
Synthesis, Characterization, and Antibacterial Properties of Silver Nanoparticles Prepared from Aqueous Peel Extract of Pineapple, Ananas comosus
Sarinya Poadang, Niti Yongvanich, and Siriporn Phongtongpasuk*Published Date : 2017-04-01
DOI : https://doi.org/10.12982/CMUJNS.2017.0010
Journal Issues : Number 2 ,April - June 2017
ABSTRACT
Biosynthesis is a simple, environmentally friendly, and cost effective approach to prepare metallic nanoparticles. This study attempted to synthesize colloidal silver nanoparticles (AgNPs) using pineapple peel extract as a reductant, as well as a stabilizer, and investigated their antibacterial activities. The synthesized AgNPs were characterized by UV-Vis spectrophotometry, X-ray diffraction spectroscopy, energy dispersive X-ray analysis, transmission electron microscopy, and Fourier transform infrared spectroscopy. The results showed that the prepared AgNPs were nearly spherical in shape with sizes ranging from 10 to 55 nm; they demonstrated a surface plasmon resonance peak at 445 nm. The biosynthesized AgNPs were crystalline in nature with face-centered cubic symmetry. However, Ag2O/AgO was observed in the synthesized AgNPs. The phytochemical present in pineapple peel extract was found to be responsible for the reduction and stabilization of the biogenic AgNPs. Furthermore, the antibacterial activity of AgNPs against selected pathogenic bacteria using disc diffusion assay revealed that the AgNPs effectively inhibited the growth of both Pseudomonas aeruginosa and Staphylococcus aureus.
Keywords: Ananas comosus, Antibacterial activity, Peel extract, Pineapple, Silver nanoparticles
INTRODUCTION
Silver, utilized as an antiseptic agent since ancient times, is of increasing interest due to its toxicity against a broad spectrum of bacterial strains, particularly in light of the rise of bacterial resistance to antibiotics (Feng et al., 2000). With the proliferation of nanotechnology in materials science, silver nanoparticles (AgNPs) have exhibited astonishing properties, leading to many electronic, catalysis, sensor, and therapeutic applications (Janardhanan et al., 2009; Som and Karmakar, 2011; Shukla et al., 2012). Due to wide-spectrum antimicrobial ability, AgNPs have been popularly used in cosmetics, clothing, consumer products, water and air purification, and biomedical applications.
Although the mechanism of AgNP’s antibacterial activity has not been clearly elucidated, the most commonly proposed mechanisms for its toxicity are:
• uptake of silver ions (Ag+) resulting in failure of ATP production and DNA replication,
• the formation of ROS induced by AgNPs and silver ions, and
• an absence of cell permeability due to AgNP damaging cell membranes (Marambio-Jones and Hock, 2010).
The size, shape, and dispersity of AgNPs are crucial for antimicrobial capacity, as the smaller the AgNPs, the greater their antimicrobial action (Baker et al., 2005). Consequently, processes for synthesizing AgNPs have been developed to control their size. Principally, AgNPs are prepared by first reducing a silver ion precursor to metallic silver nuclei, then stabilizing the particles using polymers or surfactants during the growth stage to prevent the agglomeration of large particles (chemical approach). The most common reducing agents are NaBH4, hydrazine, and formaldehyde. The drawbacks of this method are the use of toxic reducing agents and that it is sometimes not reproducible (Grzelczak and Liz-Marzan, 2014). In contrast, light-mediated reduction of silver ions by gamma or UV radiation (physical approach) is an environmentally-friendly and convenient method for large-scale production of AgNPs with mono-dispersed particles; however, this method can be costly and potentially dangerous (Phu et al., 2010). Recently, biosynthesizing AgNPs using plant extracts has emerged as a simple, environmentally-friendly alternative to the classic chemical and physical methods described above. As bioactive compounds in plant extracts are employed as the reducing as well as stabilizing agent, toxic reagents are avoided, and the resultant AgNPs are biocompatible (Mittal et al., 2013).
