
Characterization of Xanthone in OSA-Black Glutinous Rice Flour Microcapsules by FTIR and XRD Methods
Paponpat Pattarathitiwat and Pilairuk IntipunyaPublished Date : 2018-10-01
DOI : https://doi.org/10.12982/CMUJNS.2018.0022
Journal Issues : Number 4 , October-December 2018
ABSTRACT
Octenyl succinic anhydride is often used to modify starch for encapsulation purposes. Xanthone and its derivatives exhibit a wide range of biological and pharmacological activities, including antimicrobial, antioxidant, and antitumor. This study investigated the effect of starch modification by octenyl succinic anhydride on xanthone encapsulation efficiency. The starch sample was added with 0.4% xanthone as core material. We analyzed the encapsulation efficiency and important characteristics of microcapsules, such as xanthone content, crystallinity of amylose, and chemical structure. Black glutinous rice flour modified by octenyl succinic anhydride showed higher encapsulation efficiency than the native black glutinous rice flour. Starch-xanthone complexation resulted in an A-type starch as identified by XRD. The FTIR pattern showed shifts in the absorbance peaks at 3,000-3,700 cm-1, which indicated that xanthone was adequately complexed in the flour structure.
Keywords: OSA-modified rice flour, Black glutinous rice, Xanthone encapsulation, FTIR, XRD
INTRODUCTION
Rice starch has increasingly been used to encapsulate edible and important substances, such as flavors, lipids, and carotenoids (Fang and Bhesh, 2010). It can act as a wall or shell material around substances to protect active ones, prolong or control their release, mask undesirable tastes, and/or improve handling. Even though native rice flours or starches are abundant and low cost, they are not efficient for some encapsulation purposes. Instead, they are usually modified by chemicals, physical treatments, enzymatic treatment, or hydrophobic modification methods to improve their encapsulating-related properties, such as viscosity, process tolerance, stability, digestion profile, and crystalline structure properties (Li, 2014). This research selected chemical modification using octenyl succinic anhydride (OSA), because this hydrophobic treatment in combination with hydrolysis creates a very active starch surface (Li, 2014). The esterified starch attracts hydrophobic molecules, such as food flavoring compounds, antimicrobial agents, and other fat-soluble compounds. As such, OSA-modified starch offers a high loading capacity of core material (Sriroth and Kuakoon, 2009).
Encapsulation of hydrophobic compounds, such as xanthone, in OSA-modified starch occurs through a process of starch-core inclusion complexation. Amylose chains form a helix around a hydrophobic core or ligand, resulting in crystalline-like structures (Song et al., 2006). The evidence of complexation into crystalline structure and chemical interactions between the starch and core compound can be analyzed by X-ray diffractometer (XRD) and Fourier transform infrared (FTIR) spectroscopy.
This study used native and OSA-modified black glutinous rice flours as wall material to encapsulate xanthone. Xanthone was selected because it has been shown to have a wide range of biological and pharmacological activities, including antimicrobial, antioxidant, and antitumor (Negi et al., 2008). Xanthone has a strong inhibition effect against Collectotrichum musae (Ungkanethiwat et al., 2010), Staphylococcus aureus (Srisuwan et al., 2009), and gram-positive bacteria (Negi et al., 2008). FTIR and XRD were employed to understand the chemical and structural changes that occurred, which could help design more effective micro encapsulation techniques and microcapsules.
MATERIALS AND METHODS
Materials
Black glutinous rice flour (Oryza sativa L. indica) was purchased from a market in Doisaket District, Chiang Mai Province, Thailand. Xanthone (Sigma-Aldrich, USA), octenyl succinic anhydride (Sigma-Aldrich, USA), and other chemicals were reagent grade and used without further purification.
Flour modification by octenyl succinic anhydride
Black glutinous rice flour (30 g) was suspended in distilled water at 35% w/w with agitation. The pH of the suspension was adjusted to 7.0 by adding 3% NaOH solution. A weighed quantity of OSA (15% v/v in absolute alcohol) was slowly added within 2 hours. After reaction, pH was adjusted to 6.5 with 3% HCl solution. The mixture was centrifuged, and washed two times with distilled water (250 ml) and two times with 70% aqueous alcohol (100 ml). The solid sample was dried at 40°C for 24 hours, followed by grinding and passing through a 180-mesh sieve (90 μm opening) (Song et al., 2006).
Encapsulation by freeze drying
The OSA-modified and native black glutinous rice flours were used as wall materials for encapsulation by inclusion complexation method. The flour sample (100 g) was dissolved in distilled water at a 1:1 ratio. Xanthone at 0.4% w/w of the dry flour sample was dissolved in 10 ml of absolute ethanol (95%) and added to the flour solution. The mixture was mixed well, followed by heating at 80°C until gel formation was completed. The sample was cooled, frozen, and freeze dried using a drying chamber (at -20°C) and a cooling unit (at -50°C) under vacuum pressure of 10-2 mbar (Marefati et al., 2015). Drying was carried out for eight hours. The freeze dried samples were ground and kept in a sealed laminated aluminum bag and stored at -18°C until further use.
Xanthone content analysis by HPLC method
A high-performance liquid chromatograph (Shimazu, Japan) equipped with an UV-detector set at 319 nm wavelength was used for the analysis of xanthone content (Thongthammachat and Jarudeelokgoon, 2011). The chromatographic separation was performed at room temperature on an HPLC column ZORBAX, Rx-C18, 4.6 x 250 mm, analytical column (Algilent Technologies, USA). The sample injection volume of 20 μl was analyzed using a mobile phase flow rate of 1 ml/min.
The standard stock solution of xanthone (internal standard) was prepared by dissolving accurately weighed xanthone, with methanol added to make a final volume of 1.0 mg/ml. The xanthone stock solution was sequentially diluted with methanol (95% purity) to prepare a series of working standard solutions in the concentration range of 0.1-0.8%. All solutions were stored at 4°C and brought to room temperature before use. From the recorded peak areas in chromatograms of each standard solution, the ratios of xanthone to internal standard were calculated and plotted against xanthone concentrations. The calibration curve was constructed from triplicate analysis of the standard solutions. The linearity was evaluated by least square regression.
Determination of xanthone in microcapsules by HPLC method
Surface xanthone content analysis. The microcapsule samples were accurately weighed into 10.0 ml volumetric flask and adjusted to the mark with methanol (95% purity), then rigorously mixed by vortex 10 times. The solution was filtered through a 0.45 μm nylon syringe filter. The chromatographic analysis was performed at room temperature by injecting 20 μl and mobile phase flow rate of 1 ml/min. The eluent was monitored at 319 nm. The mobile phase consisted of methanol:water (95:5% v/v). The surface xanthone content was calculated from peak area as compared to the standard calibration curve. All samples were analyzed in duplicate.
