
In vitro Study on the Changes in Digestive Fluid Characteristics as Affected by Consumption of Dietary Fiber from Lime Residues
Saranya Jongaroontaprangsee, Naphaporn Chiewchan*, Pawadee Methacanon, Sakamon DevahastinPublished Date : 2019-08-23
DOI : 10.12982/cmujns.2016.00011
Journal Issues : Number2 ,May - August 2016
ABSTRACT
Lime (Citrus aurantifolia Swingle) residues, byproducts of juice extraction, contain significant amounts of dietary fiber and pectin, and hence offer significant potential to be transformed into functional dietary fiber powder. This study investigated the changes in digestive fluid characteristics and digested dietary fiber from lime residues after each step of in vitro digestion. The results showed that pectin in dietary fiber powder was released from the fiber matrix, leading to a significant increase in the viscosity of the gastric juice. Swollen dietary fiber powder with larger pore sizes was observed during gastric digestion; the mean particle size increased almost two-fold upon gastric digestion (from 44 μm to 76 μm). On the other hand, only slight modification in the fiber microstructure and pectin properties occurred during intestinal digestion.
Keywords: Citrus byproducts, Dietary fiber, In vitro digestion, Pectin
INTRODUCTION
Dietary fiber is an edible part of plants or analogous carbohydrates that is resistant to digestion and absorption in the human small intestine, but completely or partially fermented in the large intestine (AACC, 2001). Dietary fiber plays an important role in maintaining the functional integrity of the gastrointestinal tract and reducing the risks of many diseases (Chesson, 2006). Dietary fiber can be classified as soluble or insoluble. The soluble fraction, which is characterized by its viscosity and water solubility, helps lower blood cholesterol and triacylglyceride concentrations, as well as attenuates post-prandial glucose response. The insoluble fraction is characterized by its physical characteristics (porosity, density) and by its ability to increase fecal bulk and decrease intestinal transit time, hence enhancing intestinal peristalsis (Gupta and Premavalli, 2011).
A number of food supplements in the form of dietary fiber powder and fiber-rich products have been developed to serve the increasing demand of health-conscious consumers. Byproducts from fruit and vegetable industries are of interest as a starting material for the production of dietary fiber, since they are inexpensive and available in large quantity. Citrus byproducts are among the most promising raw materials, as residues account for as much as 50% of the original amount of the whole fruit (Lario et al., 2004). These residues also possess high-quality dietary fiber, with a balanced proportion of soluble and insoluble fractions (Larrauri et al., 1996; Grigelmo-Miguel and Martín-Belloso, 1998; Gorinstein et al., 2001). Pectin, an important soluble dietary fiber of citrus peels, has been reported to possess positive physiological effects related to human digestion. The higher viscosity nature of pectin results in decelerated diffusion of glucose and helps postpone absorption and digestion of carbohydrates, thus lowering postprandial blood glucose (Dikeman and Fahey, 2006). The cholesterol-lowering properties of pectin have also been reported (Kang et al., 2009).
In this study, lime (Citrus aurantifolia Swingle) residues after juice extraction were used as the test material. These byproducts are abundant in Thailand and have been proposed as an excellent raw material containing high amounts of dietary fiber (~80%), with equal portions of insoluble and soluble dietary fibers (Wuttipalakorn et al., 2009), as well as flavonoids (Jongaroontaprangsee et al., 2014). The processing method can affect fiber quality; Jongaroontaprangsee et al. (2014) reported that higher retention of bioactive compounds in dietary fiber powder from lime residues was noted when the residues were subject either to vacuum drying or low-pressure superheated steam drying at 80°C and at 10 kPa comparing to hot-air drying at the same temperature. They also stated that dietary fiber powder prepared by vacuum drying at 80°C has the potential to reduce blood glucose and cholesterol levels, as it exhibited high glucose and bile acid retardation indices.
To understand the physiological effects of dietary fiber from lime residues on health, this study investigated the changes in the digestive fluid as well as fiber characteristics during digestion. These findings may help design and engineer a fiber matrix structure with optimal rheology and possibly controlled release of important bioactive compounds during digestion.
