
Characterization of Bio-oils from Jatropha Residues and Mixtures of Model Compounds
Prapaporn Prasertpong, Chawannat Jaroenkhasemmeesuk, Nakorn Tippayawong* and Yoothana ThanmongkhonPublished Date : 2017-04-01
DOI : https://doi.org/10.12982/CMUJNS.2017.0011
Journal Issues : Number 2 ,April - June 2017
ABSTRACT
This study analyzed the composition of actual bio-oils produced by fast pyrolysis of jatropha and compared these with model bio-oils prepared based on typical biomass as reported in the literature. Bio-oils were found to consist primarily of organic acids, phenols, ketones, and alcohols, which were modeled using acetic acid, phenol, acetone, and ethanol, respectively. Properties of both the real and model bio-oils were subsequently characterized. While the physico-chemical characteristics of these bio-oils varied widely, long chain acids, such as oleic and palmitic acids, accounted for over 70%. Real bio-oils had higher energy content, density, viscosity, acidity, and flash and pour points than the model oils, because of the variety of large and heavy molecules present in the real bio-oils compared to the light compounds were used to represent the model oils.
Keywords: Biomass, Fast pyrolysis, Fuel property, Renewable energy
INTRODUCTION
Biomass offers an abundant and environmentally friendly source of clean energy that can help mitigate greenhouse gas emissions (Chen et al., 2014). For liquid fuel production, Biomass can be converted to liquid fuel in a number of ways (Bridgwater, 2012), with fast pyrolysis a promising one (Pattiya et al., 2007; Jaroenkhasemmeesuk; Tippayawong, 2015).
Fast pyrolysis is a thermal decomposition process occurring in a continuous flow system in the absence of oxygen (Laird et al., 2009). Bio-oil is the main product from fast pyrolysis. Direct applications of bio-oils are mainly limited by their high viscosity, high water and ash content, low heating value, instability, and high corrosiveness (Xui and Shahbaz, 2012). The unfavorable properties of biooils are caused by a complex mixture of water and various organic compounds, such as carboxylic acid, phenols, ketones, aldehydes, alcohols, and oxygenated oligomers that can react with themselves to form larger molecules. The properties and portion of each compound in bio-oils depend on biomass type and pyrolysis conditions, as shown in Table 1.
Table 1. Characteristics of bio-oils from fast pyrolysis of various biomass feedstocks compared to petroleum.
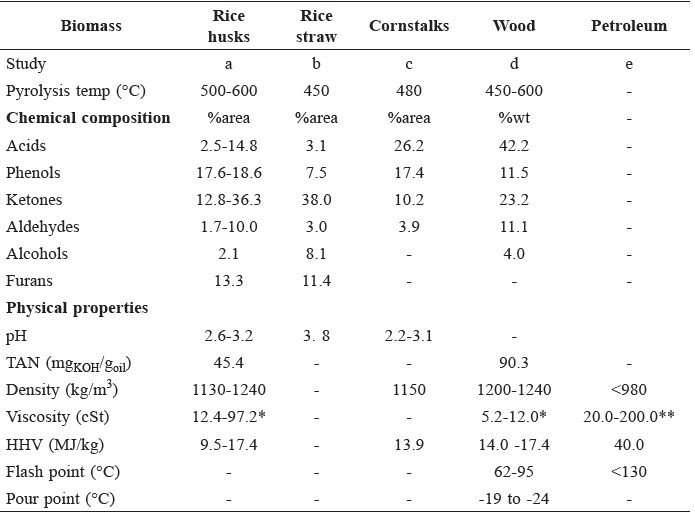
Note: *at 40°C, ** at 80°C, a = (Zhang et al., 2006; Tang, 2009; Song et al., 2010; Fukuda, 2015), b = (Fukuda, 2015), c = (Cui et al., 2010), d = (Oasmaa and Czernik, 1999; Xu et al., 2009; Xu et al., 2012 Tanneru et al., 2014; Zhang et al., 2015), e = (Chen et al., 2014)
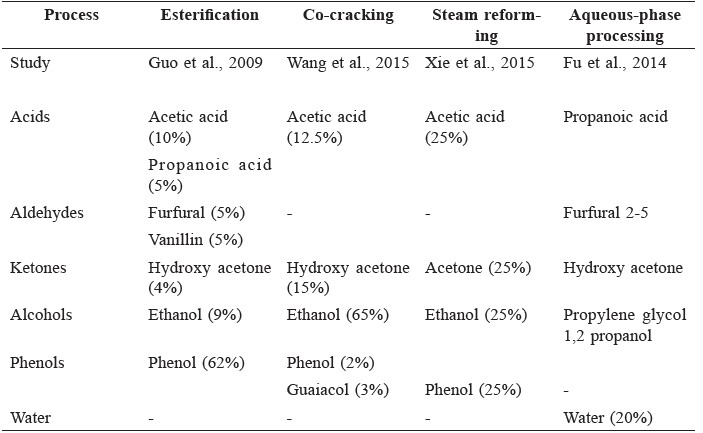
