
Detection of a Non-animal Source of Glycosaminoglycans from Edible Mushrooms in Northern Thailand
Kanyamas Choocheep* and Nantawadee NathipPublished Date : 2018-07-01
DOI : https://doi.org/10.12982/CMUJNS.2018.0015
Journal Issues : Number 3 , July-September 2018
ABSTRACT
Several kinds of cultivated or local edible mushrooms exist in northern Thailand. While their nutritional features have been extensively analyzed, the presence of phytochemicals and glycosaminoglycans has yet to be investigated. Thus, in this study, we examined the presence of phytochemicals and glycosaminoglycans from 10 cultivated or local mushrooms: milk white russula, hygroscopic earthstar, log white fungi, log black fungi, shitake mushroom, rosy russula, sajor-caju mushroom, Jew's ear, bolete, and straw mushroom. Phytochemical analysis revealed the presence of carbohydrates, amino acids, and flavonoids in the extracts from most of the mushrooms. We detected glycosaminoglycans in all of the extracts using dimethylmethylene blue (DMMB) dye-binding assay and UVVis spectrophotometry. Hygroscopic earthstar contained the most glycosaminoglycans and sajor-caju mushroom the least. However, we were unable to distinguish the types of glycosaminoglycans, which should be considered for future study. In summary, the phytochemicals found here are crucial non-animal dietary sources of carbohydrates, amino acids, and antioxidants. In addition, the glycosaminoglycans detected in the mushroom extracts in this study potentially offer a non-animal source of glycosaminoglycans that may have clinical applications, apart from their nutritive value.
Keywords: Mushrooms, Phytochemicals, Glycosaminoglycans, Dye-binding assay, Spectrophotometry
INTRODUCTION
Mushrooms are classified in the kingdom of fungi (Whittaker, 1969). Some edible species have been recognized for their health benefits (Flegg and Maw, 1997). Mushrooms have high nutritional value (Mattila et al., 2001; Barros et al., 2008; Reis et al., 2012; Carneiro et al., 2013); they are low-fat, high in protein, and contain vitamins, minerals, and trace element constituents with nutraceutical composition. Of the nutraceuticals, homopolysaccharides are the best known molecules; they play an essential role as an immunomodulator (Manzi and Pizzoferrato, 2000; Mayell, 2001; Vetvicka and Yvin, 2004), as well as exhibit anticancer, antibacterial, antioxidant, and anti-inflammatory properties (Yu et al., 2009; Zhang et al., 2011; Chang and Wasser, 2012; Finimundy et al., 2013). Therefore, mushrooms are not only a component of a balanced diet, but also provide key medicinal uses in the treatment of certain diseases.
Besides homopolysaccharides, most carbohydrates that have been found in mushrooms are monosaccharides, disaccharides, or sugar derivatives, such as, glucose, fructose, sucrose, inositol, mannitol, and sorbitol (Sanmee et al., 2003; Reis et al., 2012; Carneiro et al., 2013). However, glycosaminoglycans (GAGs), another type of polysaccharide, have yet to be
investigated in mushrooms. GAGs are unbranched heteropolysaccharides containing a repeating disaccharide unit. They are composed of sugar acid and amino sugar; usually, one sugar is an uronic acid and the other is either N-acetylglucosamine (GlcNAc) or N-acetylgalactosamine (GalNAc). Various kinds of GAGs, including chondroitin sulfate, dermatan sulfate, heparin, heparan sulfate, and keratan sulfate (Kjellén and Lindahl, 1991; Varki et al., 2009) are bound to sulfate. Thus, the negative charge in various positions along the GAG chains is due to the sugar acid residues and/or sulfate groups (Prydz and Dalen, 2000). GAGs have been known to play many roles in both physiological and pathological conditions or diseases, including signaling activity during chondrocyte differentiation (Choocheep et al., 2010), angiogenesis, anti-coagulation, axonal growth, wound healing, pulmonary and vascular diseases, and tumor progression (Gandhi and Mancera, 2008). Additionally, chondroitin sulfate and hyaluronic acid have been reported to suppress cartilage degradation for the treatment of osteoarthritis (Monfort et al., 2005). Heparin also has important roles as an anti-coagulant for the treatment of thrombosis, thrombophlebitis and embolism (Gandhi and Mancera, 2008). Other GAGs, such as heparan sulfate, can act as receptors for proteases and protease inhibitors, or cooperate with integrins and other cell adhesion receptors to facilitate cell activities (Sarrazin et al., 2011), and keratan sulfate plays an essential role in visual functions (Pomin, 2015).
Mushrooms are a traditional ingredient in Thai cuisine. In addition, several kinds of cultivated or local edible mushrooms exist in northern Thailand, such as Astraeus hygrometricus, Lentinus polychrous, Phaeogyroporus portentosus, and Russula species (Sanmee et al., 2003; Valverde et al., 2015). Although the nutritive value of these mushrooms has been investigated, the presence of phytochemicals and GAGs has not been examined. Hence, the aim of this study was to examine the presence of phytochemicals and GAGs from 10 cultivated or local edible mushrooms.
MATERIALS AND METHODS
Collection and preparation of mushroom extracts
The mushrooms were bought from markets in Wiang Pa Pao district, Chiang Rai province, or Mueang district, Chiang Mai province, both in northern Thailand. Ten fresh fruiting bodies of cultivated or local edible mushrooms – milk white russula, hygroscopic earthstar, log white fungi, log black fungi, shitake mushroom, rosy russula, sajor-caju mushroom, Jew's ear, bolete, and straw mushroom – were weighed, cut into small pieces, and ground by grinding machine. Then, 100 grams of each mushroom sample was extracted as previously described (Nakano et al., 2000), with some modifications. Briefly, the mushrooms were soaked in a water bath of deionized water of pH 4.5 at 37°C for 18 hours. Then, the extracts were boiled for 10 minutes, filtered through a muslin cloth and Whatman filter papers, and centrifuged. Next, the supernatant of the extracts was collected, and the same extracts were pooled together. These crude mushroom extracts were then freeze-dried to yield a powder, and stored at -20°C until use.
Yield of mushroom extracts powder
The yield of each mushroom extract was calculated as follows: yield (%) = (weight of fresh mushrooms/weight of mushrooms powder) x 100.
Phytochemical screening
Qualitative phytochemical analysis (Tiwari et al., 2011; Yadav et al., 2011; Vennila et al., 2012) was used to estimate the presence of carbohydrates, proteins, phenols, tannins, and flavonoids. About 200 mg of each mushroom extract powder was dissolved in 20 ml of deionized water and subjected to the phytochemical tests.
Detection of carbohydrates.
