
Repellent Efficacy of Catnip (Nepeta cataria) Essential Oil and Its Topical Formulations Against Aedes aegypti
Nataya Sutthanont*, Elsa Van De Perre, Saw Aung Htun, and Phurich SaetungPublished Date : November 13, 2025
DOI : https://doi.org/10.12982/NLSC.2026.020
Journal Issues : Number 1, January-March 2026
Abstract This study evaluated the repellent efficacy of catnip (Nepeta cataria) essential oil and its potential for development into safe topical products. Pure catnip oil was first tested against Aedes aegypti using the Arm-in-Cage (AIC) method, providing complete protection (CPT) for 220.00 ± 6.32 minutes, thereby confirming its strong intrinsic repellent activity. To create user-appropriate products, a 20% dilution of catnip oil was incorporated into two topical prototypes: a spray and a gel. Both were assessed using the AIC method, with repellency recorded at 30-minute intervals over a 3-hour exposure period. The spray demonstrated a mean repellency of 84.70 ± 2.68%, while the gel maintained a slightly higher repellency of 91.83 ± 2.09%, with CPTs of 110.00 ± 20.00 and 130.00 ± 14.83 minutes, respectively. Skin patch testing confirmed that both formulations were well tolerated, with no irritation observed. To the best of our knowledge, this is the first study to evaluate catnip essential oil formulated into topical spray and gel prototypes and to assess both their repellency and skin tolerability in human volunteers. These findings indicate that catnip oil is an effective natural mosquito repellent and that 20% spray and gel formulations can provide safe, reliable protection against Ae. aegypti.
Keywords: Aedes aegypti, Catnip essential oil, Nepeta cataria, Topical repellent, Arm-in-Cage method
Graphical Abstract:
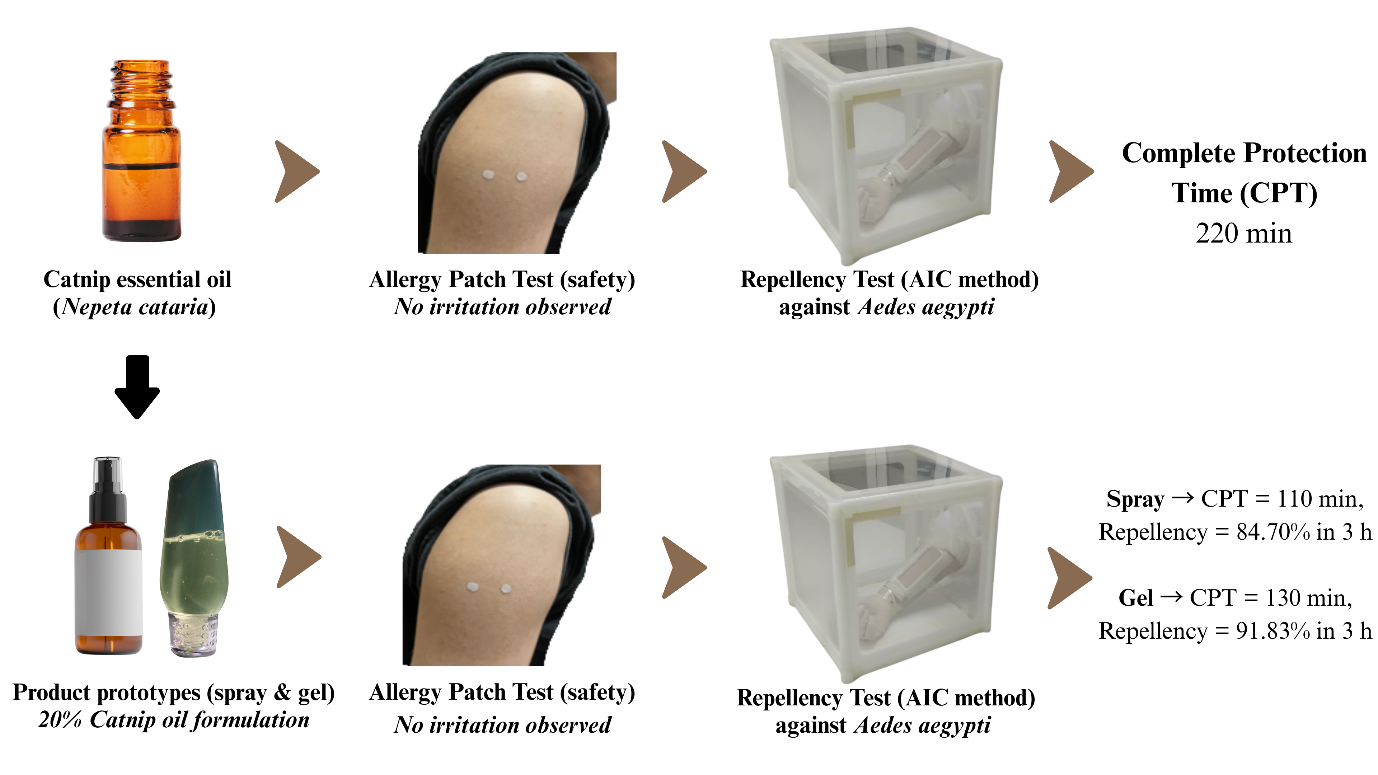
Funding: This research project has been supported by Mahidol University (Fundamental Fund: fiscal year 2025 by National Science Research and Innovation Fund (NSRF)).
citation: Sutthanont, N., Perre, E.V.D., Htun, S.A., and Saetung, P. 2026. Repellent efficacy of catnip (Nepeta cataria) essential oil and its topical formulations against Aedes aegypti. Natural and Life Sciences Communications. 25(1): e2026020.
