
Cytotoxic Steroids from the Bark of Aglaia argentea (Meliaceae)
Kindi Farabi, Desi Harneti, Nurlelasari, Rani Maharani, Ace Tatang Hidayat, Unang Supratman*, Khalijah Awang and Yoshihito ShionoPublished Date : 2017-10-01
DOI : https://doi.org/10.12982/CMUJNS.2017.0024
Journal Issues : Number 4 ,October-December 2017
ABSTRACT
The study aimed to find a potential anticancer agent by isolating and identifying the chemical structure of compounds from Aglaia argentea and testing their cytotoxic effects against P-388 murine leukimia cells. Five steroids – stigmast-5-en-3β-ol (β-sitosterol) (1), stigmast-5-en-3β-ol-3β-oleate (β-sitosterol oleate) (2), stigmast-5-en-3β-ol-3-O-(6′-O-oleoyl)-β-D-glucopyranoside (sitoindoside II) (3), stigmast-5-en-3β-ol-3-O-β-D-glucopyranoside (β-sitosterol glucoside) (4), stigmast-5,22-dien-3β-ol-3-O-β-D-glucopyranoside (stigmasterol glucoside) (5) – were isolated from the bark of Aglaia argentea. The chemical structures of 1-5 were identified with spectroscopic data, including IR, NMR (1H, 13C, DEPT 135°, HMQC, HMBC, 1H-1H COSY) and HRTOFMS, as well as by comparing with previously reported spectral data. All compounds were evaluated for their cytotoxic effects against P-388 murine leukemia cells. Compounds 1-5 showed cytotoxicity against P-388 murine leukemia cell with IC50 values of 12.45 ± 0.050, 85.25 ± 0.050, >100, 52.27 ± 0.031 and 62.52 ± 0.076 μg/mL, respectively.
Keywords: Aglaia argentea, Cytotoxic activity, Meliaceae, P-388 murine leukemia cells, Sterol
INTRODUCTION
Sterols, a type of steroid, are an important class of bioorganic molecules similar to cholesterol in structure and found widely in plants, animals, and fungi (Saeidnia et al., 2014). They include β-sitosterol, campesterol, stigmasterol, and cycloartenol (Ostlund et al., 2002). Sterols, especially β-sitosterol, have been reported to have the following activities or effects: anti-inflammatory (Prieto et al., 2006), inducing apoptosis (Ju et al., 2004; Park et al., 2007; Chai et al., 2008), chemoprotective or chemopreventive (Ovesna et al., 2004), hypocholesterolemic
(Zak et al., 1990), angiongenic (Moon et al., 1999), anti-diabetic (Jamaluddin et al., 1994; Gupta et al., 2011; Radika et al., 2013), and anti-oxidant (Vivancos and Moreno, 2005; Baskar et al., 2012).
Aglaia is the largest genus of the family Meliaceae; it includes more than 100 species, distributed mainly in India, Indonesia, Malaysia and parts of the Western Pacific (Pannell, 1992; Inada et al., 2001). Aglaia argentea, also known as langsat hutan in Indonesia, is a higher plant traditionally used to moisturize the lungs, reduce fever, and treat contusions, coughs and skin diseases (Hidayat and Hutapea, 1991; Mabberley et al., 1995; Muellner et al., 2010). Previous phytochemical studies on the genus Aglaia have revealed the presence of a variety of compounds with interesting biological activities, including recoglamides (Ishibashi et al., 1993; Wu et al., 1997; Nugroho et al., 1999), triterpenoid bisamides (Brader et al., 1998), dammarane-type triterpenoids (Roux et al., 1998; Khalit et al., 1999; Xie et al., 2007; Zhang et al., 2010; Harneti et al., 2012), and cycloartane-type triterpenoids (Khalit et al., 1999; Awang et al., 2012).
As part of our studies on anticancer candidate compounds from Indonesian Aglaia plants, we isolated and described cytotoxic triterpenoids from the bark of A. smithii and A. eximia (Harneti et al., 2012; 2014), as well as a lignan and bisamides from the bark of A. eximia (Sianturi et al., 2015; 2016). In the further screening for cytotoxic compounds from Indonesian Aglaia species, we found that n-hexane and ethyl acetate extracts of the bark of A. argentea exhibited a cytotoxic activity against P-388 murine leukemia cells with IC50 of 26.72 ± 0.02 and 15.48 ± 0.03 μg/mL. We report herein the isolation and structure elucidation of five steroids together with their cytotoxic activity against P-388 murine leukemia cells.
MATERIALS AND METHODS
General
Melting points were measured on an electrothermal melting point apparatus and are uncorrected. The IR spectra were recorded on a Perkin-Elmer spectrum-100 FT-IR in KBr. Mass spectra were obtained with a Synapt G2 mass spectrometer instrument. NMR data were recorded on a JEOL ECZ-600 spectrometer at 600 MHz for 1H and 150 MHz for 13C, with TMS as internal standard. Column chromatography was conducted on silica gel 60 (Kanto Chemical Co., Inc., Japan). TLC plates were precoated with silica gel GF254 (Merck, 0.25 mm) and detection was achieved by spraying with 10% H2SO4 in ethanol, followed by heating.
Plant material
The bark of A. argentea was collected in Bogor Botanical Garden, Bogor, West Java Province, Indonesia in June 2015. The plant was identified by the staff of the Bogoriense Herbarium, Bogor, Indonesia and a voucher specimen (No. Bo-1288718) was deposited at the herbarium.