Using agricultural waste in the biosynthesis of AgNPs offers an especially ecofriendly option; however, few have studied this option – mango peel (Yang and Li, 2013) and pineapple leaf (Emeka et al., 2014). Pineapple (Ananas comosus L.) peels, a waste product of manufacturing canned pineapples and pineapple juice, are a rich source of bioactive compounds, including cellulose, hemicellulose, carbohydrates, proteins, phenolic acids, and flavonoids (Ketnawa et al., 2012). These biomolecules have the potential to reduce metal ions and stabilize the formed particles during their growth. Hence, we sought to synthesize AgNPs using an aqueous peel extract of pineapple and subsequently evaluate the resultant nanoparticles’ antibacterial properties in an attempt to develop a particularly ecofriendly approach to producing silver nanoparticles.
MATERIAL AND METHODS
Materials
Pineapple (Ananus comosus L. cv. Phuket) was purchased from a local fruit market in Nakornpathom Province, Thailand. All reagents were analytical grade and purchased from Sigma-Aldrich. The experiments were performed in triplicate. Double distilled water was used for all experiments.
Pineapple peel extract (PPE)
Pineapple peels (30 g) were added to 600 ml double distilled water and crushed by a blender. The extract was filtered through Whatman no.1 filter paper and centrifuged at 10,000 rpm for 15 min. The supernatant was collected and stored at 4°C for further experiments.
Synthesis of silver nanoparticles
Briefly, 220 ml of pineapple peel extract was mixed with 110 ml of 10 mM silver nitrate solution. The reaction mixture was magnetically stirred at room temperature for 24 hours. The particles were then collected by centrifugation at 12,000 rpm for 15 min at 4°C. To remove excess silver ions, the silver colloids were washed three times with double distilled water, then lyophilized and stored in screw-capped vials under ambient condition for characterization.
Phytochemical analysis
Total phenolic content (TPC). The total phenolic content of pineapple peel extract was determined using Folin-Ciocalteu reagent following a slightly modified method of Ainsworth (Ainsworth and Gillespie, 2007). A volume of 0.25 ml of pineapple peel extract was mixed with 1.25 ml of Folin-Ciocalteu reagent (diluted 1:10 with deionized water) and neutralized with 1 ml of sodium carbonate solution (7.5% w/v). The reaction mixture was incubated at room temperature for 30 min with intermittent shaking for color development. The absorbance of blue solution was recorded at 760 nm using UV-Vis spectrophotometer. TPC was determined from the equation of a calibration curve prepared with various concentrations of gallic acid and expressed as milligram gallic acid equivalent per gram sample (mg GAE/g sample)
Total flavonoid content (TFC). The assay of total flavonoid content was adapted from Abu-Bakar et al. (2009). First, 0.5 ml of pineapple peel extract was mixed with 2.25 ml of distilled water. Then 0.3 ml of 5% NaNO2 was added. The reaction mixture was incubated for 6 min, then 0.3 ml of 10% AlCl3.6H2O was added and left to stand for 5 min. The solution was neutralized by 1 ml of 1 M NaOH. The absorbance of the solution was measured at 510 nm. TFC was determined using a standard curve of rutin. The mean of three readings was used and expressed as mg of rutin equivalent per gram sample (mg RE/g sample).
Protein analysis. Quantitative estimation of protein was carried out using Bradford assay (Bradford, 1976). A volume of 10 μl of pineapple peel extract was introduced into an Eppendrof tube followed by 200 μl of Bradford reagent. The tube was vortexed and incubated at room temperature for 5 min. Absorption at 595 nm was measured using a microplate reader. A standard curve was prepared with different concentrations of bovin serum albumin (BSA). The results were shown as mg BSA/g sample.
Reducing sugar content. Reducing sugar content was quantified by DNS method as described by Miller with slight modifications (Miller, 1959). The reaction mixture consisted of 1 ml of pineapple peel extract and 3 ml of DNS reagent. The solution was incubated at 95°C for 10 min. After cooling, the absorbance was measured at 550 nm using spectrophotometer. Different concentrations of glucose were used to create a calibration curve. The result was displayed in mg glucose/g sample.
Ferric reducing power assay. Reducing capacity of pineapple peel extract was evaluated by FRAP assay (Benzie and Strain, 1996). Briefly, 200 μl of pineapple peel extract was mixed with 1.8 ml of FRAP solution containing 300 mM acetate buffer pH 3.6, 10 mM TPTZ solution in 40 mM HCl and 20 mM FeCl3.6H2O solution and incubated for 4 min at room temperature. The absorbance was recorded at 593 nm using a spectrophotometer. An array of known concentrations of FeSO4 was prepared for a standard curve. The results were represented as mg FeSO4/g sample.
