
Effects of Foliar Application of Zinc on Grain Yield and Zinc Concentration of Rice in Farmers’ Fields
Piyawan Phuphong Ismail Cakmak Bernard Dell, and Chanakan Prom-u-thai*Published Date : 2018-07-01
DOI : https://doi.org/10.12982/CMUJNS.2018.0013
Journal Issues : Number 3 , July-September 2018
ABSTRACT
Three field experiments were conducted on farms in Chiang Mai, Thailand in 2015 to evaluate the effects of: (i) foliar application of Zn on the grain yield and grain Zn concentration of rice and (ii) using Zn-enriched seeds in the next cropping on growth and yield. Zn was applied by foliar spraying 0.5% ZnSO4 at three different growth stages: booting, flowering, and early milk stages. Foliar spraying of Zn improved the grain Zn concentration by 41% in one field, and an average of 30% across the three fields. The foliarsprayed Zn did not, however, affect the grain yield in any of the fields. The Zn-enriched seeds also did not affect the grain yield of the plants in the farmers’ fields in the next cropping, probably because of the high amount of soluble Zn already in the experimental fields. Clearly, the foliar application of Zn significantly increased grain Zn concentration, but had no effect on grain yield.
Keywords: Foliar Zn fertilization, Farmers’ field, Grain Zn concentration, Rice, Seed zinc enrichment
INTRODUCTION
Zinc deficiency is a major malnutrition problem, resulting in severe health complications, including growth retardation and impaired immune system, combined with increased risk of infection, DNA damage, and alterations in mental function (Hotz and Brown, 2004; Gibson et al., 2007). The recommended daily intake of Zn is only 16 mg per day (National Research Council, 1989), but many commonly consumed foods do not provide this amount, especially in the developing world where cereal-based foods that are low in Zn predominate diets (Gibson et al., 2007; Cakmak and Kutman, 2017). For example, in South and Southeast Asia where rice is the staple diet, more than one-half a billion people have been estimated to be affected by inadequate Zn intake, especially pre-school children and women (Hotz and Brown, 2004; Gibson et al., 2007; Black et al., 2008). In northeastern Thailand, 57% of pre-school children have low amounts of serum Zn (Thurlow et al., 2005). Thailand consumes high amounts of rice that is low in Zn (Gibson et al., 2007; Phattarakul et al., 2012). The average content of Zn in rice grown in Thailand is only 28.7 mg per kg (range 17.3- 59.2) in brown rice and 20.6 mg per kg (range 9.6-40.2) in white rice across several regions tested (Saenchai et al., 2012; Jaksomsak et al., 2014; Panomjan et al., 2016). It has been suggested that increasing the grain Zn concentration by up to 50 mg per kg would benefit human health through improved diet and agriculture through better seed germination and seedling vigor (Welch and Graham, 2004; Cakmak, 2008; Prom-u-thai et al., 2010).
In crop plants, Zn has a diverse range of critical functions that affect several physiological processes, including enzyme activation, protein synthesis, detoxification of reactive oxygen species gene expression and regulation, and reproductive development (pollen formation) (Cakmak, 2000; Chang et al., 2005; Marschner, 2012). Thus, biofortifying rice with increased Zn, either through plant breeding and/or fertilizer strategies, would contribute to both better plant growth and the nutritional quality of its grain (Bouis, 2002; Cakmak and Kutman, 2017). Soil Zn application affects grain Zn less than foliar fertilization, as shown in rice and wheat (Cakmak et al., 2010; Phattarakul et al., 2012). In rice, foliar application of Zn is a particularly advantageous method to enhance grain Zn concentration compared with soil application, as it avoids the complex soil interactions that limit Zn uptake through a plant’s roots (Mabesa et al., 2013). The efficiency of soil Zn fertilization in increasing the grain Zn concentration in cereal depends largely on the soil type and fertility (Cakmak, 2008). When rice was grown in different types of soil, up to 90% difference was observed in the grain Zn concentration in the same rice varieties (Graham et al., 1999). Wissanu et al. (2007) also found a significant variation in grain Zn concentration (e.g., from 8 mg kg-1 to 47 mg kg-1) for a given rice variety when grown in different soil Zn fertility. These findings indicate that environmental conditions have a significant impact on grain Zn concentration. In the case of foliar application of Zn, the type of soil has a minor effect on the biofortification of grains with Zn through foliar spray. Zinc concentration in brown rice (whole caryopsis with husk removed) was increased by 25% by foliar application of Zn in 17 field trials conducted in five different countries (China, India, Lao PDR, Thailand, and Turkey) with soil pH ranging from 4.8 to 8.8 and DTPA-extractable Zn from 0.5 mg kg−1 to 6.5 mg kg−1 (Phattarakul et al., 2012). Most of the studies focusing on biofortification of cereals with Zn have been conducted on research farms of universities or research institutions. It would, therefore, be interesting to further investigate how foliar application of Zn affects grain Zn accumulation under different soil conditions in farmers’ fields. Low Zn in seeds adversely affects not only human health, but also seed germination and seedling vigor, especially under Zn-deficient soil conditions (Yilmaz et al., 1998). While high concentrations of Zn in rice seed greatly improved the growth and development of seedlings (Prom-u-thai et al., 2012; Boonchuay et al., 2013), these studies were not investigated under farmers’ field conditions, either.