Extraction
The dried bark (2.5 kg) was extracted with methanol (12 L) at room temperature for 5 days. After removal of the solvent under vacuum, the viscous concentrated extract of MeOH (133.5 g) was first suspended in H2O and then partitioned with n-hexane, EtOAc, and n-BuOH, successively. Evaporation resulted in crude extracts of n-hexane, EtOAc, and n-BuOH. All the extracts were tested for their cytotoxic activity against P-388 murine leukemia cells.
Determination of cytotoxic activities
The P-388 cells were seeded into 96-well plates at an initial cell density of approximately 3 x 104 cells cm-3. After 24 h of incubation for cell attachment and growth, varying concentrations of samples were added. Before adding, the compounds were dissolved in DMSO at the required concentration. The subsequent six desired concentrations were prepared using PBS (phosphoric buffer solution, pH = 7.30 - 7.65). Control wells received only DMSO. The assay was terminated after a 48 h incubation period by adding MTT reagent [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; also named thiazol blue] and the incubation was continued for another 4 h during which the MTT-stop solution containing SDS (sodium dodecyl sulphate) was added and another 24 h incubation was conducted. Optical density was read by using a micro plate reader at 550 nm. IC50 values were taken from the plotted graph of percentage live cells compared to control (%), receiving only PBS and DMSO, versus the tested concentration of compounds (μg/mL). The IC50 value is the concentration required for 50% growth inhibition. Each assay and analysis was run in triplicate and averaged.
RESULTS
Extraction and isolation
The crude extracts of n-hexane (26.3 g), EtOAc (12.4 g), and n-BuOH (12.6 g) were tested for their cytotoxic activity against P388 murine leukemia cells and showed cytotoxic activity with IC50 values of 26.72 ± 0.02, 15.49 ± 0.03, and 85.67 ± 0.02 μg/mL, respectively. The n-hexane soluble fraction (26.3 g) was fractionated by vacuum liquid chromatography on silica gel 60 using a gradient n-hexane and EtOAc to give nine fractions (A–I), combined according to TLC results. Fraction A (6 g) was chromatographed on a column of silica gel, eluted successively with a gradient of n-hexane–CH2Cl2 (10:0–1:1) to give ten subfractions (A01–A10). Subfraction A03 was chromatographed on a column of silica gel, eluted with n-hexane:CHCl3 (9:1) to give 2 (74.5 mg). Fraction C (2.68 g) was chromatographed on a column of silica gel, eluted successively with a gradient of n-hexane–EtOAc (10:0–4:1) to give nine subfractions (C01-C9). Subfraction C03 was recrystallized in n-hexane, to give 1 (188.6 mg). The EtOAc soluble fraction (12.4 g) was fractionated by column chromatography on silica gel using a gradient n-hexane and EtOAc to give eight fractions (J–Q), combined according to TLC results. Fraction K (927.6 mg) was chromatographed on a column of silica gel, eluted successively with a gradient of n-hexane–EtOAc (10:0–0:10) to give 3 (106.3 mg). Fraction P (3.86 g) was chromatographed on a column of silica gel, eluted successively with a gradient of CHCl3-Me2CO (10:0–4:1), to give seven subfractions (P01-P07). Subfraction P05 was chromatographed on a column of silica gel, eluted with n-hexane-Me2CO (10:0-0:10) to give 4 (5 mg) and 5 (3 mg).
Stigmast-5-en-3β-ol (1). White needle-like crystals; m.p. 134-136 °C; IR (KBr) νmax 3424, 2937, 2870, 1464, 1379, 1056 cm-1; 1H-NMR (CDCl3, 600 MHz) see Table 1; 13C-NMR (CDCl3, 150 MHz), see Table 1; TOFMS (negative ion mode) m/z 413.0811 [M-H]-, (calcd. C29H49O-, m/z 413.3789).
Stigmast-5-en-3β-ol-3β-oleate (2). White waxy solid; IR (KBr) νmax 2937, 2870, 1710, 1620, 1464, 1379, 1056 cm-1; 1H-NMR (CDCl3, 600 MHz) see Table 1; 13C-NMR (CDCl3, 150 MHz), see Table 1; TOFMS (positive ion mode) m/z 679.6013 [M+H]+, (calcd. C47H83O2 +, m/z 679.6388).
Stigmast-5-en-3β-ol-3-O-(6′-O-oleoyl)-β-D-glucopyranoside (3). White waxy solid; IR (KBr) νmax 3425, 3342, 1707, 1566, 1292, 1020 cm-1; 1H-NMR (CDCl3, 600 MHz), see Table 1; 13C-NMR (CDCl3, 150 MHz), see Table 1.
Stigmast-5-en-3β-ol-3-O-β-D-glucopyranoside (4). White amorphous powder; m.p. (decomposed); IR (KBr) νmax 3433, 1639, 1461, 1380, 1053 cm-1; 1H-NMR (pyridine-d5, 600 MHz), see Table 2; 13C-NMR (pyridine-d5, 150 MHz), see Table 2.
Stigmast-5,22-dien-3β-ol-3-O-β-D-glucopyranoside (5). White amorphous powder; m.p. (decomposed); IR (KBr) νmax 3450, 1630, 1445, 1370, 1050 cm-1; 1H-NMR (pyridine-d5, 600 MHz), see Table 2; 13C-NMR (pyridine-d5, 150 MHz), see Table 2.