Encapsulated xanthone content analysis. The microcapsule sample obtained after surface extraction was put in a 10.0 ml volumetric flask and methanol (95% purity) was added to the marked volume. The flask was sealed with paraffin film and stored for one night at room temperature. The solution was filtered through a 0.45 μm nylon syringe filter. Chromatographic
analysis was performed as carried out for surface xanthone content analysis. All samples were analyzed in duplicate.
Encapsulation efficiency (EF). The encapsulation efficiency was calculated using xanthone content following the equation of Thongthammachat and Jarudeelokgoon (2011) as follows:

Determination of crystallinity of microcapsules
Microcapsule samples were subjected to crystallinity analysis using X-ray diffractometer (XRD). The samples were scanned with Cu-Kα radiation at 35 kV and 20 mA. The intensity was recorded at the rate 3°/min between 5° to 60° (2θ) (Haque and Roos, 2005).
Analysis of chemical structure
The change in chemical structure of the starch was quantitatively analyzed using a Fourier transform infrared (FTIR) spectroscope. Infrared light absorbance of each sample was measured over the wavelengths between 400 to 4,000 cm-1.
Analysis of physical structure
The morphology of flour was examined using a scanning electron microscope (SEM, JEOL JSM-5910LV, Japan) operated at 15 kV accelerating voltage. Then, samples were fixed on the SEM stub, which were subsequently coated with gold to provide a reflective surface for the electron beam. The gold-coated samples were then viewed under the microscope.
RESULTS
Xanthone content in microcapsules
The calibration curve for the xanthone content was linear over the concentration range from 0.01% to 0.10%. The linear regression equation of the calibration curve was Y = 3E+08X, with R2 of 0.9979. The linear calibration curve for xanthone is shown in Figure 1.
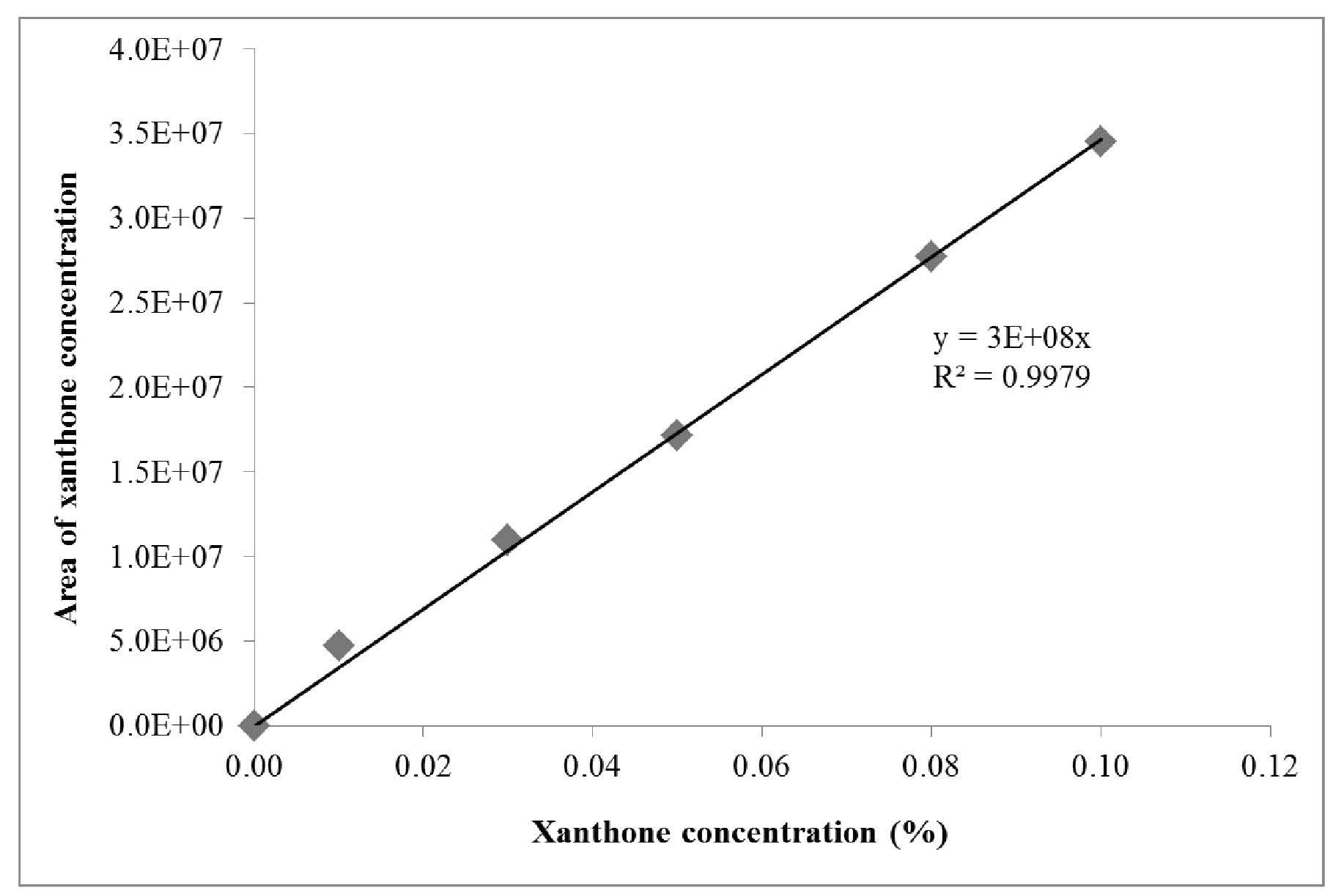
Figure 1. Calibration curve of xanthone standard with concentration ranging from 0.01% to 0.10%.
The microcapsule with OSA-modified black rice flour as wall material showed higher xanthone content than that with native black rice flour (Table 1). With the same amount of xanthone added to the flours (0.4%), the encapsulation performance of the native and OSA-modified black glutinous rice flours differed. The native rice flour sample contained significantly higher surface xanthone content (P ≤ 0.05), but less encapsulated xanthone content (P ≤ 0.05) than the OSA-modified rice flour; as a result, the native rice flour had significantly lower encapsulation efficiency (P ≤ 0.05). The encapsulation efficiency of xanthone in OSA-modified black glutinous rice flour and native rice flour was 25.00 and 23.75%, respectively. The encapsulation efficiency was calculated from the encapsulated xanthone content. The surface xanthone content was considered non-encapsulated; it is susceptible to undesirable deterioration due to direct exposure to the surrounding environment.
Table 1. Xathone content and encapsulation efficiency.
