
A Simple Device for Collecting Exhaled Breath Condensate (EBC) to Study Inflammatory Biomarkers of PM10 Exposure in Thai Schoolchildren
Waraphan Phornwisetsirikun, Tippawan Prapamontol*, Somporn Chantara, Prasak Thavornyutikarn and Somrak Rangkakulnuwat*Published Date : 2019-08-23
DOI : 10.12982/cmujns.2016.0004
Journal Issues : Number1 ,January - April 2016
ABSTRACT
This study developed a portable device to collect exhaled breath condensate (EBC) and used it to collect EBC samples from schoolchildren exposed to ambient PM10. The developed device was validated, including investigating the effect of collecting duration and breathing patterns on EBC volume, with five healthy volunteers. All five volunteers tolerated the device well, completing the EBC collection procedure without difficulty. Collecting normal tidal breathing for 10 minutes yielded the required EBC volume. We conducted a follow-up study with 104 healthy schoolchildren from two different primary schools in Chiang Mai, Thailand. We measured exhaled H2O2 concentrations in both the rainy and dry season; ambient PM10 was significantly higher in the dry season. In the dry season, the mean exhaled H2O2 concentration was significantly higher in both groups (p<0.05). This study showed that the developed EBC collector device was cost effective, safe, rapid, and simple to use and exhaled H2O2 could be used as a biomarker for elevated PM10 exposure before clinical symptoms appeared.
Keywords: Exhaled breath condensate collector device, Inflammatory marker, Schoolchildren, Chiang Mai, PM10
INTRODUCTION
Previous studies have shown that airborne particulate matter less than 10 μm in aerodynamic diameter (PM10) is a complex mixture of many pollutants –chemicals and transition metals, that are capable of redox cycling and that can stimulate inflammatory responses, especially in children (Kelly, 2003; Schwartz, 2004). Evidence has suggested that organic components deposited on the particle surface play an important role in mediating the toxic effect and inducing oxidative stress in the lungs, especially when antioxidant defenses have been overwhelmed. Airway inflammation plays an important role in the pathophysiology of various respiratory diseases. Respiratory tract health is traditionally assessed using airway biopsies, bronchoalveolar larvage (BAL) fluid, and bronchoscopy. Although these techniques provide direct information about the degree of airway inflammation, they are invasive, difficult, and not suitable for repeated use in children (Montushi and Barnes, 2003).
However, the precise mechanism of the relationship between ambient PM10 and respiratory health remains unclear, partly because of the lack of a non-invasive, biological sample, collection procedure for assessing lung inflammation. Recently, research has focused on analyzing biomarkers in exhaled breath. Exhaled breath condensate (EBC) is a new matrix for monitoring airway inflammation and oxidative stress markers of various respiratory conditions (Montushi and Barnes, 2003). EBC contains a number of volatile and non-volatile compounds derived from the respiratory surface, such as hydrogen peroxides, lipid peroxidation-derived products, and protein carbonyl groups (Taylor, 2011). In addition, EBC collection is non-invasive compared to bronchoscopy or induced sputum; it also facilitates repeated measurements in the same individual, a significant advantage over some of the other methodologies available for measuring inflammatory responses. Hydrogen peroxide (H2O2) is an oxidant produced by the alveolar membrane and can be measured in exhaled air. H2O2, a marker of oxidative stress, is pathologically indicative of lung inflammation (van Beurden et al., 2002; Murata et al., 2014). Recently, numerous researchers have explored the utility of studying airway inflammation and oxidative stress in the context of pollution exposure (Jansen et al., 2005), as well as clinical monitoring (Antus and Kardos, 2015; Corradi et al., 2015; Garcia-de-la-Asuncion et al., 2015). EBC has been proposed as a simple, non-invasive tool for measuring airway inflammation. The biomarkers in EBC include H2O2, which has been proposed as a method for assessing the health effects of air pollution in exposed populations (Doniec et al., 2005; Epton et al., 2008).
