
Effects of Drying Techniques on Selected Functional Properties and Bioactive Compounds of Dietary Fiber from the Outer Leaves of Cabbage
Nuannarat Tawatsinlapasorn, Thitima Kuljarachanan, Naphaporn Chiewchan* and Sakamon DevahastinPublished Date : 2017-01-01
DOI : 10.12982/cmujns.2017.0002
Journal Issues : Number 1 ,January - March 2017
ABSTRACT
The outer leaves of cabbage (Brassica oleracea L. var. capitata), which are usually discarded during processing or selling at the market, have been reported as a good raw material for producing functional dietary fiber powder. This study investigated the effects of different drying techniques, i.e., hot-air drying, vacuum drying and low-pressure superheated steam drying at 80°C, on selected functional properties and bioactive compounds of dietary fiber powder from the outer leaves of cabbage. The results showed that vacuum drying improved water retention capacity and swelling capacity of the dietary fiber powder compared to the hot-air dried sample. Neither the pressure level (5 and 10 kPa absolute pressure) nor steam injection before vacuum drying at 10 kPa affected the water retention or swelling capacities of the powder. No significant differences in the oil holding capacity (OHC) were observed among the samples prepared using different drying schemes. Vacuum-dried samples contained the highest contents of glucosinolates and phenolics. Overall, the results showed that powder undergoing vacuum drying at 80°C at 5 kPa possessed good functional properties and contained the most glucosinolates and phenols.
Keywords: Glucosinolates, Functional properties, Low-pressure superheated steam drying, Phenols, Vacuum drying
INTRODUCTION
White cabbage (Brassica oleracea L. var. capitata) is one of the most popular Brassica vegetables, commonly consumed raw or processed. The outer leaves of white cabbage, which are usually discarded during processing or selling at the market, are a rich source of dietary fiber (41-43% on a dry weight basis) (Jongaroontaprangsee et al., 2007) as well as various bioactive compounds, including glucosinolates and phenolics (Tanongkankit et al., 2010).
Jongaroontaprangsee et al. (2007) reported the potential of transforming the outer leaves of cabbage into dietary fiber powder containing associated bio-active compounds. Tanongkankit et al. (2010) studied the effects of processing steps, i.e., preparation and blanching method in combination with hot air drying at 80°C, on the retention of antioxidants during the production of dietary fiber powder. They recommended steam blanching the whole leaves of cabbage and then slicing, before hot-air drying at 80°C; this combination yielded a final product that retained the most antioxidants and displayed the highest antioxidant activity. Tanongkankit et al. (2012) and Tanongkankit et al. (2015) performed further investigations that suggested vacuum drying at 80°C retained even more antioxidants and glucosinolates. Tanongkankit et al. (2011) studied the effect of hot-air drying on sulforaphane, a hydrolysis product of glucosinolates and a potent food-derived anticarcinogen (Vig et al., 2009), in the outer leaves of cabbage. They concluded that sulforaphane was susceptible to heat, and to maximize its retention, the cabbage leaves should be subjected to hot-air drying at 60°C. Although processing’s effects on the major phytochemicals in the outer leaves of cabbage have been reported previously, information on functional properties, such as hydration properties and oil holding capacity have not been reported. Different drying schemes may affect the functional properties of the dietary fiber powder differently.
The functional properties of dietary fiber are important if using as an ingredient in foods. Adding fiber to food may change the consistency, texture, rheological behavior, and sensory characteristics of the final products (Dhingra et al., 2012). Hydration properties, i.e., water retention capacity and swelling capacity, play an important role in achieving a desirable texture (Thebaudin et al., 1997). Thickening, gelling, and water retention capacity contribute to the stabilization of the food structure, i.e., dispersions, emulsions, and foams (Thebaudin et al., 1997). OHC is also an important property, helping to retain fat in cooked meat products (Thebaudin et al., 1997).
Among the many steps involved in the production of dietary fiber powder, drying is one of the most important. Drying of dietary fiber at higher temperature may cause partial degradation of some dietary fiber components and alter its hydration properties, i.e., water retention, swelling, and fat absorption capacities of the fiber (Larrauri, 1999; Garau et al., 2007). Drying also causes cell wall damage, leading to the collapse of cell structure in the dried sample (Deng and Zhao, 2008). Gong et al. (2007) reported that the drying method significantly affected the bulk density of dietary fiber powder from cabbage.
