
Optimization of Vancomycin Dosing Regimens to Achieve Therapeutic Targets in Critically Ill Patients in Thailand
Thitinat Dedkaew*, Tim Roy Cressey, Chaicharn Phothirat, Romanee Chaiwarith, Baralee Punyawudho and Aroonrut LucksiriPublished Date : 2019-08-23
DOI : 10.12982/cmujns.2016.00010
Journal Issues : Number2 ,May - August 2016
ABSTRACT
It is essential to rapidly achieve therapeutic vancomycin concentrations in critically ill patients with gram-positive bacterial infections. This study aimed to determine optimal vancomycin dosing regimens for critically ill patients. Using an external dataset – therapeutic drug monitoring concentration data from 51 critically ill patients receiving vancomycin, we validated a population pharmacokinetic model to describe vancomycin plasma concentrations over time. The final population pharmacokinetic model was used to simulate different vancomycin doses for patients with varying degrees of renal function in order to determine the percentage of patients achieving therapeutic targets. Based on simulations, less than 90% of patients achieved a vancomycin trough concentration (Ctrough) ≥ 15 mg/L following administration of the standard vancomycin maintenance doses of 1000 mg every 12 hr (for patients with a creatinine clearance (CrCL) ≥ 50 mL/min) or 1000 mg every 24 hr (for those with CrCL 30-50 mL/min). Model predictions showed that to ensure ≥ 90% of patients achieve a target vancomycin Ctrough, 1250 mg every 6 hr (for CrCL ≥ 50 mL/min) and 1000 mg every 8 hr (for CrCL 30-50 mL/min) are needed. In conclusion, alternative vancomycin dosing regimens may improve the percentage of attaining target vancomycin Ctrough at steady state in critically ill patients in Thailand. However, routine monitoring of vancomycin Ctrough and serum creatinine are also recommended to ensure therapeutic and toxicity outcomes.
Keywords: Vancomycin, Critically ill patients, Simulations, Dosing regimens
INTRODUCTION
Vancomycin is a tricyclic glycopeptide antibiotic widely used to treat gram-positive bacterial infections in critically ill patients. Vancomycin is primarily eliminated through glomerular filtration; therefore, factors affecting renal function, such as body weight, age, serum creatinine, and disease can result in changes in vancomycin pharmacokinetics and consequently impact efficacy and/or toxicity (Scaglione and Paraboni, 2008; Varghese et al., 2010). Race or ethnicity has not been found to significantly influence the pharmacokinetics of drugs predominantly eliminated via the kidney by glomerular filtration with low plasma protein binding, such as vancomycin (Johnson, 1997).
Previous studies have shown that the commonly used vancomycin dose of 1000 mg every 12 hr may not provide adequate serum concentrations to achieve the recommended 24-hour Area Under the Concentration-Time Curve (AUC24)/Minimum Inhibitory Concentration (MIC) breakpoint or a trough concentration (Ctrough) of more than 10 mg/L in Intensive Care Unit (ICU) patients (Mahoney et al., 2006; del Mar Fernandez et al., 2007). Pharmacokinetics data of vancomycin in critically ill patients are limited and currently no specific guidelines for vancomycin dosing exist for this population. Determining the optimal dosage for these patients is often based on individual clinical expertise.
Vancomycin has both time-dependent bacterial killing activity and moderate Post-Antibiotic Effect (PAE) that are dependent on serum concentrations (i.e., peak concentration) (McKinnon and Davis, 2004). An AUC24/MIC ratio of > 400 has been proposed as an efficacy threshold for vancomycin to treat Staphylococcus aureus infections. There is a strong relationship between vancomycin Ctrough and AUC24/MIC in adults who have a creatinine clearance (CrCL) ≥ 100 mL/min (Pai et al., 2014). For organisms with MIC ≤ 1 mg/L, steady-state vancomycin Ctrough of 15 to 20 mg/L are correlated with an AUC24/MIC ratio ≥ 400. Vancomycin Ctrough between 15 to 20 mg/L could achieve a > 90% probability of achieving an AUC24/MIC > 400 for S. aureus with MIC ≤ 1 mg/L (Pai et al., 2014). Therefore, therapeutic drug monitoring of the vancomycin Ctrough could help to ensure optimal vancomycin treatment (James and Gurk-Turner, 2001).
Our objective was to determine the optimal vancomycin dosage regimens for critically ill patients in Thailand with various degrees of renal function. Different vancomycin dosage regimens were simulated using a validated population pharmacokinetic model, and the percentage of patients achieving target efficacy concentrations was estimated.
MATERIAL AND METHODS
We have recently reported a population pharmacokinetic model to describe vancomycin concentrations over time in critically ill patients (Dedkaew et al., 2015). This model was developed using a total of 299 vancomycin serum concentrations from 138 ICU patients. The inclusion criteria for these patients were: (a) older than 18 years old; (b) had indication for vancomycin use; and (c) stable serum creatinine (SCr) (less than 0.5 mg/dL change if SCr < 2 mg/dL and less than 20% change if SCr ≥ 2 mg/dL) during the study. Exclusion criteria were: (a) history of hypersensitivity to vancomycin or any component of the formulation; (b) no body weight value measured within the last month; (c) received vancomycin treatment within the last 14 days before enrollment; (d) pregnant; (e) morbidly obese (Body Mass Index, BMI ≥ 35 kg/m2; (f) received or planning to receive renal replacement therapy; or (g) a Simplified Acute Physiology Score (SAPS II) > 52. One hundred and thirteen patients (81.9%) were on a mechanical ventilator and eighty-eight patients (63.8%) received vasoactive drugs (e.g., dopamine, dobutamine).