Although a variety of devices to collect EBC samples are commercially available, they are expensive and not suitable for field study. The developed EBC collecting device was based on the similar principle as the system of Dean et al. (2007), but with some modifications. The condensation chamber was constructed of polypropylene, following the recommendation of Horvath (2005) for EBC collection devices.
This study aimed to develop a portable device to collect EBC samples and, using these samples, assess airway inflammation in urban and highland schoolchildren exposed to ambient PM10 in northern Thailand.
MATERIAL AND METHODS
Development and validation of a portable EBC collecting device
The portable EBC collecting device (Figure 1) consists of a mouthpiece with a one-way valve in which inspiratory and expiratory air are separated. The mouthpiece is connected to a flexible plastic tube (30 cm in length and 2 cm in internal diameter) that allows subjects to find a comfortable position. The tube connects to a 50 mL polypropylene collecting tube that acts as a sampling container. Polypropylene is a thermoplastic polymer used in variety of healthcare and laboratory applications, including syringes, tubing, hospital disposables, test tubes, beakers, and pipettes (Sastri, 2014). The collecting tube is placed inside a stainless steel chamber and designed to connect with a second one-way valve that allows the excess air in an expired breath to flow toward the top. A rubber ring between the flexible plastic tube and the hole in the stainless steel chamber creates an airtight connection. The stainless steel chamber contains liquid nitrogen in order to cool down the collecting tube.
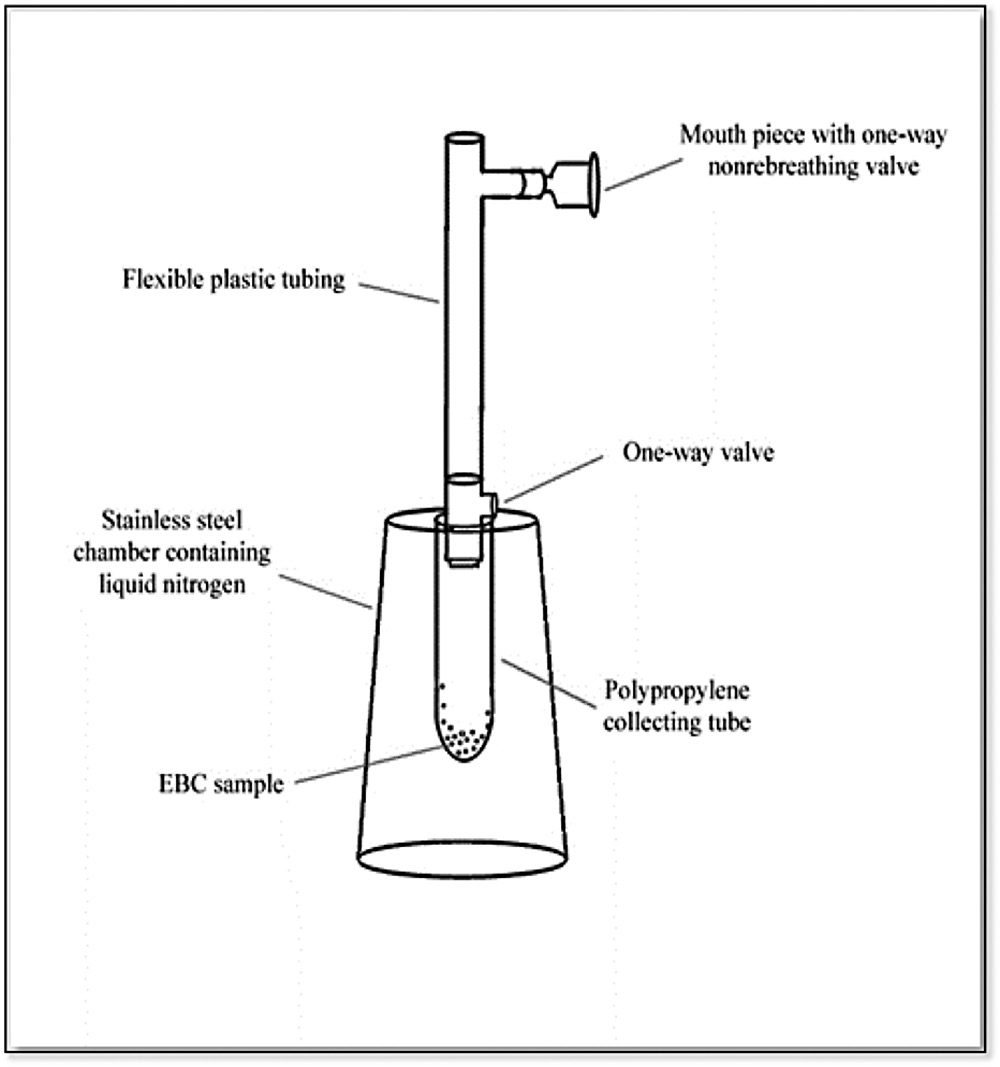
Figure 1. Schematic diagram of the developed portable EBC collecting device.