Characterization of nanoparticles
The reduction of silver ions was monitored by recording the UV-Vis spectrum in wavelengths ranging from 200-800 nm by using a Biochrom Libra S22 spectrophotometer. A Rigaku RINT2000 model X-ray diffractometer (XRD) set at a voltage of 40 kV and a current of 30 mA using CuKα radiation (1.5406 Å) in the range of 30-90° in 2θ angles with a scan speed of 2°/min was used to analyze the crystalline structure and crystallite size. The size, morphology, and elemental composition of the synthesized AgNPs were investigated using TEM equipped with EDX (Tecnai G2 20 S-Twin, USA). The sample was dispersed in deionized water and dropped on a carbon-coated copper grid. The images were obtained by operating at an accelerating voltage of 120 kV. To determine the presence of functional groups of pineapple peel extract and analyze the surface of AgNPs, Fourier transform infrared spectrophotometer (FTIR) studies were carried out using a Nicolet 6700 FTIR spectrophotometer in the range of 4,000-400 cm-1. The samples were prepared using KBr pellet technique. The surface charge and stability of AgNPs were measured by zeta potential (ZetaPlus, BrookHaven Instrument Corp., USA). Samples were prepared by mixing silver nanoparticles with deionized water at a ratio of 1:10 in a total volume of 1 ml. Then, the sample was loaded into a zeta cell for analysis.
Antibacterial activity of synthesized AgNPs
The antibacterial activity of pineapple peel extract and AgNPs was evaluated on two human pathogenic strains – Pseudomonas aeruginosa and Staphylococcus aureus – by disc diffusion assay. Briefly, 100 μl of tested bacterial strain with 105 CFU/ml was swabbed uniformly on nutrient agar plate using a sterile cotton swab. Later, sterile paper discs of 6 mm diameter containing 20 μl of pineapple peel extract (0.5 mg/ml), 20 μl of AgNPs (0.5 mg/ml) and 10 μg/disc of Gentamicin were placed in the plate and incubated overnight at 37°C. The diameter of the inhibition zone was measured in millimeters. The antibacterial activity was evaluated by the size of the clear zone; the greater the zone of inhibition, the better the antibacterial ability.
RESULTS
Phytochemical composition in pineapple peel extract
The phytochemical analysis of pineapple peel extract was quantitatively determined by colorimetric assay as depicted in Table 1. The result demonstrated that pineapple peel extract was composed of various phytochemical compounds, including TPC, TFC, reducing sugar, and protein. Reducing sugar was present in the highest quantity at 23.64±0.003 mg glucose/g sample of pineapple peel extract, and protein the least (1.26±0.013 mg BSA/g sample). In addition, the compounds were able to reduce Fe3+ to Fe2+ (shown as FRAP value of 32.63±0.019 mg FeSO4/g sample).
Table 1. Phytochemical analysis of pineapple peel extract.

Characterization of PPE-synthesized AgNPs
Color change was visually observed after adding pineapple peel extract to AgNO3 solution (Figure 1A). The color of the solution changed from light pale yellow to reddish brown during the first 30 min. The specific optical properties, or Surface Plasmon Resonance (SPR), of the AgNPs precipitated the color change. The UV-Vis absorption spectra obtained from the solution demonstrated a characteristic absorption band of AgNPs at about 445 nm (Figure 1B). This suggested the formation of AgNPs from the bioreduction of AgNO3 to AgNPs. However, this absorption band was absence in the spectra of the pineapple peel extract and AgNO3 solution.
The XRD pattern of the AgNPs obtained after the reduction using pineapple peel extract is shown in Figure 2A. The XRD peaks at 2θ degree of 38.14°, 44.38°, 64.48°, 77.19°, and 81.82° can be indexed to the (111), (200), (220), (311) and (222) crystalline planes of the face-centered cubic structure of metallic silver. The pattern demonstrates a good match with the Joint Committee on Power Diffraction Standard (JCPDS) card number 43-0783. However, unidentified phases, marked with asterisks in Figure 2A, were detected at 2θ values of 32.17°, 46.47°, 54.80°, and 57.46°. These peaks could indicate the possible presence of Ag2O/AgO. In addition, the average crystallite size of biosynthesized AgNPs was estimated to be 4.46 nm using Debye-Scherrer equation (Emeka et al., 2014):
D = 0.9λ/βcosθ (1)
where D is the diameter of crystallite size (nm), λ is the wavelength of X-ray (0.15406 nm), β is full width at half maximum (FWHM), and θ is the diffraction angle in radians.