• Molisch’s test. Two ml of each mushroom extract sample was added by two drops of 10% (w/v) α-naphthol in 95% ethanol and mixed well. Then, 1 ml of concentrated H2SO4 was slowly added along the sides of the test tubes. The formation of a purple ring at the junction of the mixture indicated the presence of carbohydrates.
• Fehling’s test. Two ml of each mushroom extract sample was added by 1 ml of both Fehling’s solution A and B. Then, the mixtures were boiled for two minutes. The formation of a red precipitate in the mixtures indicated the presence of sugars.
Detection of proteins and amino acids.
• Biuret’s test. Two ml of each mushroom extract sample was added by 0.2 ml of both 1 M NaOH and biuret reagent. The formation of a blue-violet color in the mixtures indicated the presence of proteins.
• Ninhydrin test. One ml of each mushroom extract sample was added by 0.2 ml of 0.25% (w/v) ninhydrin in acetone. Then, the mixtures were boiled for two minutes. The formation of a purple color in the mixtures indicated the presence of amino acids.
Detection of tannins. Two point five ml of each mushroom extract sample was added by two drops of 1% (w/v) gelatin. The formation of a white precipitate in the mixtures indicated the presence of tannins.
Detection of flavonoids. Two point five ml of each mushroom extract sample was added by two drops of 10% (w/v) lead acetate. The formation of a yellow precipitate in the mixtures indicated the presence of flavonoids.
Detection of phenolic compounds. Two point five ml of each mushroom extract sample was added by two drops of 5 % (w/v) ferric chloride (FeCl3). The formation of a blue-green color in the mixtures indicated the presence of phenols.
Measurement of glycosaminoglycan levels
The levels of sulfated GAGs in mushroom extracts were determined by 1,9-dimethylmethylene blue (DMMB) dye-binding assay (Farndale et al., 1982) using chondroitin sulfate (C4384, Sigma-Aldrich, USA) as standard. Briefly, 50 μl of either the extracts or standards were added into 96-well plates. Next, 200 μl of DMMB solution was added and mixed. Then, the absorbance was read at 630 nm. The levels of GAGs were estimated by the calibration curve of chondroitin sulfate standards against their known concentrations.
Scanning UV-Vis spectrophotometric analysis
The mushroom extracts were examined by spectrum mode on a UV-Vis spectrometer (UV2401, Shimadsu, Japan). The chondroitin sulfate standard and the extracts at 1 mg/ml in deionized water were scanned and analyzed from 190 to 500 nm.
RESULTS
Appearance of the 10 edible mushrooms
The appearance of the 10 fresh edible mushrooms, including 5 cultivated mushrooms; log white fungi, shitake mushroom, sajor-caju mushroom, Jew's ear, straw mushroom; and local mushrooms; milk white russula, hygroscopic earthstar, log black fungi, rosy russula, and bolete are shown in Figure 1.
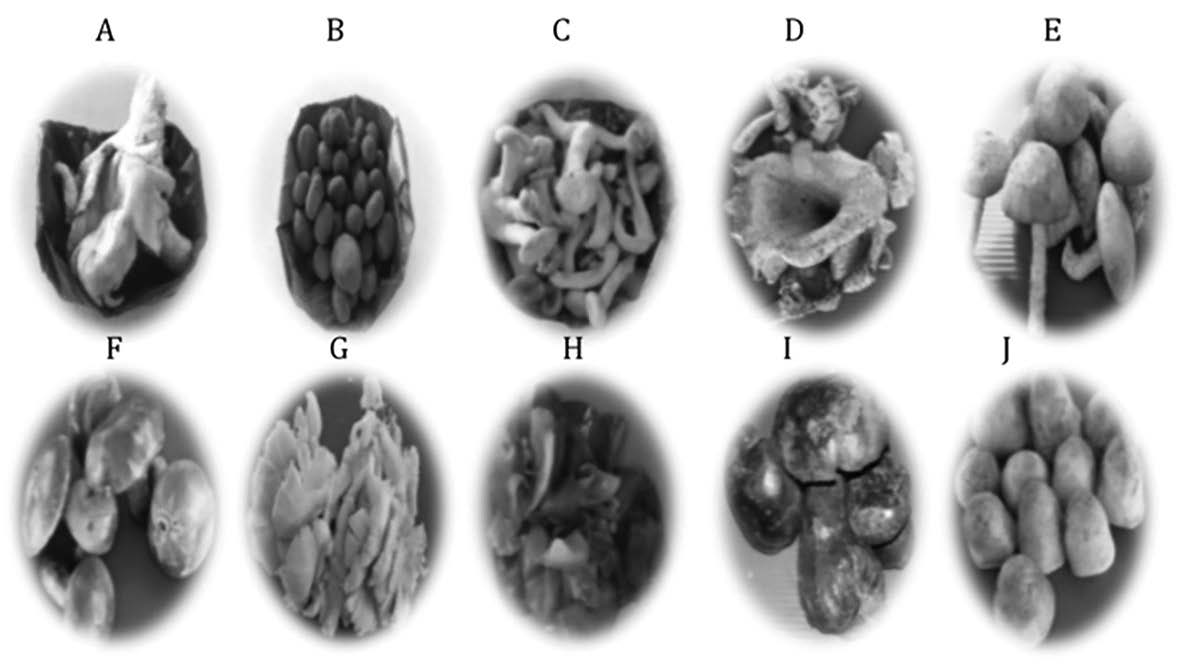
Figure 1. Gross appearance of 10 fresh edible mushrooms.
Note: A, Milk white russula; B, Hygroscopic earthstar; C, Log white fungi; D, Log black fungi; E, Shitake mushroom; F, Rosy russula; G, Sajor-caju mushroom; H, Jew's ear; I, Bolete; J, Straw mushroom.
Yields of the mushroom extract powders
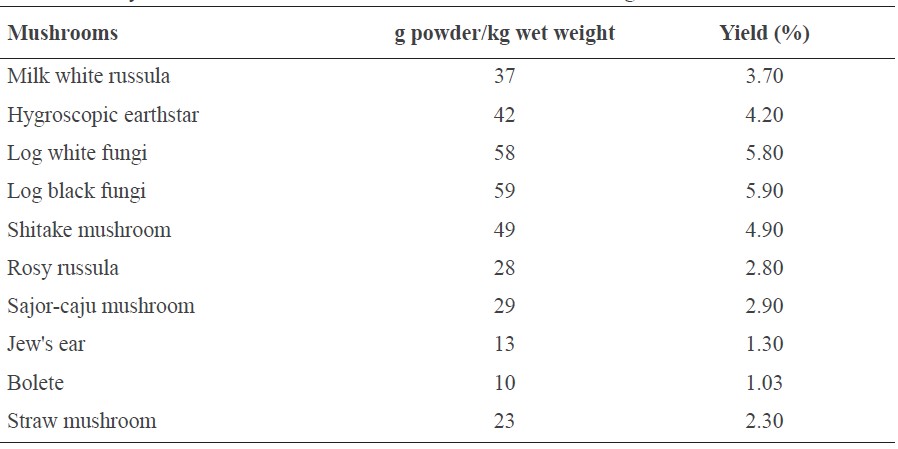
The amount and yield of the mushroom extract powders per 1 kg fresh mushrooms are shown in Table 1. Log black fungi and log white fungi had notably high yields, followed by shitake mushroom, hygroscopic earthstar, milk white russula, sajor-caju mushroom, rosy russula, straw mushroom, Jew's ear, and bolete.