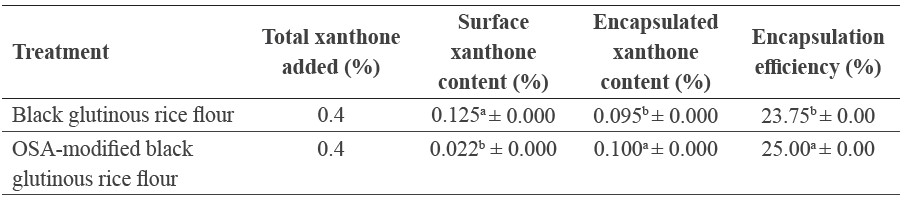
Note: Data presented in the table are mean ± SD from duplicate measurements. Different superscripts in the same column indicate statistical difference among mean values at the 95% confidence level (P ≤ 0.05).
Crystallinity of amylose in microcapsules by XRD
The XRD patterns were used to examine the crystalline structure of the microcapsules of xanthone in rice flours. The results were related to the encapsulation efficiency. Native black glutinous rice flour and OSA-modified black glutinous rice flour showed peak intensity at 2θ around 15°, 17°, and 23° (Figures 2 and 3). Esterification using OSA did not change the crystalline pattern of the black glutinous rice flour. In both Figures 2 and 3, the intensity decreased at 2θ around 15°, 17°, 18°, 20°, and 23° for the microcapsule samples as compared to XRD patterns of the wall materials. The peak intensity of the xanthone microcapsules in native flour was lower than that of the microcapsules in OSA-modified flour (Figure 4). This could mean that microcapsule in the native flour existed mostly in an amorphous state, with less helical complexation. A better xanthone inclusion complexation with the amylose in OSA-modified rice flour occurred due to substitution of the hydroxyl groups in the starch by the ester group in the OSA. The crystallinity is shown in Table 2.
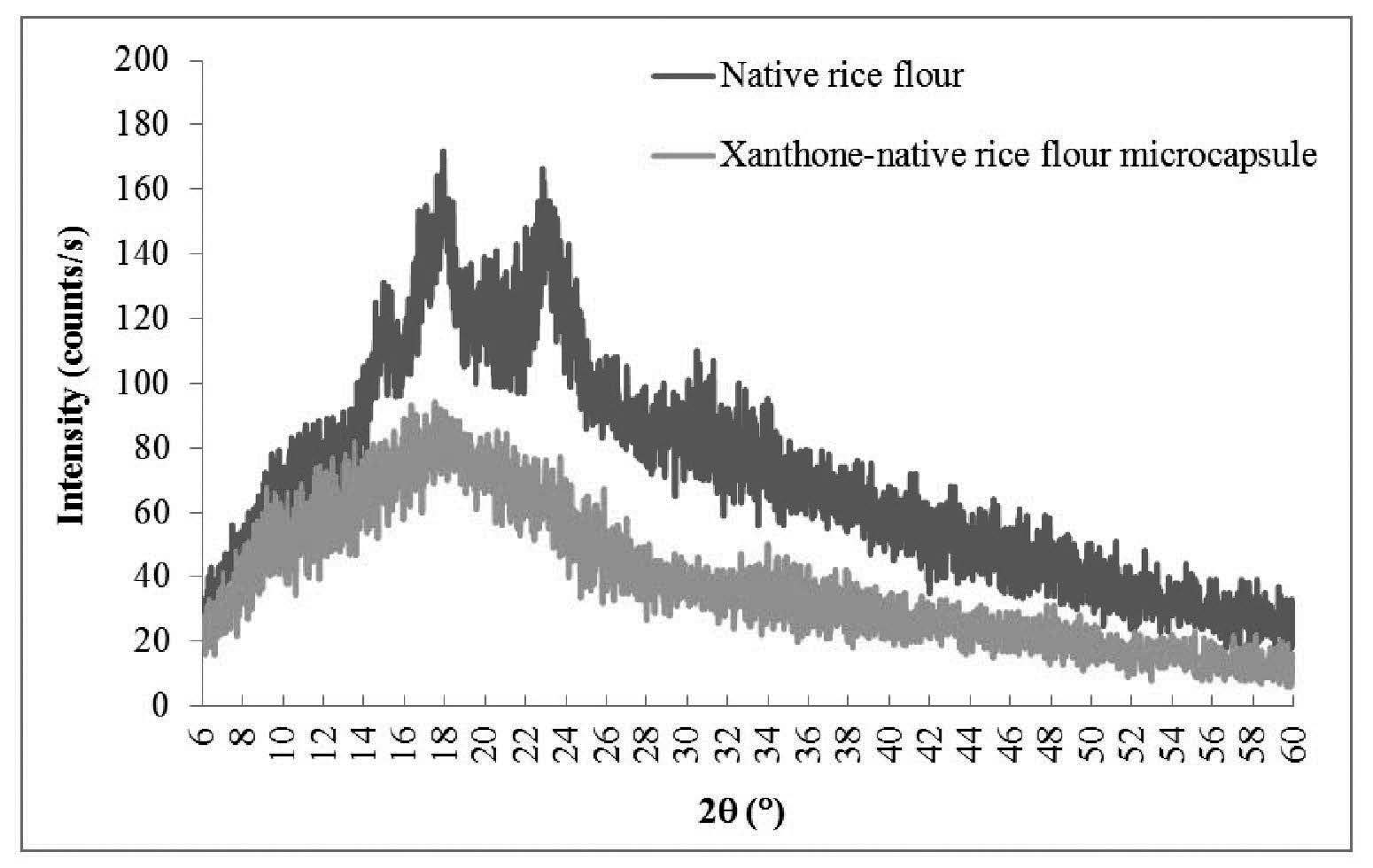
Figure 2. XRD patterns of native black glutinous rice flour and xanthone microcapsules in native black glutinous rice flour.