The present study evaluated the effect of (i) foliar application of Zn on grain Zn concentration and yield and (ii) the role of Zn-enriched seeds in plant growth and yield in three different farmers’ fields with high variation in soil fertility.
MATERIALS AND METHODS
Study location
The study was conducted during two crop seasons (July to December) in 2015 and 2016 under field conditions at three different farms in Hang Dong District, Chiang Mai Province, Thailand. Soil samples at each location were collected and analyzed for several chemical and physical parameters. Farmers at each location grew rice (Oryza Sativa L.) variety ‘RD 14’. The seeds were derived from the Rice Seed Center at Chiang Mai, Thailand. The seedlings were prepared in the farmers’ fields; seedlings that were approximately 30 days old were transplanted into the 20×20 m2 plots with three replications at each location. The fields were permanently flooded under 0.1-0.2 m of water until maturity. Nitrogen fertilizer was applied by taking into consideration the farmers’ practice. Briefly, urea (46–0–0) fertilizer was applied in two split applications at 300 kg ha-1. One-half of the N was broadcasted by hand 30 days after transplanting, along 15 kg P2O5 ha-1 and 15 kg K2O ha-1, as it was the optimal rate for rice production in this area, and the other half of the N was applied 30 days later.
Foliar application of zinc
The farmers’ plots at each location were arranged in randomized complete block designs with three independent replications. The treatments consisted of non-foliar application of Zn (Zn0) and foliar application of Zn (Zn+). In the case of the foliar Zn treatment, Zn was applied at the rate of 0.5% ZnSO4.7H2O at three different growth stages: (i) booting, (ii) flowering, and (iii) early milk stage. The spray solution was prepared by dissolving ZnSO4 in deionized water. The foliar application was carried out by evenly spraying the solution until most of the shoot parts were wet and the solution had just begun to drip from the leaves. The spraying was always conducted in the late afternoon (~5 pm). The non-foliar Zn treatment consisted of spraying with deionized water. At maturity, the grain yield and the yield components (straw dry weight, clum length, panicle length, 1000 seed weight, number of filled grain and tiller) of 1 m2 were evaluated. Brown rice (husk manually removed by hand) was analyzed for Zn concentration to compare the non-foliar and foliar treatments.
Sowing enriched Zn seeds
The Zn-enriched (Zn+) and Zn non-enriched (Zn0) seeds of the variety RD 14 used in the next field experiment were derived from the foliar spray experiment described above. The Zn-enriched seeds contained 18-21 mg Zn kg−1 and the Zn non-enriched seeds 15-16 mg Zn kg−1. The seeds were sown on a seedbed for seedling preparation. The 30-day-old seeds were then transplanted into the plots of 20×20 m2 by using standard farmers’ practice with three replications. This second field experiment used the same basal fertilizer applications and soil management practices as the first experiment. At grain maturity, the grain yield was determined; brown rice was used for the analysis of the Zn concentration.
Chemical analysis
The soil fertility characteristics were determined from soil samples collected from a depth of 30 cm. The following soil parameters were analyzed: pH (measured in 1:1, soil:water), organic matter, available phosphorus (by Bray II), extractable potassium, and diethylenetriaminepentaacetic acid (DTPA)-extractable Zn. The seed Zn concentration was analyzed in brown rice (without husk) through atomic absorption spectrophotometer after the dry-ashing method (Zarcinas et al., 1987).
