
The Effects of Nitrogen as NO3- and NH4+ on the Growth and Symbiont (Anabaena azollae) of Azolla pinnata R. Brown
Arunothai Jampeetong, Thunyachol Sripakdee, Tanaporn Khamphaya and Sutthathorn ChairuangsriPublished Date : 2019-08-23
DOI : 10.12982/cmujns.2016.0002
Journal Issues : Number1 ,January - April 2016
ABSTRACT
The growth, morphology, and symbiont (Anabaena azollae) of Azolla pinnata R. Brown were investigated under different external N-supply regimes to inform the plant’s potential in wastewater treatment. Azolla pinnata plants were supplied with nitrogen as NO3- or NH4+ at four different concentrations (0, 0.5, 1, and 5 mM) and incubated in a greenhouse for 14 days. The relative growth rates of NO3- -fed plants were not significantly different between treatments, but decreased significantly at the highest NH4+ concentration. Moreover, the NO3- concentration did not affect root number or length. The highest NH4+ concentration (5 mM) decreased both the root length and number of symbionts (Anabaena azollae) in the mature leaves of Azolla pinnata. Because Azolla pinnata continued to grow well with supplied NO3- and NH4+, and retained their ability to absorb nitrogen, they offer potential for treating wastewater, except at the highest NH4+ concentration, which led to toxicity.
Keywords: Azolla pinnata, Anabaena azollae, Heterocyst, NH4+ toxicity, Symbiont
INTRODUCTION
Azolla pinnata R. Brown is a free-floating aquatic fern belonging to the family Azollaceae. It is widely distributed in Asia and along the coast of tropical Africa (Wagner, 1997). The plant consists of alternately arranged leaves on a prostrate, floating rhizome, with one or two roots hanging in the water column. The leaf is bilobed, consisting of a chlorophyllous floating dorsal lobe and a colorless and partially submerged ventral lobe. A cavity in the ventral leaves houses symbiotic cyanobacteria, Anabaena azollae (Pabby et al., 2003). This symbiont fixes N2 from the atmosphere and produces a high N level in the plant tissue of Azolla pinnata, making the plant useful as green manure for rice fields, where it has been used for several centuries (Shi and Hall, 1988; Peters and Meeks, 1989; Forni et al., 2001; de Macale and Vlek, 2004).
More recently, Azolla spp. have been used in water treatment (Kitoh et al., 1993; Forni et al., 2001; Nahlik and Mitsch, 2006; Costa et al., 2009). Several studies have shown that Azolla spp. can both grow in and remove nutrients from wastewater (Reddy and DeBusk, 1985; Kitoh et al., 1993; Vermaat and Hanif, 1998; Costa et al., 2009). In general, two forms of inorganic nitrogen – NH4+ and NO3- – are commonly found in wastewater at concentrations of 1 to 5 mM (Kadlec and Wallace, 2009). Both the form and concentration of N may affect plant growth, morphology, and the symbiont. A previous study by Ito and Watanabe (1983) showed that NO3- and NH4+ at concentrations of 1 mM did not inhibit the acetylene reduction activity of the symbiont Anabaena azollae in the leaves of Azolla pinnata; however, they did not determine its effects on the growth and morphology of the plants.
Several studies have shown that many aquatic or wetland plants, such as Phragmites australis (Cav.) Trin. ex Steudel and Salvinia natans (L.) All., prefer NH4+ over NO3-, but that NH4+ is toxic at high concentrations (Kitoh et al., 1993; Britto and Kronzucker, 2002; Tylova et al., 2005; Cao et al., 2009; Jampeetong and Brix, 2009a, b; Jampeetong et al., 2012). Because symbionts furnish Azolla pinnata with nitrogen, external nitrogen in wastewater may affect the symbiotic relationship and subsequent plant growth. To study this, we examined the growth, morphology, and symbiotic response of Azolla pinnata R. Brown to two different forms – NH4+ and NO3- – and concentrations of inorganic nitrogen; the results can be applied to developing better water treatment systems.
MATERIALS AND METHODS
Plant material and experimental set up
Azolla pinnata was obtained from a natural pond at Chiang Mai University, Chiang Mai, Thailand. The plants were cleaned and grown on a standard N- and P-free growth medium prepared according to Smart and Barko (1985), to which 0.5 mM of NO3- or NH4+, 100 μM KH2PO4, and a commercial plant micronutrient solution (Tropica, Egaa, Denmark) (1 mL: 10 L growth solution) were added. The pH was adjusted to 6.6±0.1.