Therefore, this research studied the effects of alternative drying techniques and conditions on the selected functional properties (water retention capacity, swelling capacity, and OHC) of dietary fiber powder from the outer leaves of cabbage. The major bioactive compounds, i.e., glucosinolates and phenolics, were also determined.
MATERIALS AND METHODS
Sample preparation
The outer leaves of white cabbage (Brassica oleracea L. var. capitata) were obtained from Pak Klong Market in Bangkok, Thailand. The fresh leaves were kept at 4°C until use. Before each experiment, the leaves were washed under running tap water and drained on a screen to get rid of excess water. The leaves were steam-blanched for 1 min (Tanongkankit et al., 2011) and subsequently cooled in cold water (~ 4°C). The blanched leaves were sliced into 5 × 0.5 cm (length × width) pieces.
Drying experiments
For hot-air drying, approximately 360 g of prepared cabbage sample was spread as a single layer on a 30 x 42 cm tray. An experiment was performed with the use of a laboratory-scale, hot-air oven (Termaks, TS8000, Bergen, Norway). Hot-air drying was conducted at 80°C at a constant air velocity of 0.6 m/s. Samples (3-5 g) were taken every 10 min to determine the moisture content following the gravimetric method at 105°C (AOAC Method 984.25; AOAC, 2000).
Vacuum drying and low-pressure superheated steam drying experiments were conducted in the same equipment designed and tested by Devahastin et al. (2004). For the vacuum-drying experiment, approximately 360 g of prepared cabbage sample was placed as a single layer on the sample sieve holder. Experiments were performed at 80°C at absolute pressure of 5 kPa and 10 kPa. For the condition of steam injection followed by vacuum drying, the drying chamber was pre-heated to 80°C. Approximately 360 g of prepared cabbage sample was then placed as a single layer on the sample sieve holder. Steam injection was immediately introduced to the sample for 1 min. After that, the absolute pressure of the drying system was adjusted to an absolute pressure of 10 kPa; it took approximately 1 min to reach the targeted pressure. The sample temperature was measured continuously using type-K thermocouples with an accuracy of ± 0.1°C, while the change of the sample mass was followed by means of a load cell. Drying time was obtained from the drying curves, where the moisture content of the dried cabbage was less than 0.1 g/g dry basis. All drying experiments were performed in triplicate. For low-pressure superheated steam drying, the experimental procedures were similar to those performed during vacuum drying at 10 kPa, but used superheated steam as the drying medium instead.
Preparation of dietary fiber powder
The dried samples obtained from different drying conditions were ground into fine powder using a grinder (Waring, 8011BU, Torrington, CT) at 18,000 rpm for 1 min. The powder was sieved using a sieve analyzer (Retsch, AS200 basic, Haan, Germany). A powder size of less than 450 μm was obtained after sieving. The samples of dietary fiber powder were vacuum-packed in aluminum packets until use.
Determination of hydration properties
The water retention and swelling capacities of the dietary fiber powder were determined following the methods of Robertson et al. (2000). For the determination of water retention capacity, 1 g of a dried sample was hydrated in 30 mL of distilled water at room temperature (~30ºC). After equilibration (for 18 h), the dry sample was centrifuged at 3000×g for 20 min and the supernatant was removed. The weight of residue was recorded both before drying (residue hydrated weight) at 105ºC and after drying (residue dry weight) until constant weight was obtained. The water retention capacity was calculated as follows:
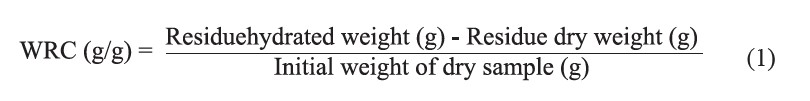
The swelling capacity was determined by hydrating 0.1 g of dry sample in 10 mL of distilled water in a calibrated cylinder (15 cm diameter) at room temperature (~30ºC). After equilibration for 18 h, the volume occupied by the settled sample was recorded. The swelling capacity was expressed as follows:
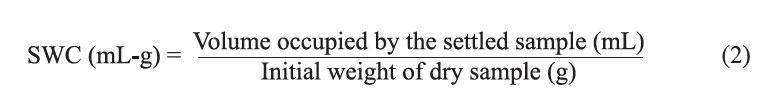
Determination of OHC
OHC was determined following the method of de Escalada Pla et al. (2012) with slight modifications. One g of dry sample was mixed with sunflower oil (30 mL) at room temperature (~30ºC). After equilibration (for 18 h), the sample was centrifuged at 1500×g for 5 min. The supernatant was decanted and the weight of the residue (pellet weight) was recorded. OHC was calculated as follows:

Determination of glucosinolates
Total glucosinolates were determined following the method of Declercq and Daun (1989) as later modified by Tanongkankit et al. (2012).