Table 1. The yields of 10 mushroom extracts calculated from 1 kg of fresh mushrooms.
Phytochemicals screening of the mushroom extracts
The phytochemical analysis of the mushroom extracts are shown in Table 2. Screening for carbohydrates by Molisch’s test detected carbohydrates in 9 of the 10 mushroom extracts, all but rosy russula. Fehling’s test detected reducing sugars in only three of the extracts: shitake mushroom, sajor-caju mushroom, and bolete. This suggested that most of the mushrooms studied here contained carbohydrates, some of which were reducing sugars.
Analysis of amino acids by ninhydrin test detected amino acids in all of the mushroom extracts, except milk white russula, bolete, and Jew's ear. This contrasted with the biuret test, which detected no proteins in any of the mushroom extracts. Analysis of tannins by gelatin test found no tannins in any of the mushroom extracts. In contrast, analysis of flavonoids by lead acetate test detected flavonoids in eight of the mushroom extracts, excluding rosy russula and Jew's ear. The ferric chloride test detected phenolic compounds in hygroscopic earthstar and shitake mushroom; thus, these two mushrooms contained both flavonoids and phenolic compounds. The results suggested that they could be a potential source of antioxidant compounds (Balasundram et al., 2006; Armassa et al., 2009; Ferreira et al., 2009; Thetsrimuang et al., 2011a, 2011b).
Thus, the 10 cultivated or local edible mushrooms studied here are potential sources of carbohydrates, amino acids, and flavonoids.
Table 2. The qualitative phytochemical screening of 10 mushroom extracts.
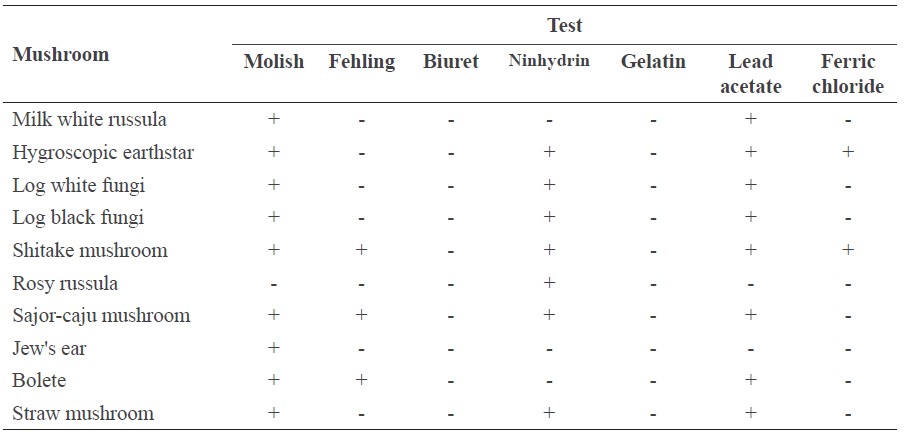
Note: (+) Positive; (-) Negative.
Glycosaminoglycan screening of the mushroom extracts
Glycosaminoglycans (GAGs) were present to some degree in all of the mushroom extracts (Figure 2). Hygroscopic earthstar contained the most, followed by log white fungi, bolete, log black fungi, shitake mushroom, rosy russula, straw mushroom, Jew's ear, milk white russula, and sajor-caju mushroom, respectively. Interestingly, the three most popular local edible mushrooms contained the most GAGs – hygroscopic earthstar (19 μg/ml), log white fungi (12 μg/ml), and bolete (11 μg/ml).
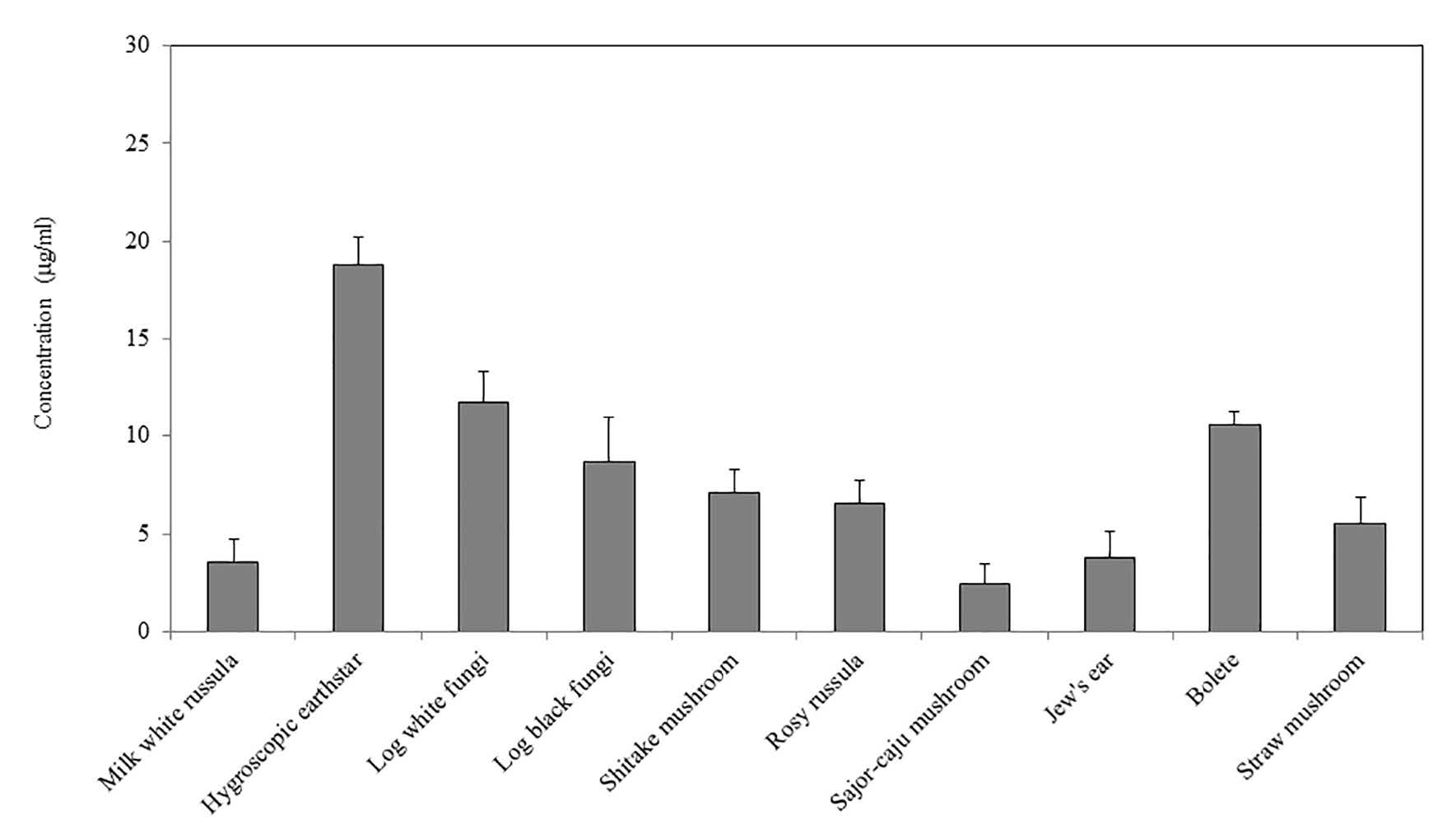
Figure 2. Dimethylmethylene blue (DMMB) dye-binding assay of glycosaminoglycans (GAGs) from 10 mushroom extracts.