The vancomycin concentration-time profiles in this population were best described by a two-compartment pharmacokinetic model. Age, body weight, and creatinine clearance (estimated using the Cockroft-Gault equation) were tested as covariates. Creatinine clearance was the only covariate that significantly influenced vancomycin pharmacokinetics.
In the current study, an additional 51 ICU patients (28 males, 23 females) with available data on retrospective therapeutic drug monitoring of vancomycin were used as an external dataset to validate the population model. Inclusion and exclusion criteria were similar to our previous study (Dedkaew et al., 2015). No patient identification could be traced back. To estimate vancomycin trough concentrations at steady state (after 3-5 doses), the serum vancomycin concentration was measured in a blood sample 2 hr before the following dose – e.g., at 58 hr or 2 hr prior to the 6th dose, if vancomycin was administered every 12 hr.
Monte Carlo Simulations were performed using Phoenix® Trial Simulatort™ software (Version 2.2.2, Certara™, St. Louis, MO, USA). Vancomycin concentration-
time profiles were simulated for 10,000 subjects using vancomycin dosage regimens received by patients included in the external validation dataset.
To determine the optimal vancomycin dosage regimens for critically ill patients with different degrees of renal function (CLCr > 50 mL/min and CLCr 30-50 mL/min), vancomycin concentration-time profiles were simulated (10,000 subjects). The percentage of patients achieving vancomycin Ctrough ≥ 15 mg/L was reported. The target Ctrough attainment was defined as ≥ 90%.
RESULTS
The characteristics of the 51 ICU patients were: mean age (± standard deviation, SD) 66.3 ± 15.1 years, body weight 62.2 ± 12.4 kg, creatinine clearance 53.9 ± 27.6 mL/min, and SAP II score 46.1 ± 4.3 points. The patients’ vancomycin dosage regimens, clinical features, and list of concomitant mechanical ventilation and vasoactive drugs are shown in Table 1.
Table 1. Clinical characteristics and vancomycin dosage regimens of the 51 ICU patients.
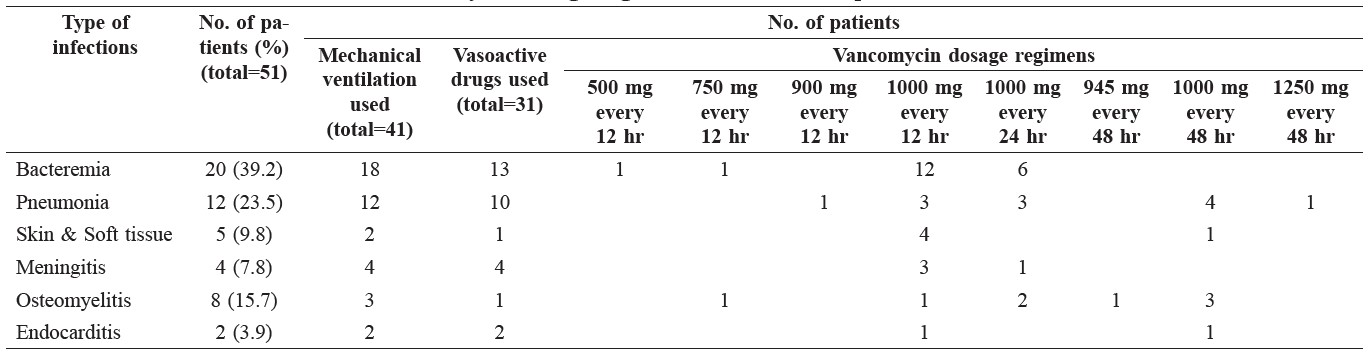
The characteristics of patients who received vancomycin dose at 1000 mg every 12 hr and 1000 mg every 24 hr are shown in Table 2.
Table 2. Characteristics of patients who received vancomycin dose at 1000 mg every 12 hr and 1000 mg every 24 hr.
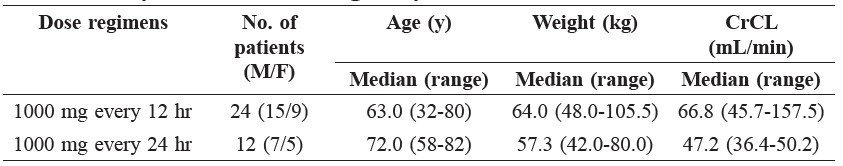
Note: CrCL is calculated Creatinine clearance using Cockcroft-Gault equation.
