
Optimization of the Production Conditions of Glutinous Rice Bran Protein Hydrolysate with Antioxidative Properties
Warintip Sarringkarin and Thunnop Laokuldilok*Published Date : 2017-01-01
DOI : 10.12982/cmujns.2017.0001
Journal Issues : Number 1 ,January - March 2017
ABSTRACT
Glutinous rice bran (GRB) is a byproduct of milling rice. Because of its high protein content, GRB can be used to produce protein hydrolysate with antioxidative properties. The antioxidant activity of protein hydrolysate depends on hydrolysis conditions. In this study, protein from GRB cv. RD6 was prepared and then subjected to proteolytic hydrolysis by alcalase. The hydrolysis conditions were optimized using response surface methodology (RSM). We investigated two independent variables: the enzyme to substrate (E/S) ratio (0.59-3.41%, w/w) and the time taken for hydrolysis to occur (45-555 minutes). The E/S ratio and hydrolysis time significantly affected the yield, DPPH radical scavenging activity, metal chelating activity, degree of hydrolysis (DH), and average molecular weight (MW) of the protein hydrolysates. The optimum conditions for hydrolysis were an E/S ratio of 2.84% and 480 minutes for hydrolysis, which obtained a yield of 40.73 ± 0.44%, an IC50 value of 0.87 ± 0.02 mg/ml in the DPPH assay, a metal chelating activity of 72.80 ± 1.79%, a DH of 22.18 ± 0.42% and a MW of 3.07 ± 0.14 kDa. GRB protein hydrolysate, produced using alcalase, could have potential applications as an ingredient in functional food products due to its high antioxidative properties.
Keywords: Glutinous rice bran, Protein hydrolysate, Alcalase, Optimization, Antioxidant activity
INTRODUCTION
Glutinous rice is widely cultivated in northern and northeastern Thailand. The major byproduct obtained from milling is bran (Onyeneho and Hettiarachchy, 1992), which is a rich source of nutrients, including protein, fiber, lipids, vitamins, minerals, and flavonoids (Juliano, 1994; Aguilar-Garcia et al., 2007). Rice bran contains 12–20% protein (Saunders, 1990) and has more lysine than other cereal bran proteins (Juliano and Ben, 1985). It is a good source of hypoallergenic protein, and thus suitable for infant food formulations (Burks and Helm, 1994). Several researchers have studied the nutraceutical and functional properties of rice bran proteins (Wang et al., 1999; Fabian and Ju, 2011), however, their antioxidative properties are less known. Furthermore, rice bran protein, with its potential uses in health foods, offers an alternative use for rice.
Protein hydrolysates are composed of a complex mixture of peptides, containing chains of different lengths, and are produced from the hydrolysis of isolated proteins using an acid or proteolytic enzyme (Manninen, 2009). Several health benefits of protein hydrolysates have been reported, such as the reduction of blood pressure, moderation of hypertension, bacteriolysis, antioxidant properties, enhancement of calcium absorption, and the prevention of tumor formation (Adebiyi et al., 2008; Aluko, 2012). The antioxidant activity results from its capacity to act as a radical scavenger, proton donor, and metal-ion chelator (Xiong, 2010; Sarmadi and Ismail, 2010). Advantageously, low molecular weight peptides are resistant to digestion in the gastrointestinal tract and, therefore, are absorbed into the blood circulatory system in their intact form (Koopman et al., 2009; Aluko, 2012). However, the antioxidant activity of protein hydrolysate depends on several factors. Adebiyi et al. (2008) reported that the antioxidant properties of protein hydrolysate were dependent on the degree of hydrolysis (DH), the amino acid sequence of the peptide, and protease specificity. The most popular method for producing protein hydrolysate is enzymatic hydrolysis, which can be achieved using various proteases (Zhang et al., 2014). Among these proteases, alcalase is one of the most efficient, and can also reduce microbial contamination and bitterness (Hoyle and Merritt, 1994; Chabeaud et al., 2009; See et al., 2011). Previous studies have shown that protein hydrolysates with antioxidant properties can be produced from the dark muscle of tuna (Kuo, 2010) and salmon (See et al., 2011), alfalfa leaves (Xie et al., 2008), hemp seeds (Girgih et al., 2011), wheat germ (Zhu et al., 2006), potatoes (Cheng et al., 2010), and rice bran (Silpradit et al., 2010). However, no one has reported producing protein hydrolysate from glutinous rice bran (GRB).
It is necessary to optimize the production conditions in order to obtain protein hydrolysates with the highest antioxidative properties. Response surface methodology (RSM) is a useful tool for optimizing food production (Hu, 1999). Therefore, the objectives of this study were to produce protein hydrolysates with antioxidative properties from proteins derived from GRB using enzymatic hydrolysis by alcalase, and to determine the optimal processing conditions for the proteolytic hydrolysis. We investigated the obtained protein hydrolysates for their DPPH radical scavenging activity, metal-ion chelating activity, and inhibition of lipid peroxidation, as well as the DH, yield, molecular weight (MW), and solubility of the GRB protein hydrolysates.
MATERIALS AND METHODS
Materials and chemicals
Glutinous rice cv. RD6 (Oryza sativa L.) was purchased from a local rice miller in Chiang Mai Province, Thailand, and stored in aluminum bags at -18ºC until use. Alcalase, an endoprotease derived from Bacillus licheniformis, was purchased from Novo Nordisk (Denmark). Linoleic acid and bovine serum albumin were purchased from Sigma-Aldrich (USA). Low molecular weight gel filtration calibration kits were purchased from GE Healthcare Life Sciences (USA).