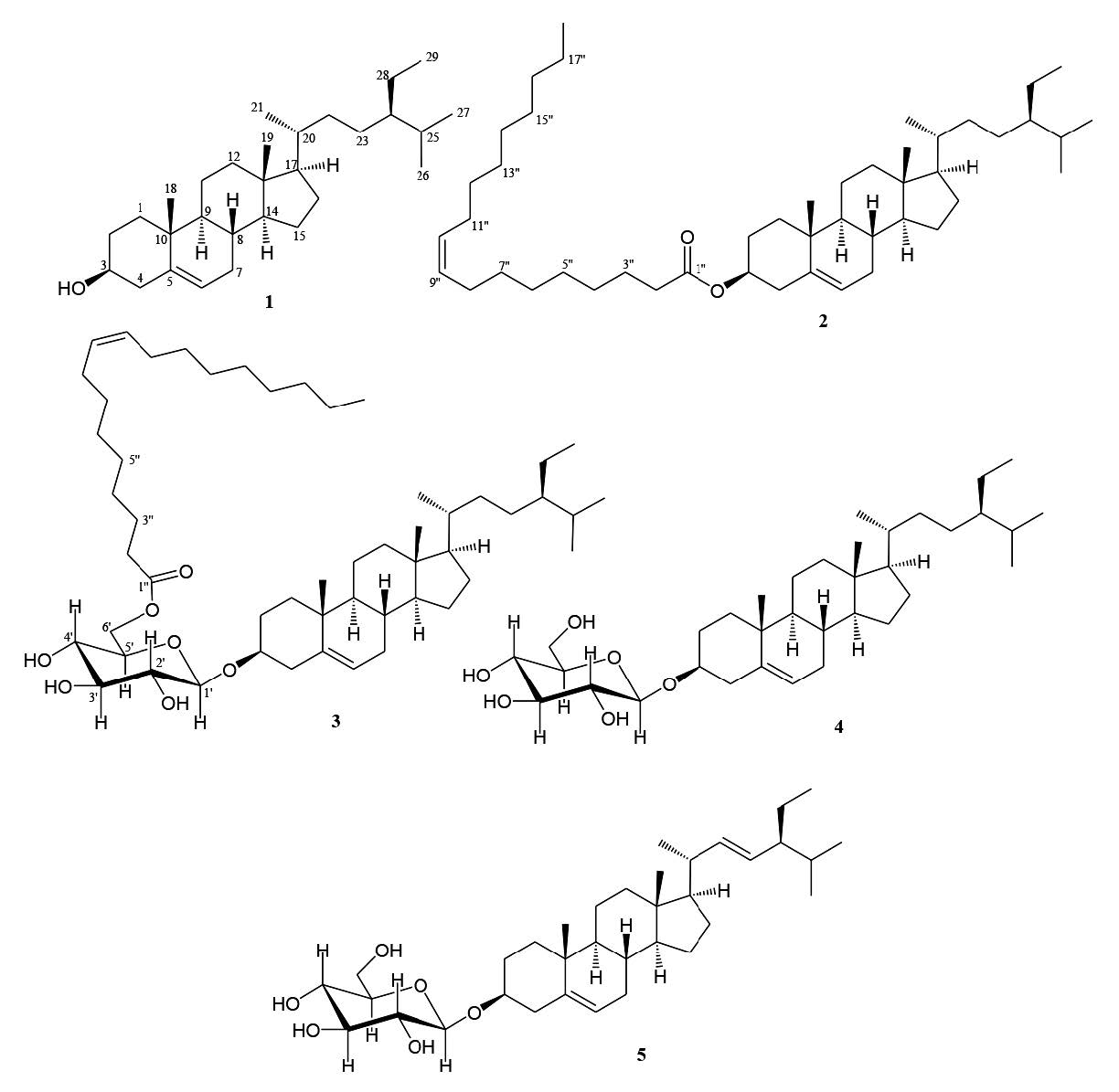
Figure 1. Structures of compounds 1-5.
Table 1. NMR data (600 MHz for 1H and 150 MHz for 13C, in CDCl3) for 1-3.
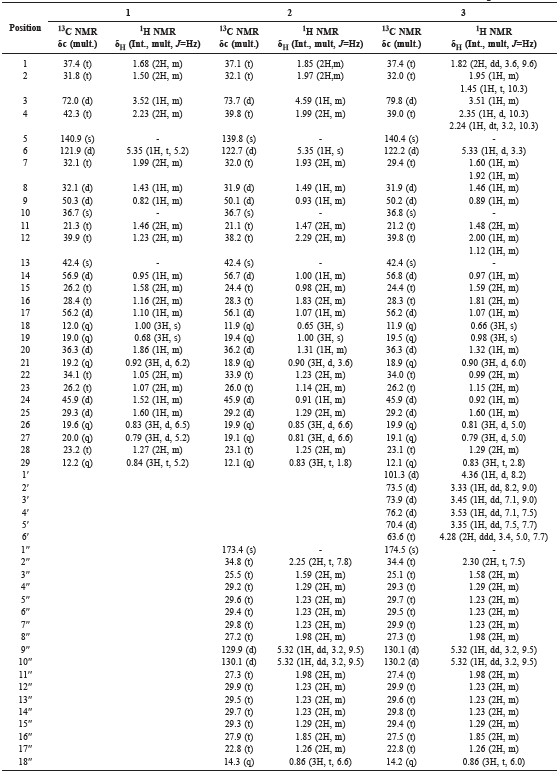
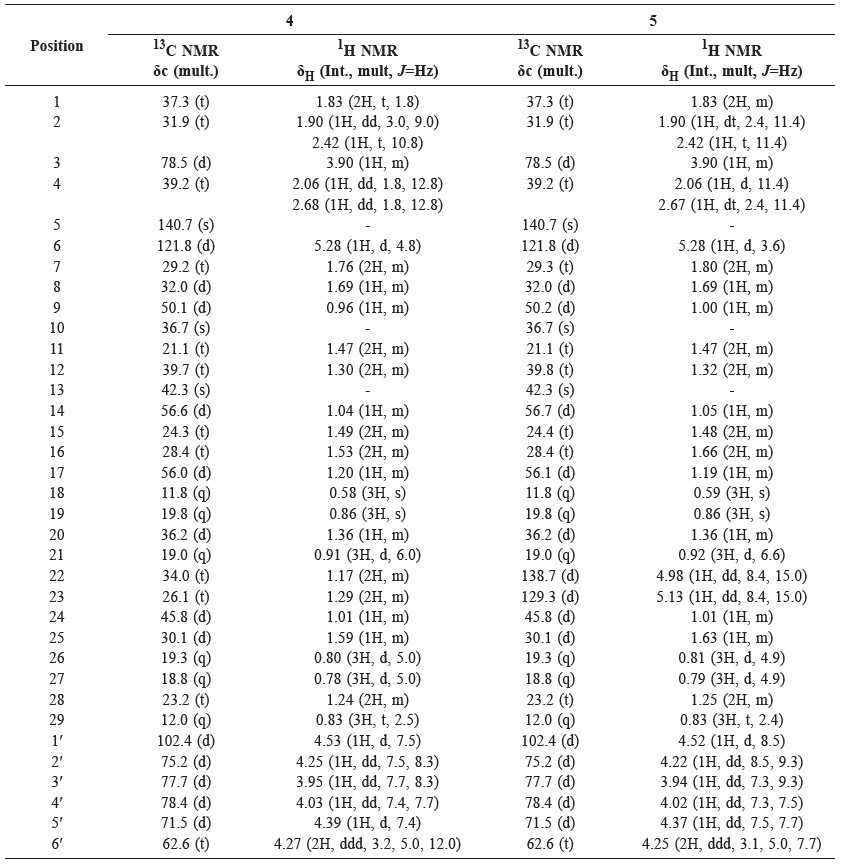
Table 2. NMR data (600 MHz for 1H and 150 MHz for 13C, in pyridine-d5) for 4 and 5.
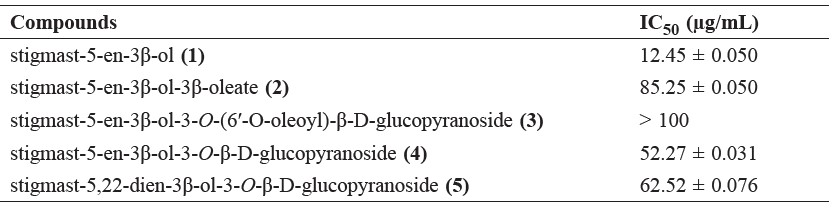
The cytotoxicity effects of the five isolated compounds against P-388 murine leukemia cells were conducted according to the method described previously (Harneti et al., 2012; Sahidin et al., 2005; Alley et al., 1988); artonin E (IC50 0.3 μg/mL) was used as the positive control (Hakim et al., 2007). The cytotoxicity activities of the isolated compounds 1-5 are shown in Table 3.
Table 3. Cytotoxicity activity of compounds 1-5 against P-388 murine leukemia cells.
DISCUSSION
The phytochemical test for the n-hexane and EtOAc extract showed the presence of steroids. By using cytotoxic assay to guide separations, the n-hexane and EtOAc fraction was separated by column chromatography over silica gel by gradient elution. The fractions were repeatedly subjected to normal-phase column chromatography and preparative TLC on silica gel GF254 yielded five cytotoxic steroids 1-5 (Figure 1).
Compound 1 was obtained as a white needle-like crystal. The TOFMS spectrum showed [M-H]+ m/z 413.0811 (calcd. m/z 413.3789), which corresponded to the molecular formula C29H50O and thus required five degrees of unsaturation, originating from one pair of C sp2 and the remaining tetracyclic stigmastane-type steroid. The IR spectra showed absorption peaks at 3,424 cm-1 (OH), 2,937 and 2,870 cm-1 (C-H sp3), 1,464 cm-1 (C=C), 1379 cm-1 (gem-dimethyl