
Identification of Antioxidants in Lamiaceae Vegetables by HPLC-ABTS and HPLC-MS
Trakul Prommajak, Sang Moo Kim, Cheol-Ho Pan, Sang Min Kim, Suthat Surawang and Nithiya Rattanapanone*Published Date : 2019-08-23
DOI : 10.12982/cmujns.2016.0003
Journal Issues : Number1 ,January - April 2016
ABSTRACT
Antioxidants in five Lamiaceae vegetables, namely lemon balm, sweet basil, clove basil, holy basil, and lemon basil, were identified by HPLC-ABTS and HPLC-MS. The major antioxidants in the samples were hydroxycinnamic acids. Rosmarinic acid exhibited the highest antioxidant activity in each sample, except clove basil, in which chicoric acid contributed the highest antioxidant activity. Lemon balm had the highest concentration of rosmarinic acid. Chicoric acid (highest in clove basil) and dihydroxy-dimethoxyflavone (highest in basil) were also important antioxidants in many Ocimum spp. Other minor antioxidants were caffeic acid, caftaric acid, syringic acid, syringic acid, rutin, and acacetin-acetylglucoside.
Keywords: Lamiaceae, Antioxidant, HPLC-ABTS, Rosmarinic acid, Chicoric acid
INTRODUCTION
Free radicals in food cause lipid peroxidation, which adversely affects its nutritional and organoleptic properties (Yen and Duh, 1994). Free radicals in the human body are associated with many degenerative diseases, e.g. cardiovascular disease, diabetes, and cancer (Boeing et al., 2012). Consumption of fruits, vegetables, and antioxidants was associated with a lower risk of cancers and cardiovascular disease (Genkinger et al., 2004).
Lamiaceae is a family of annual or perennial aromatic herbs. It contains 236 genera that can be grown under a variety of soil and climatic conditions worldwide, with the exception of the cold regions of high latitude (Harley et al., 2004). Lavender, thyme, peppermint, patchouli, rosemary, and sage are used to extract volatile aromatic oil or prepare perfumes. Some plants are used for medicinal purposes. For example, the leaves of Ocimum sanctum are used to treat the common cold. Flowers of O. basillicum are used to treat digestive and renal diseases. Leaves and tubers of some Lamiaceae plants are used as food (Reddy et al., 2004).
Lamiaceae plants also contain antioxidative phenolic compounds that effectively scavenge free radicals. The major antioxidants in the plants of this family are hydroxycinnamic acids and flavonoids. Rosmarinic acid has also been found, notably in many Lamiaceae plants, e.g. basil, sage, lemon balm, peppermint, thyme, lavender, rosemary, marjoram, hyssop, savory, and oregano (Zgórka and Głowniak, 2001; Dorman et al., 2003). The other hydroxycinnamic acids responsible for the antioxidant capacity of Lamiaceae plants include caffeic acid, chlorogenic acid, and ferulic acid (Chen and Ho, 1997). Chicoric acid, another hydroxycinnamic acid, was recently found in basil and had not yet been identified in other Lamiaceae plants (Lee and Scagel, 2009). Lamiaceae plants also contain flavonoids. Apigenin and luteolin derivatives have been identified in germander, sage, thyme, savory, marjoram, and oregano (Dorman et al., 2003; Valant-Vetschera et al., 2003).
Online HPLC-antioxidant assay is a technique developed for simultaneous determination of compounds and their antioxidant capacity. The compounds eluted from a column react with an oxidant probe, e.g. 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) radical cation (ABTS•+), and decrease the absorbance of the chromophore. This method avoids the decomposition of the compounds that occurs in a conventional process (He et al., 2010).
Lemon balm, basil, clove basil, holy basil, and lemon basil are common culinary herbs in Thailand. To the best of our knowledge, the profiles of the phenolic compounds with their antioxidant capacity of these plants cultivated in Thailand have not been reported. Therefore, the objective of this study was to identify and quantify the bioactive compounds that are responsible for antioxidant activity in Lamiaceae vegetables from Thailand using LC-ABTS and LC-MS assays.
MATERIAL AND METHODS
Materials
Lemon balm (Melissa officinalis L.), sweet basil (Ocimum basilicum L.), clove basil (Ocimum gratissimum L.), holy basil (Ocimum sanctum L.), and lemon basil (Ocimum × citriodorum) were purchased from a local market in Chiang Mai Province, Thailand during August-September 2012. Methanol and water for HPLC analysis were purchased from Fisher (Seoul, Korea). 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and potassium persulfate
were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of vegetable extracts
The edible parts of vegetables, without bruises or visual defects, were selected and washed in water for about 1 min. The vegetables (10 g) were extracted in 60% ethanol (50 ml) using a blender (Model MR 4050 CA, Braun, Spain) for 30 sec. Then, the extracts were filtered through a double layer of muslin cloth and centrifuged at 2,500xg for 20 min. The supernatant was kept at -80°C until analysis. Extraction was performed in duplicates.
Identification and quantification of antioxidants
Separation of the compounds in the crude vegetable extracts was performed by Agilent 1200 series HPLC (Agilent Technologies, Santa Clara, CA, USA) using the method described by He et al. (2010) with slight modification. Sample (10 μl) was eluted by 100% methanol (solvent A) and methanol/water/formic acid (20/80/0.25, v/v/v) (solvent B) through a Kromasil 100-5C18 (250×46 mm) column with a flow rate of 0.7 ml/min and column temperature of 30°C. Gradient elution began with the initial condition of 100% B, then changed to 90% B at 6.25 min, 80% B at 12.5 min, 55% B at 50 min, and 20% B at 68.75 min, before changing back to 100% B at 75 min. The compounds were detected by diode array detector (DAD) at 210, 254, 280, 330, and 520 nm.
The compounds that escaped from DAD were subjected to online-ABTS analysis. ABTS reagent (2 mM ABTS and 3.5 mM potassium persulphate) was prepared and kept in a dark condition at room temperature for 16 h to stabilize the radical before use. The reagent was pumped at a flow rate of 0.5 ml/min to react with the eluate, before passing through a multiple wavelength detector (MWD) for detection of ABTS at 734 nm. The presence of antioxidants in a sample resulted in negative peaks in the chromatogram.
Antioxidant concentrations were quantified from calibration curves. Flavonoids were quantified as the molar equivalent of quercetin. Other phenolic compounds were quantified as the molar equivalent of caffeic acid. Antioxidant capacities of the compounds were calculated from the area of the negative peak and expressed as Trolox equivalents.
For HPLC-MS analyses, compound separation and UV-Vis detection were performed using the same conditions as HPLC-ABTS. Atmospheric pressure ionization-electrospray (API-ES) ionization was performed by an Agilent 6120 quadrupole mass spectrometer in both positive and negative modes. Capillary voltages were 4,000 V for positive and 3,500 V for negative ionization. The drying gas flow rate was 9 L/min, the drying gas temperature was 350°C, and the nebulizer pressure was 35 psig. Mass spectra were recorded in the range of m/z 100-1,500. The compounds were identified by comparing molecular weights, fragments, and UV absorption with that reported in the literature.