Preparation of GRB protein
The GRB protein was prepared according to the procedure described by Adebiyi et al. (2008) with some modifications. The glutinous rice was milled for 30 seconds using a laboratory rice miller (NW250; Natrawee Technology, Thailand). The rice bran fraction was collected and defatted using hexane (1:3, w/v) for 30 minutes. The defatted GRB was then extracted with 0.05 M NaOH (1:10, w/v) for 1 hour. The slurry was centrifuged at 2100 × g (Sorvall Super T21; GMI, USA) at 4ºC for 15 minutes. The supernatant was then collected and the pH was adjusted to 4.0 with 1.0 N HCl. After incubation at 4ºC for 1 hour, the obtained sediment was washed twice with deionized water and the pH was adjusted to 7.0 with 0.2 M NaOH. The sediment was dehydrated using lyophilization (Freeze dryer; Labconco, USA). Finally, the GRB protein powder was collected and stored at 5ºC until use.
Enzymatic hydrolysis
The GRB protein (50 g) was mixed with 0.2 M sodium phosphate (pH 7.5) at a ratio of 1:5 (w/v). Alcalase was added to the mixtures at various enzyme to substrate (E/S) concentrations ranging from 0.59-3.41%, which were then incubated at 60ºC between 45-555 minutes, depending on the hydrolysis time. After incubation, the enzyme was inactivated by heating at 95ºC for 10 minutes. The mixtures were centrifuged at 4730 × g at 4ºC for 30 minutes. The supernatants were collected and the pH was adjusted to 7.0 with 0.2 M NaOH. This solution was dehydrated by lyophilization. The powdered protein hydrolysate was collected and stored at 5ºC until analysis.
Degree of hydrolysis
The DH was determined according to the method described by Adler-Nissen (1979) with some modifications. Samples of 0.25 ml hydrolysate were mixed with 2 ml of 0.2 M phosphate buffer (pH 8.2). Afterwards, 2 ml of 0.1% 2,4,6-trinitrobenzenesulfonic acid (TNBS) solution was added to the mixtures and incubated at 50ºC for 60 minutes. To stop the hydrolysis, 4 ml of 0.1 N HCl was added to each of the mixtures, and the absorbance was measured at 340 nm. The number of free amino groups was deduced from a L-leucine standard, and the hydrolysis degree (%) was calculated using the following equation:
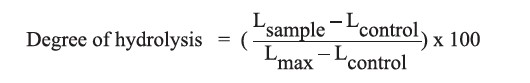
Where, Lsample is the number of amino groups in the sample after hydrolysis, Lcontrol is the number of amino groups in the original GRB protein, and Lmax is the number of amino groups in the original GRB protein after hydrolysis.
Protein solubility
The determination of protein solubility was carried out following the method described by Lowry et al. (1951) with some modifications. The protein hydrolysate solution (1 mg/ml) was mixed with 5 ml of copper ion solution (combination of 0.05% CuSO4.5H2O, 1% sodium tartrate, 2% Na2CO3, and 0.1 N HCl) and left for 10 minutes. Folin reagent was added to the mixture, which was then incubated in the dark for 30 minutes. The absorbance was determined at 750 nm against a deionized water blank. The protein solubility (%) was calculated using the equation below. Bovine serum albumin was used as a standard protein for comparison.
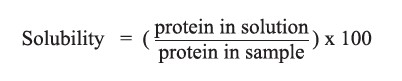
Average molecular weight by size exclusion chromatography
The average MW of the protein hydrolysate was determined using the method described by Delgado et al. (2011) with some modifications. High-performance liquid chromatography (Shimadzu, Kyoto, Japan) was performed using a SRT-C SEC-100 size exclusion column (5 μm, 4.6 × 300 mm, Sepax Technologies, USA), which was connected to a photodiode array detector (Shimadzu). The sample (3 mg) in the mobile phase solution (50 mM phosphate buffer, pH 7.0, in 150 mM sodium chloride) was filtered through a 0.45 μm syringe filter prior to HPLC injection. The flow rate was set at 0.25 ml/minute. The injection was 10 μl and the detector was monitored at 280 nm. The MW of protein hydrolysate was calculated using a low molecular weight gel filtration calibration kit (GE Healthcare Life Sciences, USA) as the protein standards, which included N-hippuryl-His-Leu (0.43 kDa), aprotinin (6.50 kDa), ribonuclease (13.70 kDa), carbonic anhydrase (29.00 kDa), ovalbumin (44.00 kDa), and conalbumin (75.00 kDa).
DPPH radical scavenging activity
The DPPH radical scavenging activity of protein hydrolysate was determined according to the method described by Zhang et al. (2014) with some modifications. The 0.25-1.00 mg/ml protein hydrolysate solutions (2 ml) were added to a 0.1 mM DPPH solution (2 ml). The mixtures were then incubated in the dark for 30 minutes. Afterward, the absorbance was determined at 517 nm against deionized water as a blank. The DPPH radical scavenging activity (%) was calculated using the following equation:
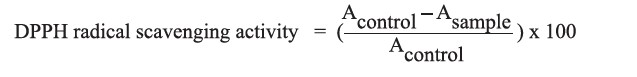
The antioxidant activity was expressed as 50% of inhibition concentration (IC50) (mg/ml).