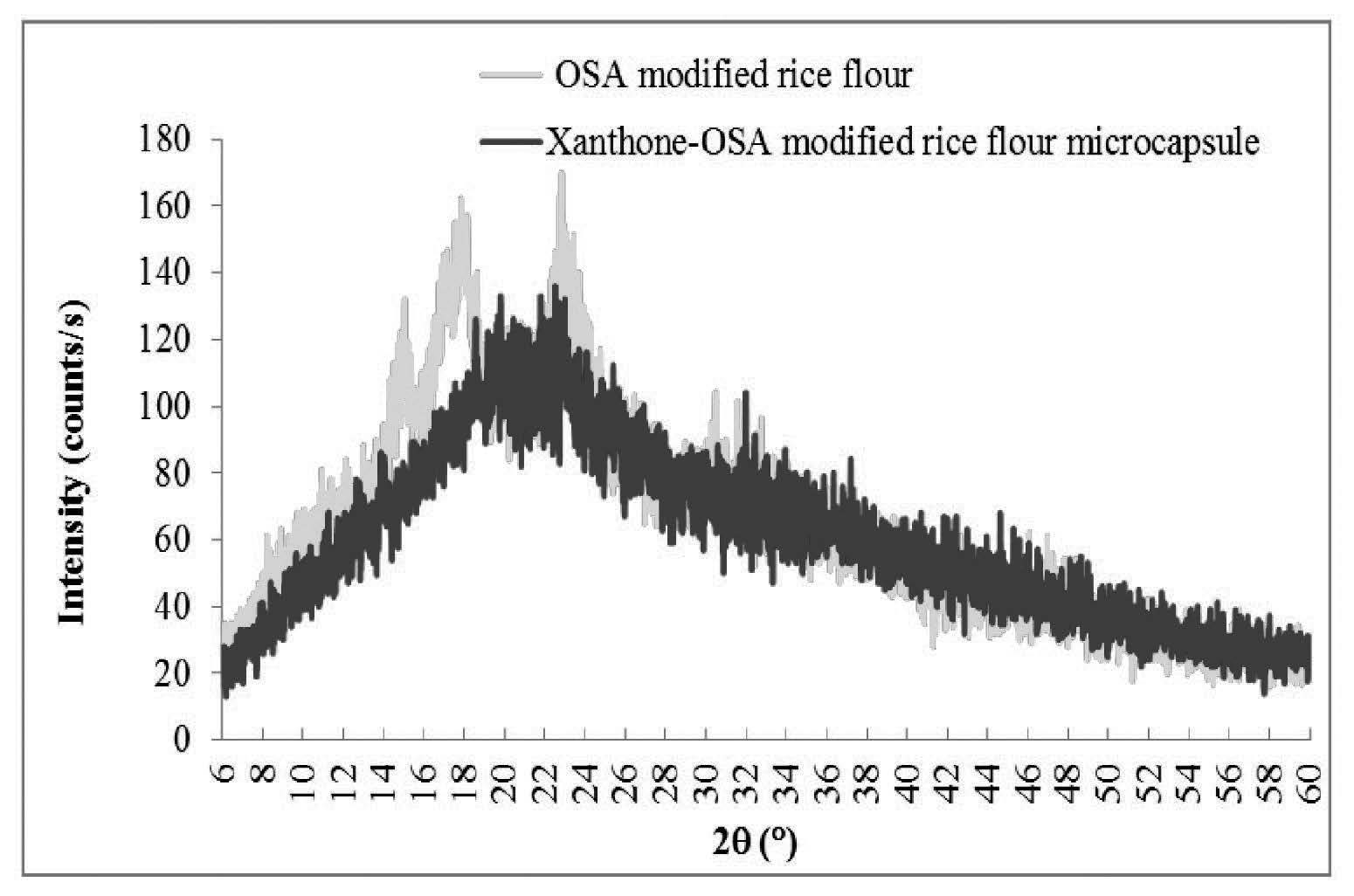
Figure 3. XRD patterns of OSA-modified black glutinous rice flour and xanthone microcapsules in OSA-modified black glutinous rice flour.
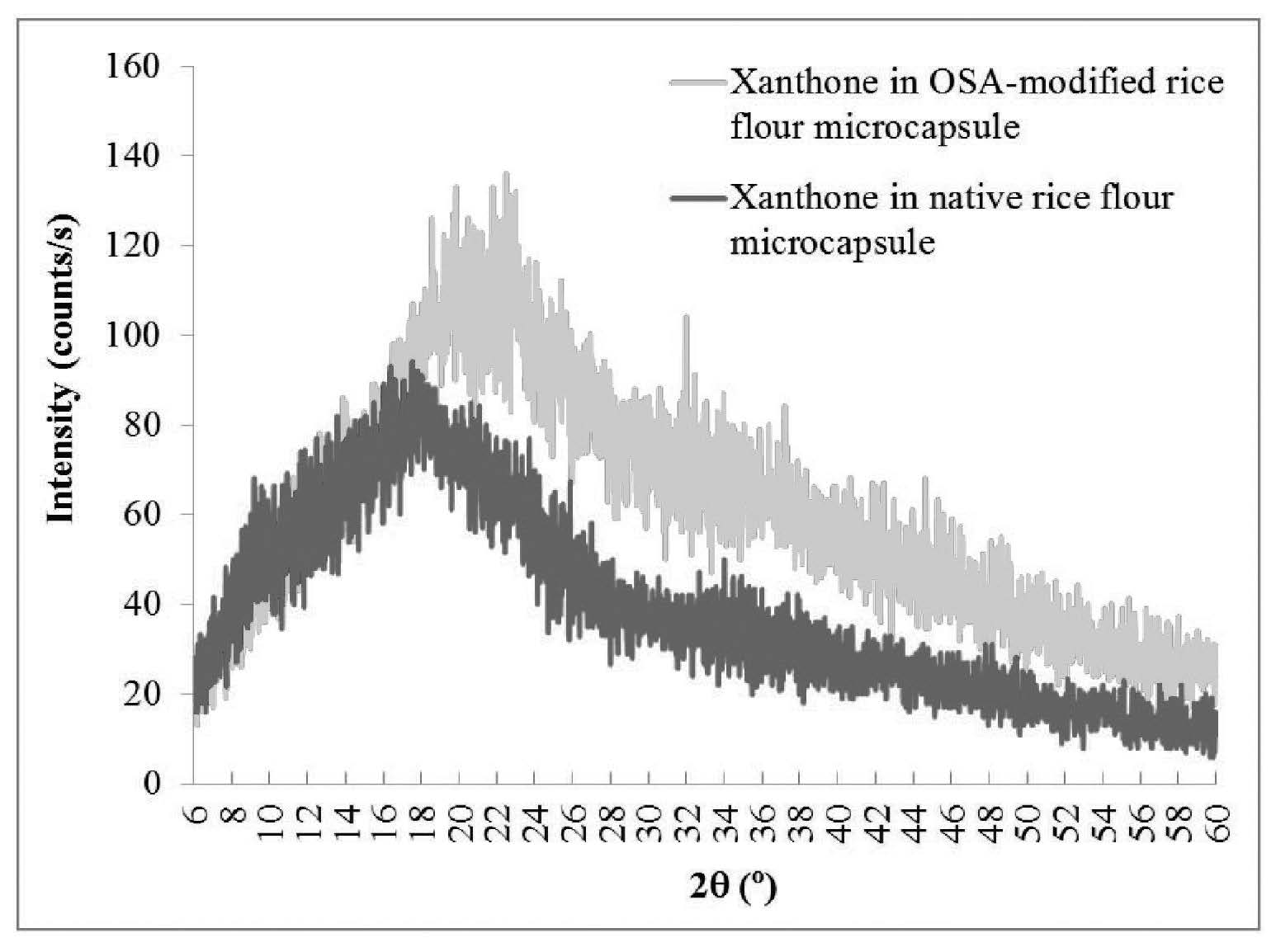
Figure 4. Comparison of XRD patterns of microcapsules of xanthone in OSA-modified black glutinous rice flour and in native rice flour.