Note: The levels of GAGs in the 10 mushroom extracts are presented as mean ± S.D.
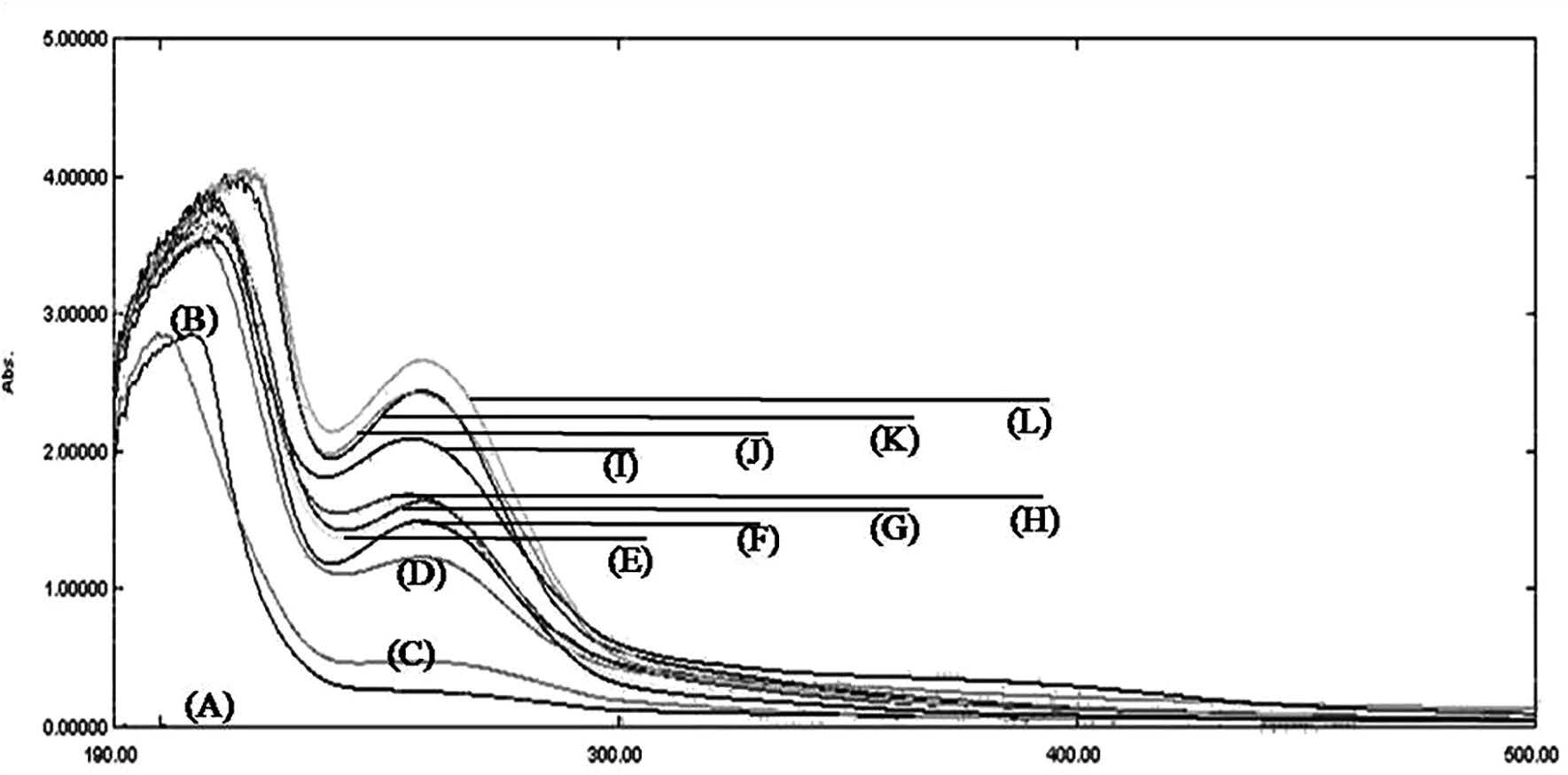
Figure 3. UV-Vis spectrophotometry pattern of glycosaminoglycans from 10 mushroom extracts. (A), baseline; (B), standard chondroitin sulfate; (C), Jew's ear; (D), rosy russula; (E), shitake mushroom; (F), sajor-caju mushroom; (G), hygroscopic earthstar; (H), milk white russula; (I), bolete; (J), log black fungi; (K), straw mushroom; (L), log white fungi.
Scanning UV-Vis spectrophotometric analysis
Scanning UV-Vis spectra of the mushroom extracts are shown in Figure 3. The absorption pattern in the mushroom extracts resembled the chondroitin sulfate standard, with two peaks around 190-300 nm in most extracts. Since GAGs polymers are composed of either iduronic acid or glucuronic acid and N-acetyl chromophores (Kjellén and Lindahl, 1991; Varki et al., 2009), the highest peaks around 190-210 nm, arising from the carboxylate chromophore of iduronate and glucuronate present in the GAGs chains, were responsible for the majority of the absorption pattern (Lima et al., 2011).