INTRODUCTION
Mosquito-borne diseases, including dengue, Zika virus, chikungunya, and yellow fever, remain major global public health concerns, particularly in tropical and subtropical regions where Aedes aegypti serves as a highly efficient vector (Thongsripong et al., 2021). Dengue alone accounts for millions of cases annually and contributes to substantial morbidity and mortality (Mbaoma et al., 2025). Although vaccines for dengue have become available, their limited accessibility and high cost underscore the continued necessity of personal protective measures such as topical repellents (Debboun et al., 2014; Shen et al., 2025). Plant products have long been utilized across various regions of the world for killing or repelling mosquitoes, either as crude extracts, essential oils, or even whole plants burned or applied directly (Peterson and Coats, 2001). With growing environmental and safety concerns over synthetic chemicals, current research has increasingly focused on developing standardized, plant-based formulations that provide effective, safe, and sustainable mosquito repellency. Chemical repellents such as N,N-diethyl-3-methylbenzamide (DEET) has long been considered a gold standard repellent owing to its proven efficacy. Nevertheless, concerns regarding skin irritation, neurotoxicity at elevated concentrations, and environmental impacts have spurred interest in botanical repellents perceived as being safer and environmentally friendly (Robbins and Cherniack, 1986).
Catnip (Nepeta cataria) essential oil has emerged as a promising natural repellent candidate, with strong short-term activity against Ae. aegypti and other arthropods such as ticks, bed bugs, dust mites, and stable flies (Peterson et al., 2002; Bernier et al., 2005; Batume et al., 2024). However, the high volatility of essential oils limits the duration of protection, necessitating optimized formulations to enhance skin retention and prolong efficacy (Cimino et al., 2021; Chulikhit et al. 2022). Consequently, the choice of essential oil concentration in topical formulations must strike a balance between repellency and user safety. Low concentrations often yield short-lived protection, while excessively high levels may increase skin irritation without offering proportional improvements in efficacy (Ellse and Wall, 2014; Rahmi et al. 2021; Vora et al., 2024). Previous studies showed that 10–15% formulations of essential oils such as clove and eucalyptus can provide 1-2 hours of complete protection (Navayan et al., 2017; Luker et al., 2023). Mid-range concentrations of 15–30% are therefore commonly adopted in commercial botanical repellents, offering a practical compromise between safety and efficacy (Khater et al., 2019; Peng et al., 2022; Fatou and Müller, 2024).
The present study was conducted to evaluate the repellent efficacy of pure catnip essential oil, using the Arm-in-Cage (AIC) method to establish its maximum baseline protection against Ae. aegypti. In addition, 20% spray and gel formulations were developed and tested to assess their practical performance and skin tolerability, with the aim of advancing catnip-based repellents as safe and feasible natural alternatives to conventional synthetic products.
MATERIALS AND METHODS
Mosquito rearing
The research was conducted at the Department of Medical Entomology, Mahidol University, Bangkok, Thailand. The Bora Bora strain of Ae. aegypti was reared under controlled laboratory conditions, maintained at 25 ± 2°C with 65 ± 10% relative humidity and a 12-hour light/dark cycle. Larvae were raised in trays containing 1,000 mL of dechlorinated water and fed daily with powdered fish food. Pupae were subsequently transferred to adult mosquito cages (20 × 20 × 30 cm) until emergence. Adult mosquitoes were provided with a 5% sugar solution. For testing, female mosquitoes aged 5–7 days, non-blood-fed, and sugar-starved for 8–12 hours were selected.
Human volunteers
Six healthy volunteers (aged 20–50 years; 3 males and 3 females) were randomly recruited to participate in all phases of the study, including patch testing and repellency evaluation. Exclusion criteria included pregnancy, lactation, use of daily medication, or a history of sensitivity to topical products. Participants abstained from perfumes, fragranced products, repellents, and tobacco use for at least 12 hours prior to and during testing. Written informed consent was obtained from all participants before enrollment.
Ingredients
Catnip (N. cataria) essential oil was obtained in June 2025 from Chemipan Corporation Co., Ltd. (Bangkok, Thailand). Cosmetic-grade excipients used for formulation were purchased from Chanjao Longevity Co., Ltd. (Bangkok, Thailand). All materials were of cosmetic or analytical grade and were stored according to the suppliers’ recommendations until use.
Skin tolerability (patch test)
Skin tolerability was assessed using a patch test method adapted from the International Contact Dermatitis Research Group (ICDRG) guidelines (Fregert, 1981; Bruze and Svedman, 2025). First, 100% catnip essential oil was applied to a small area of the upper arm and observed at 30 minutes, 48 hours, and 96 hours. The same protocol was subsequently applied to the spray and gel formulations (20% essential oil) before repellency testing. Observations focused on signs of erythema, edema, itching, or blistering.
Six volunteers participated in the test with undiluted catnip essential oil and the two prototype formulations (spray and gel). For each product, a 10 µL aliquot was applied onto a 0.6 cm-diameter filter paper and fixed to the upper arm using a waterproof wound dressing (3M Nexcare™) for 48 hours. Reactions were evaluated at 30 minutes and 48 hours by a trained investigator under dermatologist supervision at the Department of Medical Entomology. A final photographic assessment was performed by participants at 96 hours. Clinical signs of erythema, pruritus, pain, edema, and vesiculation were scored according to ICDRG criteria (Table 1). If any positive reaction occurred, volunteers were instructed to immediately wash the site with water, notify the study supervisor, and seek medical care. Any participant showing reactions was withdrawn from further testing.