Determination of total phenolic content (TPC)
The TPC was determined using Folin-Ciocalteu reagent (Yu et al., 2005). Five grams of each sample was shaken in a shaker (New Brunswick Scientific, Innova 4230, Edison, NJ) with 50 mL of acetone–deionized water solution (1:1, v/v) at 120 rpm for 15 h at ambient temperature (~30ᵒC). The acetone extract was filtrated and kept in the dark at room temperature until further analysis. Fifty microliters of the extract was diluted with 3 mL of distilled water; 250 μL of Folin-Ciocalteu reagent and 750 μL of 20 % (w/v) sodium carbonate solution were then added. The absorbance was measured at 765 nm using an ultraviolet-visible (UV Vis) scanning spectrophotometer (Shimadzu, UV 21101 PC, Kyoto, Japan) after 2 h. TPC was estimated using gallic acid as the standard. The result was expressed as milligrams of gallic acid equivalent per one hundred grams of sample (dry basis).
Statistical analysis
The experiments were designed to be completely random. The data were subjected to analysis of variance (ANOVA) and were presented as mean values with standard deviations. Differences between mean values were established using Duncan’s multiple range tests; values were considered significant at a confidence level of 95%. All statistical analyses were performed using SPSS software (version 17) (SPSS Inc., Chicago, IL). All experiments were performed in triplicate.
RESULTS
Drying characteristics of the outer leaves of cabbage
The changes in moisture content are shown in Figure 1. Moisture content of the fresh cabbage sample was 11.14 ± 0.66 g/g dry basis. After blanching, the moisture content increased to 13.28 ± 3.08 g/g dry basis. In the case of hot-air drying and vacuum drying, the moisture content of the samples decreased rapidly early, after which it decreased much more slowly until reaching equilibrium. Vacuum drying dried the samples faster than hot-air drying at the same temperature. Vacuum drying at 5 kPa was faster than at 10 kPa.
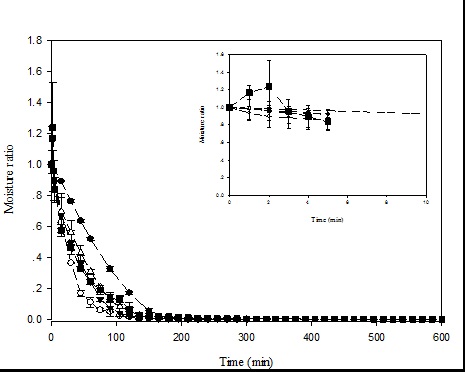
Figure 1. Drying curve of the outer leaves of cabbage under different drying conditions at 80°C: Hot-air drying (●); vacuum drying at 5 kPa (O); vacuum drying at 10 kPa (▼); steam injection followed by vacuum drying at 10 kPa ( ); low-pressure superheated steam drying at 10 kPa (■).
); low-pressure superheated steam drying at 10 kPa (■).
Additional steam pretreatment before vacuum drying at 10 kPa (SVD) was also conducted. Steam injection for 1 min in the same dryer resulted in an increase in moisture content of the sample; the sample moisture content was 14.54 g/g dry basis after steaming. Once the vacuum started, water at the sample surface was suddenly evaporated, leading to an accelerated decrease of moisture content early, after which the drying characteristics
Table 1. Time to dry the outer leaves of cabbage to a final moisture content of 0.06-0.09 g/g dry basis.

were similar to vacuum drying at 10 kPa. For low-pressure superheated steam drying, an increase in moisture content of the samples was observed during the first few minutes of drying (see inserted graph in Figure 1). Afterwards, a decrease in moisture content of the sample was observed, but slower than occurred in the case of vacuum drying at the same pressure. Required drying times to obtain the final moisture content of less than 0.1 g/g dry basis are given in Table 1.