DISCUSSION
Several studies have reported that different extraction techniques yielded varying degrees of chemical and biological components (Sasidharan et al., 2010). The yield in this study was rather low, although we used the extraction method specific to GAGs or GAGpeptides (Nakano et al., 2000). This is probably due to the naturally low occurrence of GAGs in these mushrooms, or their geographic origin and phytochemical content (Ncube et al., 2008).
According to Sanmee et al. (2003), rosy russula contained carbohydrates; however, we found none using phytochemical analysis. This inconsistency may be due to limitations of the extraction or detection methods used. Most mushrooms are high in protein, providing a good dietary source (Sanmee et al., 2003; Valverde et al., 2015; Li et al., 2017). Nevertheless, we
only detected amino acids in some of the mushroom extracts. A non-optimal pH can affect protein structure, leading to protein dysfunction or liberating free amino acids. For example, an acid environment can denature proteins through non-enzymatic hydrolysis (Marcus, 1985; Lauer et al., 2016). This may explain our results, as we extracted the mushrooms with acidic water (Nakano et al., 2000); this condition may have degraded the proteins, resulting in release of free amino acids. Furthermore, the absence of either amino acids or proteins in the mushroom extracts could be due to the sensitivity of the qualitative methods used in this study. Given we did find free amino acids in some of the extracts, and the limitations discussed above, it remains possible that the mushrooms of northern Thailand could still be a good source of non-animal proteins, especially for vegetarians.
Besides their nutritional value, mushrooms contain biologically active compounds with beneficial properties (Valverde et al., 2015; Filipa et al., 2017). For instance, numerous mushroom extracts have been shown to contain antioxidants, including phenolic compounds, vitamin C, vitamin E, and carotenoid (Ferreira et al., 2009). Phenolic compounds are aromatic hydroxylated molecules that can be found in either simple or complex phenolic molecules. The phenolics are composed of many compounds, such as phenolic acids, flavonoids, tannins, and lignans (Cote et al., 2010; D’Archivio et al., 2010), that generate antioxidant activity. This activity is related to their capacity to act as reducing agents, scavenge free radicals, quench singlet oxygen, or chelate metal ions (Balasundram et al., 2006; Ferreira et al., 2009). Furthermore, several studies have shown that the presence of phenolic compounds in mushroom extracts correlated with antioxidant activity, which depended on the type of mushroom, stage of development, age of the fresh mushroom, storage conditions (Guillamón et al., 2010), or preparation procedures. For example, the methanolic extract and crude polysaccharides of log black fungi (L. polychrous) found in northern and northeastern Thailand exhibited antioxidant activity and an inhibitory effect on breast cancer cells (Armassa et al., 2009; Thetsrimuang et al., 2011a; 2011b). Aqueous and butanol extracts of sajor-caju mushroom (P. sajor-caju) have shown high antioxidant activity linked to their total phenolic content (Kanagasabapathy et al., 2011). These results indicate that the extraction method used with different mushroom species affect the levels of active compounds and their range of bioactive properties. Based on our current results, we found no tannins in any of the mushroom extracts, but we detected flavonoids in eight of the mushroom extracts (all but rosy russula and Jew's ear), and detected phenolic compounds only in hygroscopic earthstar and shitake mushroom. These findings are thought to be the consequence of using acidic water (Nakano et al., 2000) for preparing mushroom extracts, as we did in this study; this method may not be suitable for extracting tannins or other phenolic compounds of some mushroom species (Tiwari et al., 2011). We did find flavonoids in most of the mushroom extracts; this finding supported the presence of catechin in shitake mushroom, log white fungi, and log black fungi (Attarat and Phermthai, 2015; Chowdhury et al., 2015). Some studies have demonstrated that bioactive compounds in mushrooms play an essential role in the human immune system by acting as immunomodulating molecules. The main groups of immunomodulating compounds are terpenes, terpenoids, lectins, fungal immunomodulatory proteins (FIPs) and polysaccharides (Enshasy and Kaul, 2013). Of these, the best known immunomodulating molecules are lentinan and schizophyllan; both are are β-1, 3-D-glucans with β-1, 6 branches and have been found in shitake mushroom and spilt gill mushroom, respectively (Reis et al., 2012). They have been used to treat gastric cancer (lentinan) and head and neck cancer (schizophyllan), in combination with chemotherapy (Ina et al., 2013; Filipa et al., 2017). In addition, other polysaccharides rich in β-1, 3- D-glucans, β-1, 4-D-glucans, β-1, 6-D-glucans, α-1, 4-D-glucans, α-1, 6-D-glucans, and glucomannan have been isolated from A. blazei; these have been shown to have immunomodulatory and antineoplastic properties (Biedron et al., 2012).
Several studies mentioned that most bioactive polysaccharides isolated from mushrooms are branched or unbranched homopolysaccharides (Biedron et al., 2012; Ina et al., 2013; Filipa et al., 2017), which usually contain long chains of glucose polymer. In our study, most of the mushroom extracts were positive with Molisch's test – a general test for detecting carbohydrates, including monosaccharides, disaccharides, and polysaccharides – indicating that the extracts contained carbohydrates. Since, GAGs are carbohydrates (heteropolysaccharides), we then specifically tested for their presence using a DMMB dyebinding assay; all of our mushroom extracts also contained GAGs. This suggested that some of the carbohydrates that were detected by Molisch's test were probably GAGs. In contrast to most of our mushroom extracts, rosy russula extracts were negative for carbohydrates using both the Molish and Fehling tests. This result also contrasted with Sanmee et al. (2003), who found monosaccharides in rosy russula. These conflicting results could be due to differences in the preparation procedures, origin and stage of the mushrooms, or detection methods.
Most GAGs are attached to a core protein to form proteoglycans; they are found predominantly in vertebrates and invertebrates. For example, aggrecan, the major proteoglycan present in cartilage (Heinegård and Axelsson, 1977), has a core protein that is covalently linked to sulfated glycosaminoglycans (GAGs) side chains, mainly chondroitin sulfate and keratan sulfate (Meyer et al., 1958). Other sulfated GAGs, such as heparan sulfate and dermatan sulfate, are also found in cartilage and several tissues (Heinegård and Axelsson, 1977; Gandhi and Mancera, 2008). These GAGs have distinct biological functions; for instance, heparan sulfate plays an essential role as co-receptors for various receptor tyrosine kinases (Miserocchi et al., 2001), while chondroitin sulfate can prevent and maintain the structural-functional activity of cartilage during inflammation (Takagaki et al., 2002; Raman et al., 2005). In addition, chondroitin sulfate, as nutraceuticals or prescribed drugs in combination with glucosamine (GlcN) sulfate, has been increasingly employed to treat the symptoms of osteoarthritis and osteoarthrosis (Bishnoi et al., 2016). These pharmaceutical preparations of chondroitin sulfate are usually obtained from animal parts, such as shark or bovine trachea cartilage (Garnjanagoonchorn et al., 2007). Thus, detecting GAGs from the mushroom extracts in this study could potentially provide non-animal sources of chondroitin sulfate and other GAGs. However, these mushroom extracts might also contain some other sulfated polysaccharides as have also been found in algae and other mushroom types (Ina et al., 2013; Filipa et al., 2017).