After the plants had acclimated for 14 days, approximately 2 grams of the ramet from the stock culture was placed in a plastic pot (4 pots per treatment) containing 2 liters of a standard N- and P- free growth medium prepared according to Smart and Barko (1985), to which 100 μM KH2PO4, and a commercial plant micronutrient solution (Tropica, Egaa, Denmark) were added. The pH was adjusted to 6.6±0.1 using HCl and NaOH. The treatments consisted of two N forms: NH4+ or NO3- prepared from (NH4)2SO4 or KNO3, respectively, at different concentrations (0, 0.5, 1, and 5 mM). The plants were incubated in the greenhouse at the Department of Biology, Faculty of Science, Chiang Mai University, Thailand. The growth medium was changed every 3 days and the plants were cleaned gently by hand. At the beginning of the experiment, plant ramets similar to experimental plants (n=10) were selected to estimate the fresh weight and dry weight ratio. The fresh weight of all plants was measured, and then they were dried until they reached a constant weight. The fresh to dry weight ratio was calculated and used for the relative growth rate calculation.
Growth and morphological study
After 14 days, root number and root length of the plants in each treatment were measured. The plants were then harvested and freeze dried. The relative growth rate (g g-1d-1) was calculated by the formula:
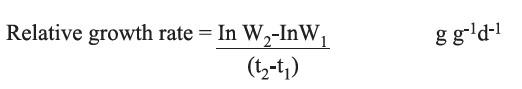
where W1 and W2 are the initial and final dry weights (g) of plant material from each pot, and t1 and t2 are the initial and final time (days).
The shoot area (cm2) was estimated from digital photos taken of each pot at the same angle and distance. The relative shoot area growth rate (RSGR, cm2cm-2 day-1) was calculated in a similar way to the relative growth rate.
Counting anabaena and heterocysts
Anabaena azollae in both young (1st in position) and mature (6th in position) leaves was determined. The leaves were broken using a needle, and then wet mount slides were made. Anabaena azollae filaments in each leaf were counted. The heterocyst frequency was measured by randomly counting 200 cells of A. azollae and recording the number of heterocysts found.
Inorganic nitrogen in the whole plant
Five milligrams of freeze-dried plant materials from each replicate were extracted with 15 mL of distilled water at 98°C in a water bath for 20 minutes. Then the NH4+ and NO3- in the extracts were analysed by a modified salicylate method (Quikchem Method no. 10-107-06-3-B; Lachat Instruments, Milwaukee, WI, USA). The absorbance of the extracts was measured at 690 and 220 nm using a UV-VIS spectrophotometer (Lambda 25 version 2.85.04, USA) to determine NH4+ and NO3-, respectively.
Statistics
The data were analyzed by one-way and two-way analysis of variance (ANOVA) using Statgraphics Plus ver. 4.1 software (Manugistics, Inc., MD, USA). The normality of the distribution and homogeneity of variance were tested using Cochran’s C-test. If necessary, data was log-transformed to ensure homogeneity of variance. Multiple comparisons of means were identified by Scheffe’s test (p<0.05).
RESULTS
Growth and morphology
Both the form and concentration of N affected relative growth rates (RGRs) of Azolla pinnata, with significant interactive effects between these two factors observed (Table 1, Figure 1a). The plants grown on the three lowest concentrations of NH4+ had higher relative growth rates than that grown on NO3-. However, increasing NO3- concentrations did not affect plant growth, whereas the highest NH4+ concentration (5 mM) decreased growth significantly. Varying the concentrations of either NH4+ or NO3- did not significantly affect relative shoot area growth rates (RSGRs) (Fig.1b).
Neither the N form nor concentration significantly affected root number (Figure 2a). However, the higher NH4+ concentrations negatively affected root length (Figure 2b), with significant interaction between N form and N concentration (Table 1).
Table 1. Degrees of freedom (d.f.), F-ratios, and significance of a two-way ANOVA of relative growth rate (RGR), relative shoot area growth rates (RSGR), root number, root length, number of Anabaena azollae, number of heterocyst, and NH4+ in the plant tissue of Azolla pinnata grown on NO3- or NH4+ at four different concentrations (0, 0.5, 1, and 5 mM) for 14 days.