Effect of drying techniques and conditions on functional properties
The water retention capacities of the samples from different drying conditions are given in Table 2. The water retention capacity of the vacuum-dried samples
was higher than the hot-air dried sample. However, reducing absolute pressure to 10 kPa and injecting steam before vacuum drying did not further improve water retention capacity of the dietary fiber powder.
Table 2. Functional properties of dietary fiber powder from the outer leaves of cabbage.
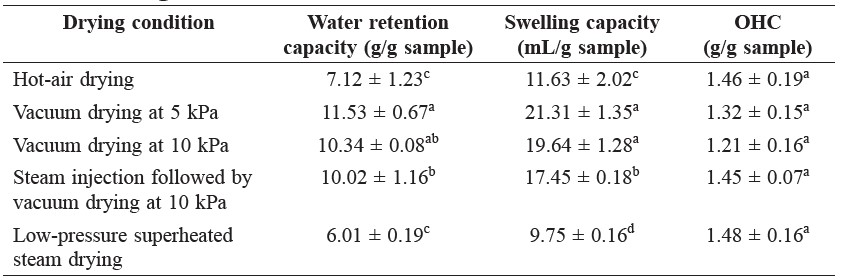
Note: Same letters in the same column indicate that values are not significantly different (p ˃ 0.05).
Vacuum drying significantly improved swelling capacity of the dietary fiber powder. Injecting steam before vacuum drying at 10 kPa yielded similar swelling capacity to that of the vacuum-dried samples. Low-pressure superheated steam drying led to poor swelling capacity.
No significant difference in OHC was observed among the dietary fiber powder samples prepared by different drying conditions.
Bioactive compounds in dietary fiber powder
Table 3 lists the total glucosinolates and TPC in dietary fiber powder from the outer leaves of cabbage prepared by different drying schemes compared with that of fresh leaves. Steam blanching did not have a significant effect on the glucosinolates and phenolic contents. Hot-air drying led to significant losses in total glucosinolates and TPC of approximately 43 and 37%, respectively, compared to the fresh sample. Vacuum-dried samples, particularly at lower absolute pressure, contained much higher contents of bioactive compounds than the hot air-dried sample. The low-pressure superheated steam drying sample lost more glucosinolates and phenols than the vacuum-dried samples.
Table 3. Bioactive compounds of fresh (steam-blanched) and dietary fiber powder (various drying treatments) samples of outer leaves of cabbage.
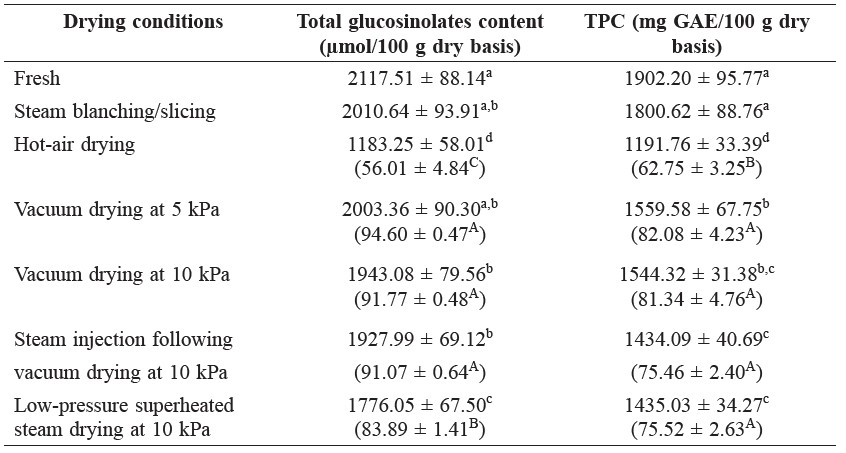
Note: Same letters in the same column indicate that values are not significantly different (p ≥ 0.05). Numbers in parentheses indicate the retention (%) of compounds as compared with the amounts in the fresh sample.
DISCUSSION
The results showed that different drying schemes required different drying times. Vacuum drying dried the samples faster than hot-air drying, due to the reduced boiling temperature of moisture at a lower pressure (Devahastin et al., 2004). Lowering the absolute pressure also lowered the boiling point of water (Methakhup et al., 2005), increasing the driving force for moisture removal from the material to the drying medium.