groups), and 1,056 cm-1 (C-O). The 1H-NMR (CDCl3 600 MHz) spectrum showed the presence of six methyl groups: two tertiary methyl groups resonating at δH 1.00 (CH3-18) and 0.68 (CH3-19); three secondary methyl groups resonating at δH 0.92 (3H, d, J = 6.2 Hz, CH3-21), 0.83 (3H, d, J = 6.5 Hz, CH3-26), and 0.81 (d, J = 5.2 Hz, CH3-27); and one primary methyl group resonating at δH 0,84 (t, J = 5.2 Hz, CH3-29); this was indicative of the presence of stigmastane-type steroid skeleton. One olefinic methine group, resonating at δH 5.35 (d, J = 5.2 Hz, H-6) and an oxymethine group resonating at δH 3.52 (1H, m, H-3) were also observed in the 1H NMR spectra. Proton pairing was also confirmed with the 1H-1H COSY spectrum (Figure 2). 1H-1H COSY cross peak observed at H-2/H-3/H-4 indicated that the hydroxy group was positioned at C-3. The cross peak also observed at H-6/H-7/H-8 indicated the position of a double bond at C-5/C-6. The 13C-NMR (CDCl3 150 MHz) and DEPT 135° spectra showed the presence of six methyl groups: one olefinic methine, one olefinic quartenary carbon, and an oxygenated methine group, resonating at δC 72.0 (C-3); this indicated that had the characteristic of a stigmastane-type steroid (Cayme and Ragasa, 2004). These functionalities accounted for one of five degrees of unsaturation. The remaining four degrees of unsaturation were consistent with the stigmastane-type steroid. A comparison of the NMR data of 1 with the data for β-sitosterol (Chaturvedula and Prakash, 2012), revealed that the structure of the two compounds were very similar; consequently, compound 1 was identified as stigmast-5-en-3β-ol (β-sitosterol); m/z 679.6013 [M+H]+, (calcd C47H83O2+, m/z 679.6388).