Semi-logarithmic plots of the simulated vancomycin concentrations (5th, 50th, and 95th percentiles) following 1000 mg every 12 hr and 1000 mg every 24 hr versus time with observed vancomycin concentrations overlaid are shown in Figures 1 and 2, respectively. The results of the other (i.e., 1000 mg every 48 hr, 750 mg every 12 hr) patients are not presented, due to the small number of patients receiving each regimen.
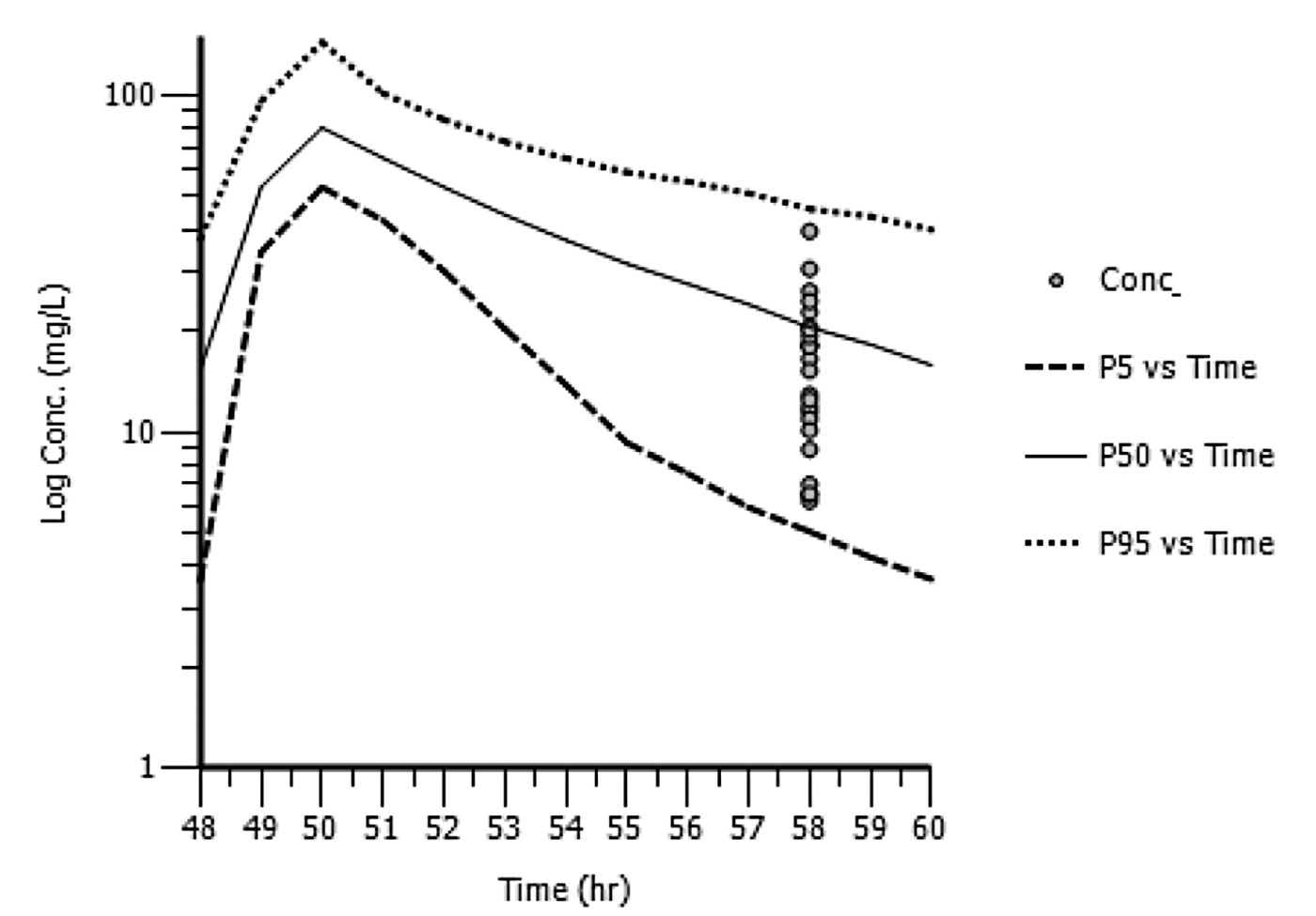
Figure 1. Semi-logarithmic plot of simulated vancomycin concentrations (5th, 50th, and 95th percentiles) following 1000 mg every 12 hr with the actual vancomycin concentrations determined at 58 hr post-dose from patients in the external dataset overlaid (N=24).
Note: conc. is simulated vancomycin concentrations; P5 is the 5th percentile of the simulated concentrations; P50 is the 50th percentile of the simulated concentrations; P95 is the 95th percentile of the simulated concentrations.