MATERIAL AND METHODS
Sample preparation
Limes (Citrus aurantiifolia Swingle) were purchased from a local market (Pracha u-tid 61 Market, Bangkok, Thailand). Limes were washed under running tap water to remove any contaminants before removal of the juice and seeds. To inactivate peroxidase and polyphenoloxidase, lime residues consisting of flavedo, albedo, segments, and juice sacs were blanched in boiling water for 5 min and then immediately cooled in cold water (4°C) before removing excess water by centrifugation at 1,440 rpm for 5 min. The blanched residues were then soaked in 95% (v/v) ethanol for 30 min to reduce the bitter flavor and centrifuged again at 1,440 rpm for 5 min (Wuttipalakorn et al., 2009). The pretreated lime residues were chopped into small pieces (particle size of 3-4 mm) before drying.
The residues were vacuum dried at 80°C at an absolute pressure of 10 kPa, according to Devahastin et al. (2004). The residues were dried until the moisture content was lower than 0.1 g/g dry basis. Moisture content of a sample was determined by gravimetric method at 105°C (AOAC Method 984.25, AOAC (2000)). The dried sample was ground into fine powder using a cyclone mill (Tecator, 1093, Höganäs, Sweden) and sieved using a sieve analyzer (Retsch, AS200 Basic, Haan, Germany) to obtain a particle size of less than 63 μm. The powder was vacuum packed in an aluminum packet and stored at room temperature (~30°C) until further analysis.
In vitro digestion
Simulated gastrointestinal digestion was performed using the method described by Briones-Labarca et al. (2011). To simulate the gastric digestion, 0.5 g of the dietary fiber powder was combined with 25 mL of deionized water. The pH of the mixture was adjusted to pH 2.0 with 6 M HCl. Six mL of pepsin solution (0.125 g of pepsin dissolving in 6 mL of 0.1 M HCl) was subsequently added before incubation in a shaking water bath (Heto, AT 110, Allerod, Denmark) at 120 rpm at 37°C for 2 h.
Once the simulated gastric digestion was finished, the pH of the mixture was adjusted to 5.0 with 1 M NaHCO3, followed by addition of 12.5 mL bile salt/pancreatin solution (containing 0.05 g pancreatin and 0.310 g bile in 12.5 mL 0.1 M NaHCO3) to simulate intestinal digestion. The digestion mixture was
incubated in a shaking water bath at 120 rpm at 37°C for 2 h. A control without the dietary fiber powder was also treated as described above. After each stage of digestion, the sample was collected and centrifuged at 5,000 rpm for 35 min at 25°C to separate the digestive fluid from the digested dietary fiber sample; each part of the sample was separately characterized.
Viscosity
The viscosity measurement of the digestive fluid was carried out using a rotational rheometer (Haake Mars III, Thermo Fisher Scientific, Karlsruhe, Germany) with cone and plate-type measuring system (1° cone angle, 60 mm diameter). The shear rates were varied from 0.01 to 1,000 s-1 (Villemejane et al., 2015). The temperature of the sample was maintained at 37°C during the measurements by a thermostat bath. The viscosity value was reported as an apparent viscosity.
To evaluate the effect of the dietary fiber powder in digestive fluid, a control condition without dietary fiber powder was measured first as the baseline.
FTIR spectroscopy
FTIR spectroscopy was performed to confirm the identity of pectin. Each sample was analyzed by an FTIR-ATR (Perkin Elmer, Spectrum One, Waltham, MA). FTIR spectra (4000-515 cm-1) were recorded with a resolution of 4 cm-1 and 64 scans. Fingerprint region for polysaccharide recognition (1800-515 cm-1) was selected. Two peak areas at 1735 and 1600 cm-1 indicated the number of esterified carboxyl and non-esterified carboxyl groups of pectin structure, respectively.
Particle size distribution
The size distribution of dietary fiber powder that had undergone gastrointestinal digestion was analyzed using a laser diffraction particle size analyzer (Malvern Instruments, Mastersizer 2000, Malvern, U.K.) equipped with Hydro 2000G. Water with a refractive index of 1.33 was used to disperse a digested dietary fiber sample from an analyzer inlet to the sample cell. The obscuration was in the range of 10-20% and the particle size was described as the volume weighed mean diameter. The size of the dietary fiber powder soaked in deionized water (not undergone digestion) was also determined and used for comparison.