The developed device was validated for: (1) technical problems, such as any discomfort while wearing the device, (2) length of time required to collect adequate EBC volume, and (3) overall acceptance of the device by the volunteer subjects. Five healthy volunteers were recruited (12, 17, 25, 40, and 44 years old). Each volunteer was asked to breathe normally into the developed device for 10 minutes to collect EBC samples. After that, they were given a 30-minute break before collecting EBC samples a second time, for 20 minutes. Validation criteria (1) and (3) were assessed by verbal communication between volunteer users and a trained research technician. For criteria (2), two EBC samples were collected and the condensate EBC volume of each sample was measured using a calibrated 1000-μL pipette.
Application of the developed portable EBC device to collect EBC samples
Study site. Two primary schools, one urban and one rural/highland, were selected as study sites. Chiang Mai Rajabhat University Demonstration School (or urban school) in Chiang Mai city represented the urban location. A recent study reported on the EBC malondialdehyde (MDA) as a biomarker of effect during elevated ambient PM10 levels at this study site (Phornwisetsirikun et al., 2014). Srinaeroo School (or highland school) represented the rural highland location.
The urban school is located in the northern part of Chiang Mai city, in the valley at about 300 m above mean sea level. This study site is adjacent to a street on one side and surrounded by workplaces and commercial areas on the other sides. The school is located within 2.5 km of the air quality monitoring station at the Yupparaj Wittayalai School in downtown Chiang Mai city. The PM10 data from this station was assumed representative of the urban participants’ exposure to PM10.
The highland school is located on Doi Suthep Mountain, about 35 km northwest of Chiang Mai city and about 1300 m above mean sea level. With no air quality monitoring station nearby, a portable airborne dust monitor (E-sampler, Met One Instruments Inc., USA) was used to collect PM10 data at the location. This portable monitor was calibrated with the air quality monitoring station at the Yupparaj Wittayalai School, before using at the highland school. The PM10 data from the portable airborne dust monitor was assumed representative of the highland participants’ exposure to PM10.
Criteria of study subjects. To be included in the study, the schoolchildren had to have attended the selected primary school for at least one year and live within 2.5 km of the air quality monitoring station for the urban participants or the portable airborne dust monitor location for the highland participants. The children had to be 10-12 years old, not diagnosed with asthma or other chronic respiratory diseases, not on long-term medication, and willing to participate in the study.
The Human Experimentation Committee of the Research Institute for Health Sciences, Chiang Mai University, Thailand, approved the study protocol (Certificate HEC approval No. 1/2010). Children and parents signed written informed consent before participation.
Study period. The study was conducted during July 2011 (rainy season) and March 2012 (dry season).
Exhaled H2O2 analysis
EBC samples were collected from the schoolchildren using the developed device, as shown in Figure 2, during both the rainy season (low PM10 level) and dry season (high PM10 level). After rinsing their mouths, subjects were instructed to form a complete seal around the mouthpiece and maintain a dry mouth during collection by periodically swallowing excess saliva. The subjects sat comfortably and wore nose clips. They were instructed to breathe normally and expire very slowly through the mouthpiece; this continued for 10 minutes to collect condensate of about 1.2 mL per subject. The collected EBC samples were immediately stored at -70°C until analysis.

