
Characterization of On-farm Rice Germplasm in an Area of the Crop’s Center of Diversity
Sansanee Jamjod, Narit Yimyam, Sittichai Lordkaew, Chanakan Prom-u-thai, and Benjavan Rerkasem*Published Date : 2017-04-01
DOI : https://doi.org/10.12982/CMUJNS.2017.0007
Journal Issues : Number 2 ,April - June 2017
ABSTRACT
The seed of rice (Oryza sativa L.) from the highlands of northern Thailand, which is located within the species’ centre of diversity, constitutes some of the world’s last local rice germplasm still retained on-farm, provides local farmers and communities with a readily accessible resource, and is a source of value-adding traits for rice breeding. This paper reports on the germplasm represented by 281 seed samples collected in 2013 from an area of the highlands between latitudes 17.76°N to 20.18°N and longitudes 97.76°E to 100.48°E. The samples were provided by farmers belonging to 10 ethnicities, in number that closely correlated with the groups’ share of the highland population (r = 0.84; P < 0.01). Compared with the slender grain rice of the lowlands, the highland germplasm was distinctive in its grain shape, and classed as large grain type in the husk, and medium grain type as de-husked, brown rice. The rice, which was predominantly of non-glutinous grain type and grown mainly as upland rice, had generally higher iron concentrations than rice in the lowlands; thus demonstrating how an on-farm rice germplasm may directly benefit local farmers and communities who consume the rice they grow. In addition, potential value-adding traits were identified in varieties and seed samples with the highest zinc density and novel rice with pigmented pericarp and high anti-oxidative capacity.
Keywords: Anthocyanin, Antioxidative activity, Iron, Rice, Zinc
INTRODUCTION
Northern Thailand, located within the center of diversity of rice (Oryza sativa L.) (Harlan, 1975), contains some of the world’s last local rice germplasm still maintained on-farm. The region’s highlands, situated at altitudes from 500 m above mean sea level, and the small low-lying areas between the mountains (Royal Decree, 2005) are rich in local rice varieties. A survey in the early 2000s found a total of 202 local rice varieties grown by 816 highland farmers belonging to 10 different ethnicities (Unthong et al., 2007). On-farm crop germplasm is a valuable resource for local communities and provides value-adding traits for the development of new rice varieties; the Conference on Biological Diversity (UN, 1992) specifically mentioned on-farm conservation as a means of preserving crop diversity. Native rice germplasm has continued to benefit rice farming in Thailand by providing traits that confer grain quality characteristics to modern varieties in the national breeding programme, and in those local varieties with better fit to particular sets of economic and ecological conditions, from a variety well-adapted to poor soils in rainfed areas of the north and northeast that produce the premium-priced, aromatic Thai Hom Mali rice, to varieties adapted to different flood regimes (Sommut, 2003; Rerkasem, 2008; BRRD, 2016), and those that have enabled farmers to respond to new market opportunities, e.g., with rice rich in bioactive compounds and fancy rice with pigmented pericarp (Boonsit et al., 2010; Saetan, 2010; Yodmanee et al., 2011; BRRD, 2012; Panomjan et al., 2016). Detailed studies of the highland rice germplasm from individual villages and sets of varieties have revealed rich genetic diversity in the variation of simple sequence repeats (SSRs) of the DNA (e.g. Pusadee et al., 2009; Pusadee et al., 2014), as well as in useful traits, from resistance to pests (Oupkaew et al., 2011) and disease (Naruebal, 2009), to adaptation to acidic soil (Laenoi et al., 2015), to endosperm density of iron (Pintasen et al., 2007) and zinc (Jaksomsak et al., 2015).
Until relatively recently, the highlands of Thailand were segregated from the rest of the country – physically because of remoteness and inaccessibility, socially and economically by racial discrimination and limited access to the market. But over the past few decades, highland agriculture has become more closely integrated with the Thai economy. Crop production that used to be primarily subsistence has become increasingly commercialized, with high-value vegetables grown in areas where irrigation is available and the market is readily accessible (Rerkasem,
2005) and rainfed maize grown in more remote areas (Punyalue et al., 2015). As has been generally noted (Brush, 1995), the economic importance of local rice germplasm varies greatly among the different highland cropping systems. In some villages, crop production is completely commercialized and rice plays a minor role in the local economy, but in other villages rice is integrated with vegetable production, and thus benefits from residual fertlizers and clean weeding applied to the vegetables (Rerkasem et al., 2002).
In this study, rice germplasm from highland farmers in northern Thailand was evaluated, with a special focus on selected grain quality characteristics.