Metal chelating activity
The metal chelating activity was determined according to the method described by Oh et al. (2006) with some modifications. The 0.8 mg/ml protein hydrolysate solutions (1.0 ml) were mixed with 2 mM FeCl2 (0.1 ml). The mixtures were incubated for 10 minutes at ambient temperature before mixing with 5 mM ferrozine (0.1 ml). Deionized water (3.0 ml) was added to the mixtures, which were then incubated for 10 minutes. Finally, the absorbance was determined at 562 nm against a deionized water blank. The deionized water was used as a control. Metal chelating activity (%) was calculated using the following equation:
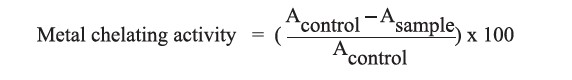
Inhibition of lipid peroxidation
The inhibition of lipid peroxidation was determined according the method described by Lingnert et al. (1979) with some modifications. The protein hydrolysate (0.2 ml) was mixed with 4.0 ml of 5.0 mM linoleic acid emulsion. This mixture was then incubated at 37°C for 8 hours. Following the incubation, 60% methanol (6.0 ml) was added to the mixture and the absorbance was determined at 234 nm. Inhibition of linoleic acid peroxidation (%) was calculated using the following equation:
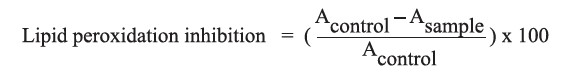
Experimental design and statistical analysis
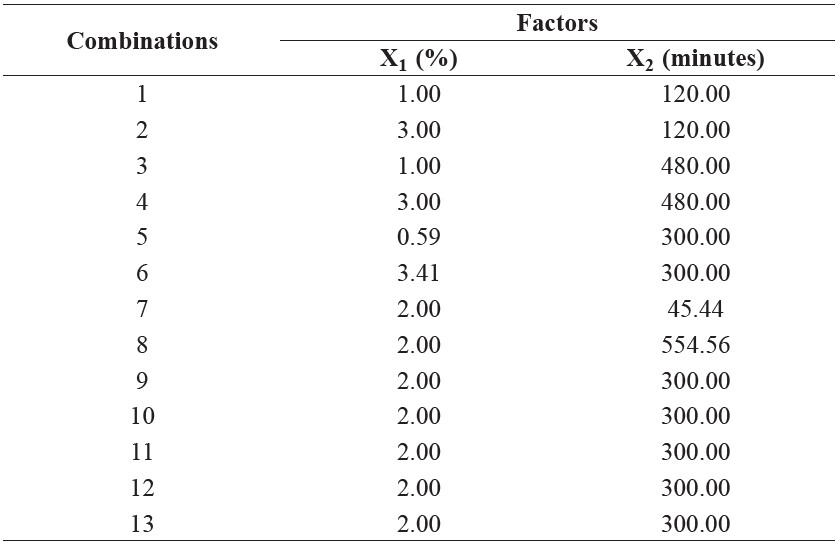
A central composite design (CCD) was used in this study. The independent variables tested were the E/S ratio, ranging from 0.59-3.41% w/w (X1) and hydrolysis time, which ranged from 45.44-554.56 minutes (X2). Five E/S concentrations and five hydrolysis times were chosen for the tests, resulting in a total of 13 combinations (Table 1). Regression models between the dependent (Y) and independent (X) variables were described by the following polynomial equation:
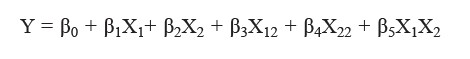
Design-Expert software (version 6.0.2; Stat-Ease Inc., USA) was used to perform all statistical analyses, including analysis of variance (ANOVA), determining the regression coefficients (R2), testing the lack of fit, determining the optimal enzymatic hydrolysis conditions (using the desirability function approach), and creating three-dimensional graphs. The predicted values were obtained using the above polynomial equation.
Table 1. Combinations of enzyme to substrate ratio and hydrolysis time used to investigate the optimal conditions for obtaining GRB protein hydrolysates.
RESULTS
Model fitting and response surface analysis
The DH (14.11-21.16%), MW (3.38-24.16 kDa), yield (31.70-43.33%), IC50 value (0.58-0.84 mg/ml) (DPPH radical scavenging activity), and metal-ion chelating activity (54.50-69.33%) of the protein hydrolysates are shown in Table 2.
Table 3 shows that the quadratic models for E/S ratio (X1) and hydrolysis time (X2) were significant (P < 0.05) for DH, MW, and metal-ion chelating activity. Furthermore, the linear regression models for X1 and X2 were statistically significant (P < 0.05) for yield and DPPH radical scavenging activity. The yield, DH, and antioxidant activity were higher for increasing X1 and X2 values (Figures 1A, B, and D, respectively). MW decreased with increasing X1 and X2 values (Figure 1C). The metal-ion chelating activity was the highest at X1 of 2% and X2 of 120 minutes (Figure 1E). No significant differences were observed for protein solubility and lipid peroxidation inhibition (P ≥ 0.05) for any of the variables.
The R2 values for equations 1, 2, 3, 4, and 5 were 0.850, 0.917, 0.480, 0.574, and 0.677, respectively, suggesting that the regression models were significant and explained the reaction well. For the lack of fit analysis, equations 1, 3, 5, and 6 were not significant (P ≥ 0.05); however, the lack of fit analysis was significant for MW (P < 0.05), indicating that other factors influenced RGB hydrolysis.