Statistical analysis
The data were subjected to combined analysis of variance (ANOVA); means that were significantly different were separated at P<0.05 by the least significant difference (LSD) at P<0.05.
RESULTS
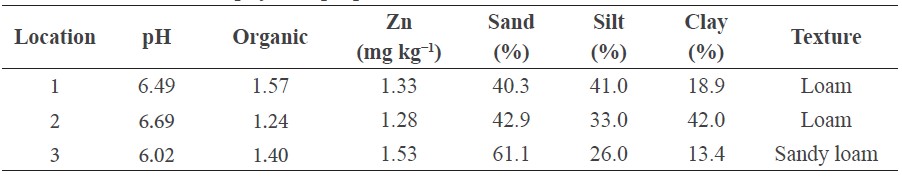
The experimental soils differed in soil pH and soil texture (Table 1). In location 3, the sand percentage was very high (about 61%). Location 3 also had higher DTPA-extractable Zn and lower soil pH compared to the other two locations. The soils were similar in organic matter content.
Table 1. Chemical and physical properties of soils in three farmers’ field locations.
Foliar application of Zn had no effect on grain yield or yield component in all farmers’ fields, except for the percentage of filled grain (Table 2). However, the Zn effect on the percentage of filled grain was not consistent. The average grain yield was 6.4 ton ha−1, which was not statistically different between the farmers’ fields.
Table 2. Yield and yield components of rice cultivar RD 14 with (Zn+) and without (Zn0) foliar application of 0.5% ZnSO4.7H2O at three different locations.
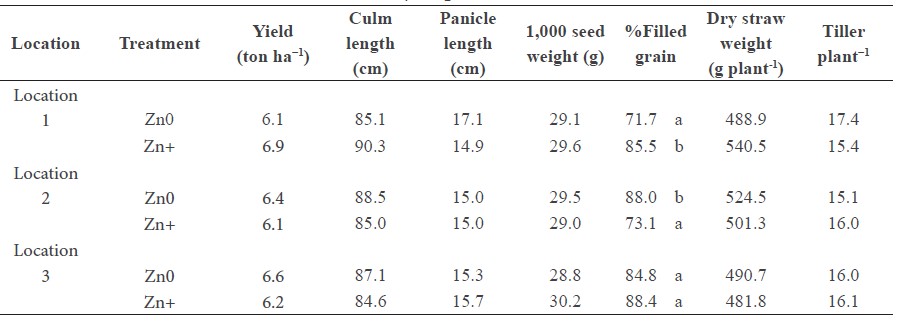
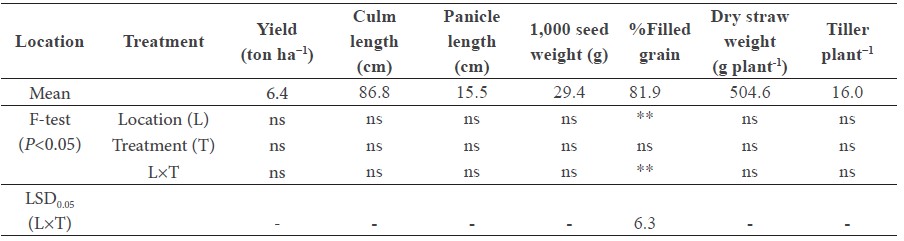
Note: Different lowercase letters indicate significant difference between Zn treatments at P<0.05; ‘ns’ indicates ‘no significant difference’ between field locations and Zn treatments at P<0.05.
The Zn concentration measured in the brown rice was significantly (P<0.05) affected by the foliar Zn treatment in the three field locations (Table 3). The foliar application of Zn increased the grain Zn concentration in all the fields compared to the controls; the increases ranged from 21% (location 3) to 41% (location 1), with an average increase in the three fields of 32% (Table 3). When Zn was not sprayed, the grain Zn concentrations in the three farmers’ fields did not differ significantly.
Table 3. Effect of foliar Zn spray in the form of ZnSO4 at a rate of 0.5% on Zn concentrations of brown rice in three farmers’ fields.