For comparison of the dietary fiber powder size between wet and dry states, the dried powder size was measured using another laser diffraction particle size analyzer (Malvern Instruments, Mastersizer 3000, Malvern, U.K.) equipped with Aero S. Air with a refractive index of 1.47 was used as the dispersion agent; distributions were made in triplicate and for each sample. The dispersion air pressure was 4.0 bar. Less than 5% obscuration was achieved throughout the entire measurement duration. The experiments were done in triplicate for each sample.
Microstructure
Microstructures of dietary fiber powder after each stage of digestion, including a control (dietary fiber powder soaked in deionized water) and dried powder, were observed. Each sample was sequentially dehydrated in aliquots of 30, 50, 70, 90, and 100% (v/v) ethanol for 10 min and then dehydrated in liquid CO2 using a critical point dryer (Samdri 780-A; Tousimis, Rockville, MD), before being coated with gold in a sputter coater. The microstructure of each sample was observed using a scanning electron microscope (JEOL, JSM-5410LV, Tokyo, Japan) at 200× and 1000× magnification.
Statistical analysis
The experiments were designed to be completely random. The data were subject to analysis of variance (ANOVA) and are presented as mean values with standard deviations. Differences between mean values were established using Duncan’s multiple range tests; values were considered significant at a confidence level of 95%. All statistical analyses were performed using SPSS software (version 17) (SPSS Inc., Chicago, IL). All experiments were performed in duplicate, unless specified otherwise.
RESULTS
The viscosity of digestive fluid during each stage of digestion was determined. Compared to the control, without dietary fiber powder, a significant increase in the viscosity of the gastric juice from 0.85 to 8.34 mPa·s (Table 1) was observed with the dietary fiber powder. The viscosity of digestive fluid significantly decreased during intestinal digestion.
Table 1. Consistency index (K), flow behavior index (n) and apparent viscosity at 1000 s-1 of digestive fluid, with and without dietary fiber powder.
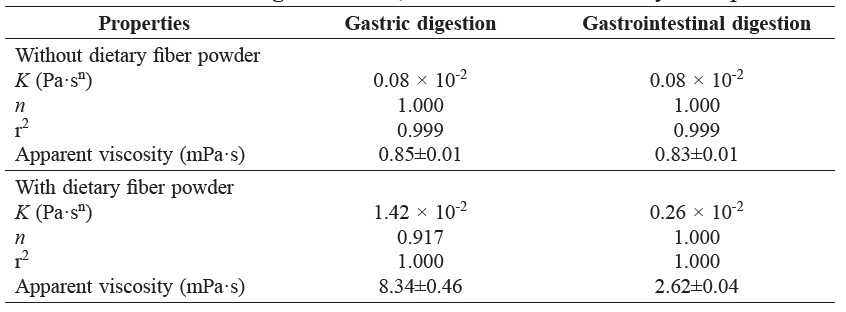
Figure 1 shows the FTIR spectrums of the fingerprint region for polysaccharide recognition (1800-500 cm-1) (Szymanska-Chargot and Zdunek, 2013). Pectin could be recovered from digestive fluid during each stage of digestion, i.e., gastric and intestinal digestions. Two distinct peaks absorbing at 1735 and 1600 cm-1, representing the fingerprint of pectin assigned for the absorption of the esterified and non-esterified carboxyl groups, respectively (Monsoor et al., 2001), could be detected.