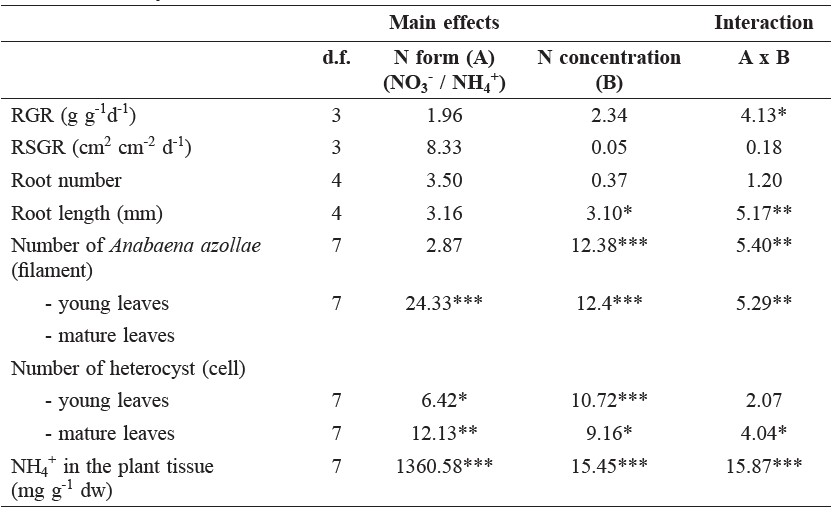
Note: *(in column) indicate significant differences between factors, * (P<0.05), ** (P<0.01), and *** (P<0.001).
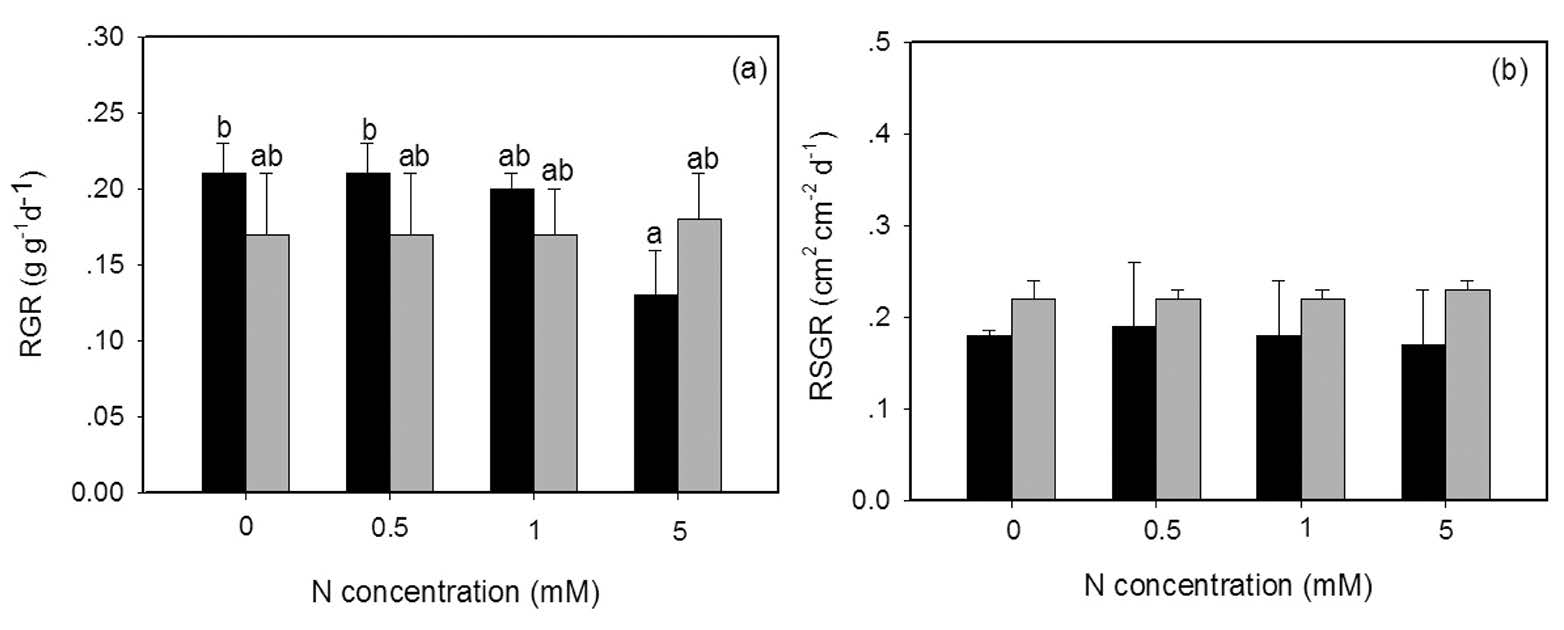
Figure 1. The relative growth rate (RGR) (a) and the relative shoot area growth rate (RSGR) (b) of Azolla pinnata (mean±SD) grown with NO3- (grey column) or NH4+ (dark column) as the nitrogen source at four different concentrations (0, 0.5, 1, 5 mM) for 14 days. Different letters above columns indicate significant differences between treatments.