To overcome these disadvantages, the physical and chemical properties of bio-oils must be improved, with a number of techniques having been proposed, including hydrogenation, hydrodeoxygenation, catalytic hydrocracking, catalytic steam reforming, and catalytic esterification (Zhang et al., 2013). To study the mechanisms of these techniques, many studies have utilized model compounds by synthesizing chemicals to represent the oxygenated compounds (such as acids, alcohols, ketones, aldehydes, and phenols) in bio-oils, as shown in Table 2.
Table 2. Example compositions of model bio-oils used in some upgrading studies.
Few in the literature have reported on the characterization of mixtures of model compounds, nor compared their representative bio-oils against real bio-oils. Hence, the objective of this work was to explore and compare the properties of real bio-oils produced by pyrolysis with model bio-oils. Bio-oils from fast pyrolysis of jatropha cake and wood (pine), as well as their representative model bio-oils were analyzed. Their characteristics based on chemical and physical properties were compared and discussed.
MATERIAL AND METHODS
Materials
Real bio-oils from jatropha cake were used in this study. They were provided by the Thailand Institute of Scientific and Technological Research. They were produced from fast pyrolysis of jatropha cake in a circulating fluidized bed pyrolyzer at 500°C. For model compounds, acetic acid, phenol, ketone, ethanol (analytical reagent grade), and 0.1 phenolphthalein solution in ethanol were obtained from Vechavit Part., Ltd. Potassium hydroxide (85% analytical reagent grade), isopropyl alcohol (99.8%) and toluene (99.5% analytical reagent grade) were purchased
from RCI Labscan Co., Ltd.
Preparation of model bio-oils
Two model oils were prepared, one with similar composition to real biooil from fast pyrolysis of jatropha cake with composition obtained from analysis, and the other similar to real bio-oil of wood (pine) with composition information obtained from the literature as shown in Table 3.
The representative mixtures of both bio-oils were prepared based on the compositions and types of compound for easy preparation. Acetic acid, phenol, ethanol, and acetone were used to represent acids, phenols, alcohol, and other compounds in real bio-oil, respectively.
Table 3. Composition of bio-oil from pine wood (Zhang et al., 2015).
Characterization of bio-oils
The compositions and relative concentrations of bio-oil constituents from jatropha cake were analyzed by gas chromatography - mass spectrometry (GC-MS; GC Algilent 7890A, MSD Algilent 5975C). The NIST and Wiley mass spectral libraries were used to identify the chemical compounds. The chromatographic separation was carried out using a DB5-MS column (30 m × 0.25 mm × 0.5 μm film thickness). The injector and detector temperatures were set at 250 and 280°C, respectively. The column temperature was programed from 40°C (hold for 5 min)
to 280°C (hold for 10 min) at a heating rate of 10°C/min.