With low-pressure superheated steam drying, the surface temperature of the sample increased rapidly, due to steam condensation on the surface; this increased the moisture content of the sample during the early period of drying. Several investigators (Kerdpiboon and Devahastin, 2007; Phungamngoen et al., 2011, for example) observed similar characteristics with low-pressure superheated steam drying.
The water retention capacity is the quantity of water that remains bound to the hydrated fiber following the application of an external force (pressure or centrifugation) (Raghavendra et al., 2006). The ability of fiber to retain water can be explained by absorption phenomena and some water is retained outside the fiber matrix (free water) (Viuda-Martos et al., 2012). Water retention capacity also relates to the capillary porous structure formed by polysaccharide chains (Zhu et al., 2014). In general, drying and heating can modify the physical properties of the fiber matrix and thus alter the fiber’s hydration properties (Bejar et al., 2011).
The water retention capacity of dietary fiber powder prepared by hot-air drying is close to those of lemon pomace (4.79 g water/ g sample) (Huang et al., 2009) and citrus by-products (9.49 g water/ g powder) (Xingjian et al., 2014), but lower than those of carrot peels (12.40-13.37 g water/ g dry weight) (Chantaro et al., 2008). The water retention capacity is very dependent on the fiber structure (Yamazaki et al., 2005), as it plays important roles on the kinetics of water uptake (Figuerola et al., 2005). The higher degree of shrinkage, as well as the collapse of the capillary porous structure, that occurred during hot-air drying at 80°C might limit the absorption of water by the fiber matrix, leading to the lower water retention capacity. Bondaruk et al. (2007) reported that hot-air drying generally resulted in more structural damage of plant tissues than vacuum drying. Krokida and Maroulis (1997) also reported that the porosity of air-dried samples was lower than that of vacuum-dried samples.
Previous research has reported that low-pressure superheated steam drying provided better quality dried products in terms of less shrinkage and higher porosity and rehydration (Devahastin et al., 2007). However, we found that the water retention capacity of the low-pressure, superheated, steam-dried sample was similar to that of the hot air-dried sample, but significantly lower than that of the vacuum-dried samples. The outer leaves of cabbage were preliminary steam-blanched for 1 min to eliminate the activity of the enzyme responsible for browning. This first steam blanching partially modified the plant structure. Once the blanched sample was subjected to low-pressure superheated steam drying, the cabbage tissues are subject to additional steam blanching due to steam condensation during the early period of drying, leading to the fiber losing its hydrophilic property (Tanongkankit et al., 2012). The softened tissues were then collapsed during low-pressure superheated steam drying. Therefore, the low-pressure superheated steam drying fiber lost the ability to hold water in its matrix.
The swelling capacity of the vacuum-dried dietary fiber powder sample was much higher than those of plum pomace (11.5-13.6 mL/ g sample) (Kosmala et al., 2013) and wheat bran (5.75 mL/ g sample) (Matin et al., 2013). The results also showed that the swelling capacity of the fiber prepared by vacuum drying was approximately twice that of the fiber prepared by hot air drying. Vacuum drying appears to preserve the microstructure of the fiber matrix better than hotair drying.
Previous research has reported that drying methods and conditions significantly affected the OHC of fiber powder (Femenia et al., 2003; Garau et al., 2007; Ganem et al., 2012). However, in our study, the OHC values did not vary significantly by drying technique; our OHC results were similar to those of wheat bran (1.21 g oil/ g sample) and barley bran (1.79 g oil/g sample) (Matin et al., 2013), but lower than those of lemon byproducts (6.60 g oil/ g sample) (Lario et al., 2004) and date fiber (Mrabet et al., 2012).
The results presented here also showed that drying retained more of the bioactive compounds (glucosinolates and TPC) than hot-air drying due to the shorter drying time and oxygen deficient environment in the drying chamber (Devahastin et al., 2004; Jongaroontaprangsee et al., 2014). Vacuum-dried samples also possessed higher glucosinolates and TPC contents than the low-pressure, superheated, steam-dried sample. The longer drying time required for low-pressure superheated steam drying may degrade more of the glucosinolates and TPC.