Optimization of enzyme hydrolysis and model validation
Yield, DPPH radical scavenging activity, and metal-ion chelating activity were the factors chosen for optimizing the processing conditions. The conditions that obtained the highest yield and antioxidant activities of protein hydrolysate were an E/S ratio of 2.84% and hydrolysis time of 480 minutes. The predicted responses for yield, IC50 values (DPPH radical scavenging activity), and metal-ion chelating activity were 41.0%, 0.63 mg/ml and 63.24%, respectively. To confirm the predicted values, a verification experiment was performed. The actual values for yield, DPPH radical scavenging activity, and metal-ion chelating activity obtained from these experiments were 40.73%, 0.87 mg/ml, and 72.80%, respectively (Table 4). These results revealed that the actual values were close to the predicted values, with small variability. Furthermore, the predicted and actual values for DH and MW were very close. The predicted values for DH and MW (calculated from equations 1 and 2) at the optimal conditions described above were 20.84% and 6.34 kDa, and the experimental values were 22.18 ± 0.42% and 3.07 ± 0.14 kDa, respectively.
Table 2. Actual level of independent variables along with the observed values for the response variables.
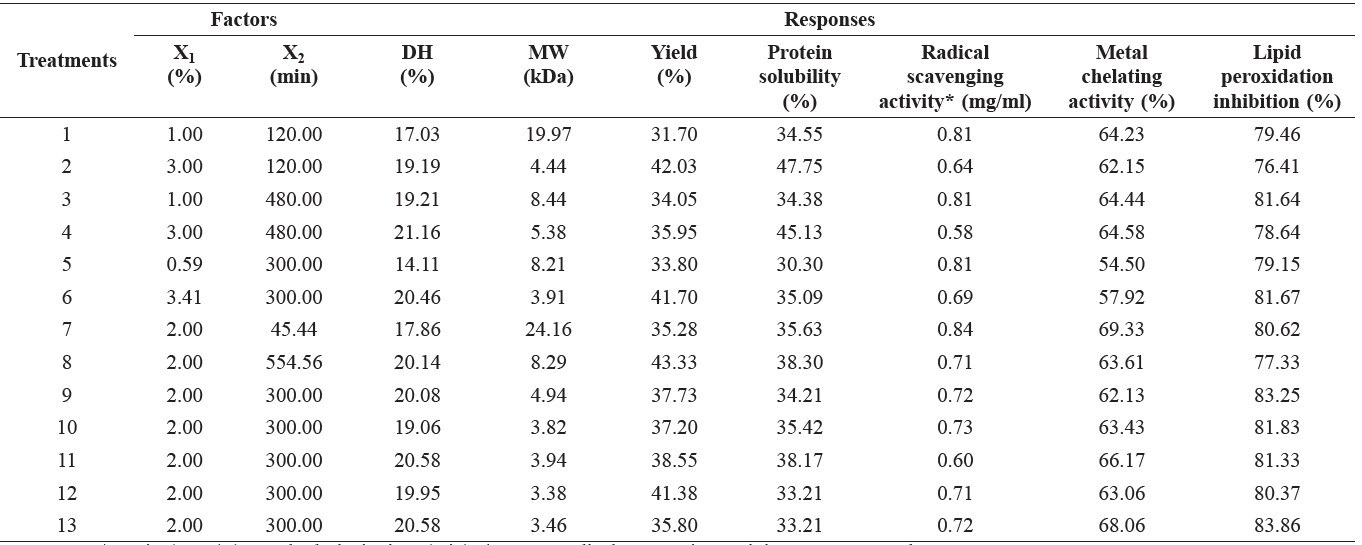
Note: X1: E/S ratio (% w/w), X2: hydrolysis time (min). * DPPH radical scavenging activity was expressed as IC50.
Table 3. Equation models and R2 of each response.
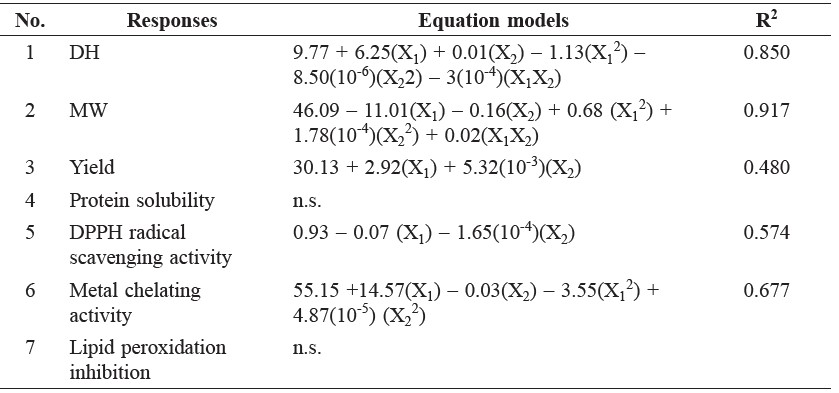
Note: Y = response, X1= E/S ratio (% w/w), X2 = hydrolysis time (min), n.s.= not significant.
Table 4. The predicted and actual values of protein hydrolysates obtained from optimum conditions.

Note: 2.84 % of E/S ratio and 480 min of hydrolysis time. *DPPH radical scavenging activity was expressed as IC50.