Figure 2. Collection of EBC samples using the developed device.
The concentration of H2O2 in EBC was measured using a spectrophotometric assay by means of horseradish peroxidase-catalyzed oxidation of tetramethylbenzidine, according to the method previously described by Gallatin and Pratch (1985). The detection limit was approximately 0.1 μM.
Data analysis
The statistical analysis was performed using the Statistical Package for the Social Sciences for Windows (SPSS, Thailand) Version 17. The differences between exhaled H2O2 levels and pulmonary function indices in the rainy and dry seasons were determined using paired t-test. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Development and validation of a portable EBC collecting device
The developed EBC collecting device was validated with five healthy volunteers; they reported no technical problems or complaints while wearing the device. EBC samples from five healthy volunteers were collected and the mean volumes by normally breathing over a period of 10 and 20 minutes were 1.24±0.07 (n=5) and 1.30±0.05 (n=5) mL, respectively (p=0.017). As 10 minutes yielded adequate volume for the biomarker test in the present study, a collection time of 10 minutes was used throughout the study. The procedure of EBC collection using the developed device was found safe, rapid, and simple to use and operate.
Application of the EBC device to collect EBC samples for investigating the airway inflammation of schoolchildren
General characteristics of the study participants. Table 1 shows the general characteristics of the participants. The participants, all between the ages of 10-12 years, had a median height of 129-144 cm and weight of 27-34 kg. However, the urban children were significantly taller and heavier than their highland counterparts, partly skewed by one overweight subject of 59 kg.
Table 1. General characteristics of the participants.
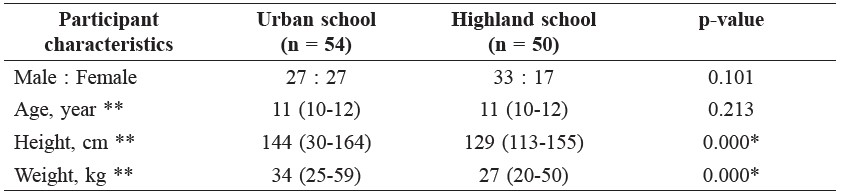
Note: *significant difference between schools (p < 0.05), ** median (range).
PM10 and exhaled H2O2 concentrations. In both schools, the 24-hour mean PM10 in the dry season was significantly higher than in the rainy season. However, it was five times higher at the urban school, while only twice as high at the highland school; this difference between these two locations was also statistically significant. Both levels of PM10 during the study period did not exceed Thailand’s 24-hour mean PM10 limit of 120 μg/m3 (Thailand Air Quality and Noise Standards, 2004). This phenomenon has been explained by the westerly wind that blows into Chiang Mai City (Wiriya et al., 2013). Also, Doi Suthep Mountain, to the west, is a National Park and open biomass burning is strictly prohibited.
At the urban school, the mean concentration of exhaled H2O2 was 0.17 μM in the rainy season and 0.21 μM in the dry season (p = 0.003). At the highland school, the mean concentration of exhaled H2O2 was 0.16 μM in the rainy season and 0.18 μM in the dry season (p=0.001). The concentrations of exhaled H2O2 in both schools increased significantly in the dry season.
Table 2. Comparison of PM10 and exhaled H2O2 concentrations of the schoolchildren from urban and highland schools between the rainy and dry seasons.
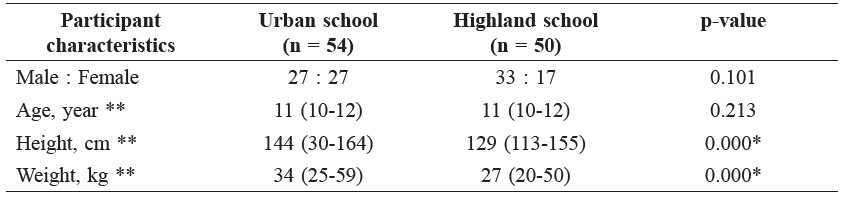
Note: *significant difference between schools (p < 0.05), ** median (range).