Anabaena and heterocyst
Both the form and concentration of N affected the amount of Anabaena azollae; the interaction between these two factors was observed (Table 1). Fewer Anabaena azollae were found in young leaves than mature leaves. In young leaves, the amount of Anabaena azollae was mildly affected by the form and concentration of N compared to mature leaves, in which high concentrations of NH4+ reduced the number of Anabaena azollae (Figure 3a, b). Similarly, heterocyst counts per 200 vegetative cells of Anabaena significantly decreased in plants fed with 5 mM NH4+; NO3- had no affect on either the young or mature leaves (Figure 3b, d). The effects of N concentration depended on the N form, as shown by the significant interaction term in the ANOVA results (Table 1).
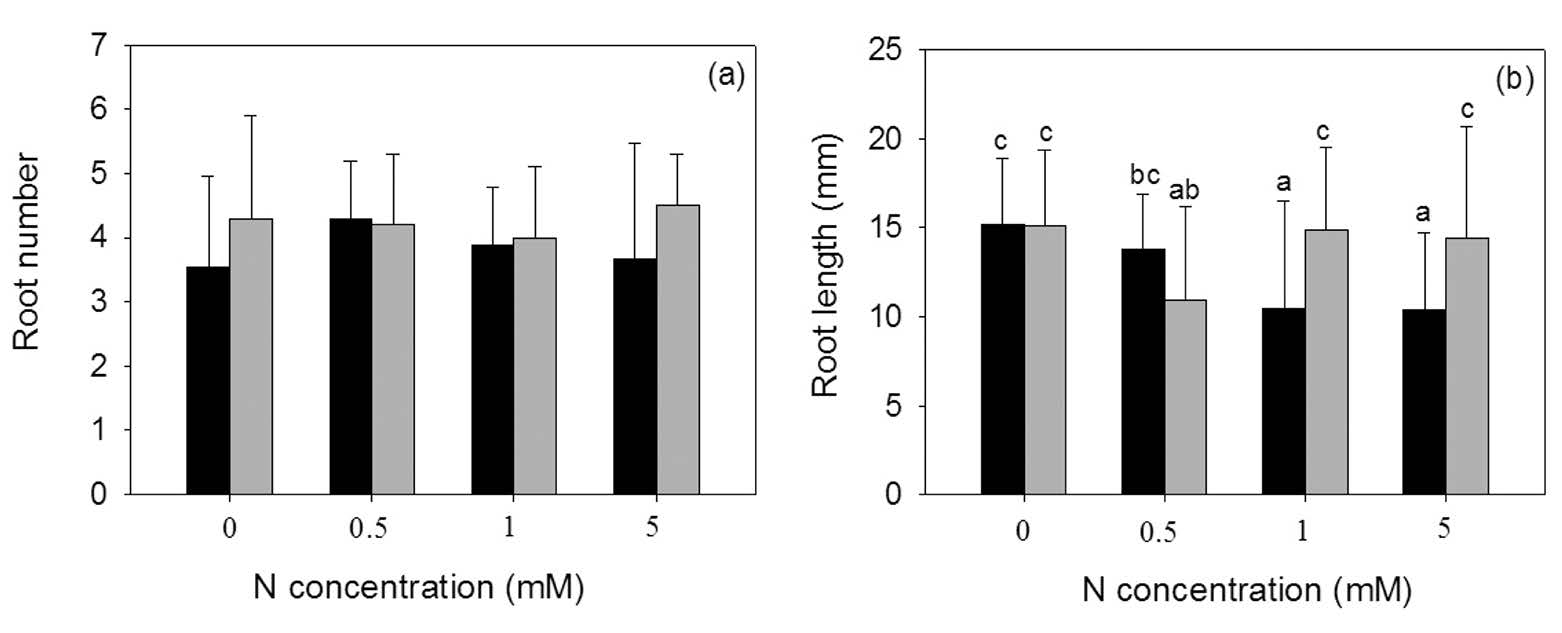
Figure 2. Root number (a) and root length (b) of Azolla pinnata (mean±SD) grown with either NO3- (grey column) or NH4+ (dark column) as the nitrogen source at four different N concentrations (0, 0.5, 1, 5 mM). Different letters above columns indicate significant differences between treatments.