Compound 2 was obtained as a white waxy solid. The TOFMS spectrum showed [M+H]+ m/z 679.6013 (calcd. C47H82O2, m/z 678.6388); the fragment ion peaks occurred at m/z 415.2132 [M+H-265], indicating the loss of a fatty acid (terminal oleate acid unit), which coresponded to the molecular formula C29H50O and thus required five degrees of unsaturation. The 1H and 13C-NMR spectrum of 2 resembled that of 1 – the main difference was that compound 2 was substituted with a fatty acid in the ester linkage. The position of the fatty acid was determined by HMBC correlation (Figure 2). The presence of a fatty acid (oleate acid) attached at C-3 in ester form was supported by HMBC correlation from oxymethine proton at H-3 (δH 4.59) to C-1′′ (δC 173.4) in the ester group, and correlation at H-2′′ (δH 2.25) to C-1′′ (δC 173.4). The position of the double bond in oleic acid at C-9′′/C-10′′ was evidenced by HMBC correlation from H-9′′ (δH 5.32) to C-6′′ (δC 29.4) and C-7′′ (δC 29.8). This correlation also supported the appearance of oleate acid in mass spectra, which appeared at molecular ion peak m/z 265. The coupling constant of H-9/H-10 was 9.5 Hz; this indicated that each proton was in cis position. Compound 2 agreed well with data from the literature (Tesemma et al., 2013; Ragasa et al., 2016), supporting its identification as stigmast-5-en-3β-ol-3β-oleate (β-sitosterol oleate).
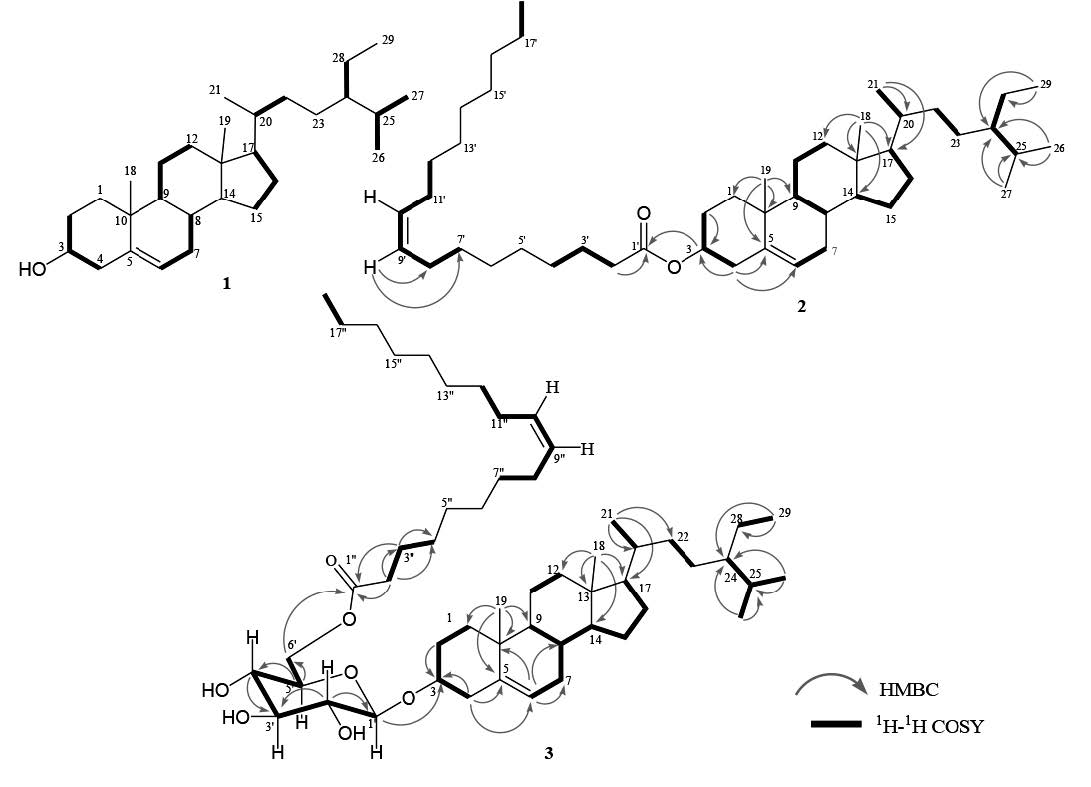
Figure 2. Selected HMBC and 1H-1H COSY correlations for 1, 2, and 3.