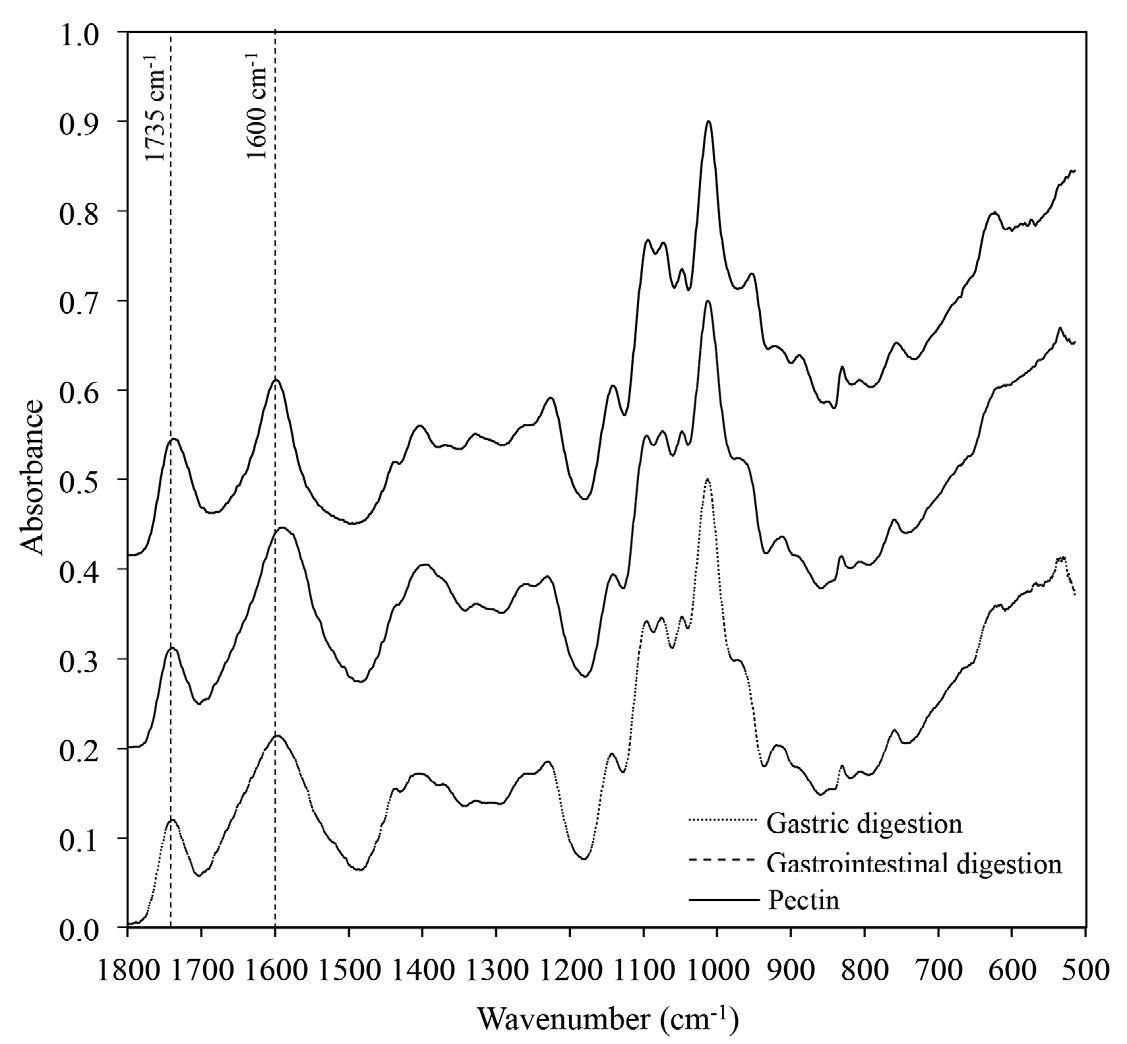
Figure 1. FTIR spectrum of pectin after gastric digestion, gastrointestinal digestion, and pectin.
Microstructures of dried dietary fiber powder and hydrated dietary fiber particles after being soaked in water and after each stage of digestion were investigated, as shown in Figure 2. The volume weight mean diameter of dried dietary fiber powder was approximately 44 μm. When lime dietary fiber swelled, size increased about 1.5-1.7 times of the original fiber particle; the particle size of fiber that had undergone digestion was larger than that swelled in water at similar time and temperature, i.e., 4 h at 37°C (Table 2).
Table 2. Particle size of dietary fiber during in vitro digestion.
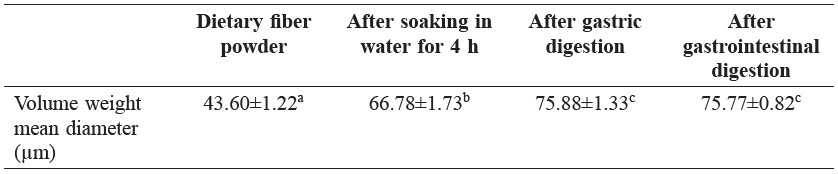
Same letters in the same row indicate that values are not significantly different (p > 0.05).