The physical properties of real bio-oil from jatropha cake and model bio-oils were analyzed by property-specific methods. The calorific value was measured using an oxygen bomb calorimeter based on ASTM D8409 standard. The viscosity was determined by Saybolt viscometer based on ASTM D88. The pH value was measured using a pH meter. The flash point was determined by Pensky-Marten closed cup (ASTM D3828). The pour point and density were measured following ASTM D79 and D369. The acid number was determined, following ASTM D974 with modification by using 0.34M of KOH in isopropyl alcohol and phenolphthalein for the indicator. They were subsequently used to compare with the physical properties of real bio-oil produced to simulate the composition of wood (pine) bio-oil obtained from the literature.
RESULTS
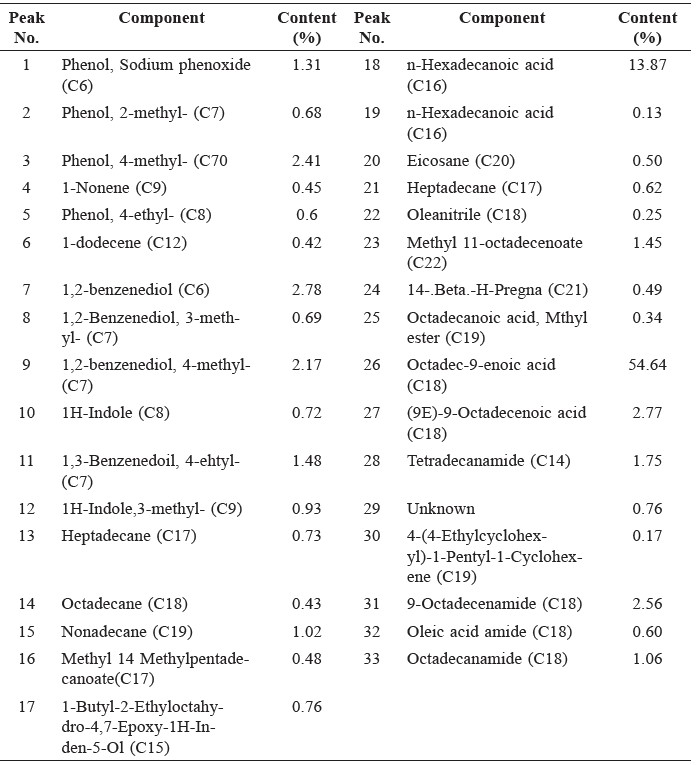
The components of bio-oil from jatropha cake analyzed by GC-MS are shown in Figure 1 and the identification of peaks from the chromatogram are summarized in Table 4.
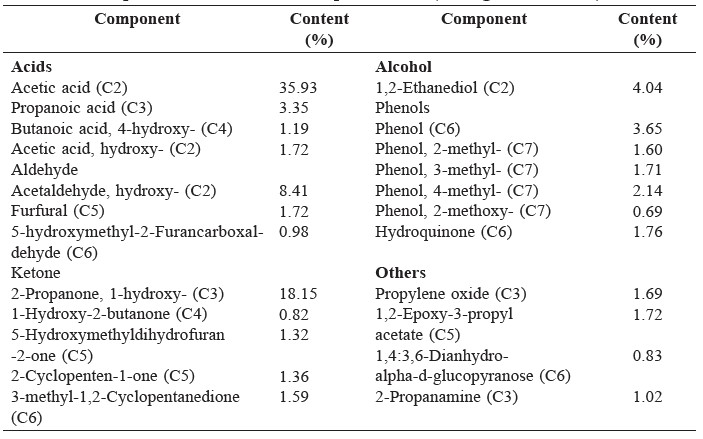
Compositions of the bio-oil from jatropha cake pyrolysis included complicated compounds – mainly acids, phenols, and amides –as well as a small amount of other compounds. Acids were the most abundant in bio-oil, comprising octadec-9-enoic acid and n-hexadecanoic acid.