MATERIALS AND METHODS
This survey, conducted in 2013, covered 41 highland villages in Chiang Mai, Chiang Rai, and Mae Hong Son provinces – an area that accounts for more than one-half of the highland population of Thailand. Seed samples of 0.5-1 kg were collected from farmers, together with information on the village’s location and elevation, farmer’s ethnicity, variety name, and whether the rice was grown as wetland rice (on flooded soil) or upland rice (on well-drained soil). The morphology and grain quality characteristics of each genotype or variety (Chang et al., 1965; Yoshida, 1981) were determined. The morphology – husk color, grain length and width, and weight of 1000 seeds – of the unhusked seeds were evaluated. The shape of rice grain in the husk was classified into large, slender, or round grain by the ratio of its length relative to width, as defined by Matsuo (Oka, 1988). The seed weight was used to classify samples by grain size: extra-small (≤ 23.0 mg per seed), small (23.1-28.0 mg per seed) medium (28.1-33.0 mg), large (33.1-38.0 mg), and extra-large (>38.0 mg). One-hundred-gram sub-samples of each sample were de-husked with a laboratory husker (model P-1, Ngek Seng Huat) to produce brown rice (de-husked caryopsis enclosed in pericarp, with embryo attached) that was evaluated visually for pericarp color and grain dimension, determined as the mean of the grain width and length of 100 seeds per sample. The brown rice grain was categorized by its length-to-width ratio of 1.1-2.0 as bold, 2.1-3.00 as medium, and > 3.00 as slender (Juliano, 1993). The common lowland varieties RD6, Khao Dawk Mali 105 (KDML105), RD15, Chai Nat 1 (CNT1), Suphan Buri 1 (SPR1), Pathum Thani 1 (PTT1), and Phitsanulok 2 (PSL2) were used as standards.
The iron (Fe), zinc (Zn), and anthocyanin concentrations and anti-oxidative capacity were determined on approximately 25% of the brown rice samples. Iron and Zn concentrations were determined by atomic absorption spectrophotometer (Hitachi Model Z-8230) after dry-ashing at 535°C for 8 h (Allen, 1961). Anthocyanin was determined by the pH-differential method (Wrolstad et al., 2001; Escribano-Bailón et al., 2004). The trolox equivalent anti-oxidative capacity (TEAC) was determined with the DPPH free radical scavenging method (Yue and Xu, 2008).
RESULTS
A total of 281 seed samples, bearing 135 distinct names, were collected from the highland area between latitudes 17.76°N to 20.18°N and longitudes 97.47°E to 100.48°E; from 211 farmers belonging to 10 ethnic groups in 41 villages at altitudes lower than 500 m to higher than 1,000 m (Table 1). Fifteen percent of the samples were from low altitudes (500 m or lower), 22% from mid-level altitudes (501-800 m), 43% from high altitudes (801-1,000 m), and 20% from very high altitudes (higher than 1000 