Statistical analysis was performed by R version 2.15.1.
RESULTS
Identification of antioxidants
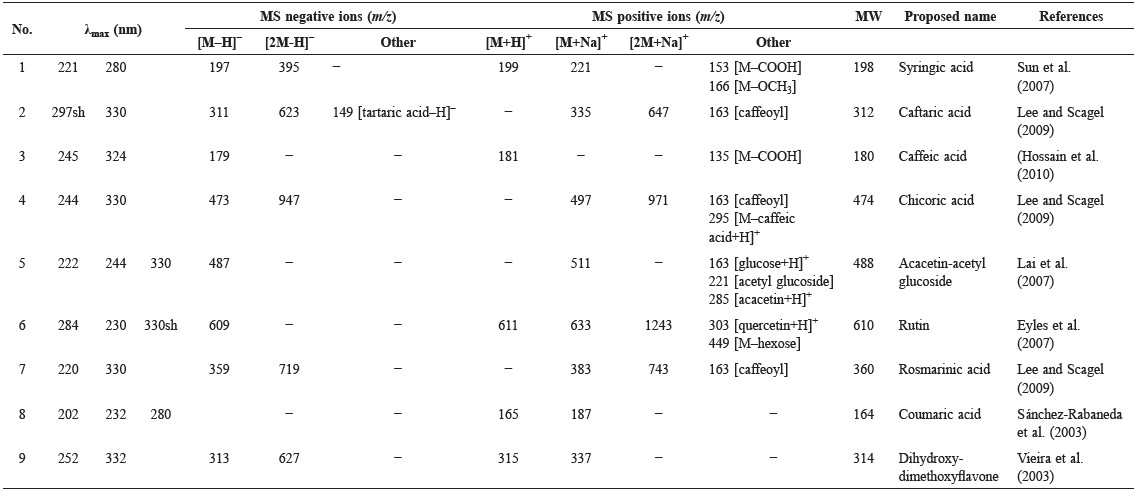
Various compounds accounted for the antioxidant activity of the ethanol extracts of the five Lamiaceae vegetables. HPLC-ABTS chromatograms revealed that the vegetables had similar antioxidant profiles (Figure 1). Mass spectra of identified compounds are shown in Figure 2. Compound 1 was identified as syringic acid (Table 1). Compounds 2, 3, 4, and 7 were hydroxycinnamic acids, identified as caftaric acid, caffeic acid, chicoric acid, and rosmarinic acid, respectively. Compounds 5, 6, and 9 were flavonoids, identified as acacetin-acetyl glucoside, rutin (quercetin rutinoside), and dihydroxy-dimethoxyflavone, respectively. For general phenolic compounds, UV absorption at 280 nm was detected. But for hydroxycinnamic acids and flavonoids, absorption maxima around 330 nm were also detected (Tomas-Barberan and Ferreres, 2012).
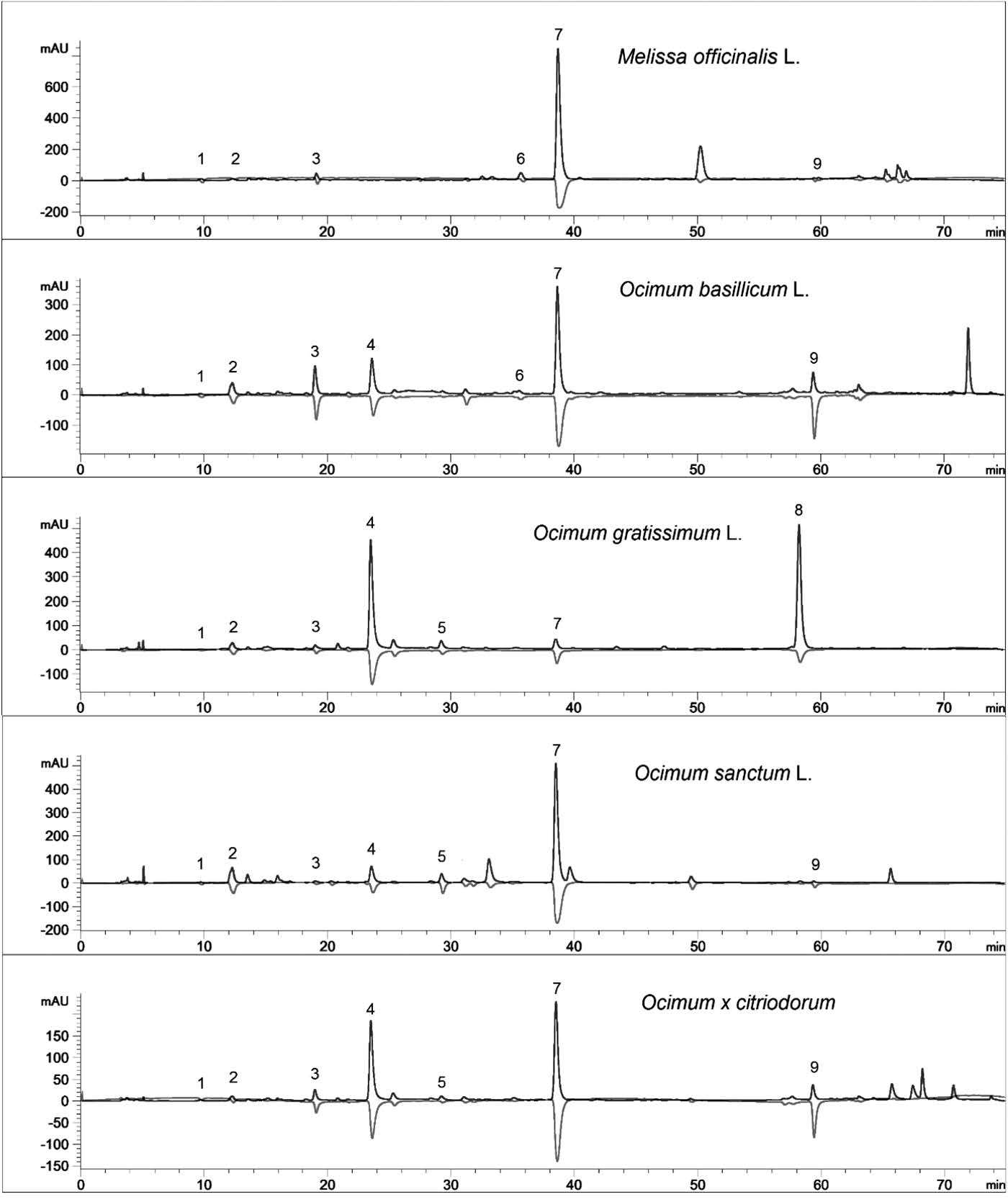
Figure 1. HPLC-ABTS chromatograms of the ethanolic extract of Lamiaceae vegetables. Positive peaks: DAD signal at 280 nm for detection of phenolic compounds. Negative peaks: MWD signal at 734 nm for detection of ABTS radical scavenging activity.
