Sepia pharaonis). The reaction was run for 90 min at pH 7.5 and 25°C.
SDS-PAGE patterns
The molecular weights of collagen hydrolysates with different DHs were estimated using protein standard and observed by SDS-PAGE patterns (Figure 3). The results showed that the molecular weights of collagen hydrolysates ranged between 6.7-10.3 kDa for 10% DH, 6.7-9.1 kDa for 20% DH, and 6.5-8.4 kDa for 30% DH. In contrast, all major components of PSC – including γ, β, and α chains and high-MW cross-linked components (HMC) – were revealed, with MWs ranging from 110 kDa to over 220 kDa.
Antioxidant activities of collagen hydrolysates
Antioxidant activities as determined by ABTS, DPPH, FRAP, and Fe2+ chelating assays of collagen hydrolysates with different DHs are shown in Table 1. Collagen hydrolysate with 20% DH and 30% DH exhibited similar ABTS radical scavenging activity (242.2 and 255.7 μmol TE/g protein, respectively) (p > 0.05), and higher than that of collagen hydrolysate with 10% DH (173.5 μmol TE/g protein) (p ≤ 0.05). The collagen hydrolysates with 10% DH, 20% DH, and 30% DH did not differ significantly in their DPPH radical scavenging activity, ferric reducing antioxidant power (FRAP), or chelating activity on ferrous ion (Fe2+), the last of which was low to indictable.
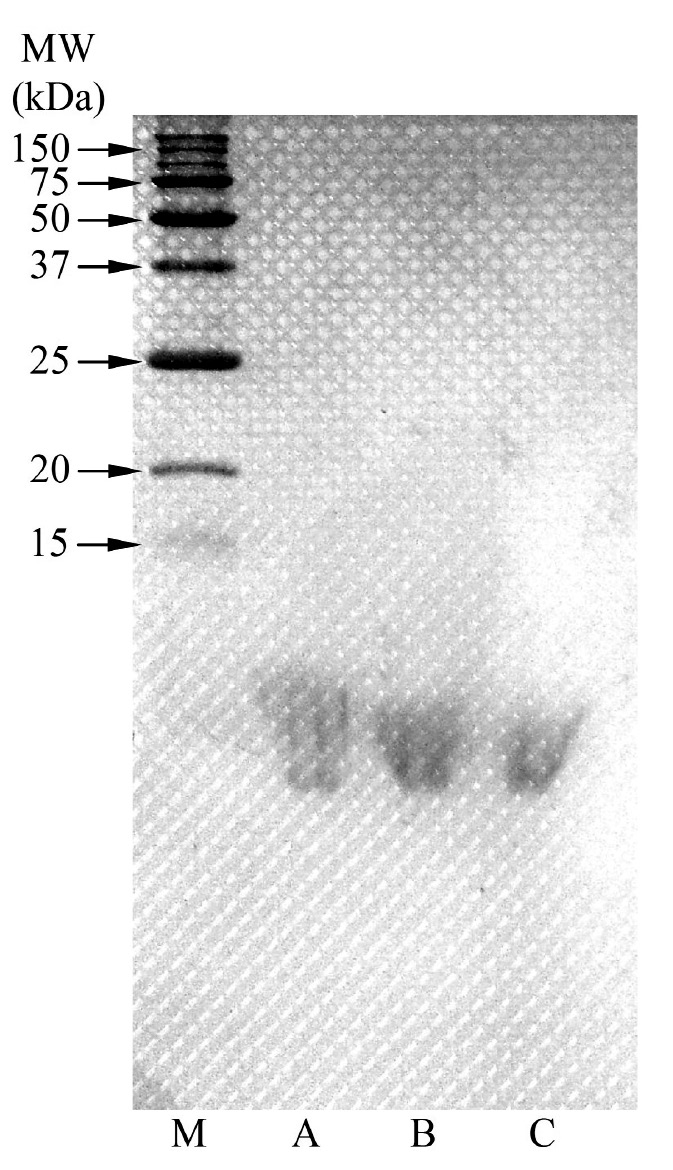
Figure 3. SDS-PAGE patterns of collagen hydrolysates (A: 10% DH, B: 20% DH, and C: 30% DH) and pepsin soluble collagen (PSC) from the skin of Pharaoh cuttlefish (Sepia pharaonis) under reducing conditions. HMC and M denote high-MW cross-linked components and molecular weight protein markers, respectively.