Table 2. Crystallinity.

Chemical structure obtained by FTIR
FTIR data showed differences in chemical conformation of rice flours and microcapsule samples (Figures 5 and 6). The FTIR pattern showed peaks at wavelengths around 860, 930, 1,015, 1,078, 1,150, 1,250, 1,540, 1,650, 2,850, 2,930, and 3,300 cm-1. Peak absorbance occurred at a wavelength of 1,750 cm-1 for the native rice flour, but shifted to a wavelength of 1,700 cm-1 for the OSA-modified rice flour (Figure 5). The FTIR pattern of OSA-modified black glutinous rice flour showed a higher peak absorbance at a wavelength of 1,000 cm-1 compared to the native rice flour. These results showed that the ester groups of OSA replaced the hydroxyl groups in the starch molecules.
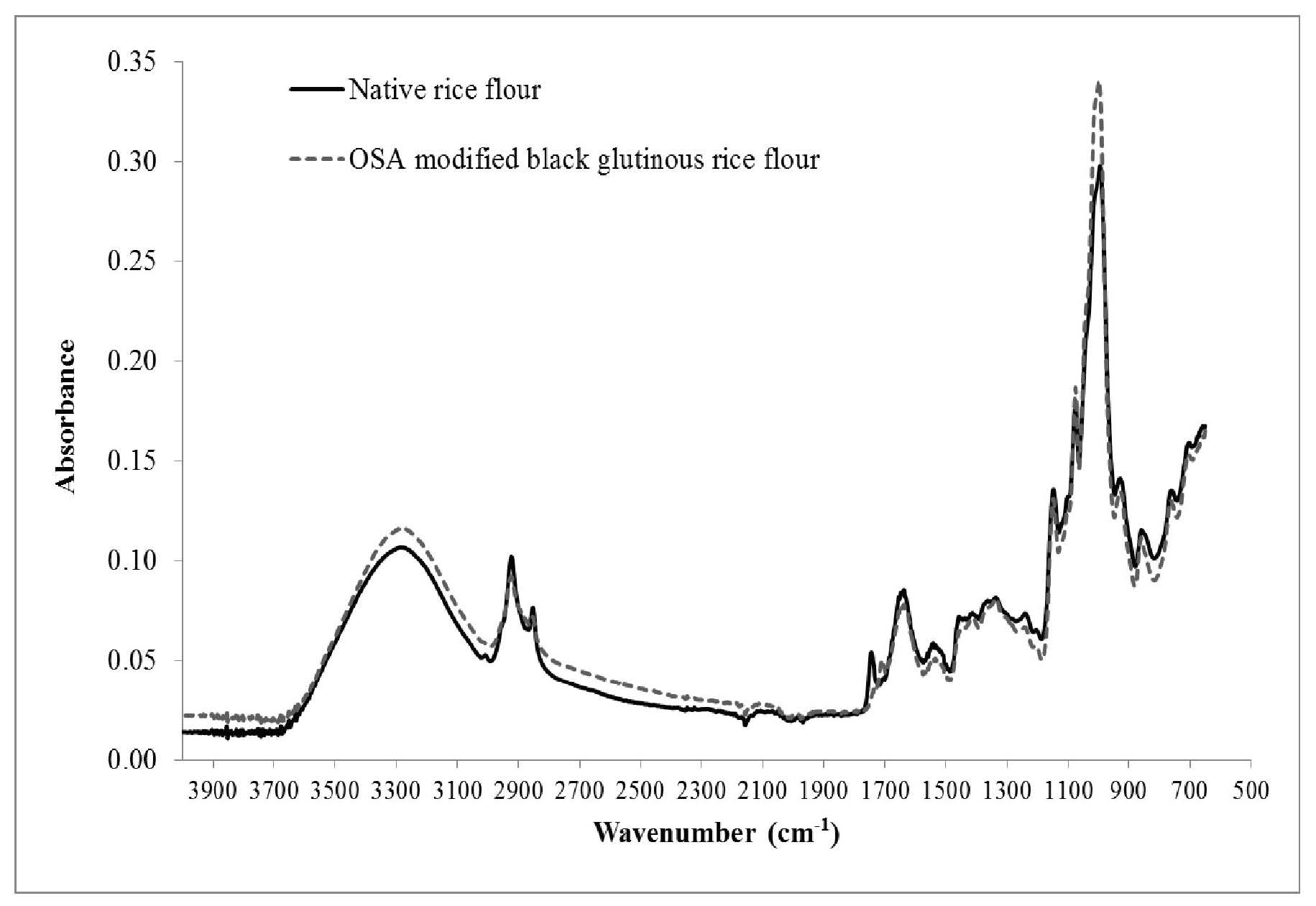
Figure 5. FTIR pattern of native and OSA-modified black glutinous rice flours.