m). The rice samples from Chiang Rai were mostly from low altitudes, Chiang Mai from mid-level altitudes, and Mae Hong Son from high to very high altitudes. Two-thirds of the seed samples were from traditional rice growers (Karen, Khamu, Lua, Thai, and Shan) and the remainder from former opium growers (Hmong, Lahu, Akha, Mien, and Lisu). Although the rice from all groups included both non-glutinous and glutinous starch types, the samples were predominantly non-glutinous, except for those from the Khamu villages, which were predominantly glutinous grain. Most of the rice – nearly all of the samples from the former opium growers and two-thirds of those from the traditional rice growers – was identified as having been grown on aerobic soil as upland rice.
Table 1. Distribution of farmers’ rice seed samples from northern Thailand by location, elevation, farmer ethnicity, endosperm starch type, and water management regime.
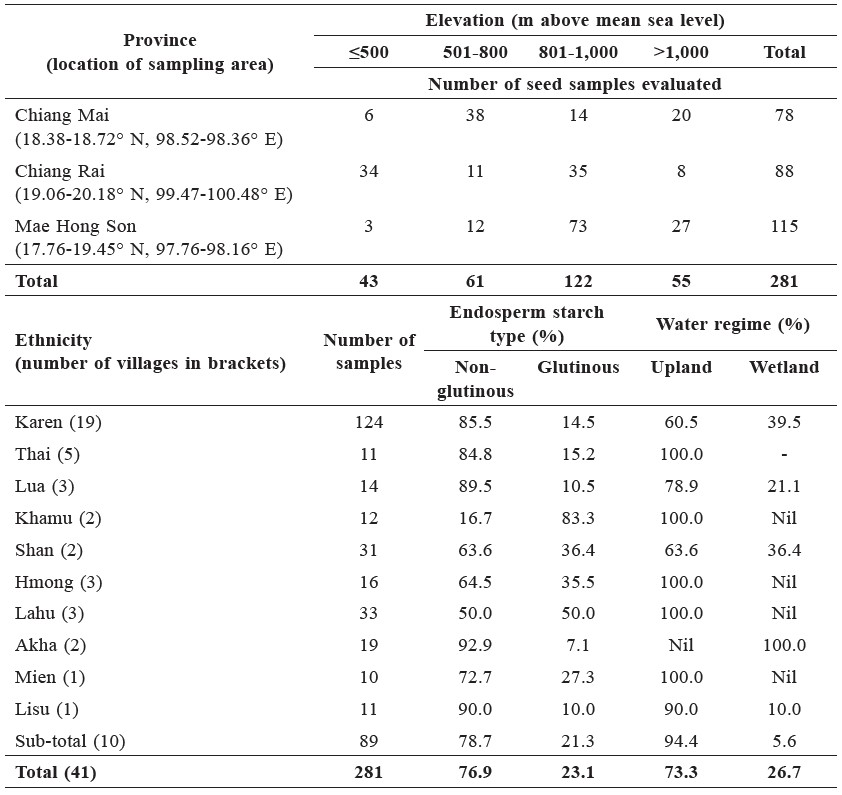
Individual seed samples were largely uniform in their husk and pericarp color (Figure 1a). Straw colored husk accounted for 51% of the samples, an admixture of straw and brown streaks on straw background 31%, brown streaks on straw background 15%, dark brown 2%, and purple 1%. Removal of the husk produced ‘brown rice’ (caryopsis with seed coat and embryo intact) with colorless pericarp (i.e., non-pigmented) in 67% of the samples, an admixture of colorless and reddish brown in 22%, purple in 8%, and reddish brown in 3%. Pericarp color was not always indicative of the husk color. For example, purple pericarp was found inside the husks of straw color, brown streaks on straw, and the admixture of the two, as well as inside purple husk. While still in the husk, most of the rice samples were classified as large grain type by the method of Matsuo (Oka, 1988), in contrast to the slender grain of common lowland varieties (Figure 1b).
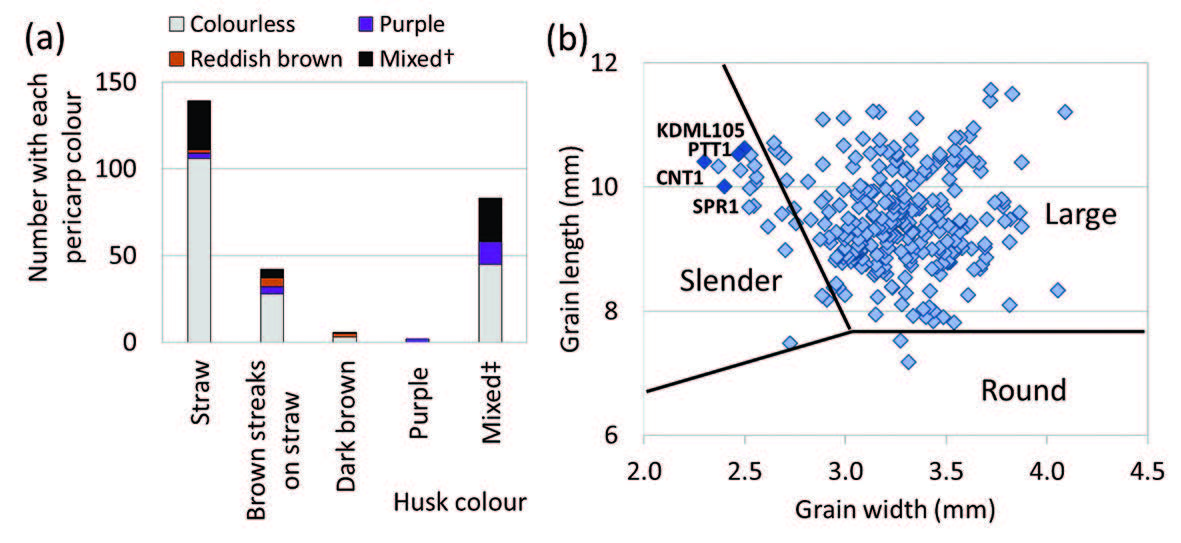
Figure 1. Distribution of seed samples with different pericarp color within each group of husk color (a), and grain shape of rice from the highlands (◆) defined by the length relative to width of grain in the husk, with lines separating grain types into large, slender, and round grain by the method of Matsuo (adapted from Oka, 1988), compared with four common lowland varieties (◆) (b).
Note: †Colorless and reddish brown; ‡straw and brown streaks on straw background.
Without the husk, the brown rice from the highlands was largely classified as medium grain shape, with 84% of the samples averaging 2.5±0.2 in grain length/width ratio, and only 8% of the samples approaching mean length/width ratio of the seven common lowland varieties at 3.5±0.2. The germplasm was somewhat diverse in its grain size, ranging from small (25%) to medium (38%) to large (32%) (Figure 2).