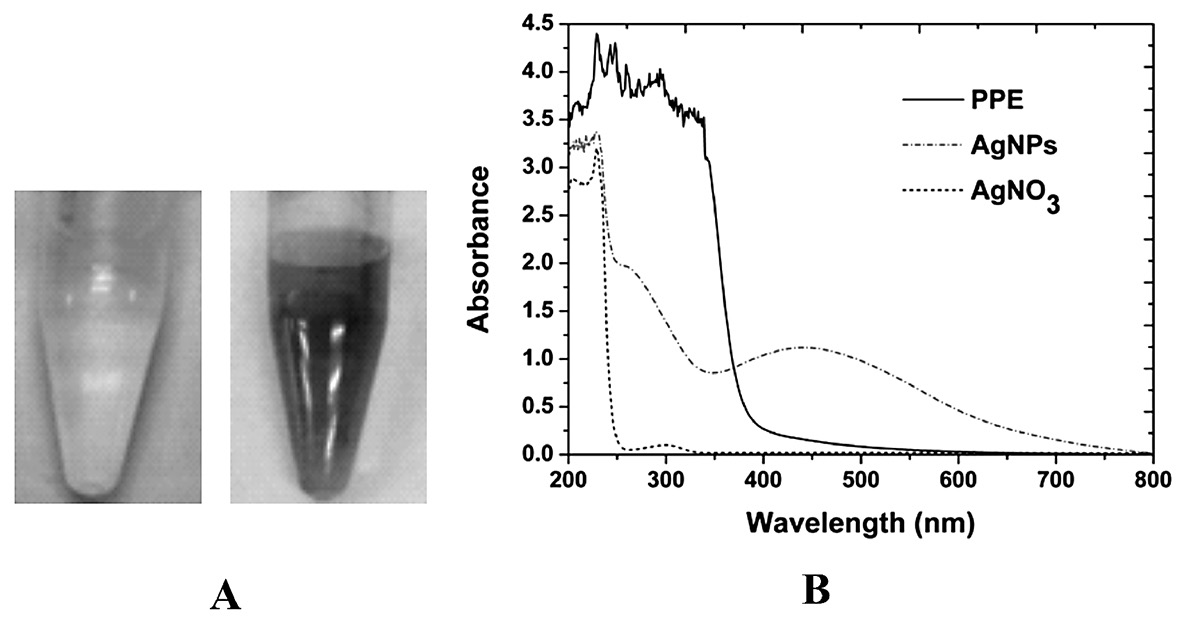
Figure 1. (A) Color change of the reaction mixture consisting of PPE and 10 mM AgNO3 at initial time (left) and 24 hours (right). (B) UV-Vis spectra of PPE, AgNPs synthesized by PPE at 24 hours, and AgNO3 solution.
Figure 2B shows the EDX analysis of prepared AgNPs. An intense signal at 3 keV is a characteristic peak of metallic silver, due to its surface plasmon resonance effect. This indicated the presence of AgNPs synthesized from pineapple peel extract. Metallic copper and carbon were also detected as a result of the grid used for sample preparation. Carbon and oxygen appeared on the spectrum due to the biomolecules on the surface of AgNPs.