Table 1. Scoring criteria for skin reactions according to ICDRG guidelines.
|
Score |
Reaction description |
|
- |
Negative reaction |
|
? |
Doubtful reaction (faint erythema only, non-specific erythema) |
|
+ |
Weak positive reaction (erythema, infiltration, possibly papules) |
|
++ |
Strong positive reaction (erythema, infiltration, papules, or small vesicles) |
|
+++ |
Extreme positive reaction (intense erythema, infiltration, coalescing vesicles) |
|
IR |
Irritant reaction (shiny, glazed, peeling, pustules, necrosis, not allergic in type) |
Repellency testing of catnip essential oil
The repellent efficacy of undiluted catnip essential oil was assessed using the AIC method under controlled laboratory conditions, following WHO guidelines (WHO, 2009). Trials were conducted on healthy six volunteers. Adult female Ae. aegypti (5–7 days old, unfed) were used in each trial. A total of 250 mosquitoes were released into a standard cage (30 × 30 × 30 cm) and allowed to acclimate for 1–2 hours before testing. Before exposure, volunteers washed their forearms with unscented soap and rinsed thoroughly with water to eliminate potential interfering substances. A rectangular area (3 × 10 cm; 30 cm2) on the inner forearm (between the wrist and elbow) was marked as the test site. One hundred microliter of 100% catnip essential oil was applied evenly to the marked area and allowed to dry for approximately 1–2 minute before exposure. The untreated contralateral forearm served as the control. Tests were conducted by exposing the control and tested forearms in the test cage containing mosquitoes. To validate mosquito activity, the control arm was first exposed to the cage for 3 minutes. If ≥10 mosquitoes landed during this period, the repellency test was continued. The treated arm was then exposed to the mosquitoes for 3 minutes at 30-minute intervals until repellency failed. Complete protection time (CPT) was defined as the interval between application of the oil and the observation of the second mosquito landing on the treated arm. In accordance with the Thai Food and Drug Administration (Thai FDA) guidelines, a minimum CPT of 120 minutes against Ae. aegypti is required for a formulation to be considered effective (TISI, 2010). All tests were conducted between 08:00 and 15:00 h, corresponding to peak biting activity of Ae. aegypti. Repellency testing was conducted at 25 ± 2 °C and 60 ± 10 % relative humidity under controlled laboratory conditions.
Formulation of topical repellents
Two topical prototypes were prepared at a concentration of 20% v/v catnip essential oil: (i) a spray and (ii) a gel. Both formulations were homogenized and stabilized at room temperature for 24 hours prior to evaluation.
Preparation of repellent spray containing catnip essential oil
The composition of the spray is shown in Table 2. Catnip essential oil (20% w/w) was blended using an emulsification approach with PEG-40 hydrogenated castor oil (10% w/w) and dipropylene glycol (DPG) (10% w/w) until uniform. 95% Ethanol (40% w/w) was then added gradually under continuous stirring to aid solubilization and prevent phase separation. Witch hazel extract (Hamamelis virginiana) was incorporated as an aqueous distillate (20% w/w) and served as the final diluent. The mixture was stirred until homogeneous. The spray was allowed to stabilize at room temperature for 24 hours before further evaluation, tested for skin tolerability, and subsequently assessed for repellency using the AIC method.
Table 2. Composition of the repellent spray.
|
Ingredients |
% w/w |
Function |
|
Catnip essential oil |
20.0 |
Active repellent ingredient |
|
PEG-40 hydrogenated castor oil |
10.0 |
Solubilizer, emulsifier |
|
Dipropylene glycol |
10.0 |
Co-solvent |
|
95% Ethanol |
40.0 |
Solvent |
|
Witch hazel aqueous extract |
20.0 |
Diluent, stabilizer |
Preparation of repellent gel containing catnip essential oil
The formulation of the gel is presented in Table 3. The gel was prepared by dispersing Carbopol 940 (0.7% w/w) in purified water (approximately 70% w/w) with disodium ethylenediaminetetraacetic acid (EDTA) (0.05% w/w) under moderate stirring until fully hydrated. Phenoxyethanol (1.0% w/w) was then incorporated as a preservative, and the dispersion was partially neutralized with triethanolamine (TEA) to form a viscous base. In parallel, the oil/solubilizer phase was prepared by mixing catnip essential oil (20% w/w) with PEG-40 hydrogenated castor oil (20% w/w), DPG (10% w/w), and light mineral oil (5% w/w) until uniform. The oil phase was added slowly into the aqueous gel base under continuous stirring at approximately 800 rpm with a propeller stirrer at room temperature until a homogeneous mixture was obtained, after which the pH was adjusted to 6.0–6.5 using TEA. The final weight was adjusted to 100% w/w with purified water. The gel was allowed to stabilize at room temperature for 24 hours prior to evaluation.
Table 3. Composition of the repellent gel.
|
Ingredient |
% w/w |
Function |
|
Catnip essential oil |
20.0 |
Active repellent ingredient |
|
PEG-40 hydrogenated castor oil |
20.0 |
Solubilizer, emulsifier |
|
Dipropylene glycol (DPG) |
10.0 |
Co-solvent |
|
Light mineral oil |
5.0 |
Carrier, enhances skin feel |
|
Carbopol 940 |
0.7 |
Gelling agent |
|
Disodium EDTA |
0.05 |
Chelating agent |
|
Phenoxyethanol |
1.0 |
Preservative |
|
Purified water |
q.s. to 100 |
Vehicle |
|
Triethanolamine (TEA) |
q.s. |
Neutralizer, pH adjustment (6.0–6.5) |
Note: q.s. = quantum satis (sufficient quantity to make up to 100% of the formulation).