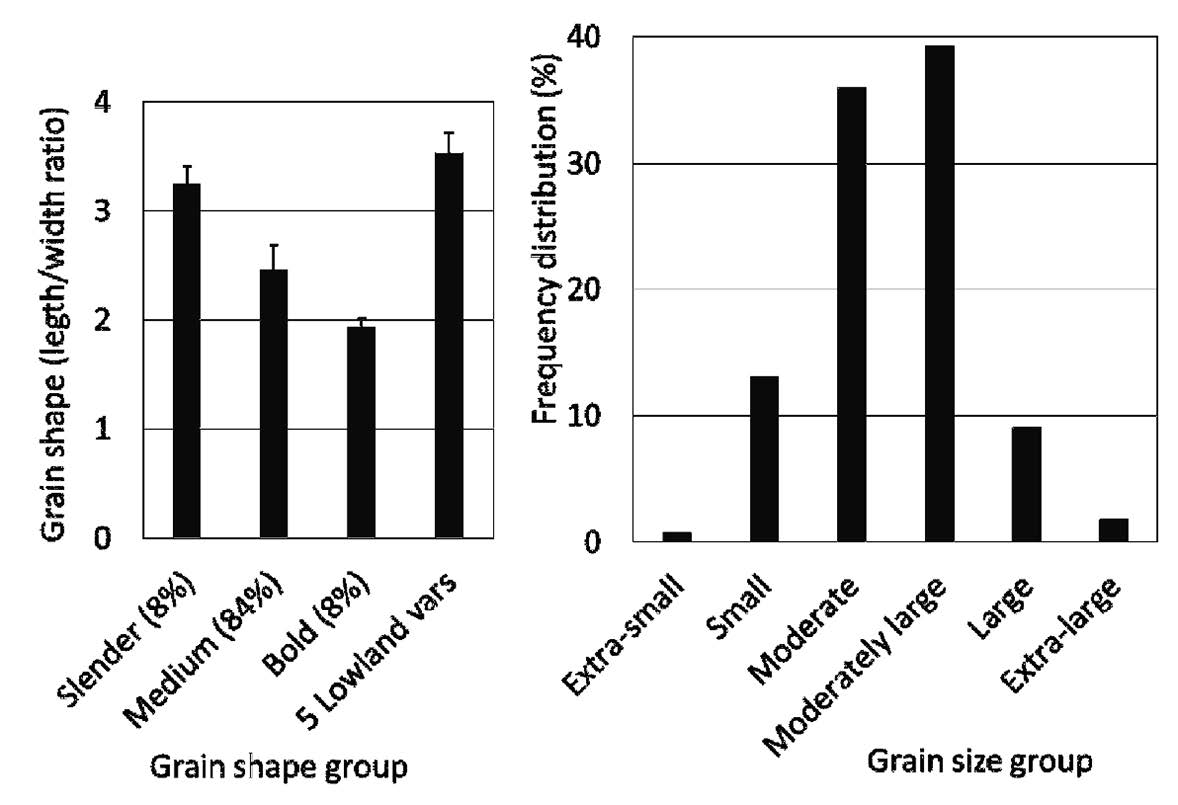
Figure 2. Grain shape of rice seed samples from highland farmers in northern Thailand compared with the mean for five common lowland varieties (left, with % of sample in each group in brackets) and frequency distribution by grain size (right).
The brown rice samples that were analysed averaged 11.3±1.8 mg Fe kg-1 (range 7.5-15.0 mg Fe kg-1), 22.5±1.8 mg Zn kg-1 (range 13.9-13.1 mg Zn kg-1), and anti-oxidative capacity of 404±449 mg trolox 100 g-1 (range 2-1,811 mg trolox 100 g-1). Most of the samples were higher in Fe and lower in Zn than the standard lowland varieties (Figure 3). Rice with moderate grain Fe (11.6±1.5 mg Fe kg-1) accounted for 54% and high Fe (13.8±0.6 mg Fe kg-1) 19% of the highland samples; only 27% were in the same low-Fe range as the average Fe content in three common lowland varieties (KDML105, CNT 1, and PTT 1). Low grain Zn (17.7±1.7 mg Zn kg-1) accounted for 17% of the samples and moderate Zn (22.4±1.6 mg Zn kg-1) 53%; only 28% of the samples had high Zn (27.1±1.3 mg Fe kg-1), within the range of Zn in lowland rice.
Figure 3. Iron and zinc concentrations of brown rice seed samples from highland farmers in northern Thailand in three groups of nutrient density† (with standard deviation bars, and % of samples in each group in brackets) compared with the means for three common lowland varieties (KDML105, CNT1, and PTT1).
Exceptionally high anti-oxidative activity was found in rice with pigmented pericarp, including those with purple pericarp rich in anthocyanin and one with a reddish brown pericarp without anthocyanin; however, samples with different pericarp pigmentation were not distinguishable by their Fe, Zn, or γ-oryzanol contents (Table 2).
Table 2. Some quality characteristics of rice with different pericarp colors.