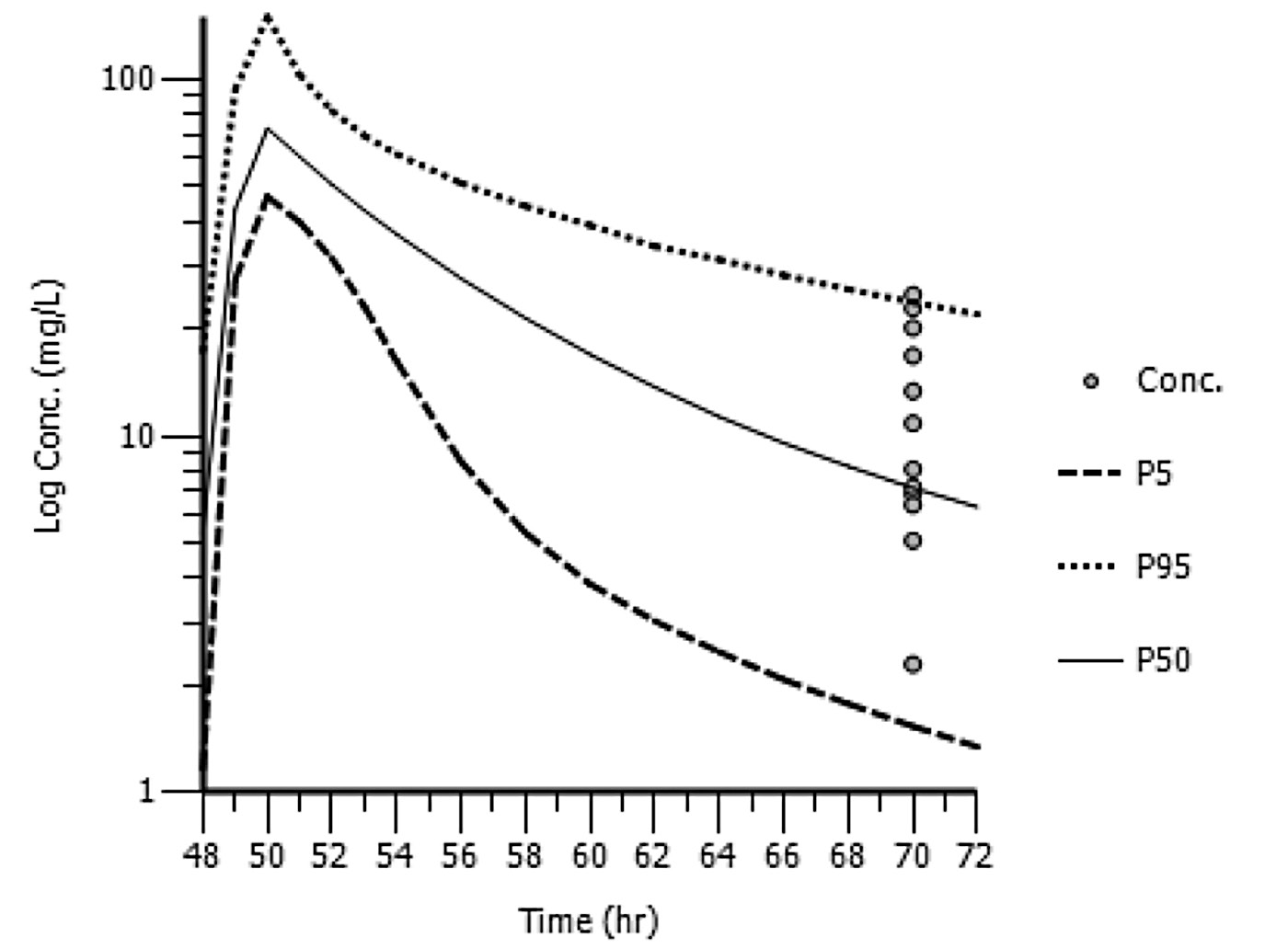
Figure 2. Semi-logarithmic plot of the simulated vancomycin concentrations (5th, 50th, and 95th percentiles) following 1000 mg every 24 hr with the actual vancomycin concentrations determined at 70 hr post-dose from patients in the external dataset overlaid (N=12).
Note: conc. is simulated vancomycin concentrations; P5 is the 5th percentile of the simulated concentrations; P50 is the 50th percentile of the simulated concentrations; P95 is the 95th percentile of the simulated concentrations.
Vancomycin doses of 1000 mg and 1250 mg every 6 hr; 750 mg, 1000 mg, 1250 mg and 1500 mg every 8 hr; and 1000 mg, 1250 mg, 1500 mg and 1750 mg every 12 hr were assessed to identify the optimal vancomycin dosage regimen for the patients with creatinine clearance ≥ 50 ml/min.