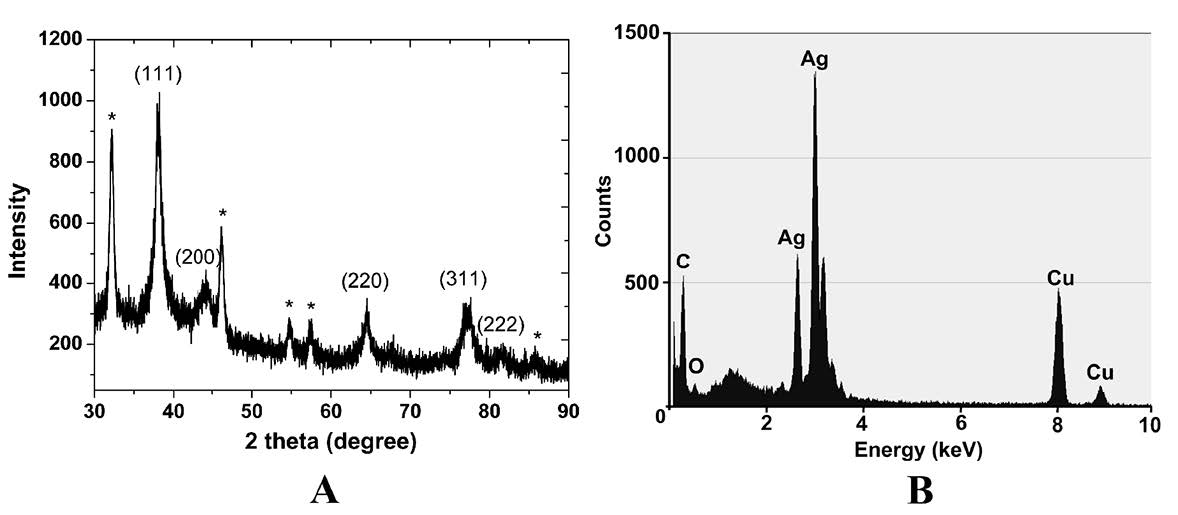
Figure 2. XRD pattern (A) and EDX analysis (B) of AgNPs prepared from PPE. Asterisks (*) indicate Ag2O/AgO impurities.
The shape and size of the synthesized AgNPs were investigated by transmission electron microscopy (TEM). The TEM image demonstrated that AgNPs were mostly spherical in shape and polydispersed with a broad range of particle size (10-55 nm), as shown in Figure 3(A-B). The average diameter size of AgNPs obtained from the micrograph was 22.19±5.62 nm.
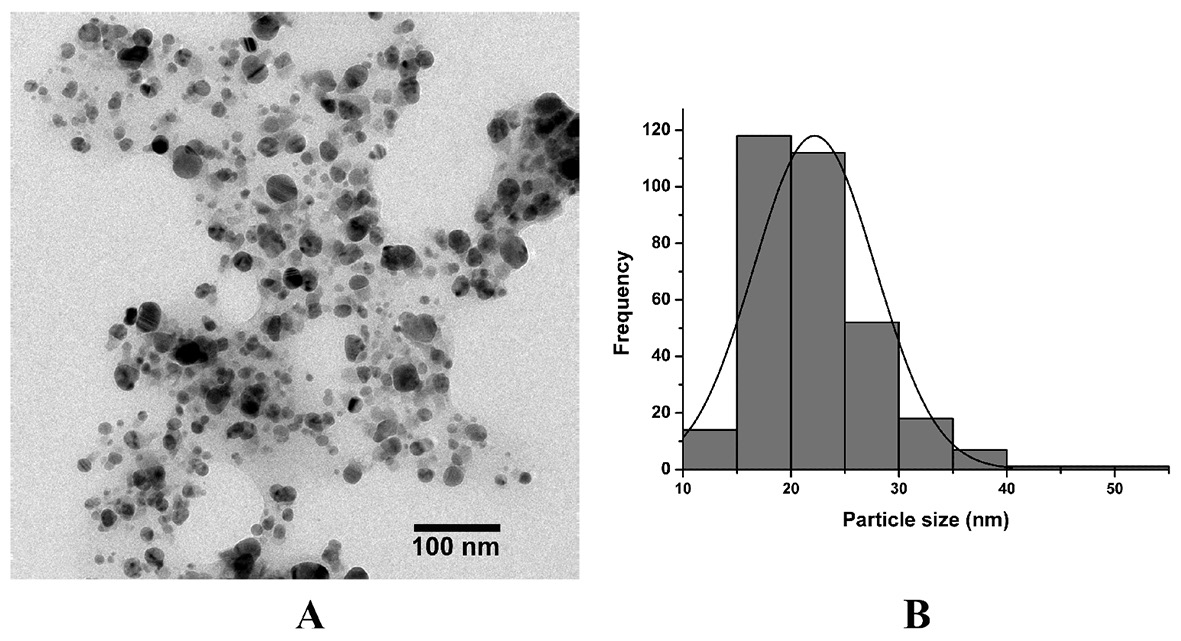
Figure 3. TEM image (A) and size distribution (B) of biosynthesized AgNPs.