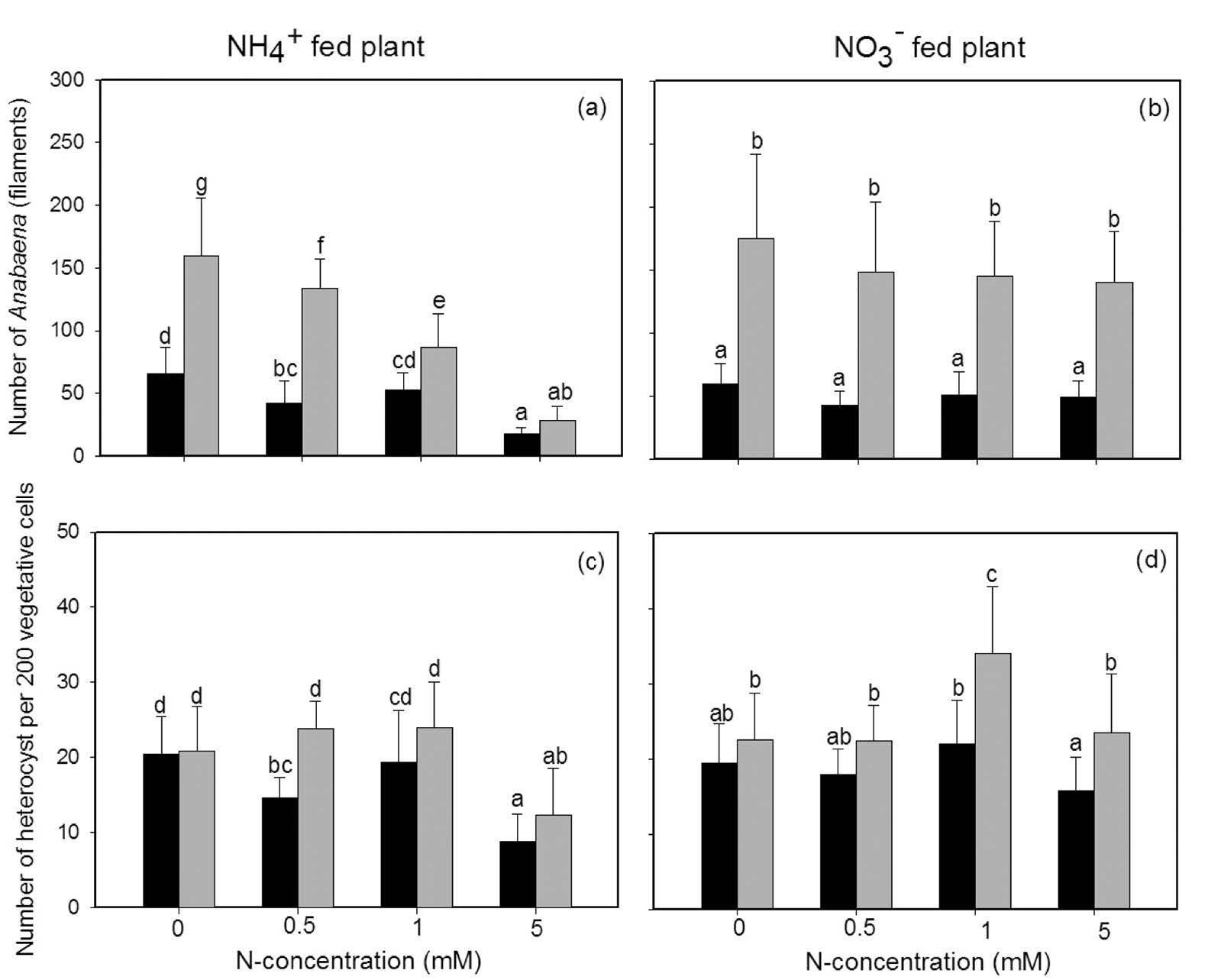
Figure 3. Number of Anabaena azollae (a, b) and heterocyst (c, d) in young leaves (dark column) and mature leaves (grey column) of Azolla pinnata (mean±SD) grown with either NH4+ or NO3- as the nitrogen source at four different N concentrations (0, 0.5, 1, 5 mM) for 14 days. Different letters above columns indicate significant differences between treatments.
Inorganic nitrogen in the whole plant
The NH4+ in the plant tissue of Azolla pinnata treated with NO3- at different concentrations did not significantly differ. In contrast, in the NH4+ -fed plants, the concentration of NH4+ in the plant tissue increased when NH4+ was supplied at high concentration (Figure 4a). NO3- in the plant tissue was unaffected in the NO3- -fed plants, even as the external concentration of NO3- increased. The NO3- in the plant tissue of the NH4+ -fed plants was not determined (Figure 4b).