Repellency testing of topical repellents
The repellency of the spray and gel formulations was evaluated using the AIC method, following the procedures described for 100% catnip essential oil. A 3 × 10 cm (30 cm2) area on the inner forearm was treated with 100 µL of each formulation and allowed to dry for 1–2 minute before exposure. Both spray and gel formulations were dispensed using a Multipette® M4 with Combitips® advanced (Eppendorf AG, Hamburg, Germany) and spread evenly over the marked area using a sterile small glass rod. The treated and untreated control arms were alternately exposed to mosquitoes for 3 minutes at 30-minute intervals over a 3-hour period. During each exposure, mosquito landings and probing attempts were recorded to assess repellency.
Percentage protection and data analysis
Repellent efficacy was expressed as the percentage protection in mosquito landings on the treated arm compared with the untreated control. The percent repellency (%R) was calculated using the formula:
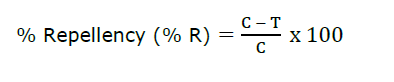
Where C is the number of mosquito landings on the untreated control arm and T is the number of mosquito landings on the treated arm. Volunteers were exposed to mosquitoes every 30 minutes for 3 hours, with each exposure lasting 3 minutes. The CPT was defined as the interval between repellent application and the second mosquito landing or probing on the treated area.
Data were summarized as mean ± standard error (SE). Comparisons of mean repellency and CPT between spray and gel formulations were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Statistical significance was set at P < 0.05. Analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA).
Ethical approvals
All experimental procedures involving human and animal subjects were reviewed and approved by the relevant institutional ethics committees. Human volunteer testing with the AIC method was conducted in accordance with the ethical standards of the Faculty of Tropical Medicine, Mahidol University (Bangkok, Thailand) and approved under Certificate of Ethical Approval No. MUTM 2024-091-01. Participants were monitored throughout the study for skin irritation or adverse reactions, and none were observed. Ethical approval for animal use was granted by the Institutional Animal Care and Use Committee (FTM-ACUC), Faculty of Tropical Medicine, Mahidol University, under Certificate No. FTM-ACUC 020/2024.
RESULTS
Physical characteristics of spray and gel formulations
The catnip spray formulation was a homogenous, light yellowish to slightly opalescent liquid. It possessed the characteristic herbal scent derived from the catnip essential oil. No phase separation or sedimentation was observed after 24 hours of stabilization. The catnip gel formulation exhibited low viscous, translucent with a pale yellow-amber color. It emitted a robust aroma characteristic of its high concentration of catnip essential oil. The gel maintained physical integrity, showing no water separation or oil separation or changes in consistency after 24 hours. Both formulations remained physically stable during the experimental period, showing no changes in color, odor, or phase separation. The pH of the spray and gel formulations was stable at 6.3 and 6.5, respectively, throughout room temperature storage.
Skin tolerability (patch test)
Patch testing was conducted on six volunteers with 100% catnip essential oil and with spray and gel formulations containing 20% catnip essential oil. No adverse skin reactions were observed at 30 minutes, 48 hours, or 96 hours in any of the tested products. None of the participants exhibited erythema, itching, pain, edema, vesiculation, or other signs of irritation during the observation period, indicating excellent skin tolerability of both the pure oil and formulated repellents.
Repellent efficacy of catnip formulations
Repellency tests were performed under controlled laboratory conditions at 25 ± 2 °C and 60 ± 10 % relative humidity, which remained stable throughout the testing period. Table 4 presents the results for the repellent efficacy of the catnip essential oil and its formulations evaluated based on mean repellency and CPT against Ae. aegypti landings. Pure, undiluted (100%) catnip essential oil was tested specifically to determine its maximum duration of complete effectiveness. The assay was terminated once the second mosquito landing occurred, resulting in a CPT of 220.00 ± 6.32 minutes. The CPT for the pure oil significantly exceeds the 120-minute benchmark required by the Thai FDA. For the 20% w/w formulations, efficacy was assessed as average repellency over a 3-hour exposure period. The gel formulation (91.83 ± 2.09%) exhibited significantly higher repellency than the spray formulation (84.70 ± 2.68%) (P < 0.05). Similarly, the gel provided a longer CPT (130.00 ± 14.83 minutes) compared with the spray (110.00 ± 20.00 minutes).
Table 4. Repellent efficacy of catnip essential oil and its formulations against Ae. aegypti.
|
Formulation |
Concentration (% w/w) |
Mean Repellency (%) (± SE) |
Complete Protection Time (CPT) (minutes, mean ± SE) |
|
Pure Essential Oil |
100 |
Not Applicable* |
220.00 ± 6.32ˣ |
|
Catnip Spray |
20 |
84.70 ± 2.68ᵇ |
110.00 ± 20.00ᶻ |
|
Catnip Gel |
20 |
91.83 ± 2.09ᵃ |
130.00 ± 14.83ʸ |
Note: Mean repellency is not applicable for the pure essential oil because its test was designed to measure CPT, not an average repellency over a fixed 3-hour period. Within each column, means followed by different superscript letters (a,b and x,y,z) are statistically significantly different from each other (ANOVA, Tukey's post hoc test, P < 0.05).