FTIR analysis identified the functional groups responsible for stabilizing AgNPs. Figure 4 represents the FTIR spectra of biosynthesized AgNPs (solid line) and pineapple peel extract (dashed line). Interestingly, the spectrum pattern of AgNPs and pineapple peel extract were similar, except for a little peak shift. For pineapple peel extract, the broad peak at 3,381 cm-1 could be attributed to O-H stretching, while the peak at 2,934 cm-1 was due to C-H stretching of methyl group. The peak located at 1,635 and 1,723 cm-1 indicated C=O stretchingof carbonyl group. The peaks at 1,252 and 1,055 cm-1 were assigned to stretching vibrations of C-N aromatic and aliphatic amines.
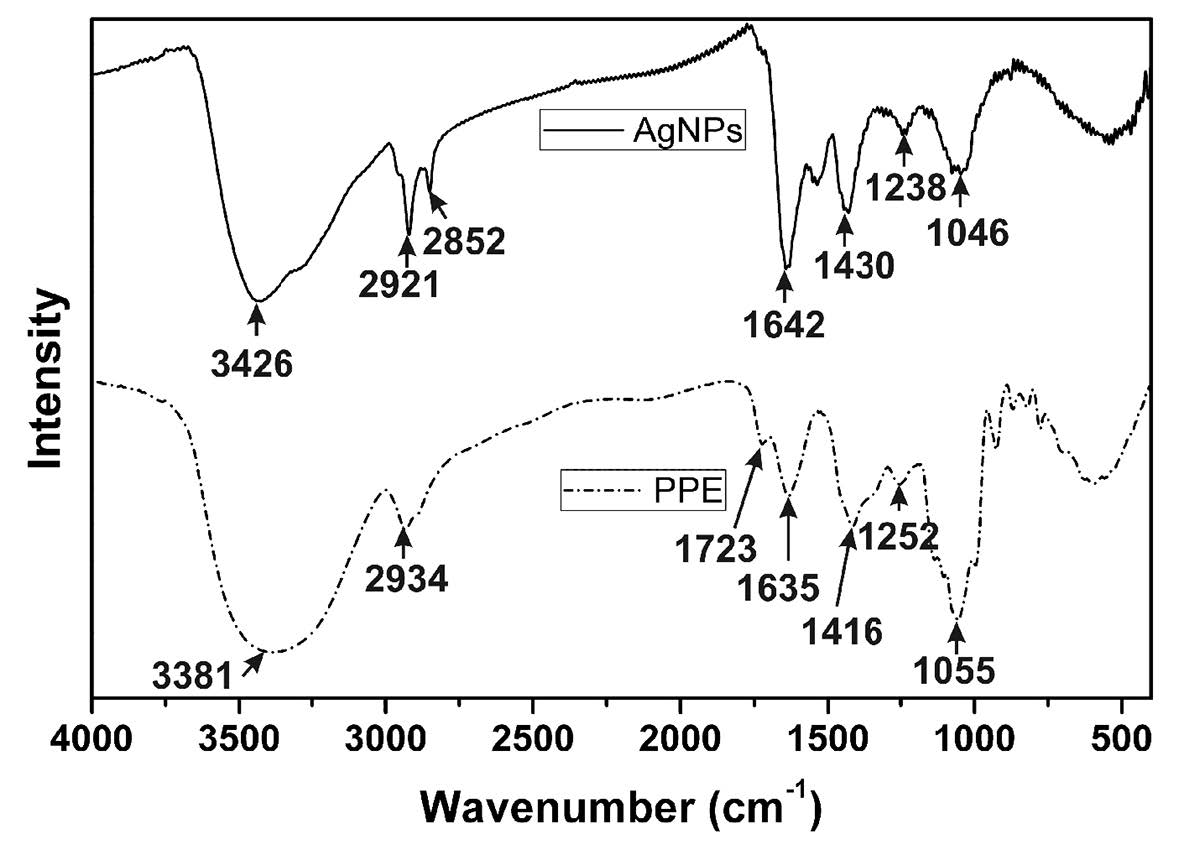
Figure 4. FTIR spectra of prepared AgNPs and pineapple peel extract.
Measuring the zeta potential (-18.69±1.31 mV) of the prepared AgNPs in an aqueous environment indicated that the synthesized AgNPs were stable against agglomeration, with a negative charge on their surface.