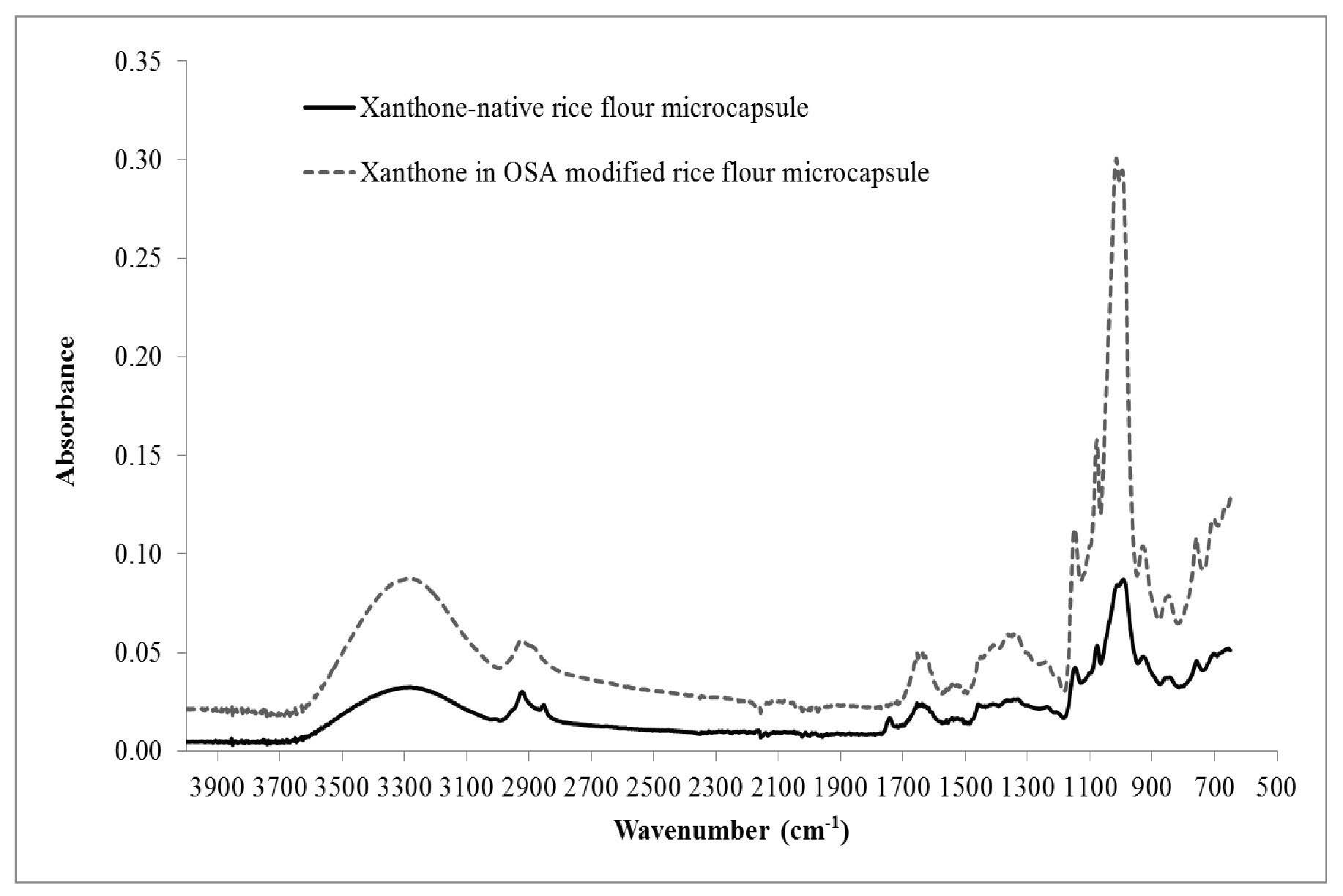
Figure 6. FTIR patterns of xanthone microcapsules in native and OSA-modified rice flours.
Figure 6 shows FTIR patterns of xanthone microcapsules encapsulated by native and OSA-modified rice flours. When the OSA-modified rice flour was used as wall material for encapsulation, higher absorbance at wavelengths around 930, 1,000, 1,150, 1,250, 1,650, and 3,300 cm-1 was detected, as compared to the pattern for microcapsules in native flour. Microcapsules of xanthone in OSA-modified rice flour did not show peak absorbance at a wavelength of 1,700 cm-1, while microcapsule in native flour showed peak absorbance at 1,750 cm-1.
Physical structure obtained by SEM
The SEM micrographs highlighted the effects of freeze drying on surface morphology of microcapsule particles (Figure 7). Freeze-dried powders exhibited slight variations in their surface morphology due to the properties of the encapsulating material. Many studies have reported a similar morphology for freeze dried microcapsules (Anandharamakrishnan et al., 2010). All freeze-dried powders produced with different modified rice flour showed a similar particle shape. Many cracks occurred on the particle surface of most freeze-dried powders, which may affect xanthone stability during storage because of oxygen permeability. SEM did not show residual xanthone on the surface of the encapsulated sample; this may due to the xanthone being included in the rice flour.
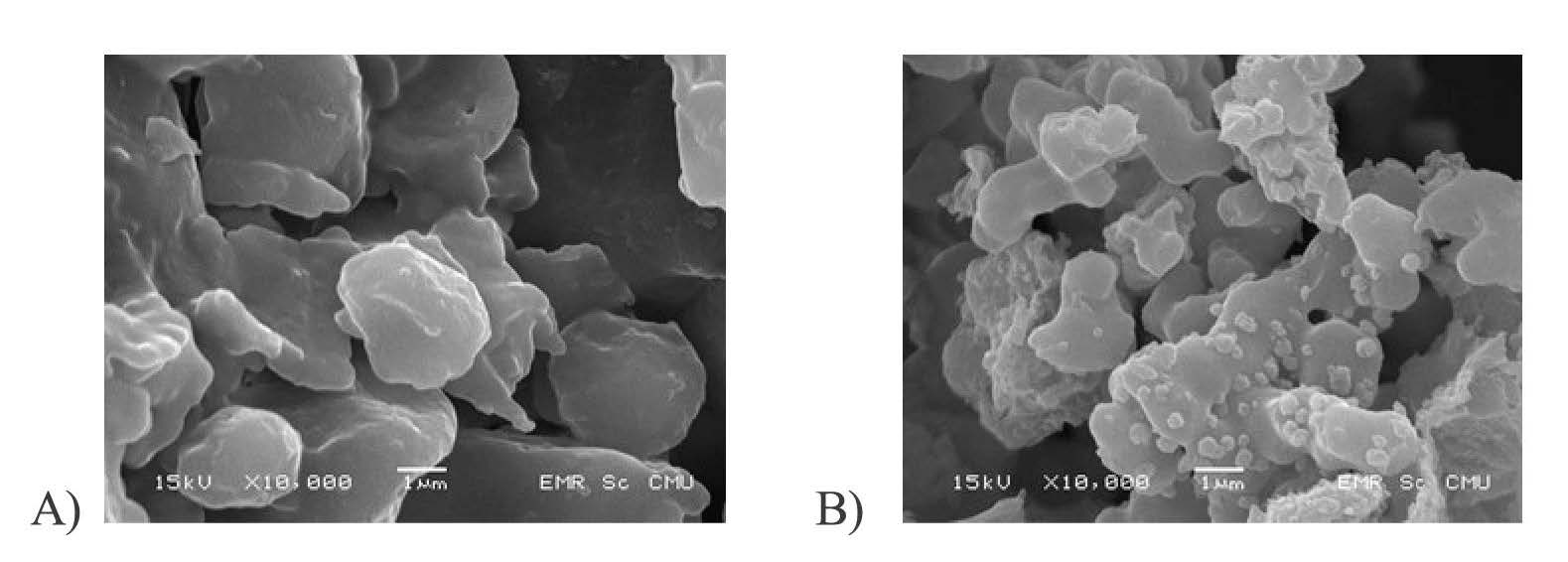
Figure 7. SEM of xanthone microcapsules in A) native and B) OSA-modified rice flours.