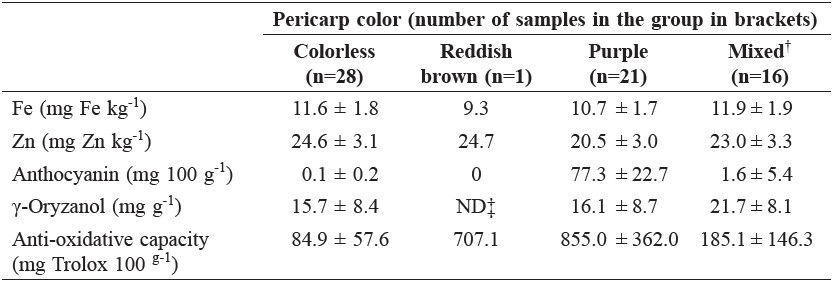
Note: †Colorless and reddish brown; ‡Not determined.
Some variation in grain quality attributes of Fe and Zn concentrations and anti-oxidative capacity was found among seed samples with the same varietal names, so that the varieties overlapped in the nutrient content of the grain, except between those with the lowest and highest Fe and Zn concentrations (Table 3). The glutinous type was also distinguishable by its much higher anthocyanin pigmentation of the pericarp, but not by Fe, Zn, or γ-oryzanol content (Figure 4).
Table 3. Selected nutritional qualities and variability of the rice seed samples sharing the same names.
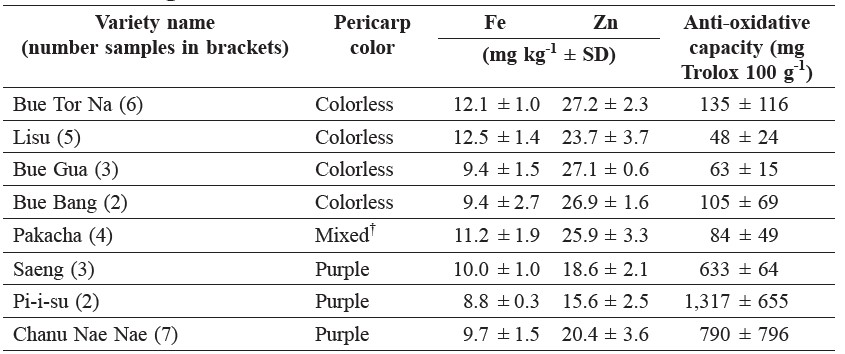
Note: †Colorless; + reddish brown.
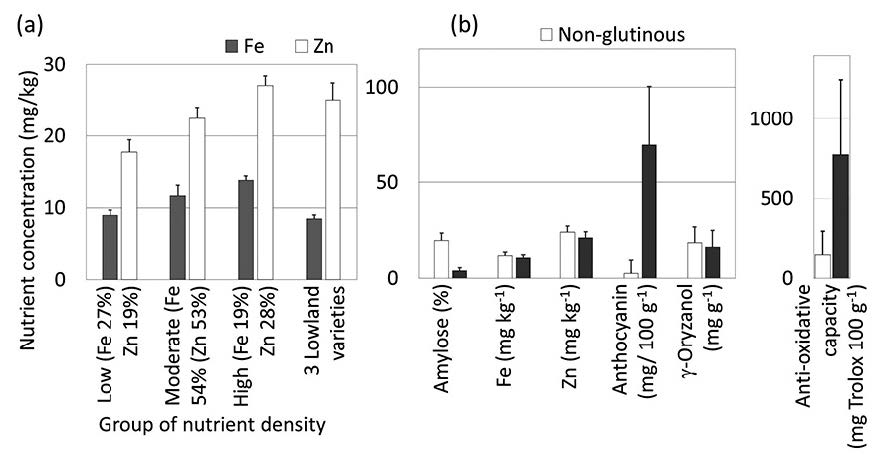
Figure 4. Selected nutritional qualities of non-glutinous and glutinous starch type rice.
DISCUSSION
The number of rice seed samples from each of the minority groups was closely correlated (r = 0.84; P < 0.01) with their share of the highland population recorded in 2009 (HRDI 2010). The minority groups included traditional rice growers (Karen, Khamu, Lua, Thai, and Shan), along with former opium growers (Hmong, Lahu, Akha, Mien, and Lisu); once their main cash crop (Kunstadter and Chapman, 1978), these groups have replaced opium with vegetables and other cash crops (Rerkasem, 2005), following strict enforcement of an opium production ban that began in the 1970s (Renard, 2001). It, therefore, appears that rice is important to the highland groups for more than its economic value alone. The dominance of non-glutinous rice in the germplasm reflects the preferences of the highland minority groups. The Khamu were the only group preferring glutinous rice, sharing this preference with the majority Thai population in the lowlands of northern Thailand, northeastern Thais, and Laotians. However, non-glutinous rice eaters maintain glutinous germplasm for special uses, such as making deserts and ceremonial purposes; and vice versa for glutinous rice eaters, although glutinous rice is generally preferred for brewing rice wine and spirits.