The antibacterial activity of green synthesized AgNPs towards selected human pathogens was evaluated by disc diffusion assay. As expected, the positive control loaded with 10 μg of gentamicin showed zones of inhibition at 14 mm and 13 mm for S. aureus and P. aeruginosa, respectively. Plain pineapple peel extract, the negative control, did not produce any clear zone. For the discs loaded with 10 μg of AgNPs, the zone of inhibition was 10 mm and 7 mm for gram-positive (S. aureus) and gram-negative bacteria (P. aeruginosa), respectively. Based on these results, the prepared AgNPs exhibited antibacterial activity against tested pathogenic bacteria, although it was better against gram-positive than gramnegative bacteria.
Table 2. Antibacterial activity of AgNPs against pathogenic bacteria using disc diffusion assay.

DISCUSSION
The bioreduction of silver ions to AgNPs was confirmed by the color transition that occurred when pineapple peel extract was added to AgNO3 solution. The UV-Vis spectra of AgNPs showed a broad plasmon band with an absorption tail in the longer visible region of electromagnetic spectrum, indicating the formation of AgNPs with widely distributed particle sizes (Chen et al., 2007). This result corresponded to the TEM image, which showed nanoscale particles ranging in size from 10 to 55 nm. The synthesized AgNPs were spherical in shape and of crystalline character with the face-centered cubic phase of metallic silver. However, a number of extra diffraction peaks were present in the XRD spectrum, and likely to be Ag2O/AgO. This result indicated an incomplete reduction of silver ions to AgNPs. To obtain more pure AgNPs, the effect of the initial pH value of the reaction mixture and the ratio of pineapple peel extract to silver salt on the synthesis of AgNPs should be further studied (Yang and Li, 2013).
The aqueous solution of pineapple peel extract consisted of phenolic acid, flavonoid, protein and reducing sugar. These chemical constituents contain various functional groups, including aldehyde, ketone, carboxyl, and hydroxyl groups, responsible for the reduction as well as stabilization of AgNPs by coating the surface of the nanoparticles (Iravani, 2011; Mittal et al., 2013). A slight shift of the peaks in the spectra of pineapple peel extract to higher wavenumber in comparison with that of AgNPs was monitored from 3381 to 3,426 cm-1 and 1,635 to 1,642 cm-1 indicating the binding of silver ions with hydroxyl and carbonyl groups, respectively. These band shifts suggested the possibility of reduction of silver ions to silver nanoparticle due to the hydroxyl and carboxyl groups of the phytochemicals in the extract (Kora and Arunachalam, 2013).
CONCLUSION
This study attempted to synthesis AgNPs in a simple, cost-effective, and ecofriendly method by using aqueous pineapple peel extracts. The nanoparticles were synthesized at ambient conditions with biomolecules in the pineapple peel extract acting as both reducing and stabilizing agents. Highly crystalline, spherical, metallic AgNPs of crystallite size approximately 4.46 nm with a trace of AgO/Ag2O were prepared. The average particle size calculated from TEM images was 22.19±5.62 nm, with a size distribution of the particles in the range of 10-55 nm. FTIR analysis showed that phytochemicals in pineapple peel extract formed a coat covering the nanoparticles to stabilize them and prevent their agglomeration. UVVis spectra and zeta potential results supported the formation and stability of the biogenic AgNPs. Furthermore, the prepared AgNPs exhibited good antibacterial activity against two selected human pathogenic bacteria.
We recommend attempting to use pineapple peel extract to completely reduce the AgNPs with an even narrower size distribution of nanoparticles for future research.
ACKNOWLEDGEMENTS
This research was supported by the Higher Education Research Promotion (HERP) and National Research University (NRU) Project of Thailand, Office of the Higher Education Commission (Grant No. SURDI-HERP 57/01/12).
REFERENCES
Abu-Bakar, M.F., M. Mohamed, A. Rahmat, and J. Fry. 2009. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chemistry. 113: 479-483.
Ainsworth, E.A., and K.M. Gillespie. 2007. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocol. 2(4): 875-877.
Baker, C., A. Pradhan, L. Pakstis, D.J. Pochan, and S.S. Ismat. 2005. Synthesis and antibacterial properties of silver nanoparticles. Journal of Nanoscience and Nanotechnology. 5(2): 244-249.
Benzie, I.F., and J.J. Strain. 1996. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Analytical Biochemistry. 239: 70-76.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72: 248-254.