Algae, another non-animal source, contains the most abundant GAG-resembling sulfated glycans (Vasconcelos et al., 2017). Brown algae contains sulfated fucans that exhibit biomedical functions in inflammation, coagulation, angiogenesis, and cell adhesion (Cumashi et al., 2007). Green and red algae also contain sulfated galactans that involve many pathophysiological systems. These sulfated glycans occur as structural components in the cell walls of algae (Chevolot et al., 2001), and their structures are maintained among phyla. Most sulfated glycans found in algae are composed of disaccharide repeating units with distinct sulfation patterns. Whereas GAG-resembling sulfated glycans of algae have been studied extensively (Vasconcelos and Pomin, 2017), very few studies have researched sulfated glycans or GAGs of local edible mushrooms found in northern Thailand. To potentially exploit the mushrooms in our study as a good source of GAGs, additional study is needed on how to obtain high yielding mushroom extracts.
GAGs levels measured by the DMMB dye-binding assay from the top two mushroom extracts (log white fungi and bolete) were highly correlated with the highest absorption peak around 190-210 nm from spectrophotometric analysis; this tentatively represented the carboxylate chromophore of iduronate and glucuronate present in the GAGs chains (Lima et al., 2011), as shown in Figure 3 (L for log white fungi; and I for bolete). Although hygroscopic earthstar contained the highest levels of GAGs according to the DMMB dye-binding assay, its absorption (G) peak at around 190-210 nm was shifted toward the absorption peaks of the shitake mushroom (E), milk white russula (H), rosy russula (D), and sajor-caju mushroom (F) extracts. The Jew's ear (C) extract had the lowest absorption pattern, consistent with the DMMB dye-binding analysis. Since DMMB dye reacts with all types of sulfated GAG chains, this assay was not able to distinguish the types of GAGs present in the mushroom extracts. Although our spectrophotometric analysis confirmed the presence of GAG chains at peaks around 190-210 nm (Lima et al., 2011), we also found additional broad peaks around 240-260 nm that were related to other aromatic compounds (de Micalizzi et al., 1998). Some ions, such as calcium or sodium, or the sulfation pattern of GAGs or other contaminants can shift the peaks (McEwen et al., 2009). These factors might have affected the correlation between our spectrophotometric analysis and DMMB dye-binding assay. Taken together, our UV-based spectroscopy confirmed the presence of GAGs, but could not distinguish their types. Further study of the GAGs found here using magnetic resonance spectroscopy (NMR) or disaccharide analyses should be performed (Santos et al., 2017).
CONCLUSION
Our results found GAGs in edible mushrooms grown in northern Thailand, providing a potentially new source of these important compounds.
ACKNOWLEDGEMENTS
We thank Aroonwan and Hem Choocheep for local edible mushrooms supply. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
Armassa, N., Poungchompu, O., Rayan, S., Leethong, S., Weerapreeyakul, N., and Machana, S. 2009. Antioxidant activity and cytotoxicity in breast cancer cells line of mushrooms extracts Lentinuspolychrous Lev. compared to Ganodermalucidum (Fr.) Karst. Isan Journal of Pharmaceutical Sciences. 5(3): 243–250. In Thai.
Attarat, J., and Phermthai, T. 2015. Bioactive compounds in three edible Lentinus mushrooms. Walailak Journal of Science and Technology. 12(6): 491–504. https://doi.org/10.14456/WJST. 2015.80
Balasundram, N., Sundram, K., and Samman, S. 2006. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chemistry. 99(1): 191–203. https://doi.org/10.1016/j.foodchem.2005.07.042
Barros, L., Correia, D.M., Ferreira, I.C.F.R., Baptista, P., and Santos-Buelga, C. 2008. Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method. Food Chemistry. 110(4): 1046–1050. https://doi.org/10.1016/j.foodchem.2008.03.016
Biedron, R., Tangen, J.M., Maresz, K., and Hetland, G. 2012. Agaricus blazei Murill -immunomodulatory properties and health benefits. Functional Foods in Health and Disease. 2(11): 428-447.
Bishnoi, M., Jain, A., Hurkat, P., and Jain, S.K. 2016. Chondroitin sulphate: a focus on osteoarthritis. Glycoconjugate Journal. 33(5): 693–705. https://doi.org/10.1007/s10719-016-9665-3
Carneiro, A.A.J., Ferreira, I.C.F.R., Dueñas, M., Barros, L., da Silva, R., Gomes, E., and Santos-Buelga, C. 2013. Chemical composition and antioxidant activity of dried powder formulations of Agaricus blazei and Lentinus edodes. Food Chemistry. 138(4): 2168–2173. https://doi.org/10.1016/j.foodchem.2012.12.036
Chang, S.T., and Wasser, S.P. 2012. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. International Journal of Medicinal Mushrooms. 14(2): 95–134. https://doi.org/10.1615/IntJMedMushr.v14.i2.10
Chevolot, L., Mulloy, B., Ratiskol, J., Foucault, A., and Colliec-Jouault, S. 2001. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydrate Research. 330(4):529-535. https://doi.org/10.1016/S0008-6215(00)00314-1
Choocheep, K., Hatano, S., Takagi, H., Watanabe, H., Kimata, K., Kongtawelert, P., and Watanabe, H. 2010. Versican facilitates chondrocyte differentiation and regulates joint morphogenesis. The Journal of Biological Chemistry. 285(27): 21114–21125. https://doi.org/10.1074/jbc.M109.096479
Chowdhury, M., Kubra, K., and Ahmed, S. 2015. Screening of antimicrobial, antioxidant properties and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Annals of Clinical Microbiology and Antimicrobials. 14(8). https://doi.org/10.1186/s12941-015 -0067-3
Côté, J., Caillet, S., Doyon, G., Sylvain, J.-F., and Lacroix, M. 2010. Bioactive compounds in cranberries and their biological properties. Critical Reviews in Food Science and Nutrition. 50(7): 666–679. https://doi.org/10.1080/10408390903044107
Cumashi, A., Ushakova, N.A., Preobrazhenskaya, M.E., D’Incecco, A., Piccoli, A., Totani, L., Tinari, N., Morozevich, G.E., Berman, A.E., Bilan, M.I., et al. 2007. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 17(5): 541–552. https://org/10.1093/glycob/cwm014
D’Archivio, M., Filesi, C., Varì, R., Scazzocchio, B., and Masella, R. 2010. Bioavailability of the polyphenols: status and controversies. International Journal of Molecular Sciences. 11(4): 1321–1342. https://doi.org/10.3390/ijms11041321
De Micalizzi, Y.C., Pappano, N.B., and Debattista, N.B. 1998. First and second order derivative spectrophotometric determination of benzyl alcohol and diclofenac in pharmaceutical forms. Talanta. 47(3): 525–530. https://doi.org/10.1016/S0039-9140(98)00080-0
El Enshasy, H.A., and Hatti-Kaul, R. 2013. Mushroom immunomodulators: unique molecules with unlimited applications. Trends in Biotechnology. 31(12): 668–677. https://doi.org/10 .1016/j.tibtech.2013.09.003
Farndale, R.W., Sayers, C.A., and Barrett, A.J. 1982. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connective Tissue Research. 9(4): 247–248.