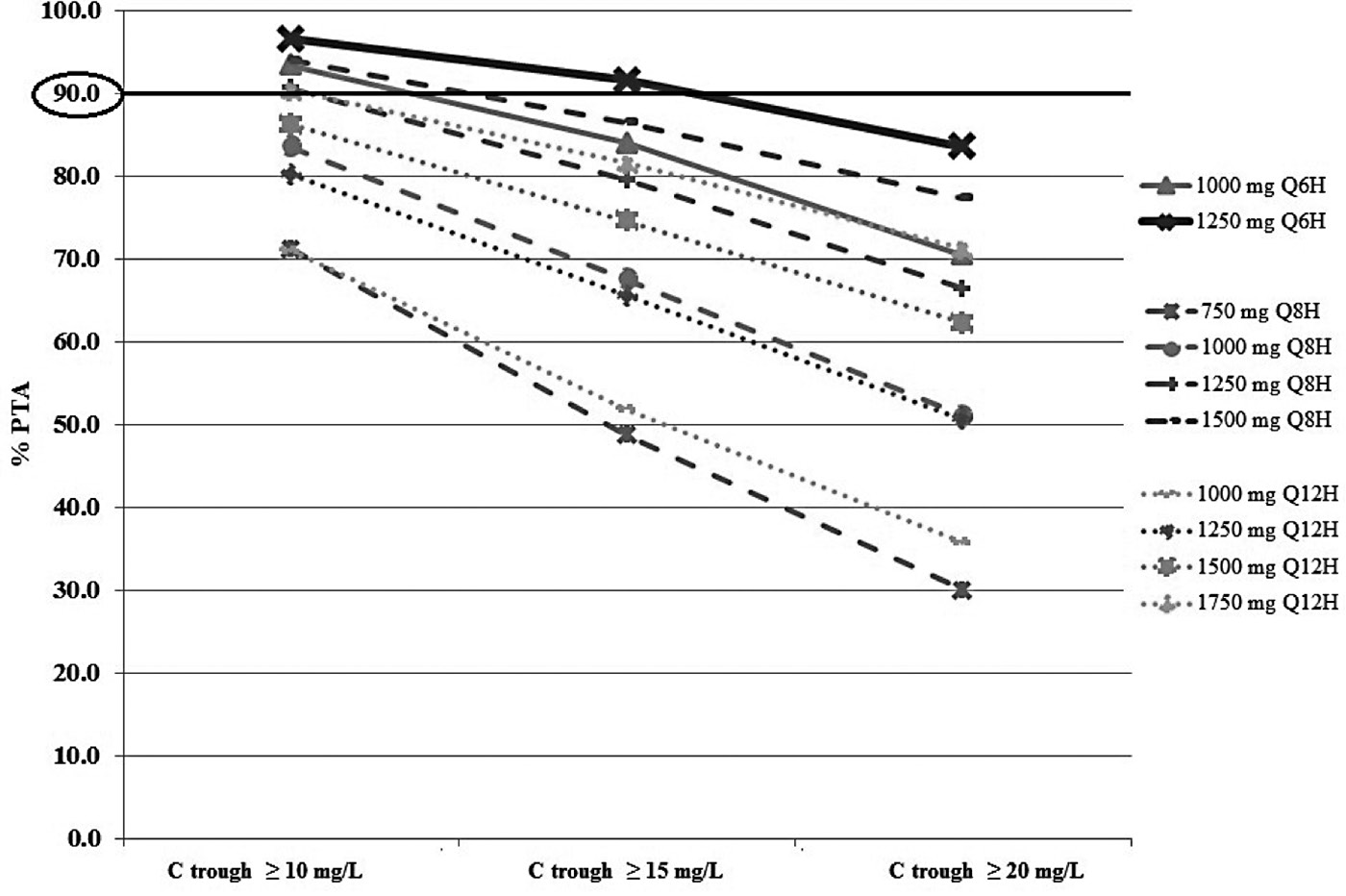
Figure 3. Percentage of target attainment (PTA) for vancomycin Ctrough > 10, 15, and 20 mg/L following different simulated dosage regimens in patients with a CrCL ≥50 mL/min.
With the recommended vancomycin dose of 1000 mg every 12 hr for patients with a CrCL ≥ 50 ml/min, a PTA ≥ 90% for a Ctrough ≥ 15 mg/L and ≥ 10 mg/L was not achieved. In order to achieve a PTA ≥ 90% using a Ctrough ≥ 10 mg/L, a vancomycin dose of 1000 to 1250 mg every 6 hr or 1250 to 1500 mg every 8 hr was required. A vancomycin dose of 1250 mg every 6 hr was the optimal regimen to achieve PTA ≥ 90% for a Ctrough ≥ 15 mg/L in ICU patients with a CrCL ≥ 50 ml/min (Figure 3).
Vancomycin concentrations following doses of 500 mg and 750 mg every 6 hr; 750 mg and 1000 mg every 8 hr; 750 mg, 1000 mg, 1250 mg and 1500 mg every 12 hr; and 750 mg, 1000 mg, 1250 mg and 1500 mg every 24 hr were assessed to identify the optimal vancomycin dosage regimen for the patients with a CrCL 30-50 ml/min (Figure 4).