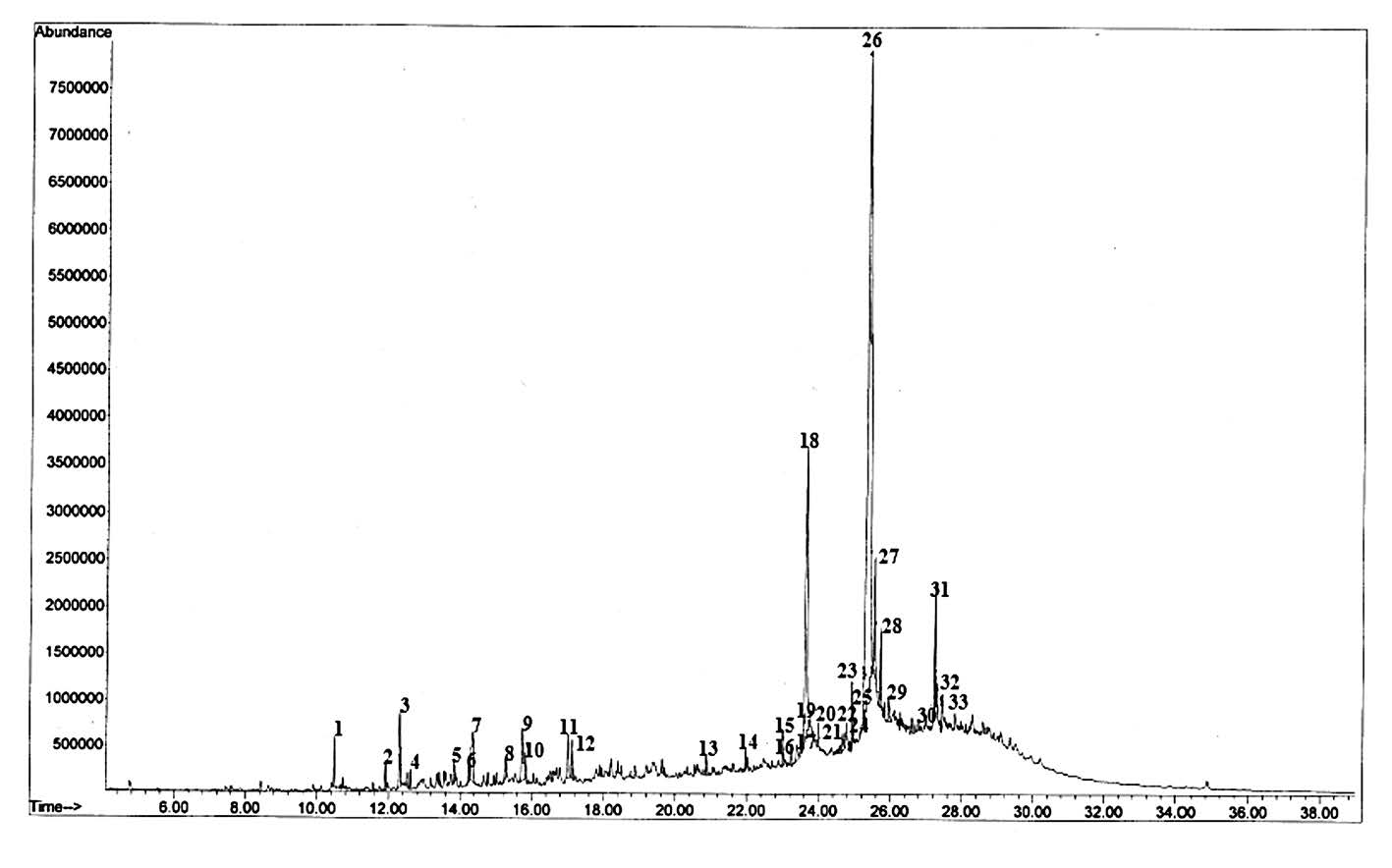
Figure 1. Typical GC-MS result of bio-oil from jatropha cake.