DISCUSSION
In this study, we found that the E/S ratio and hydrolysis time significantly influenced the DH of GRB protein hydrolysis (Figure 1A). Determining the optimum E/S ratio maximizes enzyme performance when sufficient substrates are available (Uhlig and Linsmaier-Bednar, 1998). This results in protein hydrolysates that contain an increased amount of free amino acids and small peptides (Salwanee et al., 2013). Similar results for the effect of E/S ratio on DH have previously been reported for other protein sources (See et al., 2011; Salwanee et al., 2013; Roslan et al., 2014). In this study, we found that the DH of the GRB protein hydrolysate was higher when the hydrolysis time was increased. Longer hydrolysis times allow for more extensive enzyme action, leading to increased cleavage of peptide bonds, and thus, a higher DH of the protein hydrolysate (Haslaniza et al., 2010). This has also been confirmed in other studies (Mukhin and Novikov, 2001; Bhaskar et al., 2008; Dong et al. 2008; Ovissipour et al., 2009).
We showed that decreased MW of the protein hydrolysate was achieved by increasing both the E/S ratio and the hydrolysis time (Figure 1B). This is similar to results from Saidi et al. (2013), who produced protein hydrolysate from a byproduct of the dark muscle of tuna using alcalase; the MW protein hydrolysates were found to be negatively related with the DH. You et al. (2009) previously reported the intra-chain cleavage of peptide bonds during hydrolysis, resulting in protein hydrolysates that were mainly composed of low MW peptides (< 3 kDa). Dong et al. (2008) reported that increasing the hydrolysis time from 0.25 to 4 hours increased peptide fractions of < 0.5-1 kDa and decreased peptide fractions of > 10 to 1 kDa for silver carp protein hydrolysate.
The yield obtained increased linearly with increasing both the E/S ratio and hydrolysis time (Figure 1C). The yield of protein hydrolysate is known to depend on its DH (Elavarasan et al., 2014). Jin et al. (2007) previously reported that increasing the E/S ratio resulted in a higher nitrogen soluble content of the supernatant from protein hydrolysates, which was due to increased proteolysis. Furthermore, protein hydrolysates with a higher DH have been shown to contain increased amounts of small peptides that were more hydrophilic (Mahmoud, 1994), resulting in greater solubility (Quaglia and Orban, 1987; Gbogouri et al., 2004), and thus, increased hydrolysate yield. Other researchers have reported similar results (Benjakul and Morrissey, 1997; Haslaniza et al., 2010). The increased hydrolysis time resulted in increased hydrolysate yield, as this allowed sufficient time in which proteolysis could occur. Other studies have confirmed this effect of hydrolysis time on the yield of protein hydrolysate (Shahidi et al., 1995; Aspmo et al., 2005; Tang et al., 2009).

Figure 1. Response surface plots of E/S ratio (%) and hydrolysis time (min) on DH (A), MW (B), yield (C), IC50 of DPPH radical scavenging activity (D), and metal-ion chelating activity (E).
It is well known that the antioxidant activities of protein hydrolysates are influenced by their amino acid sequence, which depends on the protease specificity (Dong et al., 2008). In this study, the antioxidant activities of protein hydrolysates produced from GRB using alcalase were verified by measuring DPPH radical scavenging activity and metal-ion chelating activity.
DPPH radical scavenging activity was assessed by determining the IC50 value, which is defined as the concentration of antioxidants that results in a 50% decrease in the initial DPPH concentration. The lower the IC50 value, the higher the radical scavenging activity. Increasing both the E/S ratio and hydrolysis time resulted in a lower IC50 value (Figure 1D). Higher amounts of DH protein hydrolysates, which contain smaller peptide molecules, have been shown to increase antioxidant activity (Xiong, 2010). The antioxidant activities of small peptides are based on the major antioxidant mechanism (Prior et al., 2005), which can occur from the actions of radical scavengers, proton donors, and/or metal-ion chelators (Xiong, 2010), which depend on the peptide composition (Zhang et al., 2014). The native proteins in rice bran are highly aggregated through hydrogen bonds and disulfide bonds; therefore, they have high molecular weights (Hamada, 1997). During protein hydrolysis, the rice bran protein unfolds and the disulfide bonds and aggregation is disrupted (Hamada, 2000). Wattanasiritham et al. (2016) found that rice bran protein hydrolysate exhibited higher antioxidant activity than in its native form. The major amino acids in rice bran protein include glutamic acid (Glu), aspartic acid (Asp), methionine (Met), tyrosine (Tyr), and serine (Ser), all of which have antioxidative properties (Wang and Gonzalez de Mejia, 2005; Bao, 2012). The non-specific cleavage of alcalase released the reactive groups of these amino acids so that they could readily act as electron donors. Tyr is composed of an electron-dense aromatic ring that exhibits strong radical scavenging activity. Thus, Tyr is a significant source of hydrogen atom transfer for the neutralization of free radicals (Chen et al., 1995; Xiong, 2010). Likewise, the carboxylic and hydroxyl side chains of Glu, Asp, and Ser all have strong electron-donating ability to DPPH radicals and are metal-ion binders (Xiong, 2010; Aluko, 2012). Furthermore, the sulfhydryl group in Met has also been reported to increase antioxidant activity when it is incorporated into peptides (Aluko, 2012). In this study, the optimal processing conditions increased the DPPH radical scavenging activity of the GRB protein hydrolysate. Similar results have previously been reported for other protein hydrolysate sources (Wu et al., 2003; Cumby et al., 2008; Amza et al., 2013).