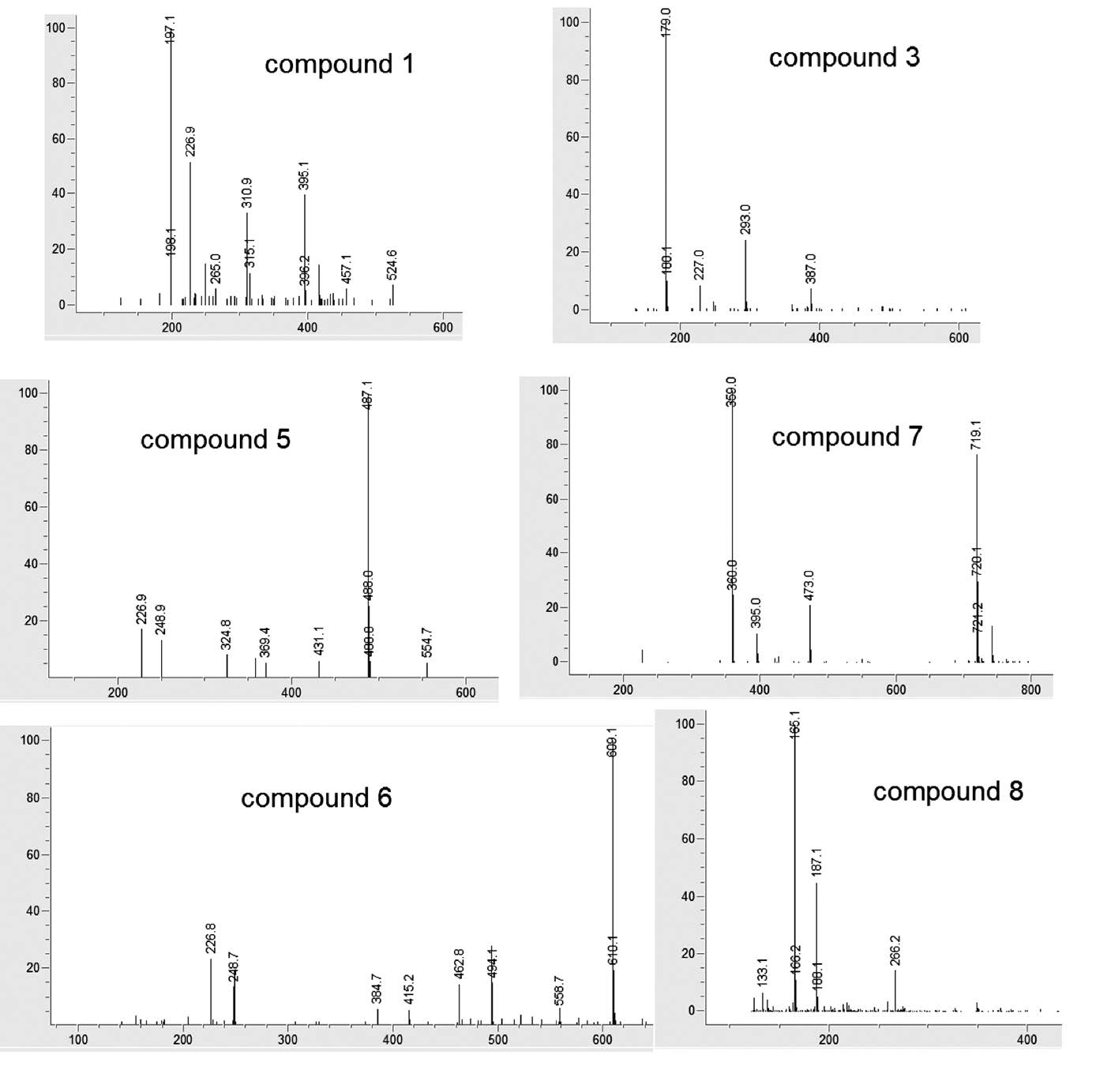
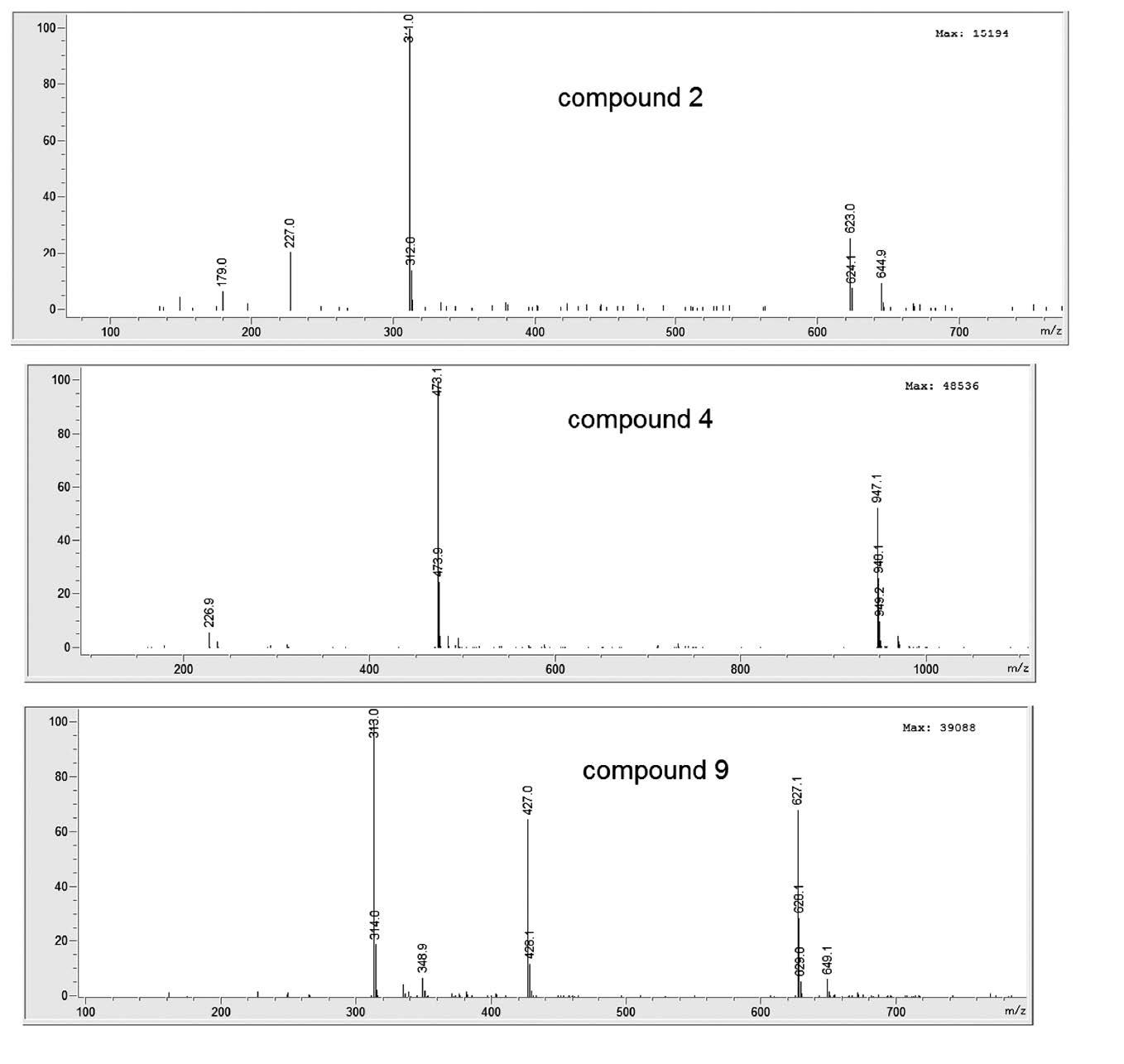
Figure 2. Mass spectra of antioxidative compounds identified from Lamiaceae vegetables. All mass spectra were from negative-ion mode, except compound 8.