Ferreira, I.C.F.R., Barros, L., and Abreu, R.M.V. 2009. Antioxidants in wild mushrooms. Current Medicinal Chemistry. 16(12): 1543–1560. https://doi.org/10.2174/092986709787909587
Finimundy, T.C., Gambato, G., Fontana, R., Camassola, M., Salvador, M., Moura, S., Hess, J., Henriques, J.a.P., Dillon, A.J.P., and Roesch-Ely, M. 2013. Aqueous extracts of Lentinula edodes and Pleurotus sajor-caju exhibit high antioxidant capability and promising in vitro antitumor activity. Nutrition Research. 33(1): 76–84. https://doi.org/
10.1016/j.nutres.2012.11.005
Filipa, S.R., Anabela, M.M., Helena, V., Patricia, M., and Isabel, C.F.R.F. 2017. Functional foods based on extracts or compounds derived from mushrooms. In Trends in Food Science & Technology. 66: 48-62. https://doi.org/10.1016/j.tifs.2017.05.010
Flegg, P.B., and Maw, G. 1997. Mushrooms and their possible contribution to the world. Mushroom Journal. 48: 395–403.
Gandhi, N.S., and Mancera, R.L. 2008. The structure of glycosaminoglycans and their interactions with proteins. Chemical Biology and Drug Design. 72(6): 455–482. https;//doi.org/10.1111/j.1747-0285.2008.00741.x
Garnjanagoonchorn, W., Wongekalak, L., and Engkagul, A. 2007. Determination of chondroitin sulfate from different sources of cartilage. Chemical Engineering and Processing. 46(5): 465–471. https://doi.org/10.1016/j.cep.2006.05.019
Guillamón, E., García-Lafuente, A., Lozano, M., D’Arrigo, M., Rostagno, M. A., Villares, A., and Martínez, J.A. 2010. Edible mushrooms: role in the prevention of cardiovascular diseases. Fitoterapia. 81(7): 715–723. https://doi.org/10.1016/j.fitote.2010.06.005
Heinegård, D., and Axelsson, I. 1977. Distribution of keratan sulfate in cartilage proteoglycans. The Journal of Biological Chemistry. 252(6): 1971–1979.
Ina, K., Kataoka, T., and Ando, T. 2013. The use of lentinan for treating gastric cancer. Anti-Cancer Agents in Medicinal Chemistry. 13(5): 681–688. https://doi.org/10.2174/1871520611313050002
Kanagasabapathy, G., Malek, S.N.A., Kuppusamy, U.R., and Vikineswary, S. 2011. Chemical composition and antioxidant properties of extracts of fresh fruiting bodies of Pleurotus sajor-caju (Fr.) Singer. Journal of Agricultural and Food Chemistry. 59(6): 2618–2626. https://doi.org/10.1021/jf104133g
Kjellén, L., and Lindahl, U. 1991. Proteoglycans: structures and interactions. Annual Review of Biochemistry. 60: 443–475. https://doi.org/10.1146/annurev.bi.60.070191.002303
Lauer, T.M., Wood, G.P.F., Farkas, D., Sathish, H.A., Samra, H.S., and Trout, B.L. 2016. Molecular Investigation of the Mechanism of Non-Enzymatic Hydrolysis of Proteins and the Predictive Algorithm for Susceptibility. Biochemistry. 55(3): 3315–3328. https://doi.org/10.1021/acs.biochem.5b01376
Li, H., Zhang, Z., Li, M., Li, X., and Sun, Z. 2017. Yield, size, nutritional value, and antioxidant activity of oyster mushrooms grown on perilla stalks. Saudi Journal of Biological Sciences. 24(2): 347–354. https://doi.org/10.1016/j.sjbs.2015.10.001
Lima, M.A., Rudd, T.R., de Farias, E.H.C., Ebner, L.F., Gesteira, T.F., de Souza, L.M., Mendes, A., Córdula, C.R., Martins, J.R.M., Hoppensteadt, D., et al. 2011. A new approach for heparin standardization: combination of scanning UV spectroscopy, nuclear magnetic resonance and principal component analysis. PloS One. 6(1): e15970. https://doi.org/10.1371 /journal.pone.0015970
Manzi, P., and Pizzoferrato, L. 2000. Beta-glucans in edible mushrooms. Food Chemistry. 68(3): 315–318. https://doi.org/10.1016/S0308-8146(99)00197-1
Marcus, F. 1985. Preferential cleavage at aspartyl-prolyl peptide bonds in dilute acid. International Journal of Peptide and Protein Research. 25(5): 542–546. https://doi.org/10.1111/j.1399-3011.1985.tb02208.x
Mattila, P., Könkö, K., Eurola, M., Pihlava, J.M., Astola, J., Vahteristo, L., Hietaniemi, V., Kumpulainen, J., Valtonen, M., and Piironen, V. 2001. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. Journal of Agricultural and Food Chemistry. 49(5): 2343–2348. https://doi.org/10.1021/jf001525d
Mayell, M. 2001. Maitake extracts and their therapeutic potential. Alternative Medicine Review. 6(1): 48–60.