DISCUSSION
We developed a cost-effective EBC collecting device for in-house and field use; each device costs about THB 600 (about USD 20) (Figure 2) and consumes liquid nitrogen for cooling of about THB 5 per sample. The device was employed in a field study of 104 primary schoolchildren in 2011-2012 in Chiang Mai. The collection procedure, which took only 20 minutes per person, was simple and did not require skilled medical staff. A trained operator was sufficient to collect the EBC samples from the subjects. Our collection procedure compared favorably with Mutlu et al. (2001), who reported that collection usually takes 5-10 minutes in adults and up to 15-20 minutes in children to obtain 1-3 mL of EBC. Furthermore, our developed device, which can be used in the field, offered similar performance to commercially available devices used in research and clinical studies, including children with respiratory diseases, which collect 1.5-2 mL of EBC in 10-15 minutes (Romieu et al., 2008; De Prins et al., 2014; Rosa et al., 2014).
Exhaled H2O2, the inflammatory biomarker used in our study, has been shown to be a significant biomarker from ambient elevated PM10 exposure. Our results here also confirm our previous findings, which reported that the EBC malondialdehyde (MDA) biomarker of oxidative stress was raised in children exposed to PM10 air pollution (Phornwisetsirikun et al., 2014). These results are also consistent with other studies of EBC biomarkers of pulmonary inflammation (Ralph et al., 2006; Gergelova et al., 2008; Chow et al., 2009).
Although possible confounding factors may exist in measuring exhaled H2O2 concentration, the present study design was a follow-up study of the same person, in order to minimize individual variability. Therefore, the present study results demonstrated that the exhaled H2O2 concentrations, as well as the MDA concentrations from our previous report (Phornwisetsirikun et al., 2014), are suitable biomarkers of elevated PM10 exposure. In conclusion, our study developed an economical device for collecting EBC that was non-invasive, safe, rapid, and simple to use. In addition, the results indicated that exhaled H2O2 concentration provided good information about inflammation in the respiratory system of children (i.e., healthy schoolchildren in the present study) before clinical symptoms appear.
ACKNOWLEDGEMENTS
The authors thank the schoolchildren for their cooperation throughout the study period, and the Research Institute for Health Sciences (RIHES), Chiang Mai University for laboratory support. This study was funded by a grant from the Center of Excellence on Environmental Health and Toxicology, Faculty of Science, Chiang Mai University and the Commission for Higher Education through the National Research University Program, Chiang Mai University, Chiang Mai, Thailand.
REFERENCES
Antus, B., and Z. Kardos. 2015. Oxidative stress in COPD: molecular background and clinical monitoring. Current Medicinal Chemistry 22(5): 627-650.
Corradi, M., D. Poli, I. Banda, S. Bonini, P. Mozzoni, S. Pinelli, R. Alinovi, R. Andreoli, L. Ampollini, A. Casalini, P. Carbognani, M. Goldoni, and A. Mutti. 2015. Exhaled breath analysis in suspected cases of non-small-cell lung cancer: a cross-sectional study. Journal of Breath Research 9(2): 027101. doi: 10.1088/1752-7155/9/2/027101
Dean, H.C., G. Jesse, and S.T. Paul. 2007. Proteomics as a method for early detection of cancer: A review of proteomics, exhaled breath condensate, and lung cancer screening. Journal of General Internal Medicine (Suppl 1): 78-84.
De Prins, S., E. Dons, M. Van Poppel, L. Int Panis, E. Van de Mieroop, V. Nelen, B. Cox, T.S. Nawrot, C. Teughels, G. Shoeters, and G. Koppen. 2014. Airway oxidative stress and inflammation markers in exhaled breath from children are linked with exposure to black carbon. Environment International 73: 440-446. doi: http://dx.doi.org/10.1016/j.envint.2014.06.017
Doniec, Z., D. Nowak, W. Tomalak, K. Pisiewicz, and R. Kurzawa. 2005. Passive smoking does not increase hydrogen peroxide (H2O2) levels in exhaled breath condensate in 9-year-old healthy children. Pediatric Pulmonology 39: 41-45.