Chen, P., L. Song, Y. Liu, and Y.E. Fang. 2007. Synthesis of silver nanoparticles by γ-ray irradiation in acetic water solution containing chitosan. Radiation Physics and Chemistry. 76(7): 1165-1168.
Emeka, E.E., O.C. Ojiefoh, C. Aleruchi, L.A. Hassan, O.M. Christina, M. Rebecca, E.O. Dare, and A.E. Temitope. 2014. Evaluation of antibacterial activities of silver nanoparticles green-synthesized using pineapple leaf (Ananas comosus). Micron. 57: 1-5.
Feng, Q.L., J. Wu, G.O. Chen, F.Z. Cui, T.N. Kim, and J.O. Kim. 2000. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of Biomedical Material Research. 52: 662-668.
Grzelczak, M., and L.M. Liz-Marzan. 2014. The relevance of light in the formation of colloidal metal nanoparticles. Chemical Society Reviews. 43(7): 2089-2097.
Iravani, S. 2011. Green synthesis of metal nanoparticles using plants. Green Chemistry. 13: 2638-2650.
Janardhanan, R., N. Karuppaiah, and T.N. Hebalkar. 2009. Synthesis and surface chemistry of nano silver particles. Polyhedron. 28: 2522-2530.
Ketnawa, S., P. Chaiwut, and S. Rawdkuen. 2012. Pineapple wastes: A potential source for bromelain extraction. Food and Bioproducts Processing. 90: 385-391.
Kora, A.J., and J. Arunachalam. 2013. Biosynthesis of silver nanoparticles by the seed extract of Strychnos potatorum: a natural phytocoagulant. IET Nanobiotechnology. 7(3): 83-89.
Marambio-Jones, C., and E.M.V. Hock. 2010. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Journal of Nanoparticle Research. 12: 1531-1551.
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 31: 426-428.
Mittal, A.K., Y. Chisti, and U.C. Banerjee. 2013. Synthesis of metallic nanoparticles using plant extracts. Biotechnology Advances. 31: 346-356.
Phu, D.V., V.T.K. Lang, N.T.K. Lan, N.N. Duy, N.D. Chau, B.D. Du, B.D. Cam, and N.Q. Hien. 2010. Synthesis and antimicrobial effects of colloidal silver nanoparticles in chitosan by γ-irradiation. Journal of Experimental Nanoscience. 5(2): 169-179.
Raut, R.W., V.D. Mendhulkar, and S.B. Kashid. 2014. Photosensitized synthesis of silver nanoparticles using Withania somnifera leaf powder and silver nitrate. Journal of Photochemistry and Photobiology B: Biology. 132: 45-55.
Shen, W., L. Feng, H. Feng, Z. Kong, and M. Guo. 2011. Ultrafine silver (II) oxide particles decorated porous ceramic composites for water treatment. Chemical Engineering Journal. 175: 592-599.
Shukla, M.K., R.P. Singh, C.R.K. Reddy, and B. Jha. 2012. Synthesis and characterization of agar-based silver nanoparticles and nanocomposite film with antibacterial applications. Bioresource Technology. 107: 295-300.
Som, T., and B. Karmakar. 2011. Nano silver: antimony glass hybrid nanocomposites and their enhanced fluorescence application. Solid State Science. 13: 887-895.
Sondi, I., and B. Salopek-Sondi. 2004. Silver nanoparticles as antimicrobial agent: a case study. Journal of Colloid and Interface Science. 275: 177-182.
Wang, X., H.F. Wu, Q. Kuang, R.B. Huang, Z.X. Xie, and L.S. Zheng. 2010. Shape-dependent antibacterial activities of Ag2O polyhedral particles. Langmuir. 26(4): 2774-2778.
Yang, N., and W.H. Li. 2013. Mango peel extract mediated novel route for synthesis of silver nanoparticles and antibacterial application of silver nanoparticles loaded onto non-woven fabrics. Industrial Crops and Products. 48: 81-88.
Sarinya Poadang1, Niti Yongvanich2 and Siriporn Phongtongpasuk1*
1 Department of Biotechnology, Faculty of Engineering and Industrial Technology, Silpakorn University, Nakornpathom 73000, Thailand
2 Department of Materials Science and Engineering, Faculty of Engineering and Industrial Technology, Silpakorn University, Nakornpathom 73000, Thailand
*Corresponding author. E-mail: phongtongpasuk_s@su.ac.th
Total Article Views