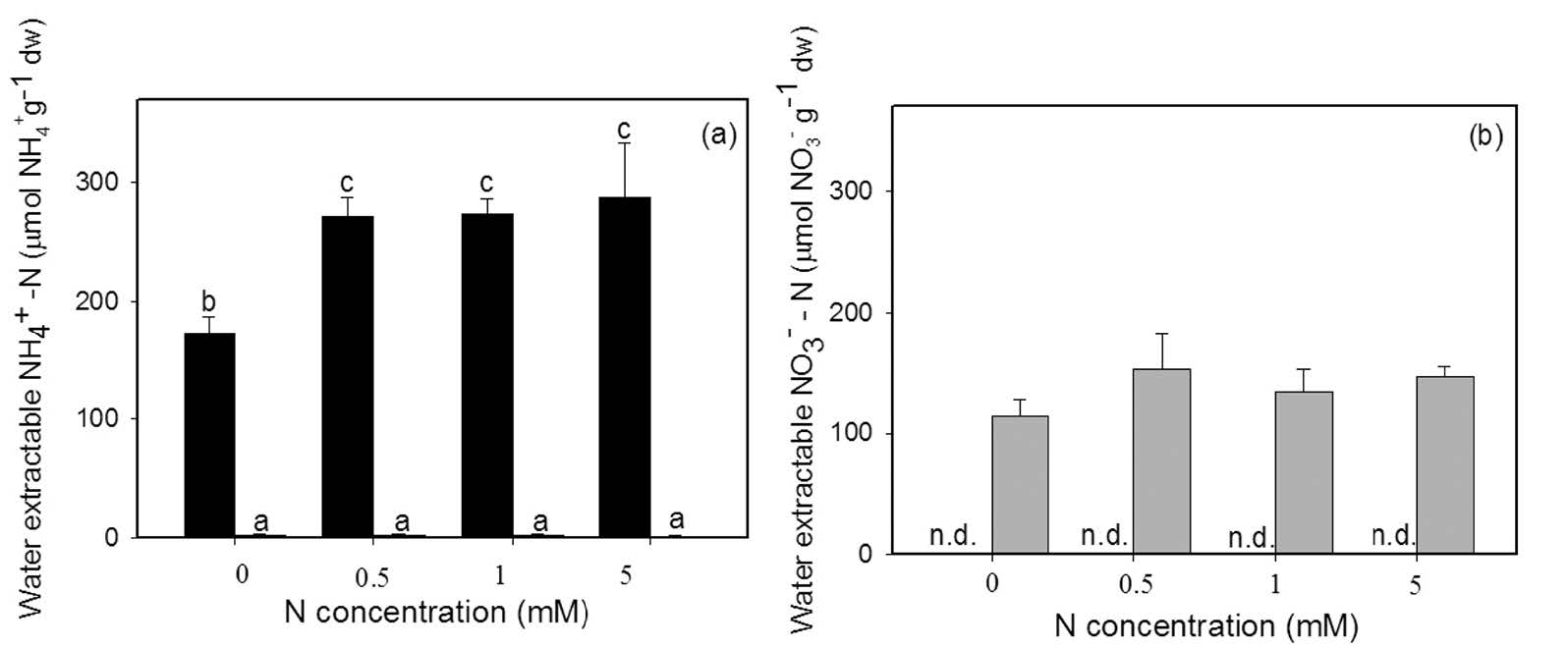
Figure 4. Water extractable NH4+ -N (a) and NO3- -N (b) in the tissue of Azolla pinnata (mean±SD) grown with either NH4+ (dark column) or NO3- (grey column) as the nitrogen source at four different N concentrations (0, 0.5, 1, 5 mM) for 14 days. Different letters above columns indicate significant differences between treatments. n.d. = not determined.