The metal-ion chelating activity of the GRB protein hydrolysate increased when the E/S ratio and hydrolysis time were approximately 2% and 120 minutes, respectively (Figure 1E). This may be associated with the observed MW and amino acid composition of the obtained GRB protein hydrolysates, which may have different modes of action, and thus, result in different metal-ion chelating activities (Nalinanon et al., 2011). Girgih et al. (2011) found that increasing the molecular weight of hemp seed protein hydrolysates increased the metal-ion chelating activity, which may due to the effects of the constituent peptides. For these constituent peptides, higher amounts of polar amino acids (such as Glu, Asp, and Ser) contained within the GRB protein hydrolysate (produced from 2% E/S ratio and 120 minutes hydrolysis) may be an important reason for the higher metal-ion chelating activity observed. These polar amino acids have side chains containing amino acids from the carboxylic (Glu and Asp) and hydroxyl (Ser) groups, which can be chelated with metal-ions (Liu et al., 2010; Xiong, 2010). Therefore, the metal-ion chelating activity of the GRB protein hydrolysates depends not only on their molecular weights, but also on other factors, such as the protein source and amino acid composition (Chen et al., 1998; Peña-Ramos and Xiong, 2001).
The optimum condition for the production of GRB protein hydrolysate was an E/S ratio of 2.84% and hydrolysis time of 480 minutes, which gave an actual yield of 40.73%, DPPH radical scavenging activity (IC50) of 0.87 mg/ml, metal-ion chelating activity of 72.80% (at 0.8 mg/ml), DH of 22.18%, and MW of 3.07 kDa. The experimental values obtained for yield, DPPH radical scavenging activity, and metal-ion chelating activity were relatively close to the predicted value, with the exception of MW. The yield for GRB protein hydrolysate was lower than that previously reported for hemp protein hydrolysate (42%), which was also produced using alcalase (E/S ratio of 5% at a hydrolysis time of 6 hours) (Tang et al., 2009). The antioxidant activity of the GRB protein hydrolysate was higher than that found for whey protein hydrolysate (produced by using an E/S ratio of 0.4 AU/g and 6 hours for hydrolysis) as determined by DPPH radical scavenging activity (IC50 = 1.3 mg/ml), which also had a lower metal-ion chelating activity (89% at 1 mg/ml) (Zhu et al., 2006). The DH obtained was higher than that previously reported for rice (cv. Dok Mali 105) bran protein hydrolysates (14.5% of DH), which were produced using the optimum condition of 1% E/S ratio and 340 minutes hydrolysis time (Silpradit et al., 2010). In addition, the optimum conditions for the production of a protein hydrolysate with antioxidative properties are still dependent on other processing variables, including the type of enzyme, temperature, and pH (Tang et al., 2009; Ren et al., 2010; Zhuang et al., 2013).
The DPPH radical scavenging activity (IC50) and metal-ion chelating activity of the native GRB protein were 10.31 mg/ml and 0.38%, respectively (data not shown). The GRB protein hydrolysate obtained from the optimal conditions had 12 and 192 times higher DPPH radical scavenging activity and metal-ion chelating activity than the native protein, respectively. These results confirm that the optimization of E/S ratio and hydrolysis time obtained a protein hydrolysate with a much higher antioxidant activity than the native protein.
CONCLUSION
Optimizing the production conditions of GRB protein hydrolysate showed that the yield, DH, MW, DPPH radical scavenging activity, and metal-ion chelating activity were significantly affected by the E/S ratio and hydrolysis time. An E/S ratio of 2.84% and hydrolysis time of 480 minutes were optimal, maximizing yield (40.73 ± 0.44%), DPPH radical scavenging activity (IC50 value of 0.87 ± 0.02 mg/ml), and metal-ion chelating activity (72.80 ± 1.79%). These conditions also produced DH of 22.18 ± 0.42% and MW of 3.07 ± 0.14 kDa. Processed under these optimal conditions, GRB protein hydrolysate is a promising antioxidant for potential use in the food and pharmaceutical industries.
ACKNOWLEDGEMENTS
This research was supported by grants funded by the National Research Council of Thailand and the Graduate School of Chiang Mai University. The authors thank the Faculty of Agro-Industry, Chiang Mai University for providing instruments.
REFERENCES
Adebiyi, A., A.O. Adebiyi, T. Ogawa, and K. Muramoto. 2008. Purification and characterization of antioxidative peptides from unfractionated rice bran protein hydrolysates. International Journal of Food Science & Technology. 43: 35-43.
Adler-Nissen, J. 1979. Enzymatic hydrolysis of food proteins. Process Biochemistry. 12-18.
Aguilar-Garcia., C., G. Gavino, M. Baragano-Mosqueda, P. Hevia, and V.C. Gavino. 2007. Correlation of tocopherol, tocotrienol, γ-oryzanol and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chemistry. 102: 1228-1232.
Aluko, R.E. 2012. Bioactive Peptides. p. 37–61. In Functional Foods and Nutraceuticals. Springer, New York.
Amza, T., A. Balla, F. Tounkara, L. Man, and H. M. Zhou. 2013. Effect of hydrolysis time on nutritional, function properties of protein hydrolysates prepared from gin (Neocarya macrophylla) seeds. International Food Research Journal. 20(5): 2081-2090.
Aspmo, S.I., S.J. Horn, and V.G.H. Eijsink. 2005. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochemistry. 40: 1957-1966.
Bao, J. 2012. Nutraceutical properties and health benefits of rice. P. 37-64. In L.Yu, R. Tsao and F. Shahidi (eds) Cereals and Pulses: Nutraceutical Properties and Health Benefits. John Wiley & Sons, Inc., New York.