Table 1. Identification of antioxidants in Lamiaceae vegetables by HPLC-MS.
Quantification of antioxidative compounds
The phenolic acid concentrations in five Lamiaceae vegetables are shown in Table 2. Of the phenolic compounds, rosmarinic acid was the most abundant in lemon balm, sweet basil, and holy basil, while chicoric acid was the most abundant in clove basil and lemon basil.
Table 2. The phenolic acid concentrations in Lamiaceae vegetables.
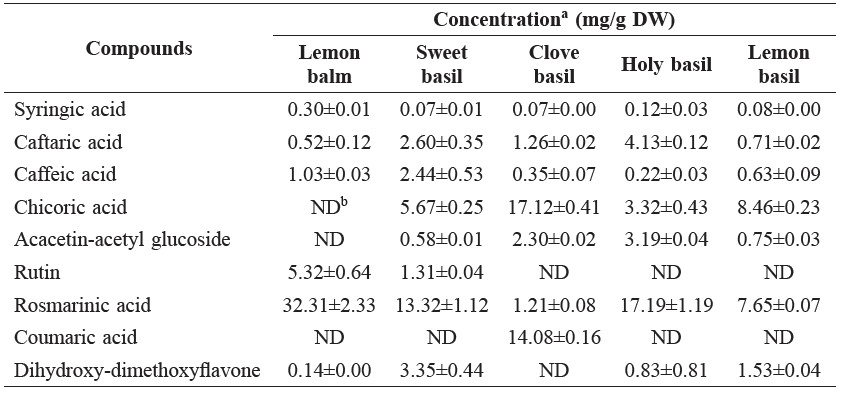
Note: aMeans of duplicate analyses. Flavonoids were quantified as molar equivalent of quercetin. Other phenolic compounds were quantified as molar equivalent of caffeic acid. bND, not detected.
Antioxidant capacity of phytochemicals
ABTS radical cation (ABTS•+) is a blue/green chromophore produced by the reaction between ABTS and potassium persulfate. It has an absorption maximum at 734 nm. In the presence of hydrogen-donating antioxidants, e.g. polyphenols and flavonoids, the radical cation becomes colorless. In an online HPLC-ABTS, it produced a negative peak in the presence of antioxidants (Koleva et al., 2001).
Rosmarinic acid possessed the highest antioxidant capacity among the compounds in the tested samples, except clove basil, in which chicoric acid contributed the highest antioxidant capacity (Table 3). Chicoric acid and dihydroxy-dimethoxyflavone were also responsible for a moderately large portion of antioxidant capacity in sweet basil and lemon basil. Caffeic acid, identified in all samples, was highest in sweet basil.
Table 3. Antioxidant capacity of phenolic compounds in Lamiaceae vegetables.
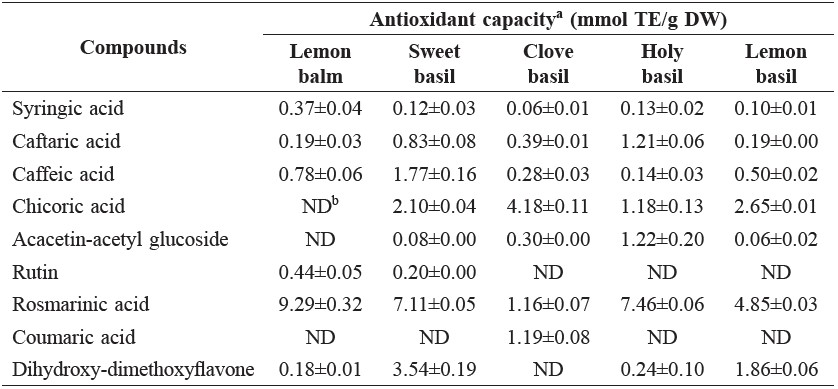
Note: aMeans of duplicate analyses. bND, not detected.
Trolox equivalent antioxidant capacity (TEAC) of antioxidants
The TEAC value is the specific antioxidant capacity of a given substance compared to the standard Trolox (Huang et al, 2005). It is calculated as the ratio between the antioxidant capacity (molar equivalent of Trolox from area of negative peak) and molarity of a compound. The antioxidant activity of the hydroxycinnamic acids decreased in the following order: rosmarinic acid > chicoric acid > caffeic acid > caftaric acid (Table 4).
Table 4. Trolox equivalent antioxidant capacity (TEAC) of phenolic compounds in Lamiaceae vegetables.
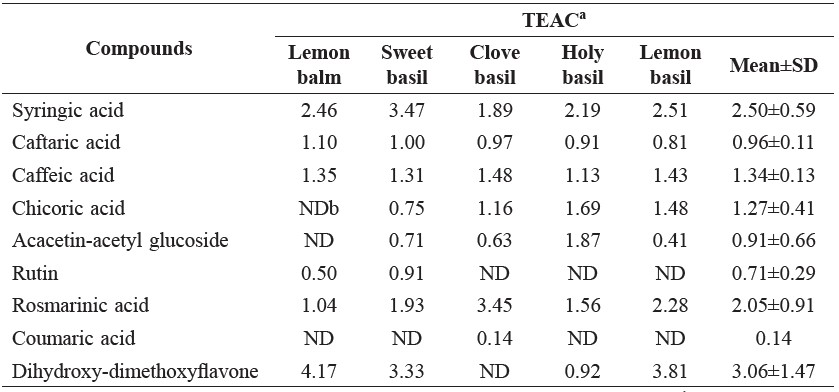
Note: aMolar of Trolox with equivalent antioxidant capacity of a 1 molar substance. bND, not detected.
DISCUSSION
Phenolic compounds are secondary metabolites in plants that play a role in plant adaptation to the environment, for example, protection against ultraviolet radiation, herbivores, pathogens, and abiotic stress (Bourgaud et al., 2001). The identified phenolic compounds were synthesized through the phenylpropanoid pathway as shown in Figure 3.