Table 1. Antioxidant activities of collagen hydrolysates from the skin of Pharaoh cuttlefish (Sepia pharaonis) prepared using collagenase from Clostridium histolyticum with different DHs.
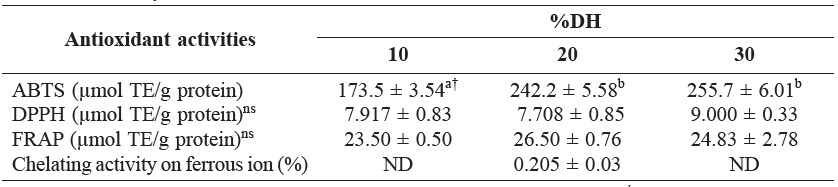
Note: Mean ± SD (n = 3). TE: Trolox equivalents. ND: not detectable. † Different letters in the same row indicate significant differences (p ≤ 0.05). ns = non-significant differences (p > 0.05).
DISCUSSION
DH of collagen hydrolysate increased rapidly during the first 90 min (reaching 35%), then slowed and leveled off (Figure 1). It possible that the presence of products or total cleavage of all susceptible peptide bonds by enzymes inhibited protein hydrolysis (Shahidi et al., 1995). The relatively high initial rate indicated that the maximum cleavage of peptide occurred within 90 min of hydrolysis. This accorded with Giménez et al. (2009b), who reported that DH of gelatin hydrolysates obtained from giant squid (Dosidicus gigas) skin gelatins by hydrolysis with alcalase increased with increasing hydrolysis time. In addition, Giménez et al. (2009a) reported that the hydrolysis of squid gelatins with alcalase resulted in ~50% DH after 3 h at 50°C. The hydrolysis rate was fast in the initial stage (15-20 min), and then gradually slowed until reaching a stationary phase when no apparent hydrolysis took place (Giménez et al., 2009a). Guerard et al. (2002) speculated that the decrease in the hydrolysis rate may be due to the formation of reaction products, the decrease in concentration of peptide bonds available for hydrolysis, enzyme inhibition, and enzyme deactivation. The DH profile with time was similar to those of protein hydrolysates from Atlantic salmon heads (Salmo salar) and yellow stripe trevally (Selaroides leptolepis) prepared by alcalase and alcalase-flavourzyme hydrolysis, respectively (Gbogouri et al., 2004; Klompong et al., 2007).
DH generally increased with increasing enzyme concentration (Figure 2). Diniz and Martin (1998) reported that the hydrolytic reaction depended on the availability of susceptible peptide bonds on which the enzyme attack was concentrated, as well as the physical structure of the protein molecules. When log10 (collagenase concentration) versus DH (%) was plotted, a linear relationship was found. This result agreed with Beak and Cadwallader (1995), Benjakul and Morrissery (1997), and Guerard et al. (2001), who reported the linear relationship between log10 (enzyme concentration) and DH for enzymatic hydrolysis of crayfish processing byproducts, Pacific whiting wastes, and yellowfin tuna wastes, respectively. In addition, Kristinsson and Rasco (2000) reported that many parameters of enzyme hydrolysis, such as substrate, enzyme-substrate ratio, temperature, and time, generally determined the DH of hydrolysate obtained.