Compound 3 was obtained as a white waxy solid. Its molecular composition C53H92O7, was established from NMR data (Table 1). The IR spectrum suggested the presence of hydroxyl (3,425 cm-1), carbonyl (1,707 cm-1), olefinic (1,566 cm-1) and ether groups (1,292 and 1,020 cm-1). The 1H and 13C-NMR spectrum of 3 resembled that of 2; the main difference was that compound 3 was substituted with sugar moiety in the ether link. The position of the fatty acid and the sugar unit were determined by HMBC and 1H-1H COSY correlations (Figure 2). The presence of oxygenated methylene at δH 4.28 (2H, ddd, J = 3.4, 5, 7, H-6′), together with an anomeric signal proton at δH 4.36 (1H, d, J = 8.2, H-1′), as well as four oxygenated methines, resonating at δH 3.33 (1H, dd, J = 8.2, 9.0, H-2′), 3.45 (1H, dd, J = 7.1, 9.0, H-3′), 3.53 (1H, dd, J = 7.1, 7.5, H-4′), and 3.35 (1H, dd, J = 7.5, 7.7, H-5′), was typical of a glucose moiety. The 13C NMR of the anomeric carbon resonated at δC 101.3 (C-1′), indicating β-glucose. The HMBC spectrum showed correlations between H-1′ (δH 4.36) to C-3 (δC 79.8), indicating that the sugar unit was linked at the C-3 position. The presence of fatty acid in ester form at C-6′ was established by correlation between H-6′ (δH 4.28) and H-2′′ (δH 2.30) to C-1′′ (δC 174.5) in an ester group. The fatty acid was identified as oleate acid based on observation of the NMR spectrum and the coupling constant of H-9/H-10, which was 9.5 Hz; this indicated that each proton was in the cis-position. Compound 3 agreed well with data from the literature (Tesemma et al., 2013; Ragasa et al., 2016), supporting its identification as stigmast-5-en-3β- O-(6′-O-oleoyl)-β-D-glucopyranoside (sitoindoside II).
Compound 4 was obtained as a white amorphous powder. Its molecular composition C35H60O6, was established from NMR data (Table 2). The IR spectrum suggested the presence of hydroxyl groups (3,433 cm-1), olefinic carbons (1,639 cm-1), gem-dimethyl group (1,461 and 1,380 cm-1), and C-O bond (1,053 cm-1). The 1H and 13C-NMR spectrum of 4 resembled that of 1; the main difference was that compound 4 was substituted with sugar moiety in the ether link. The presence of oxygenated methylene at δH 4.27 (2H, ddd, J = 3.2, 5.0, 12.0 Hz, H-6′), together with an anomeric signal proton at δH 4.53 (1H, d, J = 7.5 Hz, H-1′), as well as four oxygenated methines resonating at δH 4.25 (1H, dd, J = 7.5, 8.3 Hz, H-2′), 3.95 (1H, dd, J = 7.7, 8.3 Hz, H-3′), 4.03 (1H, dd, J = 7.4, 7.7 Hz, H-4′), and 4.39 (1H, d, J = 7.4 Hz, H-5′), was typical of a glucose moiety. The 13C NMR of anomeric carbon resonated at δC 102.4 (C-1′), indicating β-glucose. Compound 4 agreed well with data from the literature (Harneti et al., 2014), supporting its identification as stigmast-5-en-3β-ol-3-O-β-D-glucopyranoside (β-sitosterol glucoside).
Compound 5 was obtained as white amorphous powder. Its molecular composition C35H58O6, was established from NMR data (Table 2). The IR spectrum suggested the presence of hydroxyl groups (3,450 cm-1), olefinic carbons (1,630 cm-1), gem-dimethyl group (1,445 and 1,370 cm-1), and C-O bond (1,050 cm-1).The 1H and 13C-NMR spectrum of 5 were very similar to 4; the main difference was that compound 5 had an additional double bond at δH 4.98 and 5.13 (each 1H, dd, J = 8.4, 15.0 Hz, H-22, H-23) and δC 138.7 (C-22) and 129.3 (C-23). Compound 5 agreed well with data from the literature (Harneti et al., 2014), supporting its identification as stigmast-5,22-dien-3β-ol-3-O-β-D-glucopyranoside (stigmasterol glucoside).
Among the five steroid compounds identified here, stigmast-5-en-3β-ol (1), with a 3-hydroxyl group, showed the strongest cytotoxicity activity, whereas stigmast-5-en-3β-ol-3β-oleate (2), stigmast-5-en-3β-ol-3-O-(6′-O-oleoyl)-β-Dglucopyranoside (3), stigmast-5-en-3β-ol-3-O-β-D-glucopyranoside (4), and stigmast-5,22-dien-3β-ol-3-O-β-D-glucopyranoside (5), with 3-glucosides, 3β-oleate, and 3β-O-(6′-O-oleoyl)-β-D-glucopyranoside, showed weak or no activity. This indicated that the presence of a 3β-hydroxyl group may be an important sructural feature for cytotoxic activity, whereas the presence of sugar and fatty acid moieties may decrease cytotoxic activity.
ACKNOWLEDGMENTS
This investigation was financially supported by the Directorate General of Higher Education, Ministry of Research, Technology and Higher Education, Indonesia (Hibah Tim Pascasarjana, 2016-2018, by US). We thank Ahmad Darmawan and Sofa Fajriah at the Research Center for Chemistry, Indonesian Science Institute, for NMR measurements and Suzany Dwi Elita at the Department of Chemistry, Faculty of Mathemathics and Natural Sciences, Bandung Institute of Technology for cytotoxicity bioassay.
REFERENCES
Alley, M.C., Scudiero, D.A., Monks, A., Hursey, M.L., Czerwinski, M.J., Fine, D.L., Abbott, B.J., Mayo, J.G., Shoemaker, R.H., and Boyd, M.R. 1988. Feasibility of drug screening with panels of tumor cell lines using a microculture tetrazolium assay. Cancer Research. 48: 589-601.