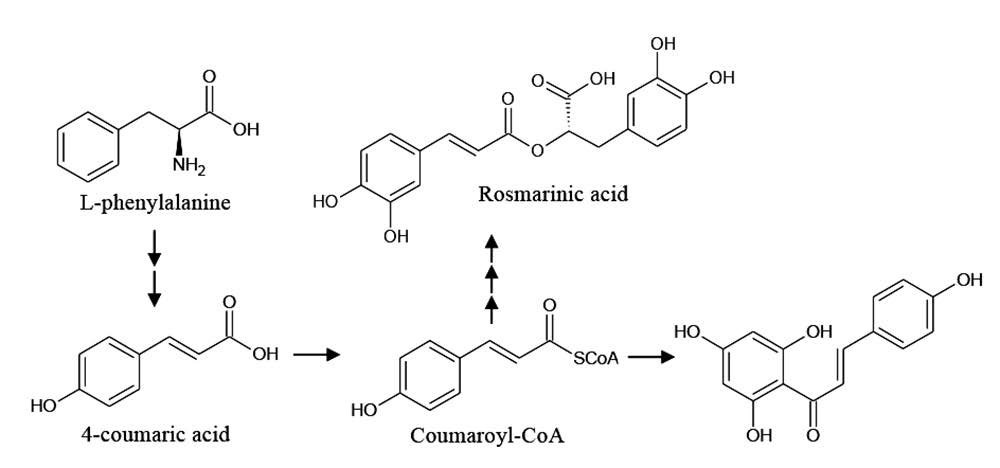
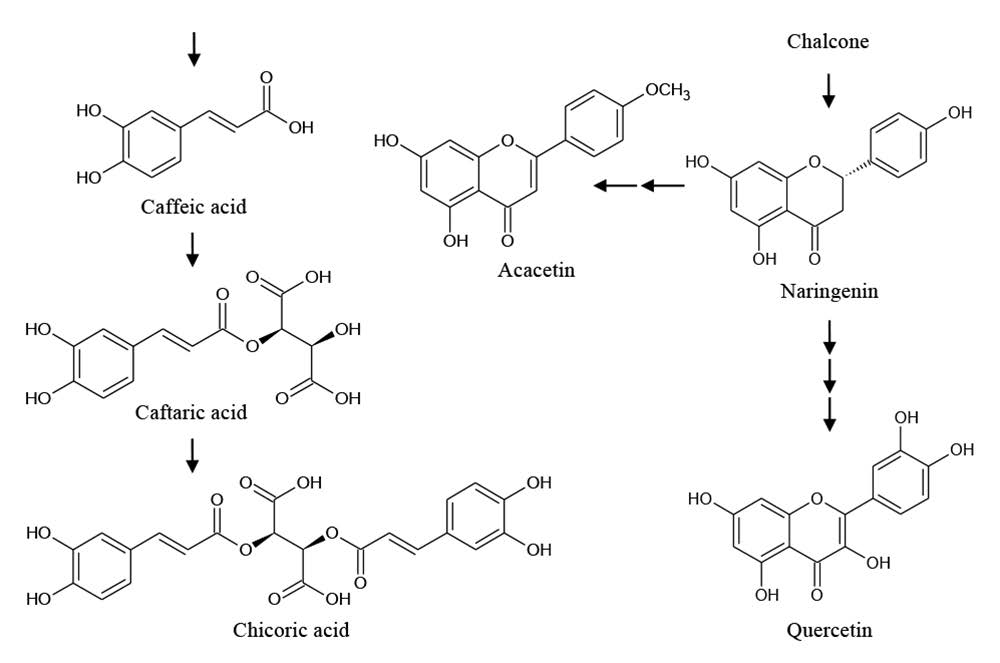
Figure 3. Phenylpropanoid pathway for synthesis of some phenolic compounds in Lamiaceae plants. Each arrow represents one enzymatic reaction.
Adapted from: Petersen et al. (2009); Saltveit (2009); and Bel-Rhlid et al. (2012).
Although the culinary herbs used in this study contained similar antioxidant compounds, their concentrations varied; this affected the total antioxidant capacity of the extracts. The variation in phenolic compounds in plants is affected by species, genotypes, climate, location, and growing conditions (Asami et al., 2003; Anttonen and Karjalainen, 2005; Scalzo et al., 2005).
The caffeic acid concentrations in the Lamiaceae plants in this study (from 0.22 mg/g DW in holy basil to 2.44 mg/g DW in sweet basil) were similar to the range found in 10 Lamiaceae plants (from 0.1 mg/g DW in lavender to 2.6 mg/g DW in sweet basil) by Zgórka and Głowniak (2001).
The concentration of caftaric acid in sweet basil (2.82 mg/g DW) found here was higher than those reported in 15 basil cultivars (from 0.09 to 0.49 mg/g DW) by Kwee and Niemeyer (2011). Caftaric acid has also been found in other plant families – 0.51-1.68 mg/g DW in manatee grass (Syringodium filiforme) (Nuissier et al., 2010) and 1.9-6.5 mg/g DW in purple coneflower (Echinacea purpuree), depending on growth temperature and light irradiation (Wu et al., 2007).
The concentration of chicoric acid in sweet basil (6.17 mg/100 g DW) found here was higher than those reported in 15 sweet basil cultivars (0.03-2.78 mg/g DW) by Kwee and Niemeyer (2011) and those reported in commercial dry basil (0.06-0.16 mg/g DW) by Lee and Scagel (2010). For other plant families, the concentration of chicoric acid has been reported as 0.94-5.26 mg/g DW for manatee grass (Nuissier et al., 2010) and 0.43-19.27 mg/g DW for Echinacea spp. (Pellati et al., 2004).
Chicoric acid has been previously identified as a major phenolic compound in Echinacea spp. (Perry et al., 2001). In addition to its antioxidant activity, this compound also has been shown to have antiviral properties by targeting human immuno-deficiency virus type 1 (HIV-1) integrase (Robinson Jr, 1998). Echinacea, indigenous to North America, has been commercialized worldwide for treating the common cold (Barrett, 2003). This study found chicoric acid, a main bioactive compound in Echinacea, in clove basil, which is widely cultivated in Southeast Asia.
Rosmarinic acid was the most abundant of the hydroxycinnamic acids found in basil (13.25 mg/g DW); others have also reported this (Lee and Scagel, 2010; Bušić et al., 2013). The rosmarinic acid concentration in basil (13.25 mg/g DW) was similar to that of sweet basil from New Zealand (10.86 mg/g DW) (Shan et al., 2005). However, this is higher than Kwee and Niemeyer (2011) found in 15 different basil cultivars (0.10 to 6.09 mg/g DW). Basil variety and nitrogen fertilization affected the concentration of the phenolic compounds. Rosmarinic acid concentrations ranged from 5.41 mg/g DW in basil cv. Sweet Thai irrigated with 5 mM nitrogen to 47.89 mg/g DW in basil cv. Dark Opal grown in the summer and irrigated with 0.1 mM nitrogen (Nguyen and Niemeyer, 2008). Rosmarinic acid content in sage (Salvia spp.), another Lamiaceae plant, has been shown to vary from 13.3 to 47.3 mg/g DW (Bandoniene et al., 2005). In addition to its antioxidant activity, rosmarinic acid also has been shown to have anti-inflammatory activity by inhibiting diesel exhaust particles-induced lung injury (Sanbongi et al., 2003) and antiviral activity against herpes simplex virus type 1 and 2 (Mazzanti et al., 2008; Astani et al., 2012).