The molecular weights and protein patterns of collagen hydrolysates with 10% DH, 20% DH, and 30% DH were estimated and observed on SDS-PAGE (Figure 3). None of the major components of PSC (HMC and the α, β, and γ chains) were observed in the collagen hydrolysates from Pharaoh cuttlefish skin treated with collagenase, suggesting the high efficiency of collagenase hydrolysis on collagen’s structure. Seifter and Harper (1971) reported that a collagenase is defined as an enzyme capable of causing hydrolytic scission of peptide bonds located in the characteristic poly-L-proline type of helical regions when the substrate is in the undenatured state. In addition, collagenase from Clostridium histolyticum (EC 3.4.24.3) specifically recognize the sequence -R-Pro-8-X-Gly-Pro-R-, where X is most often a neutral amino acid (SIGMA-ALDRICH®, 2015). Therefore, collagenase can be effectively used for the production of collagen hydrolysate from Pharaoh cuttlefish skin.
Antioxidant activities of collagen hydrolysates with different DHs tested by using different methods are shown in Table 1. Collagen hydrolysate exhibited high ABTS radical scavenging activity. Generally, hydrolysates contain peptides or proteins, which are hydrogen donors and can react with radicals to convert them to
more stable products, thereby terminating the radical chain reaction (Khantaphant and Benjakul, 2008). Nalinanon et al. (2011) found that hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna with 20% DH had higher scavenging activities against ABTS than 10% and 30% DH. ABTS assay is an excellent tool for determining the antioxidant activity of hydrogen-donating compounds (scavengers of aqueous phase radicals) and of chain-breaking antioxidants (scavenger of lipid peroxyl radicals) (Binsan et al., 2008). In addition, Nalinanon et al. (2011) postulated that antioxidative compounds with high ABTS radical-scavenging activity were most likely hydrophilic. DPPH radical scavenging activities ranging from 7.7-9.0 μmol TE/g protein were found in collagen hydrolysates from cuttlefish skin. This result suggested that the hydrolysates contained amino acids or peptides that were electron donors and could react with DPPH radicals to convert them to a more stable product. DPPH is a stable free radical in ethanol that shows a maximal absorbance at 517 nm. When DPPH encounters the proton-donating substance, the radical would be scavenged by changing color from purple to yellow and the absorbance is reduced (Shimada et al., 1992). The ferric reducing antioxidant power (FRAP) is a method for measuring antioxidant potential. Collagen hydrolysates from cuttlefish skin also exhibited reducing power (Table 1), suggesting that collagen hydrolysate from Pharaoh cuttlefish could also function as electron donor to the free radicals. Reducing power is a measurement that provides an estimation of a compound’s ability to reduce ferric iron (III) to ferrous iron (II), and is determined using a redox-linked colorimetric reaction (Alemán et al., 2011). Moure et al. (2006) reported that ferric reducing antioxidant power measures the reducing capability of the ferric ion and has been correlated to the radical scavenging capacity. Giménez et al. (2009a) reported that squid hydrolysates treated by alcalase showed approximately a two-fold higher ferric reducing ability than the corresponding gelatins. The hydrolysis of gelatin chains into peptides may give rise to the release of sequences with antioxidant properties and the exposition of hidden amino acid residues and side chains with antioxidant activity (Giménez et al., 2009a). From the result, it was suggested that collagen hydrolysate from Pharaoh cuttlefish could also function as electron donor to the free radicals. However, the results indicated that collagen hydrolysates had low potential on chelating ability towards iron. This result was in contrast to the studies of Nalinanon et al. (2011) and Klompong et al. (2007), who reported that the chelating activity on Fe2+ of protein hydrolysates improved as the degree of protein hydrolysis increased. Some antioxidants do not convert free radicals to more stable product, but slow the rate of oxidation by several different mechanisms. One of the most important mechanisms of action of secondary antioxidants is chelation of pro-oxidant metals (Huang et al., 2012). Fe2+ ion is the most powerful pro-oxidant among various species of metal ions (O’Brien, 1969). Fe2+ ion can catalyze the generation of reactive oxygen species, hydroxyl radical (OH•), by which the lipid peroxidation chain reaction is accelerated (Stohs and Bagchi, 1995).