Awang, K., Loong, X., Leong, K.H., Supratman, U., Litaudon, M., Mukhtar, M.R., and Mohamad, K. 2012. Triterpenes and steroids from the leaves of Aglaia exima (Meliaceae). Fitoterapia. 83: 1391-1395. https://doi.org/10.1016/j.fitote.2012.10.004
Baskar, A.A., Al Numair, K.S., Paulraj, M.G., Alsaif, M.A., Al Muamar, M., and Ignacimuthu, S. 2012. β-Sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. Journal of Medicinal Food. 15: 335-343. https://doi.org/10.1089/jmf.2011.1780
Brader, G., Vajrodaya, S., Greger, H., Bacher, M., Kalchhauser, H., and Hofer, O. 1998. Bisamides, lignans, triterpenes, and insecticidal cyclopenta[b] benzofurans from Aglaia species. Journal of Natural Products. 61: 1482-1490. https://doi.org/10.1021/np9801965
Cayme, J., and Ragasa, C. 2004. Structure elucidation of β-stigmasterol and β-sitosterol from Sesbania grandiflaora (Linn). Pers. and β-carotene from Heliotropium indicum Linn by NMR spectroscopy. Journal of Kimika. 20: 5-12.
Chai, J.W., Kuppusamy, U.R., and Kanthimathi, M.S. 2008. Beta-sitosterol induces apoptosis in MCF-7 cells. Malaysian Journal of Biochemistry Molecular Biology. 16: 28-30.
Chaturvedula, V.S.P., and Prakash, I. 2012. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. International Current Pharmaceutical Journal. 1: 239-242.
Gupta A., Sharma, A.K., Dobhal, M.P., Sharma, M.C., and Gupta, R.S. 2011. Antidiabetic and antioxidant potential of β-sitosterol in treptozotocin-induced experimental hyperglycemia. Journal of Diabetes. 3: 29-37. https://doi.org/10.1111/j.1753-0407.2010.00107.x
Hakim, E.H., Achmad, S.A., Juliawaty, L.D., Makmur, L., Syah, Y.M., Aimi, A., Kitajima, M., Takayama, H., and Ghisalberti, E.L. 2007. Prenylated flavonoids and related compounds of the Indonesian Artocarpus (Moraceae). Journal of Natural Medicines. 61(2): 229-236. http://dx.doi.org/10.007/s11418-006-0048-0
Harneti, D., Supriadin, A., Ulfah, M., Safari, A., Supratman, U., Awang, K., and Hayashi, H. 2014. Cytotoxic constituents from the bark of Aglaia eximia (Meliaceae). Phytochemistry Letters. 8: 28-31. https://doi.org/10.1016/j.phytol.2014.01.005
Harneti, D., Tjokronegoro, R., Safari, A., Supratman, U., Loong, X., Mukhtar, M. M., Mohamad, R., Awang, K., and Hayashi, H. 2012. Cytotoxic triterpenoids from the bark of Aglaia smithii. Phytochemistry Letters. 5: 496-499. https://doi.org/10.1016/j.phytol.2012.04.013
Hidayat, S.S., and Hutapea, J.R. 1991. Indonesian Medicinal Plants (II), Research and Development Agency. Ministry of Health. Jakarta. Indonesia. pp. 15-21.
Inada, A., Sorano, T., Murata, H., Inatomi, Y., Darnaedi, D., and Nakanishi, T. 2001. Diamide derivatives and cycloartanes from the leaves of Aglaia elliptical. Chemical and Pharmaceutical Bulletin. 49(9): 1226-1228. https://doi.org/10.1248/cpb.49.1226
Ishibashi, F., Satasook, C., Isman, M.B., and Towers, G.H.N. 1993. Insecticidal 1H-cyclo-pentatetrahydro[b]benzofurans from Aglaia odorata. Phytochemistry. 32: 307-310. https://doi.org/10.1016/s0031-9422(00)94986-0
Jamaluddin F., Mohamed, S., and Lajis, M.N. 1994. Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of β-sitosterol and stigmasterol. Food Chemistry. 49: 339-345. https://doi.org/10.1016/0308-8146(94)90002-7
Ju, Y.H., Clausen, L.M., Allred, K.F., Almada, A.L., and Helferich, W.G. 2004. β-sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells In vitro and in ovariectomized athymic mice. Journal of Nutrition. 134: 1145-1151.
Khalit, M.M., Martin, T., Najdar, H., Gaspard, G., Sevenet, T., Awang, K., Hadi, H., and Pais, M. 1999. Cytotoxic 3,4-secoapotirucallanes from Aglaia argentea Bark. Jounal of Natural Products. 62: 868-872. https://doi.org/10.1021/np990013u
Mabberley, D.J., Pannel, C.M., and Sing, A.M. 1995. Flora Malesiana: Series I. Vol 12. Leiden. Netherlands. pp. 1-407.
Moon E.J., Lee, Y.M., Lee, O.H., Lee, M.J., Lee, S.K., and Chung, M.H. 1999. A novel angiogenic factor derived from Aloe vera gel: beta-sitosterol, a plant sterol. Angiogenesis. 3: 117-123.
Muellner, A.N., Greger, H., and Pannell, C.M. 2010. Genetic diversity and geographic structure in Aglaia elaegnoidea (Meliaceae, Sapindales), a morphologically complex tree species, near the two extremes of its distribution. Blumea. 54: 207-216. https://doi.org/10.3767/000651909X476175
Nugroho, B.W., Edrada, R.A., Wray, V., Witte, L., Bringmann, G., Gehling, M., and Proksch, P. 1999. An insectisidal rocaglamida derivates and related compounds from Aglaia odorata (Meliaceae). Phytochemistry. 51: 367-376. https://doi.org/10.1016/S0031-9422(98)00751-1
Ostlund, R.E., Mcgill, J.B., Zeng, C., Covey, D.F., Stearns, J., and Stenson, W.F. 2002. Gastrointestinal absorption and plasma kinetics of soy 5-phytosterols and phytostanols in humans. American Journal of Physiology and Endocrinol Metabolism. 282: 911-916. https://doi.org/10.1152/ajpendo.00328.2001
Ovesna Z., Vachalkova, A., and Horvathova, K. 2004. Taraxasterol and beta-sitosterol: new naturally compounds with chemoprotective/chemopreventive effects. Neoplasma. 51: 407-414.