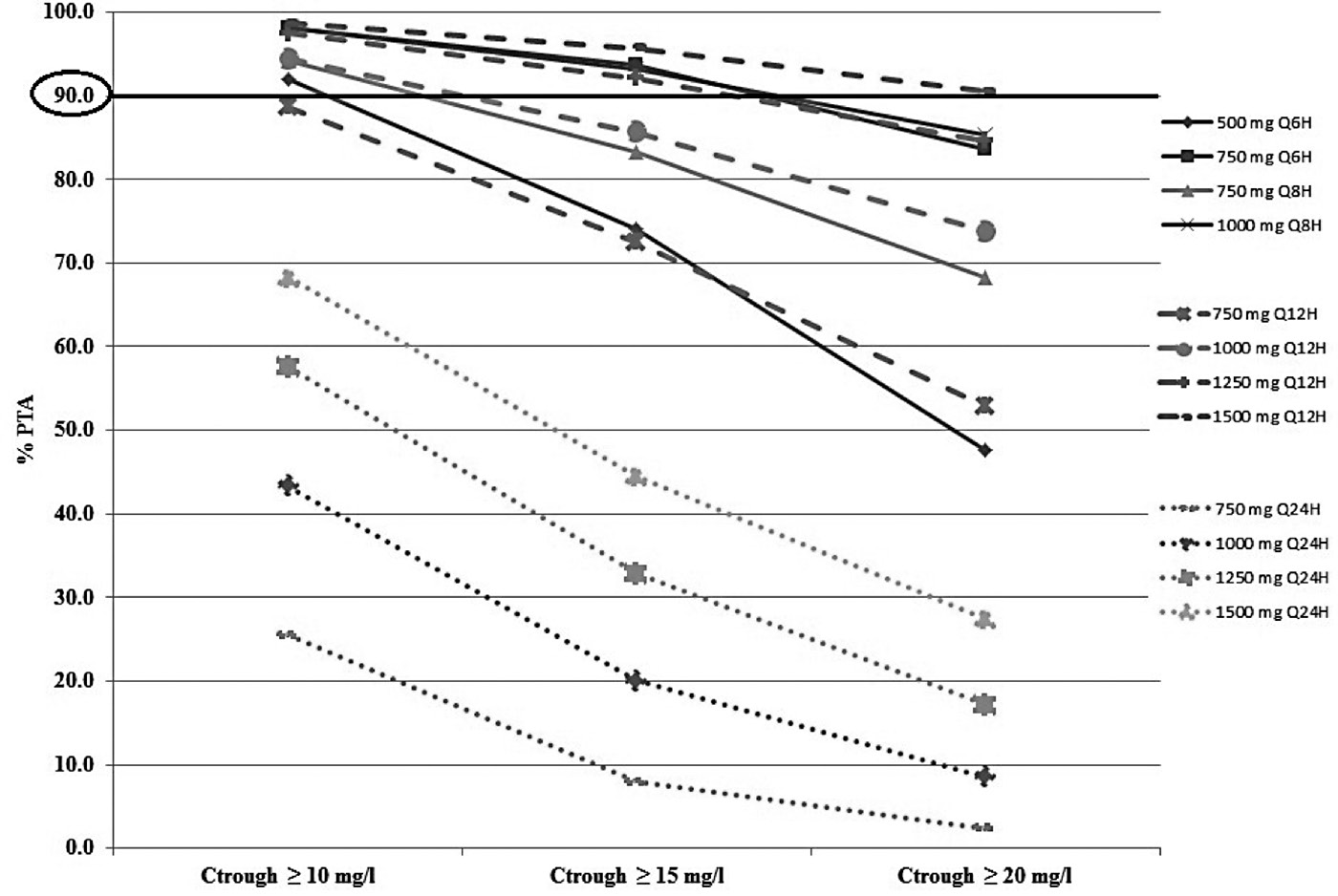
Figure 4. Percentage of target attainment (PTA) for vancomycin Ctrough > 10, 15, and 20 mg/L following different simulated dosage regimens in patients with a CrCL 30-50 ml/min.
DISCUSSION
In Thailand, a vancomycin dosage of 15 mg/kg every 12 hr has been recommended since 2007 in the Clinical Practice Guidelines for prevention and management of hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) in adults with normal renal function (IDAT, T.S.T, TSCCM, and ICST, 2007).
A report has shown that critically ill Thai patients receiving vancomycin at standard dosage (1000 mg every 12 hr) did not achieve the recommended trough concentrations (Patanwala et al., 2009). Several countries in Europe have modified their vancomycin dosage regimens for ICU patients to 1,000 mg every 8 hr (Cruciani et al., 1996). Moreover, it has been shown that using 1,000 mg of vancomycin every 12 hr leads to a 33% risk of failure to obtain the recommended AUC24/MIC breakpoint for S.aureus infections (del Mar Fernandez et al., 2007). Mahoney et al. (2006) reported that 61.4% of ICU patients with bacteremia and pneumonia receiving vancomycin using a nomogram did not achieve trough concentrations of >10 mg/L.
The majority of pharmacokinetic studies of vancomycin have been performed in healthy volunteers using multi-compartment pharmacokinetics models. Creatinine clearance, body size, and age have been reported as significant covariates affecting vancomycin pharmacokinetic parameters. In our study, vancomycin serum concentration time profiles were best described by a two-compartmental model with first-order elimination. A strong correlation between CrCL and vancomycin clearance was observed in our study, corresponding with Robert et al. (2011). However, Revilla et al. (2010) found that CrCL and age influenced vancomycin clearance; while Llopis-Salvia (2006) showed that CrCL and total body weight influenced vancomycin clearance.