DISCUSSION
Encapsulation efficiency of xanthone was calculated as the percentage of encapsulated xanthone content per total xanthone added. The surface xanthone content is considered nonencapsulated; this is susceptible to deterioration and undesirable reactions (Tonon et al., 2012). Higher encapsulation efficiency was obtained when OSA-modified glutinous rice flour was used as the wall material for encapsulation, because the flour contained an added ester group at the –OH site that assisted entrapment of core material by hydrophobic force (Song et al., 2006). Such hydrophobic force may enable effective inclusion complexation of xanthone in the amylose component of the rice flour (Song et al., 2006; Li, 2014). Retention and release of encapsulated compounds in food carbohydrates depend on the physicochemical properties of compounds and type of carbohydrates. High hydrophobicity and the presence of an alcohol group will increase the retention of the compounds (Okano et al., 2001; Wulff et al., 2005; Blaszczak et al., 2007). Because of this, native black glutinous rice flour, which is less hydrophobic, showed higher surface content of xanthone than OSA-modified rice flour.
Starches can be divided into two types: crystalline (type A) and non-crystalline (type B). Crystalline starches bind compounds less than non-crystalline types. Crystalline starches have low encapsulation efficiency because their starch granules are packed in a monoclinic unit cell. In contrast, non-crystalline starches are polymorphic with a more open structure (Naknean and Meenune, 2010). Black glutinous rice flour is classified as A-type polymorphic; which was confirmed by the XRD data (Figure 3). The A-type starch has a peak intensity at 2θ of 15°, 17°, and 23° (Song et al., 2006). We also found these intensity peaks for both native and modified glutinous rice flours, which agreed with Song et al. (2006) and Wang and Wang (2002), who found that OSA did not change the crystalline pattern of starch. However, we found that peak intensity decreased after chemical modification. This could be because the crystalline structure transformed into an amorphous structure. The heat treatment required to modify rice flour melted the crystalline region and transformed it into rubbery state. Upon cooling, the rubbery state transformed into an amorphous, dry state. This structural transformation significantly changed the XRD patterns (Dufresne, 2014). Therefore, the OSA-modified black glutinous rice flour had a more amorphous structure than the native black glutinous rice flour, although it retained its A-type (crystalline) structure. The encapsulated samples also showed less intensity at 2θ of 15°, 17°, 18°, 20°, and 23°, as compared to the non-encapsulated rice flour. From Figure 4, microcapsules of native rice flour were more amorphous than the sample with modified flour; this indicated less inclusion complex formation. Because OSA-modified flour is more hydrophobic, it can form more inclusion complex with xanthone, hence showing high intensity at specific 2θ. However, the native rice and OSA-modified flour structures displayed small amounts of a V-pattern, as shown by the peaks at 2θ of 12.9° and 19.8°. The encapsulated sample did not have this V-pattern. Therefore, it can be suggested that xanthone inclusion complexation either did not form a V-pattern structure or it formed in insufficient quantity to be detected by the analytic method (Luo et al., 2016).
From the FTIR patterns, a peak at wavelengths between 1,015-1,160 cm-1 indicated the presence of C-O and C-O-C bonds in polysaccharides (Simsek et al., 2015). A peak around 1,510 cm-1 represented C=C aromatic rings of polyphenols (Lazano et al., 2015). A peak at wavelengths between 1,078 cm-1 and 1,149 cm-1 indicated the presence of α-1,4 glycosidic bonds (Falade and Christopher, 2015); a peak at 1,270 cm-1 indicated C-O bonds of polyphenol aromatic rings. Absorption at wavelengths of 1,640 cm-1 and 2,930 cm-1 represented the existence of bound water and C-H2 bonds in the sample (Zhang et al., 2011; Joshi et al., 2013). The peak found at a wavelength between 3,000-3,700 cm-1 indicated an interaction that could reflect the presence of either a complex from intermolecular hydrogen bonding (Yu and Huang, 2010) or O-H bond stretching vibration (Li et al., 2016). As shown in Figures 5 and 6, the peak absorbance of the native flour samples were lower than that of the OSA-modified samples due to less complexation. In our experiment, modifying flour with OSA resulted in ester groups replacing the hydroxyl groups. Therefore, we detected carbonyl groups at wavelengths of 1,150, 1,080, 1,000, and 930 cm-1. Li et al. (2013) also reported that OSA-modified starch
showed carbonyl (C=O) stretching vibration of ester group at wavelengths around 1,725 cm-1. From Figure 4, new absorption at about 1,700 cm-1 was shown; this indicated that the OSA
had been successfully incorporated into the starch structure. In the microcapsules of xanthone in the OSA-modified flour, the absorption at about 1,750 cm-1 disappeared, but absorption at 1,700 cm-1 increased, perhaps due to complexation of xanthone in an amylose helix at the carbonyl of the ester group.
CONCLUSION
Modification of black glutinous rice flour with octenyl succinic anhydride improved the encapsulation efficiency of xanthone. Hydrophobicity arising from replacement of the hydroxyl group in the flour by the ester group in OSA enabled better xanthone-amylose inclusion complexation. FTIR patterns confirmed the inclusion complexation of xanthone with amylose. XRD patterns showed that the black glutinous rice flour was an A-type (crystalline) starch with its crystallinity shown at specific 2θ. OSA modification of black rice flour caused the starch’s crystalline structure to partially transform into an amorphous structure, which facilitated the xanthone-starch complexation. Because rice flour is readily available and inexpensive, its modification for encapsulation purposes offers promise in food and other industries.
ACKNOWLEDGEMENTS
This research was funded by the National Research Council (NRC) of Thailand in 2012. Appreciation is extended to Chiang Mai University’s Faculty of Agro-Industry; Lanna Rice Research Center; and Science and Technology Service Center, Faculty of Science, for the support they provided with research facilities and analytical instruments.