Pannell, C.M. 1992. Taxonnomic monograph of the genus Aglaia Lour. (Meliaceae). Kew Bulletin Additional Series XVI; HMSO: Kew, Richmond, Surrey, UK.
Park, C., Moon, D.O., Rhu, C.H., Choi, B.T., Lee, W.H., Kim, G.Y., and. Choi, Y.H. 2007. β-sitosterol induces antiproliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biological & Pharmaceutical Bulletin. 30: 1317-1323.
Prieto, J.M., Recio, M.C., and Giner, R.M. 2006. Anti-inflammatory activity of β-sitosterol in a model of oxazolone induced contact-delayed-type hypersensitivity. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 5: 57-62.
Radika, M.K., Viswanathan, P., and Anuradha, C.V. 2013. Nitric oxide mediates the insulin sensitizing effects of β-sitosterol in high fat diet-fed rats. Nitric Oxide. 32: 43-53. https://doi.org/10.1016/j.niox.2013.04.007
Ragasa, C.Y., Ng, V.A.S., and Shen, C. 2016. Chemical constituents of Moringa oliefera Lam. seeds. International Journal of Pharmacognosy and Phytochemical Research. 8(3): 495-498.
Roux, D., Martin, T., Adeline, T., Sevenet, T., Hadi, H., and Pais, M. 1998. Foveolins A dan B, dammarane triterpenes from Aglaia foveolata. Phytochemistry. 49(6): 1745-1748. https://doi.org/10.1016/S0031-9422(98)00305-7
Saeidnia, S., Manayi, A., Gohari, A.R., and Abdollahi, M. 2014. The story of beta-sitosterol-A review. European Journal of Medicinal Plants. 4(5): 590-609. https://doi.org/10.9734/ EJMP/2014/7764
Sahidin, H.E.H., Juliawaty, L.D., Syah, Y.M., Din, L.B., Ghisalberti, E.L., Latip, J., Said, I.M., and Achmad, S.A. 2005. Cytotoxic properties of oligostilbenoids from the tree bark of Hopea dryobalanoides. Z. Naturforsch. 60c: 723-727.
Sianturi, J., Harneti, D., Darwati, Mayanti, T., Supratman, U., and Awang, K. 2016. A New (-)-5′,6-dimethoxyisolariciresinol-(3″,4″-dimethoxy)-3α-O-β-D-glucopyranoside from the bark of Aglaia eximia (Meliaceae). Natural Product Research. 30(19): 2204-2208. http://dx.doi.org/10.1080/14786419.2016.1160233
Sianturi, J., Purnamasari, M., Darwati, D. Harneti, Mayanti, T., Supratman, U., Awang, K., and Hayashi, H. 2015. New bisamide compounds from the bark of Aglaia eximia (Meliaceae). Phytochemistry Letters. 13: 297-301. https://doi.org/10.1016/j.phytol.2015.07.003
Tesemma, M., Adane, L., Tariku, Y., Muleta, D., and Demise, S. 2013. Isolation of compounds from acetone extract of root wood of Moringa stenopetala and evaluation of their antibacterial activities. Research Journal of Medicinal Plants. 7(1) : 32-47. https://doi.org/10.3923/rjmp.2013.32.47
Vivancos, M., and Moreno, J.J. 2005. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radical Biology and Medicine. 39: 91-97. https://doi.org/10.1016/j.freeradbiomed.2005.02.025
Wu, T.S., Liou, M.J., Kuoh, C.S., Teng, C.M., Nagao, T., and Lee, K.H. 1997. Cytotoxic and antiplatelet aggregation principles from Aglaia ellipfolia. Journal of Natural Products. 60: 606-608. https://doi.org/10.1021/np970163+
Xie, B.J., Yang, S.P., Chen, H.D., and Yue, J.M. 2007. Agladupols A-E, Triterpenoids from Aglaia duperreana. Journal of Natural Products. 70: 1532-1535. https://doi.org/10.1021/np0702842
Zak A., Zeman, M., Vitkova, D., Hrabak, P., and Tvrzicka, E. 1990. Beta-sitosterol in the treatment of hypercholesterolemia. Casopis Lékaru Ceských. 129: 1320-1323.
Zhang, F., Wang, J.S., Gu, Y.C., and Kong, L.Y. 2010. Triterpenoids from Aglaia abbreviata and their cytotoxic activities. Journal of Natural Products. 73: 2042-2046. https://doi.org/10.1021/np100599g
Kindi Farabi1, Desi Harneti1, Nurlelasari1, Rani Maharani1,2, Ace Tatang Hidayat1,2, Unang Supratman1,2*, Khalijah Awang3 and Yoshihito Shiono4
1 Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang Km 21, Jatinangor 45363, Indonesia
2 Central Laboratory of Universitas Padjadjaran, Jl. Raya Bandung-Sumedang Km 21, Jatinangor 45363, Indonesia
3 Department of Chemistry, Faculty of Science, University of Malaya, Kuala Lumpur 59100, Malaysia
4 Department of Food, Life, and Environmental Science, Faculty of Agriculture, Yamagata University, Tsuruoka, Yamagata 997-8555, Japan
*Corresponding author. E-mail: unang.supratman@unpad.ac.id
Total Article Views