CONCLUSION
Collagen hydrolysate with antioxidant activities was successfully prepared from pepsin soluble collagen (PSC) by hydrolysis using collagenase from Clostridium histolyticum. Collagen hydrolysate with 20% DH exhibited the highest ABTS radical scavenging activity and also showed antioxidant activities toward DPPH, FRAP, and chelating activity on ferrous ion. Thus, collagen hydrolysate from Pharaoh cuttlefish skin showed potential as an alternative bioactive compound.
ACKNOWLEGEMENTS
This research was supported by the Thailand Research Fund (TRF) and the Office of the Higher Education Commission (Project No. MRG5680116 to Sitthipong Nalinanon). The authors would like to express their appreciation to King Mongkut’s Institute of Technology Ladkrabang for its support.
REFERENCES
Adler-Nissen, J. 1979. Determiantion on the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. Journal of Agricultural and Food Chemistry. 27(2): 1256-1262.
Alemán, A., E. Pérez-Santín, S. Bordenave-Juchereau, I. Arnaudin, M.C. Gómez-Guillén, and P. Montero. 2011. Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Research International. 44(4): 1044-1051. doi: 10.1016/j.foodres.2011.03.010
Beak, H.H., and K.R. Cadwallader. 1995. Enzymatic hydrolysis of crayfish processing s. Journal of Food Science. 60(5): 929-935.
Benzie, I.F.F., and J.J. Strain. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 239(1): 70-76. doi: 10.1006/abio.1996.0292
Benjakul, S. and M.T. Morrissey. 1997. Protein hydrolysates from Pacific whiting solid wastes. Journal of Agricultural and Food Chemistry. 45(9): 3423-3430.
Binsan, W., S. Benjakul, W. Visessanguan, S. Roytrakul, M. Tanaka, and H. Kishimura. 2008. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chemistry. 106(1): 185-193. doi: 10.1016/j.foodchem.2007.05.065
Boyer, R.F., and C.J. McCleary. 1987. Superoxide ion as a primary reductant in ascorbate-mediated ferritin iron release. Free Radical Biology and Medicine. 3(6): 389-395.
Diniz, F.M., and A.M. Martin. 1998. Influence of process variables on the hydrolysis of shark muscle protein. Food Science and Technology International. 4: 91-98. Fisheries Foreign Affair Division. 2015. Data export fishery products. http://www.fisheries.go.th (12 October 2015).
Gbogouri, G.A., M. Linder, J. Fanni, and. M. Parmentier. 2004. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. Journal of Food Science. 69(8): C615-C622.
Giménez, B., A. Alemán, P. Montero, and M.C. Gómez-Guillén. 2009a. Antioxi-dant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chemistry. 114(3): 976-983. doi: 10.1016/j.foodchem.2008.10.050
Giménez, B., J. Gómez-Estaca, A. Alemán, M.C. Gómez-Guillén, and M.P. Montero. 2009b. Improvement of the antioxidant properties of squid skin gelatin films by the addition of hydrolysates from squid gelatin. Food Hydrocolloids.23(5): 1322-1327. doi: 10.1016/j.foodhyd.2008.09.010
Guérard, F., L. Dufossé, D. De La Broise, and A. Binet. 2001. Enzymatic hydrolysis of proteins from yellowfin tuna (Thunnus albacares) wastes using Alcalase. Journal of Molecular Catalysis B: Enzymatic. 11(4-6): 1051-1059. doi: 10.1016/S1381-1177(00)00031-X
Guerard, F., L. Guimas, and A. Binet. 2002. Production of tuna waste hydrolysates by a commercial neutral protease preparation. Journal of Molecular Catalysis
B: Enzymatic. 19-20: 489-498. doi: 10.1016/S1381-1177(02)00203-5
Huang, S.S., J.S. Deng, H.J. Chen, Y.H. Lin, and G.J. Huang. 2012. A novel trypsin inhibitor from sweet potato (Ipomoea batatas Lam.) leaves and its synthesized peptides with antioxidant activities in vitro. Botanical Studies. 53: 215-222.