CONCLUSION
This study investigated the effects of different drying techniques on the drying kinetics of cabbage. Vacuum drying at 5 kPa required the shortest drying time. Vacuum-dried powder exhibited good hydration properties, i.e., water retention capacity and swelling capacity, compared with hot air-dried powder and low-pressure, superheated, steam-dried powder. The studied drying conditions did not affect OHC. Vacuum drying at 80°C at 5 kPa could be used to prepare functional dietary fiber powder from the outer leaves of cabbage.
ACKNOWLEDGEMENTS
The authors express our sincere appreciation to the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission and the National Research Council of Thailand for their financial support.
REFFERENCES
AOAC. 2000. Official methods of analysis (17th ed.). The Association of Official Analytical Chemists, Gaithersburg, M.D.
Bondaruk, J., M. Markowski, and W. Blaszczak. 2007. Effect of drying condition on the quality of vacuum-microwave dried potato cubes. Journal of Food Engineering. 81: 306-312.
Chantaro, P., S. Devahastin, and N. Chiewchan. 2008. Production of antioxidant high dietary fiber powder from carrot peels. LWT-Food Science and Technology.
41: 1987-1994.
de Escalada Pla, M.F., P. González, P. Sette, F. Portillo, A.M. Rojas, and L. N.Gerschenson. 2012. Effect of processing on physico-chemical characteristics of dietary fiber concentrates obtained from peach (Prunus persica L.) peel and pulp. Food Research International. 49: 184-192.
Deng, Y., and Y. Zhao. 2008. Effect of pulsed vacuum and ultrasound osmopretreatment on glass transition temperature, texture, microstructure and calcium penetration of dried apples (Fuji). LWT-Food Science and Technology. 41: 1575-1585.
Devahastin, S., P. Suvarnakuta, S. Soponronnarit, and A.S. Mujumdar. 2004. A comparative study of low-pressure superheated steam and vacuum drying of a heat-sensitive material. Drying Technology. 22: 1845-1867.
Dhingra, D., M. Michael, H. Rajput, and R.T. Patil,.2012. Dietary fiber in foods: A review. Journal of Food Science and Technology. 49: 255-266.
Femenia, A., P. García-Pascual, S. Simala, and C. Rosselló. 2003. Effects of heat treatment and dehydration on bioactive polysaccharide acemannan and cell wall polymers from Aloe barbadensis Miller. Carbohydrate Polymers. 51: 397-405.
Figuerola, F., M.L. Hurtado, A.M. Estévez, I. Chiffelle, and F. Asenjo. 2005. Fiber concentrates from apple pomace and citrus peel as potential fiber sources for food enrichment. Food Chemistry. 91: 395-401.
Garau, M.C., S. Simal, C. Rosselló, and A. Femenia. 2007. Effect of air drying temperature on physicochemical properties of dietary fiber and antioxidant capacity of orange (Citrus aurantium cv. Canoneta) by-products. Food Chemistry. 104: 1014–1024.
Ghanem, N., D. Mihoubi, N. Kechaou, and N.B. Mihoubi. 2012. Microwave dehydration of three citrus peel cultivars: Effect on water and oil retention capacities, color, shrinkage and total phenols content. Industrial Crops and Products. 40: 167-177.
Gong, Z., M. Zhang, and J. Sun. 2007. Physicochemical properties of cabbage powder as affected by drying method. Drying Technology. 25: 913-916.
Huang, S.C., T.S. Liao, T.C. Cheng, H.Y. Chang, S. M. Hwang, and D.F. Hwang. 2009. In vitro interactions on glucose by different fiber materials prepared from mung bean hulls, rice bran and lemon pomace. Journal of Food and Drug analysis. 17: 307-314.
Jongaroontaprangsee, S., W. Tritrong, W. Chokanaporn, P. Methacanon, S. Devahastin, and N. Chiewchan. 2007. Effects of drying temperature and particle size on hydration properties of dietary fiber powder from lime and cabbage by-products. International Journal of Food Properties. 10: 887-897.
Jongaroontaprangsee, S., N. Chiewchan, and S. Devahastin. 2014. Composition profiles and functional properties of dietary fiber powder from lime residues: effects of pretreatment and drying methods. Drying Technology. 32: 484-493.