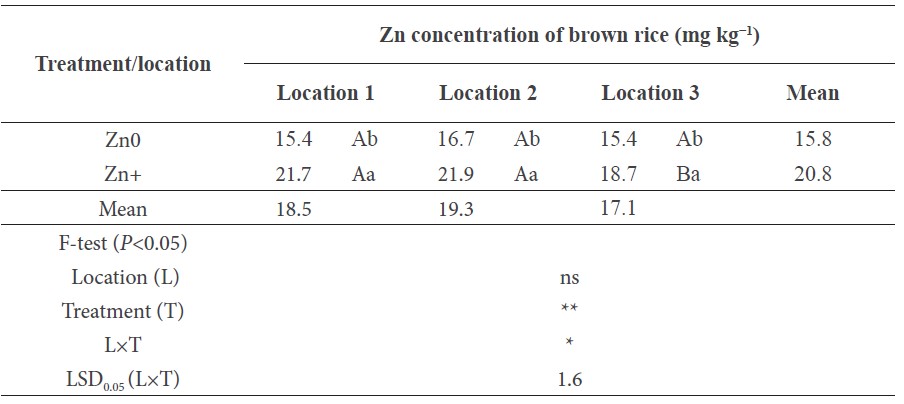
Note: The data are the means of three independent replications. Different uppercase letters indicate significant difference between field locations and lowercase letters indicate significant difference between Zn treatments at P<0.05; ‘ns’ indicates ‘no significant difference’ between field locations and Zn treatments at P<0.05.
In the next experiments, Zn-enriched and non-enriched rice seeds were sown to study the role of high concentrations of seed Zn on plant yield. In all three locations, seeds with higher Zn did not affect the grain yield (Table 4). Similarly, the Zn-enriched seeds did not affect the Zn grain concentrations in the mature grain.
Table 4. Grain yield of rice grown from Zn-enriched seed by foliar application of Zn.
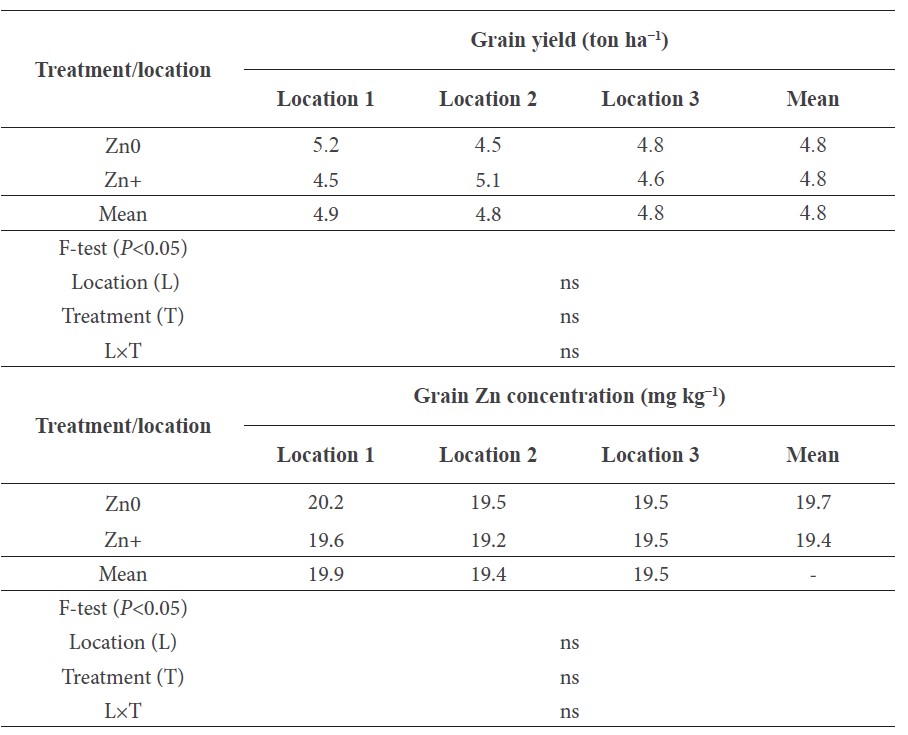
Note: ‘Zn0’ indicates that the plants were derived from low-Zn seeds (15-16 mg kg−1) and ‘Zn+’ indicates that the plants were derived from high-Zn seeds (18-21 mg kg−1). The data are the means of three independent replications. ‘ns’ indicates ‘no significant difference’ between field locations and Zn treatments at P<0.05.
DISCUSSION
The results obtained from this study confirmed that foliar application of Zn improved grain Zn in rice grown under farmers’ field conditions (Table 3). Previous studies have shown a similar affect, but most were conducted under controlled conditions at experimental research units of universities or institutes (Stomp et al., 2011; Phattarakul et al., 2012; Ram et al., 2016). The present results showed that increases in grain Zn also occurred under farmers’ field conditions with different soil chemical and physical properties.