Table 4. Identification of peaks from GC/MS result and composition of bio-oil from jatropha cake produced by fast pyrolysis.
The components of the prepared model oils were obtained and compared with the data of the real bio-oils as shown in Table 5. Model bio-oil for jatropha cake was composed of acetic acid, phenol, and ketone to represent acids, phenols and other compounds in real bio-oil. Model bio-oil for wood (pine) contained acetic acid, phenol, ketone, and ethanol.
Table 5. Composition of real and model bio-oils.
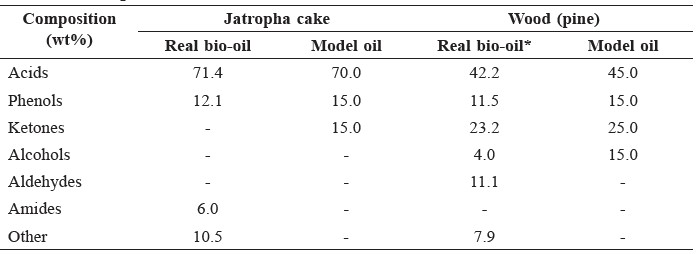
Note: *data derived from Zhang et al., 2015.
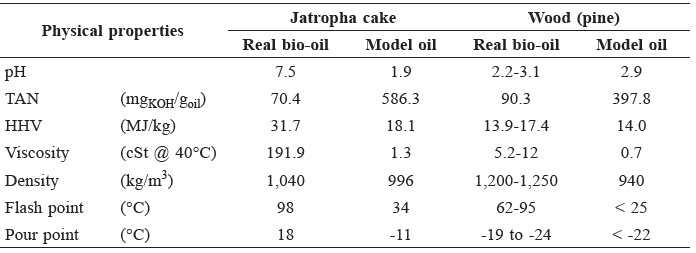
The properties of the real and model bio-oils are shown in Table 6. The physical properties of bio-oil from jatropha cake differed from those of bio-oil from typical biomass. Bio-oils from jatropha cake had higher HHV, density, viscosity, and flash and pour points than the wood bio-oils. The pH of bio-oil from jatropha cake was higher than the wood bio-oil, even though it has a high acid content.
Table 6. Properties of real and model bio-oils.
DISCUSSION
The compositions of real bio-oils from jatropha cake and typical bio-oil from wood (pine) are shown in Tables 3 and 4. Both bio-oils have different percentages and molecular weights of each main component due to largely different biomass types. Bio-oils from jatropha cake contained long chain fatty acids, phenols, amides, and other aromatic hydrocarbons. Fatty acids were the main component of bio-oil; this was consistent with the findings of Sricharoenchaikul and Atong (2009). The large molecules of fatty acids, amides, and other hydrocarbon compounds may have been derived from decomposition of triglycerides oil in jatropha cake (Kim et al., 2013), while the phenolic compounds present may have been from decomposition of lignin (Kongkasawan et al., 2016). Bio-oils from wood consisted of several compounds, including acids, phenols, aldehydes, alcohols, and small amounts of other compounds. Acid was the main component of the wood bio-oil, with acetic acid dominating. It may have come from the decomposition of hemicelluloses (Drzezdzon and Larcher, 2013).
Real and model bio-oils from jatropha cake appeared to have different molecular weight compounds in each functional group. Acids in real bio-oil contained large molecules and high carbon number (C16-C18), whereas the model bio-oil had smaller molecules (C2). Moreover, we used ketone in the model bio-oil to represent amides and other compounds that were not mentioned in the literature. For typical biomass, such as wood, real and model bio-oils appeared to have similar short chain compounds in each functional group, but real bio-oils still contained more complex compounds.