Kosmala, M., J. Milala, K. Kolodziejczyk, J. Markowski, M. Zbrzeznink, and C. M.G.C. Renard. 2013. Dietary fiber and cell wall polysaccharides from plum (Prunus domestica L.) fruit, juice and pomace: comparison of composition and functional properties for three plum varieties. Food Research International. 54: 1787-1794.
Krokida, M.K., and Z.B. Maroulis. 1997. Effect of drying method on shrinkage and porosity. Drying Technology. 15: 2441-2458.
Lario, Y., E. Sendra, J. García-Pérez, C. Fuentes, E. Sayas-Barberá, J. Fernández-López, and J.A. Pérez-Alvarez. 2004. Preparation of high dietary fiber powder from lemon juice by products. Innovative Food Science and Emerging Technologies. 5: 113-117.
Larrauri, J.A. 1999. New approaches in the preparation of high dietary fiber powders from fruit by-products. Trends in Food Science and Technology. 10: 3-8.
Methakhup, S., N. Chiewchan, and S. Devahastin. 2005. Effects of drying methods and conditions on drying kinetics and quality of Indian gooseberry flake. LWT-Food Science and Technology. 38: 579-587.
Phungamngoen, C., N. Chiewchan, and S. Devahastin. 2011. Thermal resistance of Salmonella enterica serovar Anatum on cabbage surfaces during drying: Effects of drying methods and conditions. International Journal of Food Microbiology. 147: 127-133.
Raghavendra, S.N., N.K. Rastogi, K. S.M.S. Raghavarao, and R.N. Tharathan. 2006. Dietary fiber from coconut residue: Effect of different treatment and particle size on the hydration properties. European Food Research and Technology. 218: 563-567.
Robertson, J.A., F.D. de Monredon, P. Dysseler, F. Guillon, R. Amado, and J.-F. Thibaut. 2000. Hydration properties of dietary fiber and resistant starch: A european collaborative study. LWT-Food Science and Technology. 33: 72-79.
Tanongkankit, Y., N. Chiewchan, and S. Devahastin. 2010. Effect of processing on antioxidants and their activity in dietary fiber powder from the outer leaves of cabbage. Drying Technology. 28: 1063-1071.
Tanongkankit, Y., N. Chiewchan, and S. Devahastin. 2011. Evolution of anticarcinogenic substance in dietary fiber powder from the outer leaves of cabbage during drying. Food Chemistry. 127: 67-73.
Tanongkankit, Y., N. Chiewchan, and S. Devahastin. 2012. Physicochemical property changes of the outer leaves of cabbage upon preparation into functional dietary fiber powder. Food and Bioproducts Processing. 90: 541-548.
Tanongkankit, Y., N. Chiewchan, and S. Devahastin. 2015. Evolution of antioxidants in dietary fiber powder produced from white the outer leaves of cabbage: Effects of blanching and drying methods. Journal of Food Science and Technology. 52: 2280-2287.
Thebaudin, J.Y., A.C. Lefebvre, M. Harrington, and C.M. Bourgeois, 1997. Dietary fibers: Nutritional and technological interest. Trends in Food Science and Technology. 8: 41-48.
Vig, A.P., G. Rampal, T.S. Thind, and S. Arora. 2009. Bio-protective effects of glucosinolates – A review. LWT - Food Science and Technology. 42: 1561-1572.
Viuda-Martos, M., Y. Ruiz-Navajas, A. Martin-Sánchez, E. Sánchez-Zapata, J. Fernández-López, E. Sendra, E. Sayas-Barberá, C. Navarro, and J.A. Pérez Álvarez. 2012. Chemical, physico-chemical and functional properties of pomegranate (Punica granatum L.) bagasses powder co-product. Journal of Food Engineering. 110: 220-224.
Zhu, F., B. Du, R. Li, and J. Li. 2014. Effect of micronization technology on physicochemical and antioxidant properties of dietary fiber from buckwheat hulls. Journal of Biocatalysis and Agricultural Biotechnology. 3: 30-34.
Nuannarat Tawatsinlapasorn, Thitima Kuljarachanan, Naphaporn Chiewchan* and Sakamon Devahastin
Advanced Food Processing Research Laboratory, Department of Food Engineering, Faculty of Engineering, King Mongkut’s University of Technology Thonburi, Bangkok 10140, Thailand
*Corresponding author. E-mail: naphaporn.rat@kmutt.ac.th
Total Article Views