Foliar application of Zn has been suggested as an effective method in correcting Zn deficiency and improving grain Zn concentration in rice (Wissuva et al., 2007; Jiang et al., 2008; Stomph et al., 2011). The Zn sprayed by foliar fertilizers is absorbed by the leaf epidermis, and remobilized and transferred into the rice grain through the phloem (Wu et al., 2010) with the contribution of several Zn-regulating transporter proteins (Li et al., 2013). These processes have been demonstrated in other crops, such as wheat, which efficiently remobilizes Zn from leaves to grain (Grewal and Graham, 1999), but the same has not always been the case with rice (Jiang et al., 2007; Wu et al., 2010). The phloem mobility of Zn in rice is less understood than in wheat, limiting attempts to maximize grain Zn enrichment (Cakmak, 2008).
The rice genotype and growth conditions have the largest effect on the agronomic effectiveness of foliar Zn spray to enhance grain Zn (Wissuwa et al., 2007; Phattarakul et al., 2012; Boonchuay et al., 2013; Mabesa et al., 2013). In good agreement with these previous studies, this study also showed that the effect of foliar Zn spray varied greatly between the three farmers’ fields (Table 3) with their different soils (Table 1). The loamy texture of the soil in location 1 and location 2 resulted in higher grain Zn concentration compared to the sandier soil in location 3, despite its higher soil Zn concentration.
Foliar application of Zn had no effect on grain yield and many of the yield components measured in this study. This was probably due to the high DTPA-extractable Zn concentrations in the soils (Table 1), which ranged from 1.3 mg kg−1 to 1.5 mg kg−1 – all well above the critical level of soil Zn deficiency at 0.5 mg kg−1 (Alloway, 2008; Mabesa et al., 2013). During field visits, we observed no visible symptoms of Zn deficiency on the plants. A previous study also reported that foliar application of Zn at various growth stages and frequencies had no effect on grain yield and yield components in rice plants (Boonchuay et al., 2013). An increase in grain yield after foliar application of Zn could be expected when plants are grown on a Zn-deficient soil, as reported in both rice and wheat (Wissuwa et al., 2007; Cakmak et al., 2010).
It has been well documented that using seeds with high Zn concentrations improved the growth and development of plants, especially under Zn-deficient soil conditions (Yilmaz et al., 1998; Prom-u-thai et al., 2012; Boonchuay et al., 2013). However, in the present study, using Zn-enriched seeds in farmers’ fields did not affect the grain yield and Zn concentration in the next crop compared to using non-enriched seeds. As with our first experiment, the high amounts of available Zn already in the soil was the likely reason.
In conclusion, foliar application of Zn improved rice grain Zn concentrations under field conditions on farms with different soil properties in Chiang Mai, Thailand. High concentrations of Zn in rice seed would help improve human nutrition and health as well as provide several agronomic benefits, including better seedling vigor and seed viability, higher yield, and reduced seed rate required for sowing, especially when plants are grown on Zndeficient soils. However, the foliar Zn spray in this study did not affect grain yield, which was probably due to the already high availability of Zn in the studied fields. Similar field experiments on farms, rather than under controlled research conditions, are needed in fields with Zn-deficient soils.
ACKNOWLEDGEMENTS
This study was financially supported by the HarvestZinc Project (www.harvestzinc.org), Thailand Research Fund (RSA6080024), and Functional Food Research Center for Wellbeing, Chiang Mai University, Thailand. The authors extend their thanks to all the farmers involved for their contribution to this research.
REFERENCES
Alloway, B.J. 2008. Zinc in soils and crop nutrition. International Zinc Association, Brussels. International Fertilizer Industry Association, Paris.