DISCUSSION
The form (NO3- or NH4+) and concentration (0, 0.5, 1 and 5 mM) of externally supplied nitrogen affected the growth and morphology of Azolla pinnata R. Brown. The effects were lower in the NO3- -fed plants. Most aquatic plants prefer inorganic nitrogen in the form of ammonium (NH4+), because of the lower energy needed for uptake and assimilation (Cedergreen and Madsen, 2002; Tylova-Munzarova et al., 2005; Jampeetong and Brix, 2009a). However, in our study, Azolla pinnata showed a negative response – low growth rate, short roots, and leaf chlorosis, especially in mature leaves – to the highest concentration (5 mM) of NH4+. Others have found similar effects of high NH4+ concentrations in other free-floating plants, such as Azolla filiculoides (Kitoh et al., 1993) and Salvinia natans (Jampeetong and Brix, 2009b). Ito and Watanabe (1983) reported that biomass decreased after Azolla pinnata was exposed to 10 mM NH4+ for 4 days, but growth and morphology data were not precisely determined. They also reported that both the form and concentration of nitrogen affected the symbiont Anabaena azollae in the leaves of Azolla pinnata; a high NH4+ concentration (10 mM) inhibited acetylene reduction activity, indicating decreased nitrogenase enzyme activity. However, they did not determine the amounts of Anabaena and heterocyst. In our results, we found that Anabaena azollae and its heterocyst decreased with increasing external NH4+ concentration, particularly in mature leaves. Maejima et al. (2001) found a similar result; both Anabaena azollae and heterocyst decreased more than 50% in mature leaves, while young leaves were not affected. In contrast, externally supplied NO3- did not affect Anabaena azollae and its heterocyst. Therefore, the plants can obtain NO3- from both externally supplied NO3- and from the atmosphere by fixing N2. Costa et al. (2009) found similar results with Azolla filiculoides grown on combined nitrogen wastewater.
Even though we did not determine the NH4+ uptake of Azolla pinnata, the NH4+ concentration in the plant tissue increased when the plants were supplied with high concentrations of external NH4+. This indicated that the ability of the plants to take up external NH4+ was not suppressed. Jampeetong et al. (unpublished
data) found that Azolla pinnata grown on 1 mM NH4NO3 showed that the uptake of NH4+ was 27 times higher than NO3-. Moreover, Cary and Weerts (1992) showed that Azolla pinnata and Azolla filiculoides can obtain nitrogen from an external supply, even though both species benefit from a symbiotic association. However, in our study, Azolla pinnata appeared not to have a mechanism to prevent over-accumulation of NH4+ in its cells. This over-accumulation has been shown to be toxic (Britto and Kronzucker, 2002). Similar results were recorded in several NH4+ intolerant species, including Thalassia hemprichii and Zostera marina (van Katwijk et al., 1997; Christianen et al., 2011).
Many aquatic plants have been used for wastewater treatment. Most species had high growth rates and high acquisition of nitrogen (Koerselman and Meuleman, 1996; Abe and Ozaki, 1998). According to our results, Azolla pinnata had high growth rates and biomass production. Hence, this species offers potential for treating various types of wastewater in constructed wetland systems.
In conclusion, both the form and concentration of N affected the growth and morphology of Azolla pinnata. The plants grown with NH4+ up to 1 mM had higher growth rates than NO3- -fed plants, but the growth rate and root length of the plants decreased at the highest concentration (5 mM). Anabaena azollae and its heterocyst also decreased in the mature leaves of the plants fed with a high NH4+ concentration, whereas the youngest leaves were not affected. Azolla pinnata
was able to take up external NO3- or NH4+, but high NH4+ concentrations may cause NH4+ toxicity and lead to plant destruction. Azolla pinnata offers potential
for removing nutrients from wastewater, but exposure to NH4+ contamination must be less than 5 mM in order to maintain plant growth and the potential of N uptake from the polluted water.
ACKNOWLEDGEMENTS
This research was supported by the Institute for the Promotion of Teaching Science and Technology (IPST), Thailand. The authors would like to thank Mr. Alvin Yashinaga for improving the English of the manuscript.
REFERENCES
Abe K., and Y. Ozaki. 1998. Comparison of useful terrestrial and aquatic plant species for removal of nitrogen and phosphorus from domestic wastewater. Soil Science and Plant Nutrition 44: 599-607.
Britto D.T., and H.J. Kronzucker. 2002. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology 159: 567-584.
Cao, T., P. Xie, Z. Li, and L. Ni. 2009. Physiological stress of high NH4+ concentration in water column on the submerged macrophyte Vallisneria natans L. Bulletin of Enviromental Contamination and Toxicology 82: 296-299.
Cary, P.R., and P.G.J. Weerts. 1992. Azolla pinnata R. Brown and Azolla filiculoides Lamarck as affected by water temperature, nitrogen and phosphorus supply, light intensity and pH. Aquatic Botany 43: 163-180.
Cedergreen, N., and T.V. Madsen. 2002. Nitrogen uptake by the floating macrophyte Lemna minor. New Phytologist 155: 285-292.
Costa M.L., M.C.R. Santos, F. Carrapico, and A.L. Pereira. 2009. Azolla-Anabaena’s behaviour in urban wastewater and artificial media-Influence of combined nitrogen. Water Research 43: 3743-3750.