Epton, M.J., R.D. Dawson, W.M. Brooks, S. Kingham, T. Aberkane, J.E. Cavanagh, C.M. Frampton, T. Hewitt, J.M. Cook, S. McLeod, F. McCartin, K. Trought, and L. Brown. 2008. The effect of ambient air pollution on respiratory health of schoolchildren: a panel study. Environmental Health 16. doi: 10.1186/1476-069x-7-16.
Gallati, H., and I. Pracht. 1985. Horseradish peroxidase: kinetic studies and optimization of peroxidase activity determination using the substrates H2O2 and 3,3´,5,5´-tetramethylbenzidine. Journal of Clinical Chemistry and Clinical Biochemistry 23: 453-460.
García-de-la-Asunción J., E. García-Del-Olmo, J. Perez-Griera, F. Martí, G. Galan, A. Morcillo, R. Wins, R. Guijarro, A. Arnau, B. Sarriá, M. García-Raimundo, and J. Belda. 2015. Oxidative lung injury correlates with one-lung ventilation time during pulmonary lobectomy: a study of exhaled breath condensate and blood. European Journal of Cardio-Thoracic Surgery doi: 10.1093/ejcts/ezv207
Hartog, J.J., J.G. Ayres, A. Karakatsani, A. Analitis, H.T. Brink, K. Hameri, R. Harrison, K. Katscuyanani, A. Kotronarou, I. Kavouras, C. Meddings, J. Pekkanen, and G. Hoek. 2010. Lung function and indicators of exposure to indoor and outdoor particulate matter among asthma and COPD patients. Occupational and Environmental Medicine 67: 2-10.
Horvath, I., J. Hunt, and P.J. Barne 2005. Exhaled breath condensate: methodological recommendations and unresolved questions. European Respiratory Journal 26: 523-548.
Jansen, K.L., T.V. Larson, J.Q. Koenig, T.F. Mar, C. Fields, J. Stewart, and M. Lippmann. 2005. Associations between health effects and particulate matter and black carbon in subjects with respiratory disease. Environmental Health Perspectives 113: 1741-1746.
Janson, C., S. Chinn, D. Jarvis, J. P. Zock, K. Toren, and P. Burney. 2001. Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community Respiratory Health Survey: a cross-sectional study. The Lancet 358(9299): 2103-2109. doi: 10.1016/s0140-6736(01)07214-2
Kelly, F.J. 2003. Oxidative stress: its role in air pollution and adverse health effects. Occupational and Environmental Medicine 60: 612-616.
Langkulsen, U., W. Jinsart, K. Karita, and E. Yano 2006. Health effects of respirable particulate matter in Bangkok schoolchildren. International Congress Series 1294: 197-200.
Linares, B., J.M. Guizar, N. Amador, A. Garcia, V. Miranda, J.R. Perez, and R. Chapela. 2010. Impact of air pollution on pulmonary function and respiratory symptoms in children. Longitudinal repeated-measures study. Pulmonary Medicine 10: 62.
Miller, M.R., J. Hankinson, V. Brusasco, F. Burgos, R. Casaburi, A. Coates, R. Crapo, P. Enright, C.P.M. vav der Grinten, P. Gustafsson, R. Jensen, D.C. Johnson, N. Maclntyre, R. McKay, D. Navajas, O.F. Pedersen, R. Pellegrino, G. Viegi, and J. Wanger. 2005. Standardisation of spirometry. European Respiratoty Journal 26: 319-338.
Montuschi, P., and P.J.Barnes. 2003. Analysis of exhaled breath condensate for monitoring airway inflammation. Trend in Pharmacological Sciences 23: 232-237.