Black, R.E., Allen, L.H., Bhutta, Z.A., Caulfield, L.E., de Onis, M., Ezzati, M., Mathers, C., and Rivera, J. 2008. Maternal and child under nutrition: global and regional exposures and health consequences. Lancet. 371: 243–260. https://doi.org/10.1016/S0140-6736(07)61690-0
Boonchuay, P., Cakmak, I., Rerkasem, B., and Prom-U-Thai, C. 2013. Effect of different foliar zinc application at different growth stages on seed zinc concentration and its impact on seedling vigor in rice. Soil Science and Plant Nutrition. 59: 180–188. https://doi.org/10.1080/00380768.2013.763382
Bouis, H.E. 2002. Plant breeding: a new tool for fighting micronutrient malnutrition. Journal of Nutrition. 132 (3): 491S–494S. https://doi.org/10.1093/jn/132.3.491S
Cakmak, I. 2000. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytology. 146: 185–205. https://doi.org/10.1046/j.1469-8137.2000.00630.x
Cakmak, I. 2008. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant and Soil. 302: 1–17. https://doi.org/10.1007/s11104-007-9466-3
Cakmak, I., Kalayci, M., Kaya, Y., Torum, A.A., Aydin, N., Wang, Y., Arizoy, Z., Erdem, H., Yazici, A., Gokmen, O., et al. 2010. Biofortification and localization of zinc in wheat grain. Journal of Agriculture and Food Chemistry. 58: 9092-9102. https://doi.org/10.1021/jf101197h
Cakmak, I., and Kutman, U.B. 2017. Agronomic biofortification of cereals with zinc: a review. European Journal of Soil Science, In press.
Chang, H.B., Lin, C.W., and Huang, H.J. 2005. Zinc-induced cell death in rice (Oryza Sativa L.) Roots. Plant Growth Regulator. 46: 261–266. https://doi.org/10.1007/s10725-005-0162-0
Gibson, R.S., Manger, M.S., Krittaphol, W., Pongcharoen, T., Gowachirapant, S., Bailey, K.B., and Winichagoon, P. 2007. Does zinc deficiency play a role in stunting among primary school children in NE Thailand? British Journal of Nutrition. 97: 167–175. https://doi.org/10.1017/S0007114507250445
Graham, R.D., Senadhira, D., Beebe, S., Iglesias, C., and Monasterio, I. 1999. Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crops Research. 60: 57–80. https://doi.org/10.1016/S0378-4290(98)00133-6
Grewal, H.S., and Graham, R.D. 1999. Residual effects of subsoil zinc and oilseed rape genotype on the grain yield and distribution of zinc in wheat. Plant and Soil. 207: 29–36. https://doi.org/10.1023/A:1004479911399
Hotz, C., and Brown, K.H. 2004. Assessment of the risk of zinc deficiency in populations and options for its control. International nutrition foundation: for UNU.
Jaksomsak, P., Sangruan, P., Thomson, G., Rerkasem, B., Dell, B., and Prom-u-thai, C. 2014. Uneven distribution of zinc in the dorsal and ventral sections of rice grain. Cereal Chemistry. 91: 124–129. https://doi.org/10.1094/CCHEM-09-13-0185-R
Jiang, W., Struik, P.C., Lingna, J., Van Keulen, H., Ming, Z., and Stomph, T.J. 2007. Uptake and distribution of root-applied or foliar-applied 65Zn after flowering in aerobic rice. Annual Apply Biology. 150: 383–391. https://doi.org/10.1111/j.1744-7348.2007.00138.x
Jiang, W., Struik, P.C., Van Keulen, H., Zhao, M., Jin, L.N., and Stomph, T.J. 2008. Does increased zinc uptake enhance grain zinc mass concentration in rice? Annual Apply Biology. 153: 135–147. https://doi.org/10.1111/j.1744-7348.2008.00243.x
Li, S., Zhou, X., Huang, Y., Zhu, L., Zhang, S., Zhao, Y., Guo, J., Chen, J., and Chen, R. 2013. Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family in maize. BMC Plant Biology. 8(13): 114-125. https://doi.org/10.1186/1471-2229-13-114
Mabesa, R.L., Impa, S.M., Grewal, D., and Johnson-Beebout, S.E. 2013. Contrasting grain-Zn response of biofortification rice (Oryza sativa L.) breeding lines to foliar Zn application. Field Crops Research. 149: 223–233. https://doi.org/10.1016/j.fcr.2013.05.012
Marschner, P. 2012. Marschner's Mineral Nutrition of Higher Plants (Third Edition). Amsterdam, Natherlands, Elsevier/Academic Press.
National Research Council. 1989. Recommended Dietary Allowances. 10th Edition. National Academies Press.