The changes in morphology of dietary fiber particles were clearly observed and consistent with the particle size results. Dietary fiber microstructure was largely modified by the gastric juice in acidic condition. Dietary fiber particles that had undergone gastric digestion swelled significantly with spongy-like characteristics, most likely as a result of cell wall disruption leading to larger porosity, followed by the release of pectin, as stated before.
DISCUSSION
A significant increase in the viscosity of gastric juice implied the changes from Newtonian to non-Newtonian fluid. Pseudoplastic behavior characteristics of pectin solutions at moderate concentration have also been observed by other investigators (Awasthi, 2011; Srivastava and Malviya, 2011).
Ulmius et al. (2011) reported that pectin could be released from the fiber matrix during gastric digestion, and the release continued in the small intestine. In our study, approximately 60% of the pectin content in dietary fiber powder was released into the gastric juice, and only a small amount of pectin was further released during intestinal digestion (data not shown). The FTIR results indicated that the increase in the viscosity of the gastric content was due to the release of pectin from the fiber matrix into the gastric juice. This resulted in a slower rate of gastric emptying of the solid food components of meals (Dikeman et al., 2006). We also observed a significant decrease in the viscosity of digestive fluid during intestinal digestion. This might be due to the addition of pancreatin and bile salt into the fluid, diluting the system. However, the viscosity of the digestive fluid with the dietary fiber powder was still higher than the control (without adding dietary fiber powder). This would help retard glucose absorption into the blood system.
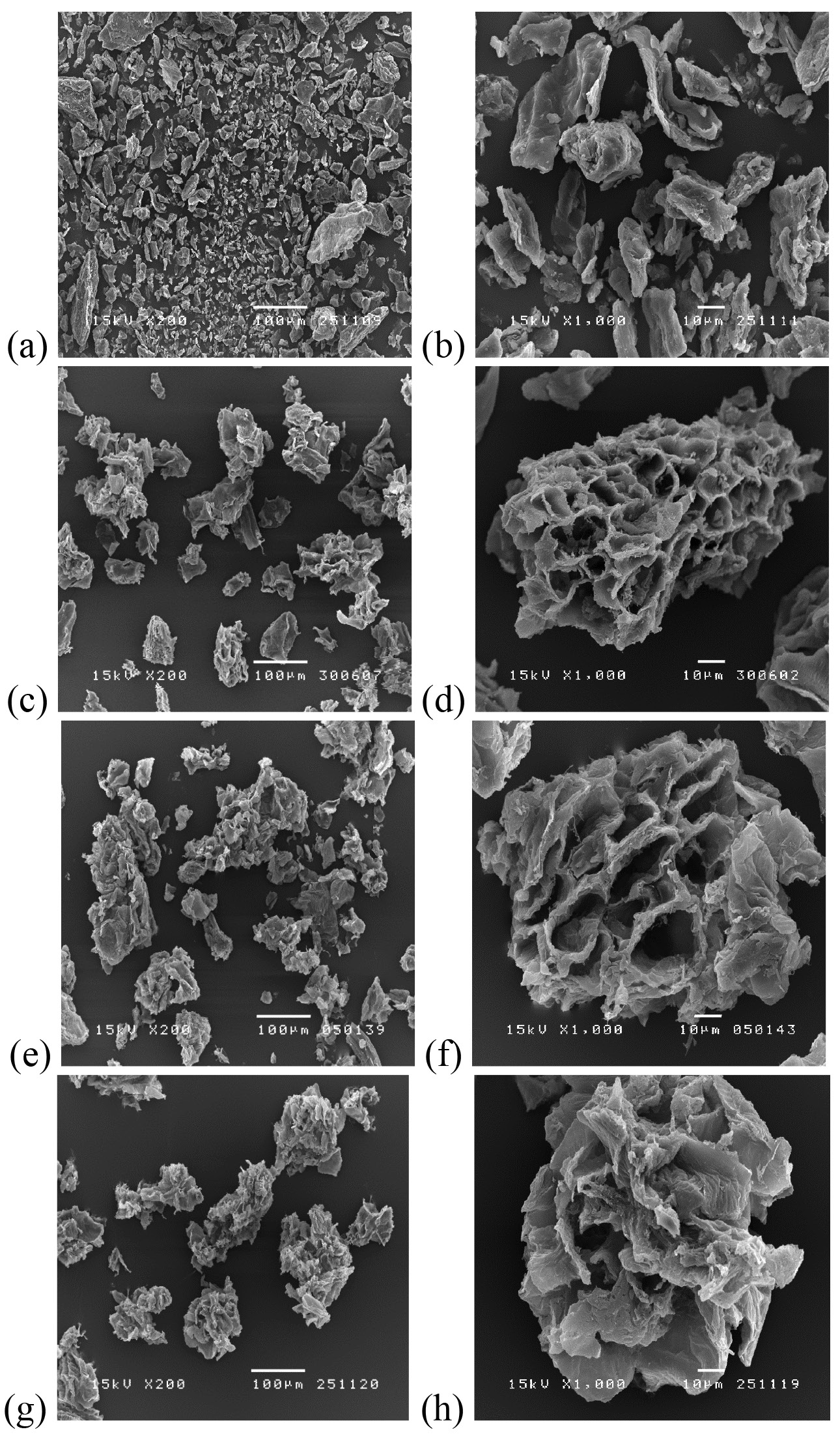
Figure 2. Microstructure of dietary fiber powder at magnifications of 200× (a, c, e, g) and 1000× (b, d, f, h) of dietary fiber powder (a-b) and after soaking in water for 4 h (c-d), after gastric digestion (e-f) and after gastrointestinal digestion (g-h).
In our study, the main tissue structure of the dietary fiber was preserved, and hindered the release of pectin. Changes in the microstructure of dietary fiber has also been shown to affect water and fat absorption (Lundberg et al., 2014). In general, polysaccharide constituents of dietary fiber are strongly hydrophilic, leading to the storage of water molecules by hydrogen bonding within the void spaces of dietary fiber (Al-Sheraji et al., 2011). In contrast, no significant