Christianen, M.J.A., T. van der Heide, T.J. Bouma, J.G.M. Roelofs, M.M. van Katwijk, and L.P.M. Lamers. 2011. Limited toxicity of NHx pulses on an early and late successional tropical seagrass species: Interactions with pH and light level. Aquatic Toxicology 104: 73-79.
de Macale, M.A.R., and P.L.G. Vlek. 2004. The role of Azolla cover in improving the nitrogen use efficiency of lowland rice. Plant and Soil 263: 311-321.
Forni, C., J. Chen, L. Tancioni, and M.G. Caiola. 2001. Evaluation of the fern Azolla for growth, nitrogen and phosphorus removal from wastewater. Water Research 35: 1592-1598.
Ito, O., and I. Watanabe. 1983. The relationship between combined nitrogen uptake and nitrogen fixation in Azolla-Anabaena symbiosis. New Phytologist 95: 647-654.
Jampeetong, A., and H. Brix. 2009a. Nitrogen nutrition of Salvinia natans: Effect of inorganic nitrogen form on growth, morphology, nitrate reductase activity and uptake kinetics of ammonium and nitrate. Aquatic Botany 90: 67-73.
Jampeetong, A., and H. Brix. 2009b. Effects of NH4+ concentration on growth, morphology and NH4+ uptake kinetics of Salvinia natans. Ecological Engineering 35: 695-702.
Jampeetong, A., H. Brix, and S. Kantawanichkul. 2012. Effects of inorganic nitrogen forms on growth, morphology, nitrogen uptake capacity and nutrient allocation of four tropical aquatic macrophytes (Salvinia cucullata, Ipomoea aquatica, Cyperus involucratus and Vetiveria zizanioides). Aquatic Botany 97: 10-16.
Kadlec, R.H., and S.D. Wallace. 2009. Treatment wetlands. 2nd edition. CRC press. New York.
Kitoh, S., N. Shiomi, and E. Uheda. 1993. The growth and nitrogen fixation of Azolla filiculoides Lam. In polluted water. Aquatic Botany 46: 129-139.
Koerselman, W., and A.F.M. Meuleman. 1996. The Vegetation N:P Ratio: a New Tool to Detect the Nature of Nutrient Limitation. Journal of Appliced Ecology 33: 1441-1450.
Maejima, K., S. Kitoh, E. Uheda, and N. Shiomi. 2001. Response of 19 Azolla strains to a high concentration of ammonium ions. Plant and Soil 234: 247-252.
Nahlik, A.M., and W.J. Mitsch. 2006. Tropical treatment wetlands dominated by free-floating macrophytes for aqulity improvement in Costa Rica. Ecological Engineering 28: 246-257.
Pabby, A., R. Prasanna, and P.K. Singh. 2003. Azolla-Anabaeba symbiosis-From traditional agriculture to biotechnology. Indian Journal of Biotechnol 2: 26-37.
Peter, G.A., and J.C. Meeks. 1989. The Azolla-Anabaena symbiosis: basic biology. Annual Review of Plant Physiology and Plant Molecular Biology 40: 193-210.
Reddy, K.R., and W.F. DeBusk. 1985. Characteristics of aquatic macrophytes cultured in nutrient-enriched water: II. Azolla, Duckweed and Salvinia. Economic Botany 39: 200-208.
Shi, D.J., and D.O. Hall. 1988. The Azolla-Anabaena association: Historical perspective, symbiosis and energy metabolism. Botanical Review 54: 345-386.
Smart, R.M., and J.W. Barko. 1985. Laboratory culture of submerged freshwater macrophytes on natural sediments. Aquatic Botany 21: 251-263.
Tylova-Munzarova, E., B. Lorenzen, H.Brix, and O. Votrubova. 2005. The effects of NH4+ and NO3- on growth, resource allocation and nitrogen uptake kinetics of Phragmites australis and Glyceria maxima. Aquatic Botany 81: 326-342.
van Katwijk, M.M., L.H.T. Vergeer, G.H.W. Schmitz, and J.G.M. Roelofs. 1997. Ammonium toxicity in eelgrass Zostera marina. Marine Ecology Progress Series 157:159-173.
Vermaat, J.E., and M.K. Hanif. 1998. Performance of common duckweed species (Lemnaceae) and the waterfern Azolla filiculoides on different types of wastewater. Water Research 32: 2569-2576.
Wagner, G.M. 1997. Azolla: A review on its biology and utilization. The Botanical Review 63: 1-26.
Arunothai Jampeetong1,2, Thunyachol Sripakdee1, Tanaporn Khamphaya1 and Sutthathorn Chairuangsri1,2*
1 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
2 Environmental Science Program, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail address: s.suwann@gmail.com
Total Article Views