Panomjan, N., Jamjod, S., Rerkasem, B., Dell, B., and Prom-u-thai, C. 2016. Variation of zinc concentration in rice caryopsis and husk among southern rice varieties grown in southern and northern Thailand. Chiang Mai University Journal of Natural Science. 15: 1-10. https://doi.org/10.12982/cmujns.2016.0001
Phattarakul, N., Rerkasem, B., Li, L.J., Wu, L.H., Zou, C.Q., Ram, H., Sohu, V.S., Kang, B.S., Surek, H., Kalayci, M., et al. 2012. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant and Soil. 361: 131–141. https://doi.org/10.1007/s11104-012-1211-x
Prom-u-thai, C., Rerkasem, B., Cakmak, I., and Huang, L. 2010. Zinc fortification of whole rice grain through parboiling process. Food Chemistry. 120: 858–863. https://doi.org/10.1016/j.foodchem.2009.11.027
Prom-u-thai, C., Rerkasem, B., Yazici, A., and Cakmak, I. 2012. Zinc priming promotes seed germination and seedling vigor of rice. Plant Nutrition and Soil Science. 175: 482–488. https://doi.org/10.1002/jpln.201100332
Ram, H., Rashid, A., Zhang, W., Duarte, A.P., Phattarakul, N., Simunji, S., Kalayci, M., Freitas, R., Rerkasem, B., Bal, R.S., et al. 2016. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant and Soil. 403: 389-401. https://doi.org/10.1007/s11104-016-2815-3
Saenchai, C., Prom-u-thai, C., Jamjod, S., Dell, B., and Rerkasem, B. 2012. Genotypic variation in milling depression of iron and zinc concentration in rice grain. Plant and Soil. 361 (1-2): 271-278. https://doi.org/10.1007/s11104-012-1228-1
Stomph, T.J., Choi, E.Y., and Stangoulis, J.C.R. 2011. Temporal dynamics in wheat grain zinc distribution: is sink limitation the key? Annual of Botany. 107: 927–937. https://doi.org/10.1093/aob/mcr040
Thurlow, R.A., Winichagoon, P., Pongcharoen, T., Gowachirapant, S., Boonpraderm, A., Manger, M.S., Bailey, K.B., Wasantwisut, E., and Gibson, R.S. 2005. Risk of zinc, iodine and other micronutrient deficiencies among school children in North East Thailand. European Journal of Clinical Nutrition. 60: 623–632. https://doi.org/10.1038/sj.ejcn.1602361
Welch, R.M., and Graham, R.D. 2004. Breeding for micronutrients in staple food crops from a human nutrition perspective. Journal of Experimental Botany. 55: 353–364. https://doi.org/10.1093/jxb/erh064
Wissuwa, M., Ismail, A.M., and Graham, R.D. 2007. Rice grain zinc concentrations as affected by genotype, native soil-zinc availability, and zinc fertilization. Plant and Soil. 306: 37–48. https://doi.org/10.1007/s11104-007-9368-4
Wu, C., Lu, L., Yang, X., Feng, Y., Wei, Y., Hao, H., Stoffella, P.J., and He, Z. 2010. Uptake, translocation, and remobilization of zinc absorbed at different growth stages by rice genotypes of different Zn densities. Journal of Agriculture and Food Chemistry. 58: 6767–6773. https://doi.org/10.1021/jf100017e
Yilmaz, A., Ekiz, H., Gültekin, I., Torun, B., Barut, H., Karanlik, S., and Cakmak, I. 1998. Effect of seed zinc content on grain yield and zinc concentration of wheat grown in zinc-deficient calcareous soils. Journal of Plant Nutrition. 21: 2257–2264. https://doi.org/10.1080/01904169809365559
Zarcinas, B.A., Cartwright, B., and Spouncer, L.R. 1987. Nitric acid digestion and multielement analysis of plant material by inductively coupled plasma spectrometry. Communication of Soil Science and Plant Analysis. 18: 131–146. https://doi.org/10.1080/00103628709367806
Piyawan Phuphong1 Ismail Cakmak2 Bernard Dell3, and Chanakan Prom-u-thai1,4*
1 Agronomy Division, Department of Plant and Soil Sciences, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand
2 Faculty of Engineering and Natural Sciences, Sabanci University, Istanbul 34956, Turkey
3 School of Veterinary and Life Sciences, Murdoch University, 90 South St, Murdoch, WA, 6150, Australia
4 Lanna Rice Research Center, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: chanakan15@hotmail.com, chanakan.p@cmu.ac.th
Total Article Views