Highlanders grow upland rice in non-flooded soil on slopes in a slashand-burn system of cultivation, and wetland rice in soil submerged under a few centimetres of water on the floor of small inter-montane valleys, on narrow strips of flat land along mountain streams and terraced fields. Traditional rice growing groups have developed wetland rice fields in the mountains wherever terrain and water supply allow, including on deposition fans at the base of slopes as the result of erosion higher up (Zinke et al., 1978). Scarcity of land suitable for wetland rice cultivation in the highlands is reflected in the predominance of the seed samples that were grown as upland rice, and supported by highland rice production statistics – wetland rice accounted for only 22% the total rice area of 70,100 ha in 2013 (Dumrongkiat, 2013). In contrast, Thailand’s main rice crop of some 13 million ha in the lowlands is invariably grown as wetland rice (OAE, 2014). Designation as upland or wetland varieties is purely agronomic, and is not always differentiated by the physiological mechanism for adaptation to aerobic or anoxic soil conditions (e.g., see Colmer, 2003). Nevertheless, adaptation to the water and nutritional conditions of aerobic soil is more likely to be found among the local rice germplasm from the highlands than from the lowlands, as has been demonstrated with adaptation to soil acidity (Laenoi et al., 2015), and seed Zn enrichment, a condition that favors good germination and early establishment in the zinc-deficient, ash-covered soil of the slash-and-burn system (Jaksomsak et al., 2015).
Rice in the lowlands of Thailand typically has slender grain, either in the rice germplasm preserved in the national gene bank (defined as slender by the scheme of Matsuo (Rerkasem and Rerkasem, 2002)), and similarly carbonized rice husk preserved in old bricks in lowland temples and ruins dating back to the 15th century (Watabe et al., 1970), or in the grain length-to-width ratio of the mega-varieties that make up Thailand’s current national production. In grain dimension, the highland rice germplasm was clearly distinct from the country’s lowland rice; the highland samples were judged to be mainly of large grain type in the husk and medium grain shape as brown rice, with 84% of the sample averaging 2.5±0.2 in the grain length-to-width ratio, compared with a ratio of 3.5±0.2 for common lowland varieties.
The nutritional quality of the farmers’ rice seed samples are indicative of their value to rice growers and eaters. First, the farming households who maintain and use the seed benefit directly, by either producing and consuming the staple rice or selling it as premium-priced, special-quality rice. Second, breeding programs can potentially benefit from the seeds’ value-enhancing traits. Although none of the highland rice samples reached the 16 mg Fe kg-1 of the high-Fe standards IR68144 (Laenoi et al., 2015) and RD29 (Palawisut et al., 2008), the higher Fe concentration in the highland rice is likely of nutritional benefit to those ethnic groups that meet most of their consumption demand from their own production. In contrast, highland groups like the former opium growers that primarily grow vegetables and other non-rice crops, purchase lowland rice to eat; like those in the lowlands, they would not derive this benefit. While no parallel direct advantage through rice consumption was observed for Zn, some accessions with exceptionally high Zn deserve a closer look as potential sources of genes for Zn-enriching traits.
The highland rice germplasm may also contribute to satisfying the growing market interest in special quality rice, e.g., varieties with pigmented pericarp and novel bioactive compounds (Boonsit et al., 2010; Saetan 2010; Yodmanee et al., 2011; BRRD, 2012; Panomjan et al., 2016). However, the strong effect of genotype by environment interaction (G x E) on special quality traits and grain Zn density (Rerkasem et al., 2015) must be taken into account. Local rice varieties are named by a highly decentralized naming system, which is much less precise
than the naming of varieties released from the national rice breeding programme. Nevertheless, consistency of quality characteristics of seed samples bearing the same name suggested that local rice varietal names could be indicative of valuable traits, including those that are not obvious to farmers, such as Fe and Zn concentrations and anti-oxidative capacity, as well as more easily observable traits captured in the varietal names, such as Pi-i-su and Bue Nermu (Karen for black glutinous rice and aromatic non-glutinous rice, respectively).
The local rice germplasm from the highlands of Thailand was found to be distinct from the rice from the country’s main production system in the lowlands. This germplasm was maintained on-farm by traditional rice growing groups as well as former opium growing groups, with the number of seed samples contributed by each group closely correlating with their share of the highland population. That the highland rice was generally higher in Fe concentration than the lowland rice suggested a direct benefit to those who grow and eat the rice. Furthermore, potential value-adding traits were identified in those varieties and seed samples with the highest Fe and Zn density, anti-oxidative capacity, and pigmented pericarp.
ACKNOWLEDGEMENTS
The authors wish to thank the farmers for generously sharing seed and information. Financial support for this research from the Agricultural Research Development Agency and the National Research University Project of Thailand’s Commission on Higher Education is gratefully acknowledged.
REFERENCES
Allan, J. 1961. The determination of zinc in agricultural materials by atomic absorption spectrophotometry. Analyst. 86: 530-534.