changes in microstructure were detected for the dietary fiber after intestinal digestion. The results suggested that gastric juice considerably modifies the structure of dietary fiber, affecting the functional properties of dietary fiber. The larger available surface area and higher porosity of dietary fiber may help improve its availability to microbial degradation in the colon (Tosh and Yada, 2010).
CONCLUSION
This study investigated the changes in digestive fluid characteristics and digested dietary fiber from lime residues after each step of in vitro digestion. The results revealed that the structure of the dietary fiber was largely modified under the severe acidic conditions during gastric digestion. Gastric digestion also effectively extracted pectin from the fiber matrix. Regulating the processing steps to modify the fiber matrix might help release pectin from plant cell walls during digestion, and improve health-related functional properties of dietary fiber. The effect of the processing and digestion conditions on the prebiotic properties of dietary fiber powder from lime residues should also be investigated to cover the overall benefits of this fiber product.
ACKNOWLEDGEMENTS
The authors express their sincere appreciation to the Research Strengthening Project of the Faculty of Engineering, King Mongkut’s University of Technology Thonburi for its financial support. Author Jongaroontaprangsee thanks the Thailand Research Fund, through its Royal Golden Jubilee Scholarship program, for supporting her doctoral study.
REFERENCES
AACC. 2001. The definition of dietary fiber. Cereal Foods World. 46: 112-126.
Al-Sheraji, S.H., A. Ismail, M.Y. Manap, S. Mustafa, R.M. Yusof, and F.A. Hassan. 2011. Functional properties and characterization of dietary fiber from Mangifera pajang Kort. Fruit pulp. Journal of Agricultural and Food Chemistry. 59: 3980-3985.
AOAC. 2000. Official methods of analysis (17th ed.). The Association of Official Analytical Chemists, Gaithersburg, M.D.
Awasthi, R. 2011. Selection of pectin as pharmaceutical excepient on the basis of rheological behavior. International Journal of Pharmacy and Pharmaceutical Sciences. 3: 229-231.
Briones-Labarca, V., C. Muñoz, and H. Maureira. 2011. Effect of high hydrostatic pressure on antioxidant capacity, mineral and starch bioaccessibility of a non conventional food: Prosopis chilensis seed. Food Research International. 44: 875-883.
Chesson, A. 2006. Dietary fiber. In A. M. Stephen, G. O. Phillips and P. A. Williams (Eds.). Food polysaccharides and their applications (pp. 629-663). Taylor and Francis, Boca Raton.