Murata, K., K. Fujimoto, Y. Kitaguchi, T. Horiuchi, K. Kubo, and T. Honda. 2014. Hydrogen peroxide content and pH of expired breath condensate from patients with asthma and COPD. COPD, 11(1): 81-87. doi: 10.3109/15412555.2013.830094
Mutlu, G.M., K.W. Garey, R.A. Robbins, L.H. Danziger, and I. Rubinstein. 2001. Collection and analysis of exhaled breath condensate in human. American Journal of Respiratory and Critical Care Medicine 164: 731-737.
Phornwisetsirikun, W., T. Prapamontol, S. Rangkakulnuwat, S. Chantara, P. Thavornyutikarn. 2014. Elevated ambient PM10 levels affecting respiratory health of schoolchildren in Chiang Mai, Thailand. Chiang Mai University Journal of Natural Sciences 113: 345-353.
Ralph, J.D., S. Norbert, G. Dan, T. Thomas, S. Constantinos, F. Kochy, C.G. Steven, and K. Michael. 2006. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environmental Health Perspectives 114: 1736-1743.
Romieu, I., A. Barraza-Villarreal, C. Escamilla-Nuñez, A.-C. Almstrand, D. Diaz-Sanchez, P. D. Sly, and A.-C. Olin. 2008. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. Journal of Allergy and Clinical Immunology 121(4): 903-909.e906. doi: http://dx.doi.org/10.1016/j.jaci.2007.12.004
Rosa, M. J., B. Yan, S. N. Chillrud, L. M. Acosta, A. Divjan, J. S. Jacobson, and M. S. Perzanowski. 2014. Domestic airborne black carbon levels and 8-isoprostane in exhaled breath condensate among children in New York City. Environmental Research 135: 105-110. doi: http://dx.doi.org/10.1016/j.envres.2014.09.003
Qian, Z., J. Zhang, L.R. Korn, F. Wei, and R.S.Chapman. 2004. Factors analysis of household factors: are they associated with respiratory conditions in Chinese children?. International Journal of Epidemiology 33: 582-588.
Sastri, V.R. 2014. 6 - Commodity Thermoplastics: Polyvinyl Chloride, Polyolefins, and Polystyrene. In Plastics in Medical Devices (Second Edition). V.R. Sastri, editor. William Andrew Publishing, Oxford. 73-120.
Schwartz, J. 2004. Air pollution and children’s health. Pediatrics, 113: 1037-1043.
Taylor D.R. 2011. Using biomarkers in the assessment of airways disease. Journal of Allergy and Clinical Immunology 128: 927-934.
Thailand Air Quality and Noise Standards. 2004. Notification of National Environmental Board No. 24, B.E. 2547 (2004) under the Enhancement and Conservation of National Environmental Quality Act B.E.2535 (1992), published in the Royal Government Gazette No. 121 Special Part 104 D dated September 22, B.E.2547 (2004).
van Beurden, W.J., P.N.R. Dekhuijzen, and F.W.J.M. Smeenk. 2003. Exhaled biomarkers in COPD: Their potential role diagnosis, treatment and prognosis. Chest Disease 57: 258-267.
Wiriya, W., T. Prapamontol, and S. Chantara. 2013. PM10-bound polycyclic aromatic hydrocarbons in Chiang Mai (Thailand): seasonal variations, source identification, health risk assessment and their relationship to air-mass movement. Atmospheric Research 124: 109-122.
Waraphan Phornwisetsirikun1,2, Tippawan Prapamontol2*, Somporn Chantara3, Prasak Thavornyutikarn3, and Somrak Rangkakulnuwat4,5*
1 Environmental Science Program and Center of Excellence on Environmental Health and Toxicology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
2 Environment and Health Research Unit, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
3 Department of Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
4 Department of Pediatrics, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
5 Pediatrics Service, Chiangmai Ram Hospital, Chiang Mai 50200, Thailand
*Corresponding authors. E-mail: tprapamontol@gmail.com, srangkak@yahoo.com
Total Article Views