REFERENCES
Anandharamakrishnan C., Rielly, C.D., and Stapley, A.G.F. 2010. Spray-freeze-drying of whey proteins at sub-atmospheric pressures. Dairy Science and Technology. 90: 321-334. https://doi.org/10.1051/dst/2010013
Blaszczak, W., Tamara, A.M., Vladimir, P.Y., and Josef, F. 2007. Effect of high pressure on binding aroma compounds by maize starches with different amylose content. Lebensmittel-Wissenschaft und–Technologie. 40: 1841–1848. https://doi.org/10.1016/j.lwt.2006.11.002
Dufresne A. 2014. Crystalline starch based nanoparticles. Current Opinion in Colloid and Interface Science. 19(5): 397-408. https://doi.org/10.1016/j.cocis.2014.06.001
Falade K.O., and Christopher, A.S. 2015. Physical, functional, pasting and thermal properties of flours and starches of six Nigerian rice cultivars. Food Hydrocolloids. 44: 478-490. https://doi.org/10.1016/j.foodhyd.2014.10.005
Fang, Z., and Bhesh, B.R. 2010. Encapsulation of polyphenols-a review. Trends in Food Science and Technology. 21(10): 510-523. https://doi.org/10.1016/j.tifs.2010.08.003
Haque, M.K., and Roos, Y.H. 2005. Crystallization and X-ray diffraction of spray-dried and freeze-dried amorphous lactose. Carbohydrate Research. 340(2): 293–301. https://doi.org/ 10.1016/j.carres.2004.11.026
Joshi, M., Aldred, P., McKnight, S., Panozzo, J.F., Kasapis, S., Adhikari, R., and Adhikari, B. 2013. Physicochemical and functional characteristic of lentil starch. Carbohydrate Polymers. 92(2): 1484-1496. https://doi.org/10.1016/j.carbpol.2012.10.035
Lazano-Vazquez, G., Lobato-Calleros, C., Escalona-Buendia, H., Chavez, G., Alvarez-Ramirez, J., and Vernon-Carter, E.J. 2015. Effect of the weight ratio of alginate-modified tapioca starch on the physicochemical properties and release kinetics of chlorogenic acid containing beads. Food Hydrocolloids. 48: 301-311. https://doi.org/10.1016/j.foodhyd.2015.02.032
Li C., Fu, X., Luo, F., and Huang, Q. 2013. Effects of maltose on stability and rheological properties of orange oil-in-water emulsion formed by OSA-modified starch. Food Hydrocolloids. 32: 79-86. https://doi.org/10.1016/j.foodhyd.2012.11.031
Li, D., Zhang, Z., and Tian, Y. 2016. Ionic liquids as novel solvents for biosynthesis of octenyl succinic anhydride-modified waxy maize starch. International Journal of Biological Macromolecules. 86(5): 119-125. https://doi.org/10.1016/j.ijbiomac.2016.01.050
Li J.Z. 2014. The Use of Starch-Based Materials for Microencapsulation. In: Gaonkar A.G., Vasisht N., Khare A.R., and Sobel R. (eds). Microencapsulation in the Food Industry. Academic Press, Amsterdam. p. 195-210.
Luo, Z., Zou, J., Chen, H., Cheng, W, Fu, X., and Xiao, Z. 2016. Synthesis and characterization of amylose-zinc inclusion comlexs. Carbohydrate Polymers. 137: 314-320. https://doi.org/ 10.1016/j.carbpol.2015.10.100
Marefati A., Sjoo, M., Timgren, M., Dejmek, P., and Rayner, M. 2015. Fabrication of encapsulated oil powders from starch granule stabilized W/O/W Pickering emulsions by freeze-drying. Food Hydrocolloids. 51: 261-271. https://doi.org/10.1016/j.foodhyd.2015.04.022
Naknean, P., and Meenune, M. 2010. Factors affecting retention and release of flavour compounds in food carbohydrates. International Food Research Journal. 17: 23-34.
Negi, P.S., Jayaprakasha, G.K., and Jena, B.S. 2008. Antibacterial activity of the extracts from the fruit rinds of Garcinia cowa and Garcinia pedunculata against food borne pathogens and spoilage bacteria. Lebensmittel-Wissenschaft und – Technologie. 41(10): 1857-1861. https://doi.org/10.1016/j.lwt.2008.02.009
Okano, L.T., Barros, T.C., Chou, D.T.H., Bennet, A.J., and Bohne, C. 2001. Complexation dynamics of xanthone and thioxanthone to ß-cyclodextrin derivatives. Journal of Physical Chemistry. 105(11): 2122-2128. https://doi.org/10.1021/jp002622c
Simsek, S., Ovando-Martinez, M., Marefati, A., Sjӧӧ, M., and Rayner, M. 2015. Chemical composition, digestibility and emulsification properties of octenyl succinic esters of various starches. Food Research International. 75: 41-49. https://doi.org/10.1016/j.foodres.2015.05.034
Song, X.Y., He, G.Q., Ruan, H., and Chen, Q.H. 2006. Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch/Starke. 58(2): 109-117. https://doi.org/10.1002/star.200500444
Sriroth, K., and Kuakoon, P. 2009. Flour technology. Kasetsart University Press, Bangkok. Srisuwan, T., Chatchawan, T., Chatchai, W., and Chomchan, A. 2009. An antibacterial plastic film made from the mangosteen peel extract. Retrieved from www.science.sut.ac.th/gradbio/stupresent/antibacterial_mangosteen.pdf
Tonon, R.V., Pedro, R.B., Grosso, C.R.F., and Hubinger, M.D. 2012. Microencapsulation of flaxseed oil by spray drying: Effect of oil Load and type of wall material. Drying Technology. 30(13): 1491-1501. https://doi.org/10.1080/07373937.2012.696227
Thongthammachat, A., and Jarudeelokgoon, S. 2011. Study of protein release from chitosan nanocapsule. Retrieved from www.intania.com/upload/NA06.pdf
Ungkanethiwat, K., Veeraboon, K., and Suphat, K. 2010. Useful of mangosteen extract for development of film anti Antract nose. Retrieved from www.agro.cmu.ac.th.
Wang Y.J., and Wang, L.F. 2002. Characterization of acetylated waxy maize starched prepared under catalysis by different alkali and alkaline-earth hydroxides. Starch/Starke. 54: 25-30. https://doi.org/10.1002/1521-379X(200201)54:1<25::AID-STAR25>3.0.CO;2-T
Wulff, G., Avgenaki, A., and Guzmann, M.S.P. 2005. Molecular encapsulation of flavors as helical inclusion complexes of amylase. Journal of Cereal Science. 41(3): 239-249. https://doi.org/ 10.1016/j.jcs.2004.06.002
Yu, H., and Huang, Q. 2010. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chemistry. 119(2): 669-674. https://doi.org/ 10.1016/j.foodchem.2009.07.018
Zhang, B., Huang, Q., Luo, F.X., Fu, X., Jiang, H.X., and Jane, J.L. 2011. Effects of octenylsuccinylation on the structure and properties of high-amylose maize starch. Carbohydrate Polymers. 84: 1276-1281. https://doi.org/10.1016/j.carbpol.2011.01.020
Paponpat Pattarathitiwat1 and Pilairuk Intipunya
1 Division of Food Science and Technology, Faculty of Agro-Industry, Chiang Mai University 50100, Thailand
2 Lanna Rice Research Center, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: pilairuk.intipunya@cmu.ac.th
Total Article Views
Article History:
Received: December 26, 2017
Revised: February 3, 2018
Accepted: March 6, 2018