Boonsit, P., P. Pongpiachan, S. Julsrigival, and D. Karladee. 2010. Gamma oryzanol content in glutinous purple rice landrace varieties. Chiang Mai University Journal of Natural Science. 9: 151-157.
Brush, S.B. 1995. In situ conservation of landraces in centers of crop diversity. Crop Science. 35: 346-354.
BRRD. 2012. Released rice varieties in 2012, Bureau of Rice Research and Development, Rice Department, Bangkok. http://www.brrd.in.th/main/index.php?option=com_content&view=article&id=669:brrd-255501&catid=70:certification& Itemid=65 (Accessed 15th April 2016)
BRRD. 2016. Rice knowledge bank: rice varieties. Bureau of Rice Research and Development, Rice Department. http://www.brrd.in.th/rkb/varieties/index.php.htm (Accessed 9th April 2016)
Chang, T.T., E.A. Bardenas, and A.C. Del Rosario. 1965. The morphology and varietal characteristics of the rice plant. IRRI Technical Bulletin 4. International Rice Research Institute, Los Banos, Philippines.
Colmer, T.D. 2003. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany. 91: 301-309.
Dumrongkiat, C. 2013. Upland rice and food security in the highlands. Rice Department, Bangkok.
Escribano-Bailón, M.T., C. Santos-Buelga, and J.C. Rivas-Gonzalo. 2004. Anthocyanins in cereals. Journal of Chromatography A. 1054: 129–141.
Harlan, J. 1992. Crop and Man, 2nd edn. Madison, WI, USA: American Society of Agronomy.
HRDI. 2010. Strategies for the development and conservation of highland natural resources on the northern highlands 10 years (2010-19). A report to the National Economic and Social Development Institute from the Highland Research and Development Institute.
Jaksomsak, P., N. Yimyam, B. Dell, C. Prom-u-thai, and B. Rerkasem. 2015. Variation of seed zinc in a local upland rice germplasm from Thailand. Plant Genetic Resources. 13: 168-175.
Juliano, B.O. 1993. Rice in human nutrition. Food and Agriculture Organization of the United Nations, Rome
Kunstadter, P., and E.C. Chapman. 1978. Problems with shifting cultivation and economic development in Northern Thailand. p. 3-23. In: P. Kunstadter, E.C. Chapman, and S. Sabhasri (Eds.), Farmers in the forest. The University Press of Hawaii, Honolulu.
Laenoi, S., N. Phattarakul, S. Jamjod, N. Yimyam, B. Dell, and B. Rerkasem. 2015. Genotypic variation in adaptation to soil acidity in local upland rice varieties. Plant Genetic Resources. 13: 206-212.
Naruebal, S. 2009. Diversity of Highland Rice Bue Polo Variety from Mae Hong Son Province. MS Thesis, Graduate School, Maejo University.
Oka, H.I. 1988. Origin of Cultivated Rice. Tokyo: Japan Scientific Society Press and Elsevier, Amsterdam.
Oupkaew, P., T. Pusadee, A. Sirabanchongkran, K. Rerkasem, S. Jamjod, and B. Rerkasem. 2011. Complexity and adaptability of a traditional agricultural system: case study of a gall midge resistant rice landrace from northern Thailand. Genetic Resource and Crop Evolution. 58: 361-372.
Palawisut, S., P. Nualsiri, P. Patirupanusara, P. Patirupanusara, A.N.L. Noenplab, N. Chiengwattana, P. Pattawatang, S. Suwanthada, S. Chairinte, S. Suttayot, J. Anawong, W. Palawisut, W. Intrarasatit, C. Wannasai, D. Ariyapruek, P. Hintang, M. Wangka, C. Deerasamee, T. Sanguansaj, V. Varamisara, S. Karnjanaphun, J. Pipatpiriyanon, T. Kongtong, W. Sriratanasak, N. Chansrisommai, C. Tayatum, D. Chettanachit, N. Nilpanit, W. Rattanakarn, R. Dhitikiatipong, and P. Aranyanart. 2008. RD29 (Chainat 80) rice variety. Thai Rice Research Journal. 2: 80-95.
Panomjan, N., S. Jamjod, B. Rerkasem, B. Dell, and C. Prom-u-thai. 2016. Variation of zinc concentration in rice caryopsis and husk among southern rice varieties grown in Southern and Northern Thailand. Chiang Mai University Journal of Natural Science. 15: 1-10.
Pintasen, S., C. Prom-u-thai, S. Jamjod, N. Yimyam, and B. Rerkasem. 2007. Variation of grain iron content in a local upland rice germplasm from the village of Huai Tee Cha in northern Thailand. Euphytica. 158: 27-34.
Punyalue, A., J. Jongjaidee, S. Jamjod, and B. Rerkasem. 2015. Reduce burning in maize production by relay cropping with legume. Agriculture and Agricultural Science Procedia. 5: 17-21.