No patient covariates were found to significantly influence the vancomycin volume of distribution in our study, similar to the results of Revilla et al. (2010). However, other studies have found that body weight influenced vancomycin volume of distribution (Robert et al., 2011; Thomson et al., 2009; Llopis-Salvia et al., 2006). Age has also been shown to be an influential covariate on vancomycin volume of distribution (Sanchez et al., 2010; Purwonugroho et al., 2012). Based on simulations, we found that higher vancomycin doses are necessary for optimal microbiological and clinical outcomes. Likewise, Roberts et al. (2011) reported that patients with higher CrCL should receive a larger daily dose. Patel et al. (2011) reported that vancomycin regimens of at least 3000 mg daily were required to achieve a PTA of approximately 80% for an MIC value of 1 mg/mL.
Our study found that critically ill patients receiving the commonly used dose of 1000 mg of vancomycin every 12 hr for patients with CrCL > 50 mL/min were unlikely to achieve vancomycin target Ctrough at steady state. Using the current common vancomycin dose for patients with a CrCL between 30-50 mL/min (1000 mg every 24 hr) did not achieve a PTA ≥ 90% for a vancomycin trough concentration ≥ 15 mg/L. Thus, higher dosing regimens of vancomycin in these populations are required to achieve the target concentrations.
The observed vancomycin trough concentrations in the external model validation dataset were well-predicted using the population pharmacokinetic model. However, Figure 1 shows a slight over-estimation and Figure 2 shows a slight under-estimation. Additional data to refine our population pharmacokinetic model may be useful.
Moise-Broder et al. (2004) indicated that an AUC0-24/MIC ratio of ≥ 400 was related to an optimal clinical outcome and is a significant pharmacodynamic parameter of the probability of achieving target attainment (PTA). However, Jeffres et al. (2006) has shown that the vancomycin AUC0-24 is correlated with vancomycin trough concentration, supporting the use of vancomycin trough concentrations to monitor efficacy and toxicity.
Although it was reported that a vancomycin Ctrough between 15 to 20 mg/L is related to the target AUC24/MIC ratio of 400, every 10 mg/L increase in vancomycin trough concentration provided a 10-fold increase in the risk of neph rotoxicity (Horey et al., 2013). Moreover, the more aggressive the vancomycin dosing, the higher the risk of nephrotoxicity and ototoxicity (Bosso et al., 2011). Consequently, it is equally important to closely monitor dose-related nephrotoxicity due to high doses of vancomycin, especially when other nephrotoxicity agents are used concomitantly (Brown et al., 2013).
A limitation of our study was the lack of full pharmacokinetic profiles within the external validation dataset. Therefore, AUC0-24, which is considered the best predictor for clinical outcomes of vancomycin, cannot be estimated. Trough serum vancomycin concentrations are recommended as an optimal monitoring parameter for routine therapeutic drug monitoring, since it is the most accurate and practical method for monitoring drug efficacy (Rybak et al., 2009). Our study aimed to predict Ctrough following various dosage regimens of vancomycin in a population with different degrees of renal function in order to determine the optimal dosage regimens. It must be noted that our data should be interpreted cautiously, if extrapolating to a different study population.
The recommended vancomycin regimens may help guide vancomycin dosing in ICU patients, especially in hospital settings that lack therapeutic drug-monitoring services. However, the patient’s renal function should be closely monitored to prevent nephrotoxicity from vancomycin.
CONCLUSION
Based on our simulations, in order to achieve a PTA of ≥ 90%, a vancomycin dose of 1250 mg every 6 hr (for CrCL ≥ 50 mL/min) and 1000 mg every 8 hr or 1250 mg every 12 hr (for those with CrCL 30-50 mL/min) is necessary. Our findings confirm that the commonly used vancomycin dosage regimens, e.g., 1000 mg every 12 hr or 1000 mg every 24 hr in critically ill patients with a CrCL above 30 mL/min or 50 mL/min, respectively, would lead to a suboptimal Ctrough.
Although a higher dose and more frequent dosage regimens of vancomycin may result in a higher probability of achieving the desired target, the higher Ctrough obtained also increases the risk of toxicity. Therapeutic drug monitoring remains necessary to ensure the safety of these modified vancomycin dosing recommendations in an ICU setting and the efficacy of vancomycin use to obtain the desirable therapeutic targets.
ACKNOWLEDGEMENTS
This research was supported by grants funded by Chiang Mai University and the Graduate School of Chiang Mai University. The authors would like to thank Certara™ USA, Inc. for the Academic License for the Phoenix® NLME 1.3 and the Pharsight® Trial SimulatorTM Version 2.2.2.
REFERENCES
Bosso, J.A., J. Nappi, C. Rudisill, M. Wellein, B.B. Bookstaver, J. Swindler, and P.D. Mauldin. 2011. Relationship between Vancomycin Trough Concentrations and Nephrotoxicity: a Prospective Multicenter Trial. Antimicrobial Agents and Chemotherapy. 55(12): 5475-5479.
Brown, D.L., C.D. Laala, and A.J. Masselink. 2013. AUC versus Peak-Trough Dosing of Vancomycin: Applying New Pharmacokinetic Paradigms to and Old drug. Therapeutic Drug Monitoring. 35: 443-449.