Syringic acid has been identified in other Lamiaceae plants, e.g., basil, oregano, rosemary, sage, spearmint, and thyme, in concentrations ranging from 0.05 to 0.12 mg/g DW (Kivilompolo and Hyötyläinen, 2007).
Aglycone of acacetin has been previously identified in many Ocimum spp., e.g., O. basillicum, O. × citriodorum, O. americanum, and O. minimum (Grayer et al., 2004).
Rutin or quercetin-3-O-rutinoside has been previously identified in Lamiaceae plants, e.g., rosemary, oregano, basil, and thyme, with concentrations ranging from 0.3 to 34.0 mg/g FW (Lee and Scagel, 2009; Hossain et al., 2010; Sofic et al., 2010). Rutin has also been identified in clove basil (Grayer et al., 2000), although it was not detected in this study.
The isomers of dihydroxy-dimethoxyflavone, including cirsimaritin (5,4’di-OH-6,7-diOMe flavone) and ladanein (5,6-diOH-7,4’-diOMe flavone), have been identified in hoary basil (O. americanum), sweet basil, and lemon basil (Grayer et al., 2004). The isomers have also been found in other Lamiaceae plants. For example, cirsimaritin and ladanein (5,6-diOH-7,4’-diOMe flavone) were identified in catmint (Nepata spp.) (Jamzad et al., 2003). Cirsimaritin and 5,4’-diOH-7,3-diOMe flavone have been identified in Teucrium spp. Cirsimaritin, ladanein, and velutin (5,4’-diOH-7,3’-diOMe flavone) have been identified in Salvia spp. (Valant-Vetschera et al., 2003).
The number and position of hydroxyl groups has been shown to influence the antioxidant activity of phenolic compounds (Arts et al., 2003). Rosmarinic acid and chicoric acid have four hydroxyl groups attached to two aromatic rings, while caffeic acid and caftaric acid have two hydroxyl groups attached to an aromatic ring. While the number and position of the hydroxyl groups attached to the aromatic rings is similar in rosmarinic acid and chicoric acid, rosmarinic acid has a higher TEAC value. The difference might due to the presence of a tartaroyl group in chicoric acid. A similar trend has also been found between caffeic acid and cafteric acid, with the latter containing a tartaroyl group.
Flavonoid aglycone (dihydroxy-dimethoxyflavone) had a higher TEAC value than flavonoid glycosides (rutin and acacetin-acetyl glucoside). Glycosylation negatively affected the antioxidant activity of flavonoids, due to a steric effect (Rice-Evans et al., 1996).
The TEAC values of the phenolic compounds found here (insert your values or range) were similar to those reported previously in the literature – 1.31 and 1.26 for caffeic acid (Cai et al., 2006; Rice-Evans et al., 1996) and 2.13 for rosmarinic acid (Nenadis et al., 2004).
The TEAC value represents the specific antioxidant capacity of a compound. The same compounds, even though analyzed from different samples, should have similar TEAC value. However, some of the same compounds extracted from different Lamiaceae plants had different TEAC values. A possible explanation for this variation is the low reaction kinetics of an ABTS assay, which takes a long time to reach the end point (Karadag et al., 2009). A normal reaction time for an ABTS assay has been reported as 6 min (Re et al., 1999). In contrast, the reaction in an online HPLC-ABTS assay takes only a few minutes before reaching a detector. Different compounds require different reaction times with the ABTS radical to reach the end point. Walker and Everette (2009) have reported end points for Trolox and ascorbic acid of within 2 s; gallic acid of more than 15 s; rutin, quercetin, and curcumin of about 1 min; and chlorogenic acid, caffeic acid, and ferulic acid of more than 1 min. Higher antioxidant concentrations prolonged the reaction rates. Gallic acid at the concentration of 50 μM required upwards of 5 min to reach steady state, while 250 μM gallic acid required 15 min (Pérez-Jiménez and Saura-Calixto, 2008). Although the reaction time between the ABTS radical and different substances may differ, each substance had the same reaction time from mixing point to detector (as determined by flow rate of HPLC). Therefore, the same substance from different samples had the same reaction time, and should respond according to their concentration.
In this study, the TEAC value of rosmarinic acid (derivative of caffeic acid) varied from 1.04 to 3.45. A plot between concentration and TEAC value of rosmarinic acid showed a negative relationship (Figure 4). The higher the concentration of rosmarinic acid in the samples, the lower the TEAC values. These results indicated that ABTS might not be appropriate for quantifying the antioxidant capacity of antioxidants with slow reaction kinetics by an online HPLC-ABTS, unless the antioxidant concentrations were low enough to reach steady state before reaching a detector.
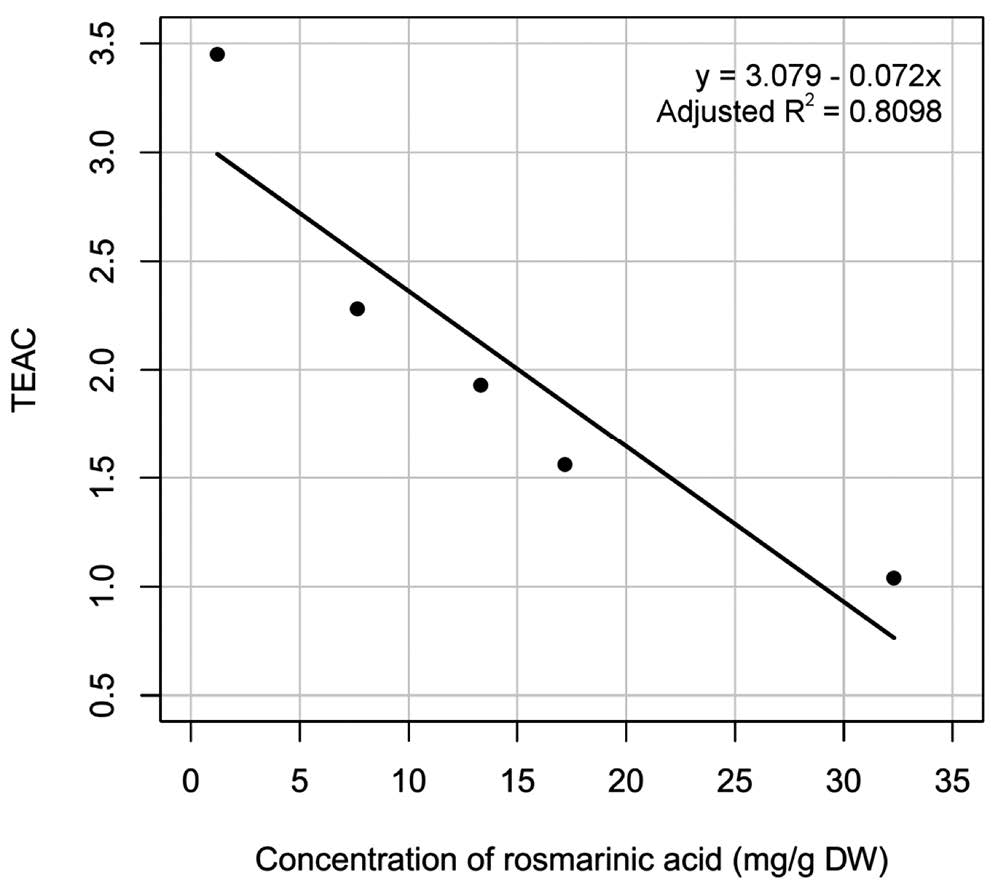
Figure 4. Relation between rosmarinic acid concentration and TEAC value obtained by HPLC-ABTS.