Pusadee, T., S. Jamjod, Y. Chiang, B. Rerkasem, and B.A. Schaal. 2009. Genetic structure and isolation by distance in a landrace of Thai rice. Proceedings of the National Academy of Sciences of the United States of America. 106: 13880-13885.
Pusadee, T., P. Oupkaew, B. Rerkasem, S. Jamjod, and B. Schaal. 2014. Natural and human-mediated selection in a landrace of Thai rice (Oryza sativa). Annals of Applied Biology. 165: 280
Renard, R.D. 2001. Opium Reduction in Thailand 1970-2000. A Thirty Years of Journey. Chiang Mai: Silkworm Books, for the United Nations International Drug Control Programme. Regional Centre for East Asia and the Pacific, Bangkok.
Rerkasem, B. 2005. Transforming subsistence cropping in Asia. Plant Production Science. 8: 273-285.
Rerkasem, B. 2008. Diversity in local rice germplasm and rice farming: a case study of Thailand. Biodiversity. 9: 49-51.
Rerkasem, B., S. Jumrus, N. Yimyam, and C. Prom-u-thai. 2015. Variation of grain nutritional quality among Thai purple rice genotypes grown at two different altitudes. Science Asia. 41: 377–385.
Rerkasem, B., and K. Rerkasem. 2002. Agrodiversity for in situ conservation of Thailand’s native rice germplasm. Chiang Mai University Journal of Natural Science. 1: 129-148.
Rerkasem, K., N. Yimyam, C. Korsamphan, C. Thong-ngam, and B. Rerkasem. 2002. Agrodiversity lessons in mountain land management. Mountain Research and Development. 22: 4-9.
Royal Decree. 2005. Establishment of the highland research and development Institute, Thailand’s Royal Decree volume 122, section 95a, October 14th, 2005.
Saetan, S. 2010. Local Rice Varieties of Southern Thailand Volume II. Patthalung Rice Research Center, Bureau of Rice Research and Development, Rice Department, Bangkok.
Sirabanchongkran, A., N. Yimyam, W. Boonma, K. Rerkasem, K. Coffey, M. Pinedo-Vasquez, and C. Padoch. 2004. Varietal turnover and seed exchange: implications for conservation of rice genetic diversity on-farm.
Sirabanchongkran, A., K. Rerkasem, N. Yimyam, W. Boonma, K. Coffey, M. Pinedo-Vasquez, and C. Padoch. 2004. Varietal turnover and seed exchange: implications for conservation of rice genetic diversity on-farm. International Rice Research Notes. 29(2): 12-14
Sommut, W. 2003. Changes in flood-prone rice ecosystems in Thailand, crop year 2000-2001. Department of Agriculture, Thailand.
UN. 1992. United Nations Convention on Biological Diversity. Rio de Janeiro, Brazil: United Nations Conference on the Environment and Development.
Unthong, A., M. Kaosa-ard, and N. Punkiew. 2007. Factors affecting upland farmers’ choice of local rice varieties in Thailand. Kasetsart University Journal of Economics. 14: 70-85.
Watabe, T., T. Akihama, and O. Kinoshita. 1970. The alteration of cultivated rice in Thailand and Cambodia. Tonan Ajia Kenkyu (The Southeast Asian Studies). 8: 36-48.
Wrolstad, R.E., T.E. Acree, H. An, E.A. Decker, M.H. Penner, D.S. Reid, S.J. Schwartz, C.F. Shoemaker, and P. Sporns. 2001. Current protocols in food analytical chemistry. Wiley, New York.
Yodmanee, S., T.T. Karrila, and P. Pakdeechanuan. 2011. Physical, chemical and antioxidant properties of pigmented rice grown in Southern Thailand. International Food Research Journal. 18: 901-906.
Yoshida, S. 1981. Fundamentals of rice crop science. International Rice Research Institute, Los Banos, Philippines.
Yue, X., and Z. Xu. 2008. Changes of anthocyanins, anthocyanidins, and antioxidant activity in bilberry extract during dry heating. Journal of Food Science. 73: 494-499.
Zinke P, S. Sabhasri, and P. Kunstadter. 1978. Soil fertility aspects of the Lua forest fallow system of shifting cultivation. p. 134-159. In: P. Kunstadter, E.C. Chapman, and S. Sabhasri (Eds.), Farmers in the forest. The University Press of Hawaii, Honolulu.
Sansanee Jamjod1,2, Narit Yimyam2,3, Sittichai Lordkaew2,4, Chanakan Prom-u-thai1,2 and Benjavan Rerkasem5*
1 Department of Plant and Soil Science, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand
2 Lanna Rice Research Centre, Chiang Mai University, Chiang Mai 50200, Thailand
3 Department of Highland Agriculture, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand
4 Center for Agricultural Resource Systems Research, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand
5 Plant Genetic Resource and Nutrition Laboratory, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: benjavan.r@cmu.ac.th
Total Article Views