DISCUSSION
This study evaluated the repellent efficacy and dermatological safety of catnip (N. cataria) essential oil, tested both as a pure oil and in two topical prototypes (spray and gel at 20% w/w). Patch testing confirmed that catnip oil was well tolerated, with no irritation observed at 30 minutes, 48 hours, or 96 hours post-application. These findings indicate that catnip-based formulations are dermatologically safe under the tested conditions, consistent with previous reports that essential oils, when appropriately diluted, are generally safe for topical use (Botham et al., 1991; Basketter et al., 1997). Undiluted catnip oil provided 220.00 ± 6.32 minutes of CPT against Ae. aegypti, exceeding the 120-minute benchmark required by the Thai FDA and confirming its strong intrinsic repellent activity (Chauhan et al., 2005; Zhu et al., 2006; Birkett et al., 2011; Qualls et al., 2021). This performance aligns with earlier reports highlighting the potency of nepetalactone, the dominant constituent of N. cataria (Peterson et al., 2002; Birkett et al., 2011; Melo et al., 2021). As a bicyclic monoterpenoid, nepetalactone functions as both a spatial repellent and a behavioral disruptor by activating mosquito olfactory and irritant receptors, including TRPA1, thereby interfering with host-seeking behavior and reducing landing and probing attempts (Bernier et al., 2005; Melo et al., 2021). The strong repellency observed reinforces nepetalactone’s role as a potent natural repellent and underscores its relevance for product development, particularly when incorporated into formulations designed to enhance skin retention and chemical stability.
The 20% spray and gel formulations showed reduced performance compared with pure catnip oil, yet both provided substantial efficacy. The gel achieved a mean repellency of 91.83 ± 2.09% with a complete protection time (CPT) of 130.00 ± 14.83 minutes, while the spray provided 84.70 ± 2.68% repellency with a CPT of 110.00 ± 20.00 minutes. These values are comparable to those reported for other essential oils. For example, citronella oil typically provides protection ranging from 30 minutes to about 2 hours, depending on concentration and formulation (Kongkaew et al., 2011; Luker et al., 2023). Comparative laboratory studies have shown that well-known botanical repellents such as eucalyptus, clove, and citronella oils often exhibit a rapid decline in efficacy when formulated into topical products against Aedes and Culex mosquitoes (Luker et al., 2023; Noguera-Gahona et al., 2025). Although the incorporation of fixatives or advanced formulation strategies can extend protection, the inherent volatility of their active constituents remains the principal limitation (Reegan et al., 2014; Mitra et al., 2019; Iovinella et al., 2022). In addition, increased perspiration and elevated skin temperature, conditions common in tropical environments, can accelerate evaporation of repellents and destabilize the surface film, further reducing their duration of action (Verhulst et al., 2011; Mapossa et al., 2020). Although this study was conducted under controlled laboratory conditions (25 ± 2 °C and 60 ± 10 % RH), these findings highlight the importance of evaluating formulation performance under realistic tropical field conditions. Despite these challenges, both prototypes developed in the present study-maintained effectiveness within the tested duration and demonstrated excellent skin compatibility, underscoring their promise as consumer-friendly, plant-based alternatives (Dolan and Panella, 2011).
In addition to efficacy, the physical appearance and formulation characteristics of topical repellent products in this study were stable during the experimental period. In this study, the spray formulation appeared as a clear solution with uniform dispersion, whereas the gel was smooth, translucent, and easy to spread. The gel utilized Carbopol 940 as a gelling agent, which, upon neutralization with triethanolamine, forms a hydrogel network capable of entrapping essential oils and slowing their evaporation (Li et al., 2022; Chelu, 2024; Maurya et al., 2024). This structural property likely contributed to the slightly longer CPT and higher repellency observed for the gel compared with the spray (Shivhare et al., 2018). The inclusion of neutralizing agents, solvents, and stabilizers ensured appropriate viscosity and pH stability (within the skin‑friendly range of 5.5–6.5), enhancing overall formulation performance (Muangsiri et al., 2023). Solvents such as ethanol and propylene glycol not only aided in dispersing the essential oil but also likely improved film formation and skin adherence, contributing to repellency through enhanced delivery (Levang et al., 1999; Ardiningsih et al., 2025). In the spray formulation, PEG-40 hydrogenated castor oil acted as a solubilizer and emulsifier, ensuring uniform dispersion of catnip oil and stable film formation on the skin (Jang et al., 2015; Fonseca-Santos et al., 2018). Witch hazel functioned mainly as a stabilizer; although it lacks intrinsic repellency, it enhanced formulation stability and skin tolerability (Wójciak et al., 2025).
Overall, this study confirms the potential of catnip oil as a natural mosquito repellent and shows that 20% spray and gel formulations can provide safe, reliable protection against Ae. aegypti. Although less durable than pure oil, their stability, ease of application, and skin safety support further development. Future work should focus on optimizing formulations to prolong efficacy, as well as conducting field trials under tropical conditions with larger volunteer cohorts and consumer acceptability studies. Together, these findings suggest that catnip oil–based products could serve as practical natural alternatives to synthetic repellents such as DEET.
CONCLUSION
This study demonstrated that catnip (N. cataria) essential oil provides strong intrinsic repellency against Ae. aegypti, with complete protection exceeding 220.00 minutes under laboratory conditions. Topical spray and gel prototypes containing 20% catnip oil delivered safe, reliable protection while maintaining favorable physical stability and dermatological safety. Although protection was shorter than pure oil, these formulations show promise as natural alternatives to synthetic repellents. Future work should focus on optimizing formulations to prolong efficacy and validating results under tropical field conditions with larger volunteer cohorts and consumer acceptability studies. To the best of our knowledge, this is the first study to evaluate catnip essential oil formulated into topical spray and gel prototypes and to assess both their repellency and skin tolerability in human volunteers.