CONCLUSION
The Lamiaceae vegetables studied here contained similar compounds that were responsible for their antioxidant activity. However, these compounds were present at different concentrations. The major antioxidants were hydroxycinnamic acids and flavonoids. Rosmarinic acid was responsible for the highest antioxidant activity in all samples, except clove basil. The rosmarinic acid content was highest in lemon balm. Other major antioxidants were chicoric acid and dihydroxy-dimethoxyflavone. Chicoric acid content was highest in clove basil. Dihydroxy-dimethoxyflavone content was highest in sweet basil. The minor antioxidants were caffeic acid, caftaric acid, syringic acid, rutin, and acacetin-acetylglucoside. In addition to antioxidant activity, Lamiaceae plants also possessed other medicinal properties. Ocimum spp., especially clove basil, contained a significant amount of antiviral chicoric acid, making it an alternative source to Echinacea.
ACKNOWLEDGEMENTS
The Thailand Research Fund, through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0260/2551), supported this research. A KIST Gangneung Institute Intramural Grant (2Z04220) supported C. Pan and S. Kim.
REFERENCES
Anttonen, M.J., and R.O. Karjalainen. 2005. Environmental and genetic variation of phenolic compounds in red raspberry. Journal of Food Composition and Analysis 18: 759-769.
Arts, M.J., J. Sebastiaan Dallinga, H.P. Voss, G.R. Haenen, and A. Bast. 2003. A critical appraisal of the use of the antioxidant capacity (TEAC) assay in defining optimal antioxidant structures. Food Chemistry 80: 409-414.
Asami, D.K., Y.-J. Hong, D.M. Barrett, and A.E. Mitchell. 2003. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. Journal of Agricultural and Food Chemistry 51: 1237-1241.
Astani, A., J. Reichling, and P. Schnitzler. 2012. Melissa officinalis extract inhibits attachment of herpes simplex virus in vitro. Chemotherapy 58: 70-77.
Bandoniene, D., M. Murkovic, and P.R. Venskutonis. 2005. Determination of rosmarinic acid in sage and borage leaves by high-performance liquid chromatography with different detection methods. Journal of Chromatographic Science 43: 372-376.
Barrett, B. 2003. Medicinal properties of Echinacea: A critical review. Phytomedicine 10: 66-86.
Bel-Rhlid, R., N. Pagé-Zoerkler, R. Fumeaux, T. Ho-Dac, J.Y. Chuat, J.L. Sauvageat, and T. Raab. 2012. Hydrolysis of chicoric and caftaric acids with esterases and Lactobacillus johnsonii in vitro and in a gastrointestinal model. Journal of Agricultural and Food Chemistry 60: 9236-9241.
Boeing, H., A. Bechthold, A. Bub, S. Ellinger, D. Haller, A. Kroke, E. Leschik-Bonnet, M.J. Müller, H. Oberritter, and M. Schulze. 2012. Critical review: vegetables and fruit in the prevention of chronic diseases. European Journal of Nutrition 51: 637-663.
Bourgaud, F., A. Gravot, S. Milesi, and E. Gontier. 2001. Production of plant secondary metabolites: a historical perspective. Plant Science 161: 839-851.
Bušić, A., A. Vojvodić, D. Komes, C. Akkermans, A. Belščak-Cvitanović, M. Stolk, and G. Hofland. 2013. Effect of different drying techniques on bioactive profile and antioxidant capacity of basil (Ocimum basilicum L.). Proceedings of Euro Food Chem XVII. Istanbul, Turkey.
Cai, Y.Z., M. Sun, J. Xing, Q. Luo, and H. Corke. 2006. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sciences 78: 2872-2888.
Chen, J.H., and C.-T. Ho. 1997. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. Journal of Agricultural and Food Chemistry 45: 2374-2378.
Dorman, H.J.D., A. Peltoketo, R. Hiltunen, and M.J. Tikkanen. 2003. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chemistry 83: 255-262.
Eyles, A., W. Jones, K. Riedl, D. Cipollini, S. Schwartz, K. Chan, D. Herms, and P. Bonello. 2007. Comparative phloem chemistry of manchurian (Fraxinus mandshurica) and two North American ash species (Fraxinus Americana and Fraxinus pennsylvanica). Journal of Chemical Ecology 33: 1430-1448.
Genkinger, J.M., E.A. Platz, S.C. Hoffman, G.W. Comstock, and K.J. Helzlsouer. 2004. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. American Journal of Epidemiology 160: 1223-1233.
Grayer, R.J., G.C. Kite, M. Abou-Zaid, and L.J. Archer. 2000. The application of atmospheric pressure chemical ionisation liquid chromatography–mass spectrometry in the chemotaxonomic study of flavonoids: characterisation of flavonoids from Ocimum gratissimum var. gratissimum. Phytochemical Analysis 11: 257-267.
Grayer, R.J., R.F. Vieira, A.M. Price, G.C. Kite, J.E. Simon, and A.J. Paton. 2004. Characterization of cultivars within species of Ocimum by exudate flavonoid profiles. Biochemical Systematics and Ecology 32: 901-913.
Harley, R.M., S. Atkins, A.L. Budantsev, P.D. Cantino, B.J. Conn, R.J. Grayer, M.M. Harley, R.P.J.D. Kok, T.V. Krestovskaja, R. Morales, A.J. Paton, and P.O. Ryding. 2004. Labiatae. p.167-275. In K. Kubitzki (ed) The Families and Genera of Vascular Plants Volume VII. Springer-Verlag, Berlin.
He, W., X. Liu, H. Xu, Y. Gong, F. Yuan, and Y. Gao. 2010. On-line HPLC-ABTS screening and HPLC-DAD-MS/MS identification of free radical scavengers in Gardenia (Gardenia jasminoides Ellis) fruit extracts. Food Chemistry 123: 521-528.