Khantaphant, S., and S. Benjakul. 2008. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comparative Biochemistry and Physiology. 151(4): 410-419. doi: 10.1016/j.cbpb.2008.08.011.
Kittiphattanabawon, P., S. Benjakul, W. Visessanguan, and F. Shahidi. 2012. Gelatin hydrolysate from blacktip shark skin prepared using papaya latex enzyme: Antioxidant activity and its potential in model systems. Food Chemistry. 135(3): 1118-1126. doi: 10.1016/j.foodchem.2012.05.080
Klompong, V., S. Benjakul, D. Kantachote, and F. Shahidi. 2007. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chemistry. 102(4): 1317-1327. doi: 10.1016/j.foodchem.2006.07.016
Kristinsson, H.G., and B.A. Rasco. 2000. Fish protein hydrolysates: production, biochemical and functional properties. Food Science and Nutrition. 40(1): 43-81.
Laemmli, U.K. 1970. Cleavage of structure proteins during the assembly of head of bacteriophage T4. Nature. 227: 680-685.
Li, B., F. Chen, X. Wang, B. Ji, and Y. Wu. 2007. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chemistry. 102(4): 1135-1143. doi: 10.1016/j.foodchem.2006.07.002
Moure, A., H. Domínguez, and J.C. Parajó. 2006. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochemistry. 41(2): 447-456. doi: 10.1016/j.procbio.2005.07.014
Nagai, T., E. Yamashita, K. Taniguchi, N. Kanamori, and N. Suzuki. 2001. Isolation and characterisation of collagen from the outer skin waste material of cuttlefish (Sepia lycidas). Food Chemistry. 72(4): 425-429. doi: 10.1016/S0308-8146(00)00249-1
Nalinanon, S., S. Benjakul, W. Visessanguan, and H. Kishimura. 2007. Use of pepsin for collagen extraction from the skin of bigeye snapper (Priacanthus tayenus). Food Chemistry. 104(2): 593-601. doi: 10.1016/j.foodchem.2006.12.035
Nalinanon, S., S. Benjakul, H. Kishimura, and F. Shahidi. 2011. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chemistry. 124(4): 1354-1362. doi: 10.1016/j.foodchem.2010.07.089
O’Brien, P.J. 1969. Intracellular mechanisms for the decomposition of a lipid peroxide. I. Decomposition of a lipid peroxide by metal ions, heme compounds, and nucleophiles. Canadian Journal of Biochemistry. 47(5): 485-492.
Seifter, S., and E. Harper. 1971. p.649-697. The collagenases. In P.D. Boyer (ed). The enzyme. Academic Press, New York.
Shahidi, F., X. Han, and J. Synowiecki. 1995. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chemistry. 53(3): 285-293. doi: 10.1016/0308-8146(95)93934-J
Shimada, K., K. Fujikawa, K. Yahara, and T. Nakamura. 1992. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry. 40(6): 945-948.
SIGMA-ALDRICH®. 2015. Product information sheet of collagenase (C0130). http://www.sigmaaldrich.com/ (29 November 2015).
Stohs, S.J., and D. Bagchi. 1995. Oxidative mechanism in the toxicity of metalions. Free Radical Biology and Medicine. 18(2): 321-336. doi: 10.1016/0891-5849(94)00159-H
Sophawan Bousopha1, Sitthipong Nalinanon1* and Chodsana Sriket2
1 Faculty of Agro-Industry, King Mongkut’s Institute of Technology Ladkrabang, Bangkok 10520, Thailand
2 Program in Food Science and Technology, Faculty of Agriculture, Ubon Ratchathani Rajabhat University, Ubon Ratchathani 34000, Thailand
*Corresponding author. E-mail: sitthipong.na@kmitl.ac.th
Total Article Views