The physical properties of the real and model bio-oils varied widely, as shown in Table 4. Bio-oils from jatropha cake appeared to have higher HHV, viscosity, and flash and pour points than wood bio-oils, possibly due to long chain compounds from decomposition of jatropha-based triglycerides, as well as from degradation of lignocellulosic materials. The high acidity of wood bio-oil resulted from its simple, short chain acids, whereas the bio-oil from jatropha cake contained large molecular acids, hence showing high pH values.
The real bio-oils tended to have much higher energy content, viscosity, and flash and pour points than the model oils, while their acid numbers were lower. This was because the model bio-oils contained simple and lighter molecules than the real ones. For similar percentage by weight, the model oils contained high molar concentrations of acid. Differences in physical properties between real and model bio-oils from jatropha cake were higher than those from wood (pine). This was because model bio-oils from jatropha cake had simpler structured compounds than real bio-oils, whereas model bio-oils from wood had similar structure to real bio-oils from wood.
Bio-oils from various biomass types and pyrolysis conditions seemed to have largely different physico-chemical characteristics. Bio-oils contained a variety of complex, long chain and heavy compounds. Acids were found to be the main constituent. Bio-oils from jatropha cake consisted of fatty acids, phenols, amides, and other aromatic hydrocarbons. Fatty acid dominated (> 70 wt%), and was comprised of octadec-9-enoic acid and n-hexadecanoic acid. The model biooils with similar representative functional groups, but different molecular weights of compounds to real bio-oils, also showed different physical properties because of different structures of the compound. The model bio-oils prepared with similar functional group compounds to real bio-oils still showed some differences in physical properties, because real bio-oils had other complex compounds that cannot be represented by simple model compounds.
ACKNOWLEDGEMENTS
This research was financially supported by the Royal Golden Jubilee (PHD 560651033) Scholarship, the Thailand Research Fund, and Chiang Mai University. The authors would like to acknowledge the Thailand Institute of Scientific and Technological Research for material support and Rajamangala University of Technology Lanna and Maejo University for equipment.
REFERENCES
Bridgwater, A.V. 2012. Review of fast pyrolysis of biomass and product upgrading. Biomass & Bioenergy. 38: 68-94.
Chen, D., J. Zhou, Q. Zhang, and X. Zhu. 2014. Evaluation methods and research progresses in bio-oil storage stability. Renewable & Sustainable Energy Reviews. 40: 69-79.
Cui, H., J. Wang, S. Wei, S. Zhuo, Z. Li, L. Wang, and W. Yi. 2010. Upgrading bio-oil by esterification under supercritical CO2 conditions. Journal of Fuel Chemistry & Technology. 38: 673-678.
Drzezdzon, B., and A. Larcher. 2013. Characterisation of biomass pyrolysis by stepwise product collection and analysis: Mallee wood. Journal of Analytical & Applied Pyrolysis. 104: 308-15.
Fu, J., G. Sun, and B.H. Shanks. 2014. Aqueous-phase processing on multi-functional compounds over platinum-rhenium supported on carbon. Energy & Fuels. 28: 2123-2128.
Fukuda S. 2015. Pyrolysis investigation for bio-oil production from various biomass feedstocks in Thailand. International Journal of Green Energy. 12: 215-224.
Guo, Z., S. Wang, and Y. Zhu. 2009. Catalytic esterification of model compounds of biomass pyrolysis oil. IEEE International Conference on Energy and Environment Technology. 2009, Oct 16-18; Guilin, China.
Jaroenkhasemmeesuk, C., and N. Tippayawong. 2015. Technical and economic analysis of a biomass pyrolysis plant. Energy Procedia. 79: 950-955.
Kim, SW., B.S. Koo, J.W. Ryu, J.S. Lee, C.J. Kim, D.H. Lee, G.R. Kim, and S. Choi. 2003. Bio-oil from the pyrolysis of palm and jatropha wastes in a fluidized bed. Fuel Processing Technology. 108: 118-124.