Benjakul, S., and M.T. Morrissey. 1997. Protein hydrolysates from pacific whiting solid wastes. Journal of Agricultural and Food Chemistry. 45: 3423-3430.
Bhaskar, N., and N.S. Mahendrakar. 2008. Protein hydrolysate from visceral waste proteins of Catla (Catla catla): Optimization of hydrolysis conditions for a commercial neutral protease. Bioresource Technology. 99: 4105-4111.
Burks, A.W., and R.M. Helm. 1994. Hypoallerginicity of rice protein. In: Presented at the annual meeting of the American Association of Cereal Chemists. Nashville, Tennessee.
Chabeaud, A., P. Dutournié, F. Guérard, L. Vandanjon, and P. Bourseau. 2009. Application of Response Surface Methodology to Optimise the Antioxidant Activity of a Saithe (Pollachius virens) Hydrolysate. Marine Biotechnology. 11: 445-455.
Chen, H. M., K. Muramoto, and F. Yamauchi. 1995. Structural analysis of antioxidative peptides from soybean β-Conglycinin. Journal of the Science of Food and Agriculture. 43: 574-578.
Chen, H.M., K. Muramoto, F. Yamaguchi, K. Fujimoto, and K. Nokihara. 1998. Antioxidant properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. Journal of Agricultural and Food Chemistry. 46: 49–53.
Cheng, Y., Y.L. Xiong, and J. Chen. 2010. Antioxidant and emulsifying properties of potato protein hydrolysate in soybean oil-in water emulsions. Food Chemistry. 120: 101-108.
Cumby, N., Y. Zhong, M. Naczk, and F. Shahidi. 2008. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chemistry. 109: 144-148.
Delgado, M.C.O., V.A. Tironi, and M.C. Añón. 2011. Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT - Food Science and Technology. 44: 1752-1760.
Dong, S., M. Zeng, D. Wang, Z. Liu, Y. Zhao, and H. Yang. 2008. Antioxidant and biochemical properties of protein hydrolysate prepared from Silver carp (Hypophthalmichthys molitrix). Food Chemistry. 107: 1485-1493.
Elavarasan, K., K.V. Naveen, and B.A. Shamasundar. 2014. Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (Catla catla) as influenced by the nature of enzyme. Journal of Food Processing and Preservation. 38: 1207-1214.
Fabian, C., and Y. H. Ju. 2011. Rice bran protein: its properties and extraction methods. Critical Review in Food Science and Nutrition. 51: 816-827.
Gbogouri, G.A., M. Linder, J. Fanni, and M. Parmentier. 2004. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. Journal of Food Chemistry and Toxicology. 69: 615-622.
Girgih, A.T., C.C. Udenigwe, and R.A. Aluko. 2011. In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. Journal of the American Oil Chemists’ Society. 88: 381-389.
Hamada, J.S. 1997. Characterization of protein factions of rice bran to devise effective methods of protein solubilisation. Cereal Chemistry. 74: 662-668.
Hamada, J. S. 2000. Characterization and functional properties of rice bran proteins modified by commercial exoproteases and endoproteases. Journal of Food Science. 65: 305-310.
Haslaniza, H., M.Y. Maskat, W.M. Wan Aida, and S. Mamot. 2010. The effects of enzyme concentration, temperature and incubation tiome on nitrogen content and degree of hydrolysis of protein precipitate from cockle (Anadara granosa) meat wash water. International Food Research Journal. 17: 147-152.
Hoyle, N.T., and J.H. Merritt. 1994. Quality of fish hydrolysates from herring (Clupea harengus). Journal of Food Science. 59: 76-79.
Hu, R. 1999. Food product design: a computer-aided statistical approach. CRC Press, New York.
Jin, S., M.Z. Mou, Z.Z. Qiang, B. Yang, and M.J. Yue. 2007. Characterization of hydrolysates derived from enzymatic hydrolysis of wheat gluten. Journal of Food Science. 72(2): 103-107.
Juliano, B. O. 1985. The rice grain and its gross composition. In B.O. Juliano (2nd ed) The American Association of Cereal Chemists. St. Paul, Minnesota, USA.
Juliano, B.O. 1994. Rice: Chemistry and Technology. The American Association of Cereal Chemists. St. Paul, Minnesota, USA.
Koopman, R., N. Crombach, A.P. Gijsen, S. Walrand, J. Fauquant, A.K. Kies, S. Lemosquet, W.H. Saris, Y. Boirie, and L.J.V. Loon. 2009. American Society for Nutrition. 90: 106-115.
Kuo, C.H. 2010. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chemistry. 122: 42-48.
Lingnert, H., K. Vallentin, and C.E. Eriksson. 1979. Measurement of antioxidative effect in model system. Journal of Food Processing and Preservation. 3: 87-103.
Liu, Q., B. Kong, Y.L. Xiong, and X. Xia. 2010. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chemistry, 118: 403-410.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 193: 265-275.
Mahmoud, M.I. 1994. Physicochemical and functional properties of protein hydrolysates in nutritional products. Food Technology and Biotechnology. 48: 89-95.
Manninen, A.H. 2009. Protein hydrolysates in sports nutrition. Journal of Nutrition and Metabolism. 6: 38.
Mukhin, V. A., and V. Yu. Novikov. 2001. Enzymatic hydrolysis of proteins from crustaceans of the Barents Sea. Applied Biochemistry and Microbiology. 37(5): 538-542.
Nalinanon, S., S. Benjakul, H. Kishimura, and F. Shahidi. 2011. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chemistry. 124: 1354-1362.