Hossain, M.B., D.K. Rai, N.P. Brunton, A.B. Martin-Diana, and C. Barry-Ryan. 2010. Characterization of phenolics composition in Lamiaceae spices by LCESI-MS/MS. Journal of Agricultural and Food Chemistry 58: 10576–10581.
Jamzad, Z., R.J. Grayer, G.C. Kite, M.S.J. Simmonds, M. Ingrouille, and A. Jalili. 2003. Leaf surface flavonoids in Iranian species of Nepeta (Lamiaceae) and some related genera. Biochemical Systematics and Ecology 31: 587-600.
Kivilompolo, M., and T. Hyötyläinen. 2007. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. Journal of Chromatography A 1145: 155-164.
Koleva, I.I., H.a.G. Niederländer, and T.A. Van Beek. 2001. Application of ABTS radical cation for selective on-line detection of radical scavengers in HPLC eluates. Analytical Chemistry 73: 3373-3381.
Kwee, E.M., and E.D. Niemeyer. 2011. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chemistry 128: 1044-1050.
Lai, J.-P., Y.H. Lim, J. Su, H.M. Shen, and C.N. Ong. 2007. Identification and characterization of major flavonoids and caffeoylquinic acids in three Compositae plants by LC/DAD-APCI/MS. Journal of Chromatography B 848: 215-225.
Lee, J., and C.F. Scagel. 2009. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chemistry 115: 650-656.
Lee, J., and C.F. Scagel. 2010. Chicoric acid levels in commercial basil (Ocimum basilicum) and Echinacea purpurea products. Journal of Functional Foods 2: 77-84.
Mazzanti, G., L. Battinelli, C. Pompeo, A. Serrilli, R. Rossi, I. Sauzullo, F. Mengoni, and V. Vullo. 2008. Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Natural Product Research 22: 1433-1440.
Nenadis, N., L.F. Wang, M. Tsimidou, and H.-Y. Zhang. 2004. Estimation of scavenging activity of phenolic compounds using the ABTS+ assay. Journal of Agricultural and Food Chemistry 52: 4669-4674.
Nguyen, P.M., and E.D. Niemeyer. 2008. Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). Journal of Agricultural and Food Chemistry 56: 8685-8691.
Nuissier, G., B. Rezzonico, and M. Grignon-Dubois. 2010. Chicoric acid from Syringodium filiforme. Food Chemistry 120: 783-788.
Pellati, F., S. Benvenuti, L. Magro, M. Melegari, and F. Soragni. 2004. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. Journal of Pharmaceutical and Biomedical Analysis 34: 289-301.
Perry, N.B., E.J. Burgess, and V.L. Glennie. 2001. Echinacea standardization: analytical methods for phenolic compounds and typical levels in medicinal species. Journal of Agricultural and Food Chemistry 49: 1702-1706.
Petersen, M., Y. Abdullah, J. Benner, D. Eberle, K. Gehlen, S. Hücherig, V. Janiak, K.H. Kim, M. Sander, and C. Weitzel. 2009. Evolution of rosmarinic acid biosynthesis. Phytochemistry 70: 1663-1679.
Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 26: 1231-1237.
Reddy, S.M., M.M. Rao, A.S. Reddy, M.M. Reddy, and S.J. Chary. 2004. University Botany-3. New Age International, New Delhi.
Rice-Evans, C.A., N.J. Miller, and G. Paganga. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine 20: 933-956.
Robinson Jr, W.E. 1998. l-Chicoric acid, an inhibitor of human immunodeficiency virus type 1 (HIV-1) integrase, improves on the in vitro anti-HIV-1 effect of Zidovudine plus a protease inhibitor (AG1350). Antiviral Research 39: 101-111.
Saltveit, M.E. 2009. Synthesis and Metabolism of Phenolic Compounds. p.89-100. In L.A.L. Rosa, E. Alvarez-Parrilla and G. A. Gonzalez-Aguilar (eds) Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability. Wiley-Blackwell, Ames.
Sanbongi, C., H. Takano, N. Osakabe, N. Sasa, M. Natsume, R. Yanagisawa, K.I. Inoue, Y. Kato, T. Osawa, and T. Yoshikawa. 2003. Rosmarinic acid inhibits lung injury induced by diesel exhaust particles. Free Radical Biology and Medicine 34: 1060-1069.
Sánchez-Rabaneda, F., O. Jáuregui, I. Casals, C. Andrés-Lacueva, M. Izquierdo-Pulido, and R.M. Lamuela-Raventós. 2003. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). Journal of Mass Spectrometry 38: 35-42.
Scalzo, J., A. Politi, N. Pellegrini, B. Mezzetti, and M. Battino. 2005. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 21: 207-213.
Shan, B., Y.Z. Cai, M. Sun, and H. Corke. 2005. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agricultural and Food Chemistry 53: 7749-7759.
Sofic, E., A. Copra-Janicijevic, M. Salihovic, I. Tahirovic and G. Kroyer. 2010. Screening of medicinal plant extracts for quercetin-3rutinoside (rutin) in Bosnia and Herzegovina. Medicinal Plants-International Journal of Phytomedicines and Related Industries 2: 97-102.
Sun, J., F. Liang, Y. Bin, P. Li, and C. Duan. 2007. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 12: 679-693.
Tomas-Barberan, F.A., and F. Ferreres. 2012. Analytical methods of flavonols and flavones. p.207-246. In Z. Xu and L.R. Howard (eds) Analysis of Antioxidant-Rich Phytochemicals. Wiley-Blackwell, Chichester.
Valant-Vetschera, K.M., J.N. Roitman, and E. Wollenweber. 2003. Chemodiversity of exudate flavonoids in some members of the Lamiaceae. Biochemical Systematics and Ecology 31: 1279-1289.
Vieira, R.F., R.J. Grayer, and A.J. Paton. 2003. Chemical profiling of Ocimum americanum using external flavonoids. Phytochemistry 63: 555-567.
Wu, C.-H., H.N. Murthy, E.J. Hahn, and K.-Y. Paek. 2007. Enhanced production of caftaric acid, chlorogenic acid and cichoric acid in suspension cultures of Echinacea purpurea by the manipulation of incubation temperature and photoperiod. Biochemical Engineering Journal 36: 301-303.
Yen, G.C., and P.D. Duh. 1994. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. Journal of Agricultural and Food Chemistry 42: 629-632.
Zgórka, G., and K. Głowniak. 2001. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. Journal of Pharmaceutical and Biomedical Analysis 26: 79-87.
Trakul Prommajak1, Sang Moo Kim2, Cheol-Ho Pan3, Sang Min Kim3, Suthat Surawang1 and Nithiya Rattanapanone1,4*
1 Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
2 Department of Marine Food Science and Technology, Gangneung-Wonju National University, Gangneung 210702, South Korea
3 Functional Food Center, Korea Institute of Science and Technology, Gangneung 210340, South Korea
4 Posthavest Technology Research Institute, Chiang Mai University, Chiang Mai 50200, Thailand
Total Article Views