ACKNOWLEDGEMENTS
The authors thank the medical physician at the Hospital for Tropical Diseases, Faculty of Tropical Medicine, Mahidol University, for valuable supervision and advice regarding the allergy patch testing. We are also grateful to the staff of the Insecticide Research Unit, Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University, for their technical support and collaboration, which made this study possible.
AUTHOR CONTRIBUTIONS
Nataya Sutthanont designed and conducted all of the experiments, secured funding, performed statistical analyses, created data visualizations, and prepared both the draft and final version of the manuscript. Elsa Van De Perre contributed to the design of the spray formulation experiments and wrote the first draft of the manuscript. Saw Aung Htun and Phurich Saetung assisted in conducting the experiments. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ardiningsih, A.R., Hardyawati, W., Isningroom, M., Darsih, C., Praharasti, A.S., Sahid, M.N.A., and Laksitorini, M.D. 2025. Xanthan gum and propylene glycol effects on physicochemical and antioxidant activities of honey-based syrup. Natural and Life Sciences Communications. 26(3): e2025043. https://doi.org/10.12982/NLSC.2025.043
Basketter, D.A., Cookman, G., Gerberick, G.F., Hamaide, N., and Potokar, M. 1997. Skin sensitization thresholds: Determination in predictive models. Food and Chemical Toxicology. 35(3-4): 417-425. https://doi.org/10.1016/S0278-6915(97)00129-4
Batume, C., Mulongo, I.M., Ludlow, R., Ssebaale, J., Randerson, P., Pickett, J.A., Mukisa, I.M., and Scofield, S. 2024. Evaluating repellence properties of catnip essential oil against the mosquito species Aedes aegypti using a Y-tube olfactometer. Scientific Reports. 14(1): 2269. https://doi.org/10.1038/s41598-024-52715-y
Bernier, U.R., Furman, K.D., Kline, D.L., Allan, S.A., and Barnard, D.R. 2005. Comparison of contact and spatial repellency of catnip oil and N,N-diethyl-3-methylbenzamide (DEET) against mosquitoes. Journal of Medical Entomology. 42(3): 306-311. https://doi.org/10.1093/jmedent/42.3.306
Birkett, M.A., Hassanali, A., Hoglund, S., Pettersson, J., and Pickett, J.A. 2011. Repellent activity of catmint, Nepeta cataria, and iridoid nepetalactone isomers against Afro-tropical mosquitoes, ixodid ticks and red poultry mites. Phytochemistry. 72(1): 109-114. https://doi.org/10.1016/j.phytochem.2010.09.016
Botham, P.A., Basketter, D.A., Maurer, T., Mueller, D., Potokar, M., and Bontinck, W.J. 1991. Skin sensitization-a critical review of predictive test methods in animals and man. Food and Chemical Toxicology. 29(4): 275-286. https://doi.org/10.1016/0278-6915(91)90025-3
Bruze, M. and Svedman, C. 2025. Clarification and modification of the international contact dermatitis research group classification of patch test reactions on behalf of the international contact dermatitis research group. Dermatitis: Contact, Atopic, Occupational, Drug. 36(5): 440–446. https://doi.org/10.1089/derm.2024.0365
Chauhan, K.R., Klun, J.A., Debboun, M., and Kramer, M. 2005. Feeding deterrent effects of catnip oil components compared with two synthetic amides against Aedes aegypti. Journal of Medical Entomology. 42(4): 643-646. https://doi.org/10.1603/0022-2585(2005)042[0643:FDEOCO]2.0.CO;2
Chelu, M. 2024. Hydrogels with essential oils: Recent advances in designs and applications. Gels. 10(10): 636. https://doi.org/10.3390/gels10100636
Chulikhit, Y., Maneenet, J., Monthakantirat, O., Daodee, S., and Khamphukdee, C. 2022. The repellent potential of herbal oils alone and in combination in mouse behavioral models (Mus musculus). Chiang Mai University Journal of Natural Sciences. 21(3): e2022049. https://doi.org/10.12982/CMUJNS.2022.049
Cimino, C., Maurel, O.M., Musumeci, T., Bonaccorso, A., Drago, F., Souto, E.M.B., Pignatello, R., and Carbone, C. 2021. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics. 13(3): 327. https://doi.org/10.3390/pharmaceutics13030327
Debboun, M., Frances, S.P., and Strickman, D. 2014. Insect repellents handbook. 2nd ed. CRC Press, Boca Raton, FL. https://doi.org/10.1201/b17407
Dolan, M.C. and Panella, N.A. 2011. A review of arthropod repellents. p. 1–19. In G.E. Paluch and J.R. Coats (eds.) Recent developments in invertebrate repellents. ACS Symposium Series. American Chemical Society, Washington, D.C. https://doi.org/10.1021/bk-2011-1090.ch001
Ellse, L. and Wall, R. 2014. The use of essential oils in veterinary ectoparasite control: A review. Medical and Veterinary Entomology. 28(3):233-243. https://doi.org/10.1111/mve.12033
Fatou, M. and Müller, P. 2024. In the arm-in-cage test, topical repellents activate mosquitoes to disengage upon contact instead of repelling them at distance. Scientific Reports. 14(1): 24745. https://doi.org/10.1038/s41598-024-74518-x
Fonseca-Santos, B., Pacheco, C., Pinto, M., and Chorilli, M. 2018. An effective mosquito-repellent topical product from liquid crystal-based tea tree oil. Industrial Crops and Products. 128: 488-495. https://doi.org/10.1016/j.indcrop.2018.11.020
Fregert, S. 1981. Manual of contact dermatitis. 2nd ed. Munksgaard, Copenhagen; Year Book Medical Publishers, Chicago.