Cruciani, M., G. Gatti, L. Lazzarini, G. Furlan, G. Broccali, M. Malena, C. Franchini, and E. Concia. 1996. Penetration of Vancomycin Into Human Lung Tissue. Journal of Antimicrobial Chemotherapy. 38: 865-869.
Dedkaew, T., T.R. Cressey, B. Punyawudho, and A. Lucksiri. 2015. Pharmacokinetics of Vancomycin in Critically Ill Patients in Thailand. International Journal of Pharmacy and Pharmaceutical Sciences. 7(9): 232-236.
del Mar Fernandez de, M., G. Garcia, N. Revilla, M.V. Calvo, A. Dominguez- Gil, and A.S. Navarro. 2007. Pharmacokinetic/pharmacodynamics analysis of vancomycin in ICU patients. Intensive Care Medicine. 33: 279-285.
Horey, A., K.A. Mergenhagen, and A. Mattappallil. 2012. The Relationship of Nephrotoxicity of Vancomycin Trough Serum Concentrations in a Veteran’s Population: A Retrospective Analysis. The Annals of Pharmacotherapy. 46: 1477-1483.
Infectious Disease Association of Thailand, Thoracic Society of Thailand, Critical Care Society of Thailand and Infections Control Society of Thailand. 2007. Thai Clinical Practice Guidelines for Management and Prevention of Adults with Hospital-acquired and Ventilator-associated Pneumonia. 1-27.
James, C.W., and C. Gurk-Turner. 2001. Recommendations for monitoring serum vancomycin concentrations. BUMC Proceedings. 14: 189-190.
Jeffres, M.N., W. Isakow, J.A. Doherty, P.S. McKinnon, D.J. Ritchie, S.T. Micek, and M.H. Kollef. 2006. Predictors Of Mortality For Methicillin-Resistant Staphylococcus aureus Health-Care-Associated Pneumonia: Specific Evaluation Of Vancomycin Pharmacokinetic Indices. Chest. 130: 947-955.
Johnson, JA. 1997. Influence of Race or Ethnicity on Pharmacokinetics of Drugs. Journal of Pharmaceutical Sciences. 86(12): 1328-1333.
Llopis-Salvia, P., and N.V. Jimenez-Torres. 2006. Population Pharmacokinetic Parameters Of Vancomycin In Critically Ill Patients. Journal of Clinical Pharmacy and Therapeutics. 31: 447-454.
Mahoney, K., L.A. Browning, L.G. Hall, and J. Janisse. 2006. An Evaluation of a Validated Vancomycin Dosing Nomogram in the Critically Ill Population: 550. Critical Care Medicine. 34(12): A153.
Pai, M.P., M, Neely, K.A. Rodvold, and T.P. Lodise. 2014. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Advanced Drug Delivery Review. 77: 50-57.
Patanwala, A.E., C.J. Norris, D.E. Nix, B.J. Kopp, and B.L. Erstad. 2009. Vancomycin Dosing for Pneumonia in Critically Ill Trauma Patients. The Journal of Trauma. 67(4): 802-804.
Patel, N., M.P. Pai, K.A. Rodvold, B. Lomaestro, G.L. Drusano, and T.P. Lodise. 2011. Vancomycin: We Can’t Get There From Here. Clinical Infectious Diseases. 52: 969-974.
Revilla, N., A. Martin-Suarez, M.P. Perez, F.M. Gonzalez, and M. del Mar Fernandez de Gatta. 2010. Vancomycin Dosing Assessment In Intensive Care Unit Patients Based On A Population Pharmacokinetic/Pharmacodynamic Simulation. British Journal of Clinical Pharmacology. 70(2): 201-212.
Roberts, J.A., F.S. Taccone, A.A. Udy, J. Vincent, F. Jacobs, and J. Lipman. 2011. Vancomycin Dosing in Critically Ill Patients: Robust Methods for Improved Continuous-Infusion Regimens. Antimicrobial Agents and Chemotherapy. 55(6): 2704-2709.
Sanchez, J.L., A.R. Dominguez, J.R. Lane, P.O. Anderson, E.V. Capparelli, and J.M. Cornejo-Bravo. 2010. Population Pharmacokinetics Of Vancomycin In Adult And Geriatric Patients: Comparison Of Eleven Approaches. International Journal of Clinical Pharmacology and Therapeutics. 48: 525-33.
Scaglione F., and L. Paraboni. 2008. Review Pharmacokinetics / pharmacodynamics of antibacterials in the Intensive Care Unit: setting appropriate dosing regimens. International Journal of Antimicrobial Agents. 32: 294-301.
Varghese, J.M., J.A. Roberts, and L. Lipman. 2010. Pharmacokinetics and pharmacodynamics in critically ill patients. Current Opinion in Anesthesiology. 23(4): 472-478.
Thitinat Dedkaew1*, Tim Roy Cressey2,3, Chaicharn Phothirat4, Romanee Chaiwarith4, Baralee Punyawudho1 and Aroonrut Lucksiri1
1 Department of Pharmaceutical Care, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand
2 Program for HIV Prevention and Treatment (PHPT/IRD URI 174), Division of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
3 Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, MA, USA
4 Department of Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: thitinat_dedkaew@cmu.ac.th
Total Article Views