McEwen, I., Rundlöf, T., Ek, M., Hakkarainen, B., Carlin, G., and Arvidsson, T. 2009. Effect of Ca2+ on the 1H NMR chemical shift of the methyl signal of over sulphated chondroitin sulphate, a contaminant in heparin. Journal of Pharmaceutical and Biomedical Analysis. 49(3): 816–819. https;//doi.org/10.1016/j.jpba.2008.12.012
Meyer, K., Hoffman, P., and Linker, A. 1958. Mucopolysaccharides of costal cartilage. Science (Washington). 128: 896-896. https://doi.org/10.1126/science.128.3329.896
Miserocchi, G., Negrini, D., Passi, A., and De Luca, G. 2001. Development of lung edema: interstitial fluid dynamics and molecular structure. News in Physiological Sciences. 16: 66–71. https://doi.org/10.1152/physiology online.2001.16.2.66
Monfort, J., Nacher, M., Montell, E., Vila, J., Verges, J., and Benito, P. 2005. Chondroitin sulfate and hyaluronic acid (500-730 kda) inhibit stromelysin-1 synthesis in human osteoarthritic chondrocytes. Drugs Under Experimental and Clinical Research. 31(2): 71–76.
Nakano, T., Igawa, N., and Ozimek, L. 2000. An economical method to extract chondroitin sulphate-peptide from bovine nasal cartilage. Canadian Biosystems Engineering. 42(4): 205–208.
Ncube, N.S., Afolayan, A.J., and Okoh, A.I. 2008. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. African Journal of Biotechnology. 7(12): 1797–1806.
Pomin, V. H. 2015. Keratan sulfate: an up-to-date review. International Journal of Biological Macromolecules. 72: 282–289. https://doi.org/10.1016/j.ijbiomac.2014.08.029
Prydz, K., and Dalen, K.T. 2000. Synthesis and sorting of proteoglycans. Journal of Cell Science. 113(2): 193–205.
Raman, R., Sasisekharan, V., and Sasisekharan, R. 2005. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chemistry and Biology. 12(3): 267–277. https://doi.org/10.1016/j.chembiol. 2004.11.020
Reis, F.S., Barros, L., Martins, A., and Ferreira, I.C.F.R. 2012. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an interspecies comparative study. Food and Chemical Toxicology. 50(2): 191–197. https;//doi.org 10.1016/j.fct.2011.10.056
Sanmee, R., Dell, B., Lumyong, P., Izumori, K., and Lumyong, S. 2003. Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chemistry. 82(4): 527–532. https://doi.org/10.1016/S0308-8146(02)00595-2
Santos, G.R.C., Piquet, A.A., Glauser, B.F., Tovar, A.M.F., Pereira, M.S., Vilanova, E., and Mourão, P.A.S. 2017. Systematic analysis of pharmaceutical preparations of chondroitin sulfate combined with glucosamine. Pharmaceuticals. 10(2): e38. https://doi.org/10.3390/ph10020038
Sarrazin, S., Lamanna, W.C., and Esko, J.D. 2011. Heparan sulfate proteoglycans. Cold Spring Harbor Perspectives in Biology. 3(7). https://doi.org/10.1101/cshperspect.a004952
Sasidharan, S., Chen, Y., Saravanan, D., Sundram, K.M., and Yoga Latha, L. 2010. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. African Journal of Traditional, Complementary, and Alternative Medicines. 8(1): 1–10. https://doi.org/10.1625/jcam.8.1
Takagaki, K., Munakata, H., Kakizaki, I., Iwafune, M., Itabashi, T., and Endo, M. 2002. Domain structure of chondroitin sulfate E octasaccharides binding to type V collagen. The Journal of Biological Chemistry. 277: 8882–8889. https://doi.org/10.1074/jbc.M106479200
Thetsrimuang, C., Khammuang, S., and Sarnthima, R. 2011a. Antioxidant activity of crude polysaccharides from edible fresh and dry mushroom fruiting bodies of Lentinus sp. strain RJ-2. International Journal of Pharmacology. 7(1): 58–65. https;//doi.org/10.3923 /ijp.2011.58.65
Thetsrimuang, C., Khammuang, S., Chiablaem, K., Srisomsap, C., and Sarnthima, R. 2011b. Antioxidant properties and cytotoxicity of crude polysaccharides from Lentinus polychrous Lév. Food Chemistry. 128: 634–639. https://doi.org/10.1016/j.foodchem.2011.03.077
Tiwari, P., Kumar, B., Kaur, M., Kaur, G., and Kaur, H. 2011. Phytochemical screening and extraction: A review. Internationale Pharmaceutica Sciencia. 1(1): 98-106.
Valverde, M.E., Hernández-Pérez, T., and Paredes-López, O. 2015. Edible mushrooms: improving human health and promoting quality life. International Journal of Microbiology. 2015. e376387. http://dx.doi.org/10.1155/ 2015/376387
Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G., Aebi, M., Darvill, A., Kinoshita, T., Packer, N.H., Prestegard, J.J., et al. (editors). 2009. Essentials of Glycobiology, 2nd ed. [internet]. Cold Spring Harbor Laboratory Press; [cited 2017 May 30]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1900/2009
Vasconcelos, A.A., and Pomin, V. H. 2017. The sea as a rich source of structurally unique glycosaminoglycans and mimetics. Microorganisms. 5(3). https://doi.org/10.3390/micro organisms5030051
Vennila, S., Mohana, S., Bupesh, G., Mathiyazhagan, K., Dhanagaran, D, Baskar, M, Amutha, S, and Leeba, B. 2012. Qualitative phytochemical screening and in vitro antioxidant activity of Helicteres isora L. Herbal Tech Industry. 14–18.
Vetvicka, V., and Yvin, J.-C. 2004. Effects of marine beta-1,3 glucan on immune reactions. International Immunopharmacology. 4(6): 721–730. https://doi.org/10.1016/j.intimp.2004.02.007
Whittaker, R.H. 1969. New Concepts of kingdoms of organisms. Science. 163(3863): 150–160. https://doi.org/10.1126/science.163.3863.150
Yadav, S., Joshi, A., Ahmed, K., and Dubey, B.K. 2011. Pharmacognostical and phytochemical evalution of ventilago calyculata. International Journal of Pharmaceutical Sciences and Research. 2(12): 3238–3242. https://doi.org/10.13040/IJPSR.0975-8232.2(12).3238-42
Yu, S., Weaver, V., Martin, K., and Cantorna, M.T. 2009. The effects of whole mushrooms during inflammation. BioMed Central Immunology [internet]. [cited 2017 May 30]; 10: 12: Available from: http://www.biomed central.com/1471-2172/10/12 https://doi.org/10.118 6/14 71-2172-10-12
Zhang, L., Fan, C., Liu, S., Zang, Z., Jiao, L., and Zhang, L. 2011. Chemical composition and antitumor activity of polysaccharide from Inonotus obliquus. Journal of Medicinal Plants Research. 5(7): 1251–1260.
Kanyamas Choocheep* and Nantawadee Nathip
Division of Clinical Chemistry, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: kanyamas.c@cmu.ac.th
Total Article Views