Iovinella, I., Caputo, B., Cobre, P., Manica, M., Mandoli, A., and Dani, F.R. 2022. Advances in mosquito repellents: Effectiveness of citronellal derivatives in laboratory and field trials. Pest Management Science. 78(12): 5106-5112. https://doi.org/10.1002/ps.7127
Jang, H.J., Shin, C.Y., and Kim, K.B. 2015. Safety evaluation of polyethylene glycol (PEG) compounds for cosmetic use. Toxicological Research. 31(2): 105-136. https://doi.org/10.5487/TR.2015.31.2.105
Khater, H., Selim, A., Murugan, K., Vaz, N., and Govindarajan, M. 2019. Commercial mosquito repellents and their safety concerns. p. 1–22. In F.H. Kasenga (ed) Malaria. IntechOpen, London. https://doi.org/10.5772/intechopen.86000
Kongkaew, C., Sakunrag, I., Chaiyakunapruk, N., and Tawatsin, A. 2011. Effectiveness of citronella preparations in preventing mosquito bites: Systematic review of controlled laboratory experimental studies. Tropical Medicine and International Health. 16(7): 802-810. https://doi.org/10.1111/j.1365-3156.2011.02781.x
Levang, A.K., Zhao, K., and Singh, J. 1999. Effect of ethanol/propylene glycol on the in vitro percutaneous absorption of aspirin, biophysical changes and macroscopic barrier properties of the skin. International Journal of Pharmaceutics. 181(2): 255-263. https://doi.org/10.1016/S0378-5173(99)00055-1
Li, M., Kamdenlek, P., Kuntanawat, P., Eawsakul, K., Porntaveetus, T., Osathanon, T., and Manaspon, C. 2022. In vitro preparation and evaluation of chitosan/pluronic F-127 hydrogel as a local delivery of crude extract of phycocyanin for treating gingivitis. Chiang Mai University Journal of Natural Sciences. 21(4): e2022052. https://doi.org/10.12982/CMUJNS.2022.052
Luker, H.A., Salas, K.R., Esmaeili, D., Holguin, F.O., Bendzus-Mendoza, H., and Hansen, I.A. 2023. Repellent efficacy of 20 essential oils on Aedes aegypti mosquitoes and Ixodes scapularis ticks in contact-repellency assays. Scientific Reports. 13(1): 1705. https://doi.org/10.1038/s41598-023-28820-9
Mapossa, A.B., Sitoe, A., Focke, W.W., Izadi, H., du Toit, E.L., Androsch, R., Sungkapreecha, C., and van der Merwe, E.M. 2020. Mosquito repellent thermal stability, permeability and air volatility. Pest Management Science. 76(3): 1112-1120. https://doi.org/10.1002/ps.5623
Maurya, R., Misro, L., Boini, T., Radhakrishnan, T., Nair, P.G., Gaidhani, S.N., and Jain, A. 2024. Transforming medicinal oil into advanced gel: An update on advancements. Gels. 10(5): 342. https://doi.org/10.3390/gels10050342
Mbaoma, O.C., Thomas, S.M., and Beierkuhnlein, C. 2025. Significance of vertical transmission of arboviruses in mosquito-borne disease epidemiology. Parasites & Vectors. 18(1): 137. https://doi.org/10.1186/s13071-025-06761-8
Melo, N., Capek, M., Arenas, O.M., Afify, A., Yilmaz, A., Potter, C.J., Laminette, P.J., Para, A., Gallio, M., and Stensmyr, M.C. 2021. The irritant receptor TRPA1 mediates the mosquito repellent effect of catnip. Current Biology. 31(9): 1988-1994.e5. https://doi.org/10.1016/j.cub.2021.02.010
Mitra, S., Rodriguez, S.D., Vulcan, J., Cordova, J., Chung, H.-N., Moore, E., Kandel, Y., and Hansen, I.A. 2019. Efficacy of active ingredients from the EPA 25(b) list in reducing attraction of Aedes aegypti (Diptera: Culicidae) to humans. Journal of Medical Entomology. 57(2): 477-484. https://doi.org/10.1093/jme/tjz178
Muangsiri, W., San, T.T., and Werawatganone, P. 2023. Development of topical mosquito repellent emulgels containing Zanthoxylum limonella essential oils. The Thai Journal of Pharmaceutical Sciences. 47(2): e7. https://doi.org/10.56808/3027-7922.2819
Navayan, A., Moghimipour, E., Khodayar, M., Vazirianzadeh, B., Siahpoosh, A., Valizadeh, M., and Mansourzadeh, Z. 2017. Evaluation of the mosquito repellent activity of nano-sized microemulsion of Eucalyptus globulus essential oil against Culicinae. Jundishapur Journal of Natural Pharmaceutical Products. 12(4): e55626. https://doi.org/10.5812/jjnpp.55626
Noguera-Gahona, M., Peña-Moreno, C., Quiñones-Sobarzo, N., Weinstein-Oppenheimer, C., Guerra-Zúñiga, M., and Collao-Ferrada, X. 2025. Repellents against Aedes aegypti bites: Synthetic and natural origins. Frontiers in Insect Science. 4:1510857. https://doi.org/10.3389/finsc.2024.1510857
Peng, Z.Y., He, M.Z., Zhou, L.Y., Wu, X.Y., Wang, L.M., Li, N., and Deng, S.Q. 2022. Mosquito repellents: Efficacy tests of commercial skin-applied products in China. Molecules. 27(17): 5534. https://doi.org/10.3390/molecules27175534