Oh, J., H. Jo, A.R. Cho, S.-J. Kim, and J. Han. 2013. Antioxidant and antimicrobial activities of various leafy herbal teas. Food control. 31: 403–409.
Onyeneho, S.N., and N.S. Hettiarachchy. 1992. Antioxidant activity of durum wheat bran. Journal of Agricultural and Food Chemistry, 40: 1496-1500.
Ovissipour, M., A. Abedian, A. Motamedzadegan, B. Rasco, R. Safari, and H. Shahiri. 2009. The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chemistry. 115(1): 238-242.
Peña-Ramos, E.A., and Y.L. Xiong. 2001. Antioxidative activity of whey protein hydrolysates in liposomal system. Journal of Diary Science. 84: 2577-2583.
Prior, R.I., X. Wu, and K. Schaich. 2005. Standardized methods for determination of antioxidant capacity of phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry. 53: 4290-4302.
Quaglia, G.B., and E. Orban. 1987. Enzymatic solubilization of proteins of sardine (Sardina pilchardus) by commercial proteases. Journal of the Science of Food and Agriculture. 38(3): 263-269.
Ren, J., H. Wang, M. Zhao, C. Cui, and X. Hu. 2010. Enzymatic hydrolysis of grass crass myofibrillar protein and antioxidant properties of hydrolysates. Czech Journal of Food Sciences. 28(6): 475-484.
Roslan, J., S.M. Mustapa Kamal, K.F.M. Yunos, and N. Abdullah. 2014. Optimization of enzymatic hydrolysis of tilapia muscle (Oreochromis niloticus) using response surface methodology (RSM). Sains Malaysiana. 43(11): 1715-1723.
Saidi, S., M-P. Belleville, A. Deratani, and R.B. Amar. 2013. Optimization of peptide production by enzymatic hydrolysis of tuna dark muscle by-product using commercial proteases. African Journal of Biotechnology. 12(13): 1533-1547.
Salwanee, S., W.M. Wan aida, S. Mamot, M.Y. Maskat, and S. Ibrahim. 2013. Effects of Enzyme Concentration, Temperature, pH and Time on the Degree of Hydrolysis of Protein Extract from Viscera of Tuna (Euthynnus affinis) by Using Alcalase. Sains Malaysiana, 42(3): 279-287.
Sarmadi, B. H., and A. Ismail. 2010. Antioxidative peptides from food proteins. Peptides. 31: 1949-1956.
Saunders, R.M. 1990. The properties of rice bran as a food stuff. Cereal Food World. 35: 632-636.
See, S.F., L.L. Hoo, and A.S. Babjj. 2011. Optimization of enzymatic hydrolysis of Salmon (Salmo salar) skin by Alcalase. International Food Research Journal. 18(4): 1359-1365.
Shahidi, F., X-Q. Han, and J. Synowiecki. 1995. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chemistry. 53: 285-293.
Silpradit, K., S. Tadakittasarn, H. Rimkeeree, S. Winitchai, and V. Haruthaithanasan. 2010. Optimization of rice bran protein hydrolysate production using alcalase. Asian Journal of Food and Agro-Industry. 3(2): 221-231.
Tang, C-H., X-S. Wang, and X-Q. Yang. 2009. Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chemistry. 114: 1484-1490.
Uhlig, H, and E.M. Linsmaier-Bednar. 1998. Industrial enzyme and their applications. John Wiley & Sons, Inc., New York.
Wang, M., N. S. Hettiarachchy, M. Qi, W. Burks, and T. Siebenmorgen. 1999. Preparation and Functional Properties of Rice Bran Protein Isolate. Journal of Agricultural and Food Chemistry. 47: 411-416.
Wang, W., and E. Gonzalez de Mejia. 2005. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Comprehensive Review in Food Science and Food Safety. 4: 63-78.
Wattanasiritham, L., C. Theerakulkait, S. Wickramasekara, C.S. Maier, and J.F. Stevens. 2016. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chemistry. 192: 156-162.
Wu, H. C., H. M. Chen, and C. Y. Shiau. 2003. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Research International. 36: 949-957.
Xie, Z., J. Huang, X. Xu, and Z. Jin. 2008. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chemistry. 111: 370-376.
Xiong, Y.L. 2010. Antioxidant peptides. In Mine, Y., Li-Chan, E., and Jiang, B. (ed) Bioactive proteins and peptides as functional foods and nutraceuticals. IFT Press, USA. p.29-41.
You, L., M. Zhao, C. Cui, H. Zhao, and B. Yang. 2009. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innovation Food Science and Emerging Technologies. 10: 235-240.
Zhang, H. J., J. Wang, B.H. Zhang, and H. Zhang. 2014. Antioxidant activities of the fractionated of protein hydrolysates from heat stable defatted rice bran. International Journal of Food Science and Technology. 40: 1330-1336.
Zhu, K., H. Zhou, and H. Qian. 2006. Antioxidant and free radical scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochemistry (Amsterdam, Netherlands). 41: 1296-1302.
Zhuang, H., N. Tang, S-T. Dong, B. Sun, and J-B. Liu. 2013. Optimisation of antioxidant peptide preparation from gluten meal. Journal of the Science of Food and Agriculture. 93: 3264-3270.
Warintip Sarringkarin1 and Thunnop Laokuldilok1,2*
1 Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
2 Lanna Rice Research Center, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: thunnop.l@cmu.ac.th
Total Article Views