Kongkasawan. J., H. Nam, and S.C. Capareda. 2016. Jatropha waste meal as an alternative energy source via pressurized pyrolysis: A study on temperature effects. Energy. 113: 631-642.
Laird, D.A., R.C. Brown, J.E. Amonette, and J. Lehmann. 2009. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels, Bioproducts & Biorefining. 3: 547-62.
Oasmaa, A., and S. Czernik. 1999. Fuel oil quality of biomass pyrolysis oils-state of the art for the end users. Energy & Fuels. 13: 914-921.
Pattiya, A., J.O. Titiloye, and A.V. Bridgwater. 2007. Fast pyrolysis of agricultural residues from cassava plantation for bio-oil production. Asian Journal on Energy & Environment. 08(02): 496-502.
Song, M., Z. Zhong, and J. Dai. 2010. Different solid acid catalysts influence on properties and chemical composition change of upgrading bio-oil. Journal of Analytical & Applied Pyrolysis. 89: 166-170.
Sricharoenchaikul, V., and D. Atong. 2009. Thermal decomposition study on jatropha curcas L. waste using TGA and fixed bed reactor. Journal of Analytical & Applied Pyrolysis. 85: 155-162.
Tang, Z., Q. Lu, Y. Zhang, X. Zhu, and Q. Guo. 2009. One step bio-oil upgrading through hydrotreatment, esterification, and cracking. Industrial & Engineering Chemistry Research. 48: 6923-6929.
Tanneru, S.K., D.R. Parapati, and P.H. Steele. 2014. Pretreatment of bio-oil followed by upgrading via esterification to boiler fuel. Energy. 73: 214-220.
Wang, S., Q. Cai, J. Chen, L. Zhang, L. Zhu, and Z. Luo. 2015. Co-cracking of bio-oil model compound mixtures and ethanol over different metal oxide-modified HZSM-5 catalysts. Fuel. 160: 534-543.
Xie, H., Q. Yu, M. Wei, W. Duan, X. Yao, Q. Qin, and Z. Zuo. 2015. Hydrogen production from steam reforming of simulated bio-oil over Ce-Ni/Co catalyst within continuous CO2 capture. International Journal of Hydrogen Energy. 40: 1420-1428.
Xu, Y., T. Wang, L. Ma, Q. Zhang, and L. Wang. 2009. Upgrading of liquid fuel from the vacuum pyrolysis of biomass over the Mo–Ni/γ-Al2O3 catalysts. Biomass & Bioenergy. 33: 1030-1036.
Xu, Y., T. Wang, L. Ma, and G. Chen. 2012. Upgrading of fast pyrolysis liquid fuel from biomass over Ru/c-Al2O3 catalyst. Energy Conversion & Management. 55: 172-177.
Xui, S., and A. Shahbaz. 2012. Bio-oil production and upgrading research: a review. Renewable & Sustainable Energy Reviews. 16(7): 4406-4414.
Zhang, L., R. Liu, R. Yin, and Y. Mei. 2013. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renewable & Sustainable Energy Reviews. 24: 66-72.
Zhang, Q., J. Chang, T. Wang, and Y. Xu. 2006. Upgrading bio-oil over different solid catalysts. Energy & Fuels. 20: 2717-2720.
Zhang, Q., Y. Xu, Y. Li, T. Wang, Q. Zhang, L. Ma, M. He, and K. Li. 2015. Investigation on the esterification by using supercritical ethanol for bio-oil upgrading. Applied Energy. 160: 633-640.
Prapaporn Prasertpong1, Chawannat Jaroenkhasemmeesuk1, Nakorn Tippayawong1* and Yoothana Thanmongkhon2
1 Department of Mechanical Engineering, Faculty of Engineering, Chiang Mai University, Chiang Mai 50200, Thailand
2 Thailand Institute of Scientific and Technological Research, Pathum Thani 12120,
Thailand
*Corresponding author. E-mail: n.tippayawong@yahoo.com
Total Article Views