Peterson, C. and Coats, J. 2001. Insect repellents-past, present and future. Pesticide Outlook. 12: 154. https://doi.org/10.1039/b106296b
Peterson, C.J., Nemetz, L.T., Jones, L.M., and Coat, J.R. 2002. Behavioral activity of catnip (Lamiaceae) essential oil components to the German cockroach (Blattodea: Blattellidae). Journal of Economic Entomology. 95(2): 377-380. https://doi.org/10.1603/0022-0493-95.2.377
Qualls, W.A., Xue, R.D., Farooq, M., Peper, S.T., Aryaprema, V., Blore, K., Weaver, R., Autry, D., Talbalaghi, A., Kenar, J., et al. 2021. Evaluation of lotions of botanical-based repellents against Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology. 58(2): 979-982. https://doi.org/10.1093/jme/tjaa244
Rahmi, D., Yunilawati, R., Jati, B., Setiawati, I., Riyanto, A., Batubara, I., and Astuti, R. 2021. Antiaging and skin irritation potential of four main Indonesian essential oils. Cosmetics. 8: 94. https://doi.org/10.3390/cosmetics8040094
Reegan, A.D., Kannan, R.V., Paulraj, M.G., and Ignacimuthu, S. 2014. Synergistic effects of essential oil-based cream formulations against Culex quinquefasciatus Say and Aedes aegypti L. (Diptera: Culicidae). Journal of Asia-Pacific Entomology. 17(3): 327-331. https://doi.org/10.1016/j.aspen.2014.02.008
Robbins, P.J. and Cherniack, M.G. 1986. Review of the biodistribution and toxicity of the insect repellent N,N-diethyl-m-toluamide (DEET). Journal of Toxicology and Environmental Health. 18(4): 503-525. https://doi.org/10.1080/15287398609530891
Shen, J., Kharitonova, E., Tytula, A., Zawieja, J., Aballea, S., Biswal, S., Sharma, M., Rungmaitree, S., Sruamsiri, R., Wallace, D., et al. 2025. Vaccination strategies, public health impact and cost-effectiveness of dengue vaccine TAK-003: A modeling case study in Thailand. PLoS Medicine. 22(6): e1004631. https://doi.org/10.1371/journal.pmed.1004631
Shivhare, R., Kamble, M., Kar Mahapatra, D., Ingole, A., Baheti, J., and Mahapatra, D.K. 2018. Development of mosquito repellant gel formulations from various natural volatile oils: Comparative study with the marketed formulation Odomos®. Journal of Drug Delivery and Therapeutics. 8: 106-110. https://doi.org/10.22270/jddt.v8i6.2031
Thongsripong, P., Hyman, J.M., Kapan, D.D., and Bennett, S.N. 2021. Human-mosquito contact: A missing link in our understanding of mosquito-borne disease transmission dynamics. Annals of the Entomological Society of America. 114(4): 397-414. https://doi.org/10.1093/aesa/saab011
Thai Industrial Standards Institute (TISI). 2010. Mosquito repellents: Thai industrial standards. Ministry of Industry, Bangkok, Thailand.
Verhulst, N.O., Qiu, Y.T., Beijleveld, H., Maliepaard, C., Knights, D., Schulz, S., Berg-Lyons, D., Lauber, C.L., Verduijn, W., Haasnoot, G.W., et al. 2011. Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS One. 6(12): e28991. https://doi.org/10.1371/journal.pone.0028991
Vora, L.K., Gholap, A.D., Hatvate, N.T., Naren, P., Khan, S., Chavda, V.P., Balar, P.C., Gandhi, J., and Khatri, D.K. 2024. Essential oils for clinical aromatherapy: A comprehensive review. Journal of Ethnopharmacology. 330: 118180. https://doi.org/10.1016/j.jep.2024.118180
WHO. 2009. Guidelines for efficacy testing of mosquito repellents for human skin. World Health Organization, Geneva, Switzerland.
Wójciak, M., Pacuła, W., Sowa, I., Feldo, M., Graczyk, F., and Załuski, D. 2025. Hamamelis virginiana L. in skin care: A review of its pharmacological properties and cosmetological applications. Molecules. 30(13): 2744. https://doi.org/10.3390/molecules30132744
Zhu, J., Zeng, X., Yanma, Liu, T., Qian, K., Han, Y., Xue, S., Tucker, B., Schultz, G., and Coats, J. 2006. Adult repellency and larvicidal activity of five plant essential oils against mosquitoes. Journal of the American Mosquito Control Association. 22(3): 515-522. https://doi.org/10.2987/8756-971X(2006)22[515:ARALAO]2.0.CO;2
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Nataya Sutthanont1, *, Elsa Van De Perre2, Saw Aung Htun2, and Phurich Saetung2
1 Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand.
2 Department of Medical Science, Faculty of Science, Rangsit University, Pathumthani 12000, Thailand.
Corresponding author: Nataya Sutthanont, E-mail: nataya.sut@mahidol.ac.th
ORCID iD:
Nataya Sutthanont: https://orcid.org/0000-0003-0198-2109
Saw Aung Htun: https://orcid.org/0000-0003-1250-2502
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: September 23, 2025;
Revised: November 5, 2025;
Accepted: November 6, 2025;
Online First: November 13, 2025