Devahastin, S., P. Suvarnakuta, S. Soponronnarit, and A.S. Mujumdar. 2004. A comparative study of low-pressure superheated steam and vacuum drying of a heat-sensitive material. Drying Technology. 22: 1845-1867.
Dikeman, C.L. and G.C. Fahey. 2006. Viscosity as related to dietary fiber: A review. Critical Reviews in Food Science and Nutrition. 46: 649-663.
Gorinstein, S., O. Martín-Belloso, Y.S. Park, R. Haruenkit, A. Lojek, M. Cíz, A. Caspi, I. Libman, and S. Trakhtenberg. 2001. Comparison of some biochemical characteristics of different citrus fruits. Food Chemistry. 74: 309-315.
Grigelmo-Miguel, N. and O. Martín-Belloso. 1998. Characterization of dietary fiber from orange juice extraction. Food Research International. 31: 355-361.
Gupta, P. and K.S. Premavalli. 2011. In-vitro studies on functional properties of selected natural dietary fibers. International Journal of Food Properties. 14: 397-410.
Jongaroontaprangsee, S., N. Chiewchan, and S. Devahastin. 2014. Composition profiles and functional properties of dietary fiber powder from lime residues: Effects of pretreatment and drying methods. Drying Technology. 32: 484-493.
Kang, H.J., J.H. Kwon, D.U. Ahn, J.W. Lee, W.K. Lee, and C. Jo. 2009. Effect of citrus pectin oligosaccharide prepared by irradiation on high cholesterol diet B6.KOR-ApoE mice. Food Science and Biotechnology. 18: 884-888.
Lario, Y., E. Sendra, J. García-Pérez, C. Fuentes, E. Sayas-Barberá, J. Fernández-López, and J.A. Pérez-Alvarez. 2004. Preparation of high dietary fiber powder from lemon juice by-products. Innovative Food Science and Emerging Technologies. 5: 113-117.
Larrauri, J.A., P. Rupérez, L. Bravo, and F. Saura-Calixto. 1996. High dietary fiber powders from orange and lime peels: Associated polyphenols and antioxidant capacity. Food Research International. 29: 757-762.
Lundberg, B., X.J. Pan, A. White, H. Chau, and A. Hotchkiss. 2014. Rheology and composition of processed citrus fiber. Journal of Food Engineering. 125: 97-104.
Monsoor, M.A. 2005. Effect of drying methods on the functional properties of soy hull pectin. Carbohydrate Polymers. 61: 362-367.
Srivastava, P. and R. Malviya. 2011. Sources of pectin, extraction and its applications in pharmaceutical industry – An overview. Indian Journal of Natural Products and Resources. 2: 10-18.
Szymanska-Chargot, M. and A. Zdunek. 2013. Use of FT-IR spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophysics. 8: 29-42.
Tosh, S.M., and S. Yada. 2010. Dietary fibers in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Research International. 43: 450-460.
Ulmius, M., A. Johansson-Persson, T.I. Nordén, B. Bergenståhl, and G. Önning. 2011. Gastrointestinal release of β-glucan and pectin using an in vitro method. Cereal Chemistry Journal. 88: 385-390.
Villemejane, C., R. Wahl, P. Aymard, S. Denis, and C. Michon. 2015. In vitro digestion of short-dough biscuits enriched in proteins and/or fibers, using a multi-compartmental and dynamic system (1): Viscosity measurement and prediction. Food Chemistry. 182: 55-63.
Wuttipalakorn, P., W. Srichumpuang, and N. Chiewchan. 2009. Effects of pretreatment and drying on composition and bitterness of high dietary fiber powder from lime residues. Drying Technology. 27: 133-142.
Saranya Jongaroontaprangsee1, Naphaporn Chiewchan1*, Pawadee Methacanon2 and Sakamon Devahastin1
1 Advanced Food Processing Research Laboratory, Department of Food Engineering, Faculty of Engineering, King Mongkut’s University of Technology Thonburi, Bangkok 10140, Thailand
2 National Metal and Materials Technology Center, Klong Luang, Pathumthani 12120, Thailand
*Corresponding author. E-mail: naphaporn.rat@kmutt.ac.th
Total Article Views

