
Plant Growth-Promoting Mechanisms of Deep-Sea Actinobacterium Tsukamurella sp. MT6.1 and Its Potential Role in Salt Stress Mitigation in Plants
May Tharaphu Thein Win, Inthira Wongchomphu, Kawiporn Chinachanta, Pharada Rangseekaew, and Wasu Pathom-aree*Published Date : November 11, 2025
DOI : https://doi.org/10.12982/NLSC.2026.011
Journal Issues : Number 1, January-March 2026
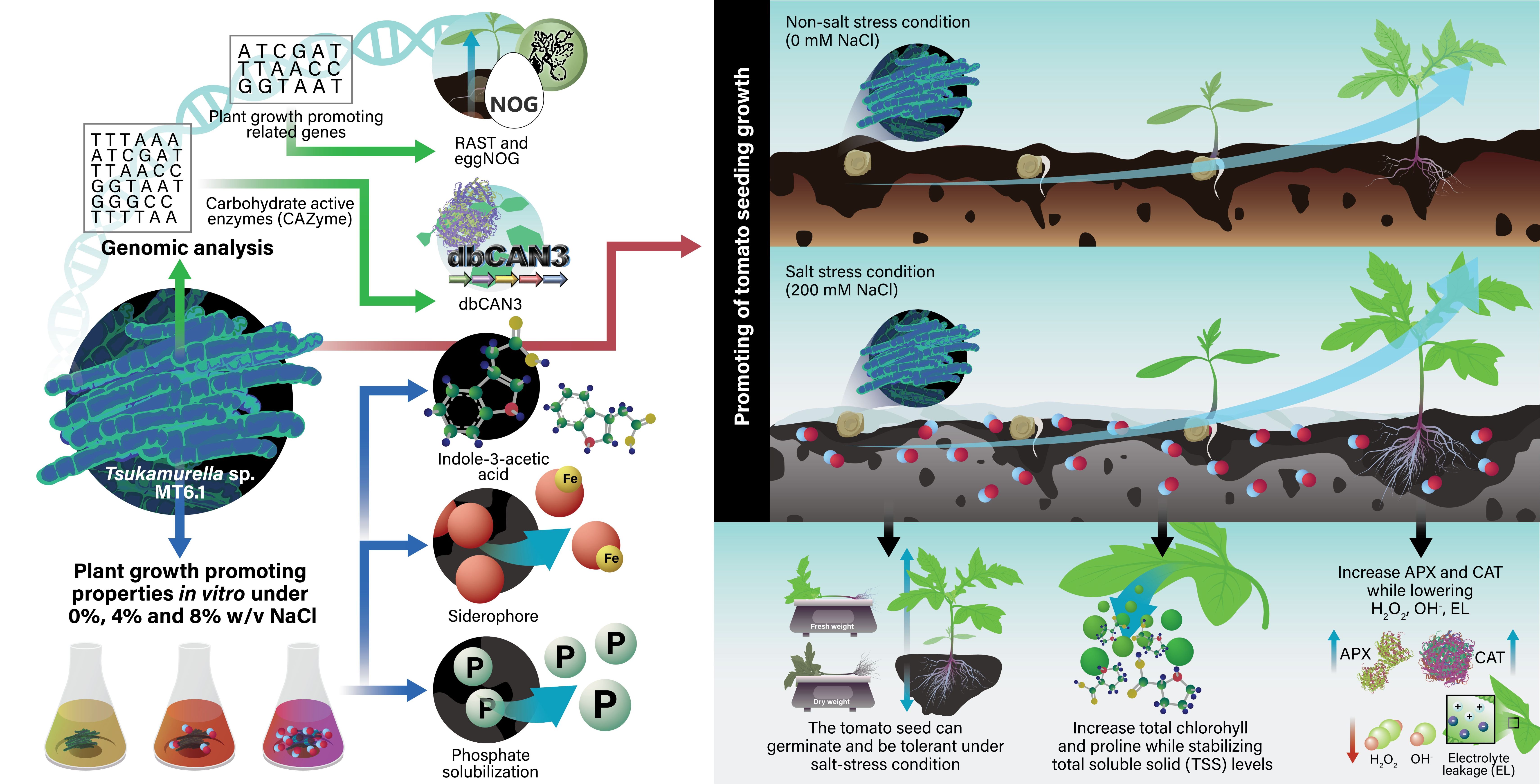
Abstract Tsukamurella sp. MT6.1, a deep-sea actinobacterium isolated from the Mariana Trench, was evaluated for its plant growth-promoting traits, and effects on tomato (Solanum lycopersicum) under salinity stress. Genome analysis of Tsukamurella sp. MT6.1 revealed genes for indole-3-acetic acid (IAA) biosynthesis, siderophore production, phosphate solubilization, potassium and nitrogen metabolism, and 102 carbohydrate-active enzymes (CAZymes), indicating versatile metabolic and plant-beneficial capabilities. In vitro assays confirmed IAA production and siderophore secretion, with phosphate solubilization notably enhanced by salt stress. Inoculation of tomato plants with Tsukamurella sp. MT6.1 enhanced shoot and root growth, fresh and dry biomass, and physiological responses under both non-stress and 200 mM NaCl conditions. Biochemical analyses showed reduced oxidative stress, increased chlorophyll content, elevated proline accumulation, and improved membrane stability in inoculated plants. Antioxidative enzyme activities, including catalase and ascorbate peroxidase, were also elevated under salt stress, further supporting stress mitigation by Tsukamurella sp. MT6.1. These findings demonstrate that Tsukamurella sp. MT6.1 possesses plant growth-promoting properties and employs multiple mechanisms to enhance plant performance, highlighting its potential as a bioinoculant to improve crop productivity under salinity stress.
Keywords: Tsukamurella sp., Plant growth promoting actinobacteria, Tomato, Salinity stress, Climate resilient agriculture
Graphical Abstract:
Funding: National Research Council of Thailand (NRCT) and Chiang Mai University (Grant Number N42A670709).
Citation: Tharaphu Thein Win, M., Wongchomphu, I., Chinachanta, K., Rangseekaew, P., and Pathom-aree, W. 2026. Plant growth-promoting mechanisms of deep-sea Actinobacterium Tsukamurella sp. MT6.1 and its potential role in salt stress mitigation in plants. Natural and Life Sciences Communications. 25(1): e2026011.
INTRODUCTION
Salinity is a growing global concern, currently affecting about 1,381 million hectares—over 10% of the world's land area—and threatening another 1 billion hectares due to climate change and poor land management (FAO, 2024). Salt stress impedes plant growth and development by inducing osmotic and oxidative stress, which disrupts essential biochemical and physiological processes as a result reduce plant productivity and quality (Parihar et al., 2014). Over 20% of the world’s cultivated land and 33% of irrigated land are already affected by high salinity, and with climate change accelerating soil salinization through rising global temperatures, increased evaporation, and reduced precipitation, salinity-affected areas are projected to expand at a rate of 10% annually, potentially reaching 50% by 2050 (Shrivastava and Kumar, 2015; Mukhopadhyay et al., 2021; Ullah et al., 2021).
To mitigate detrimental impacts of salinity in agriculture, various strategies, including biological, chemical, and physical approaches, have been explored (Ullah et al., 2021; Ondrasek et al., 2022). Among these, biological approaches, such as the development of salt-tolerant crop varieties through conventional breeding or genetic engineering, are often costly, labor-intensive, and time-consuming (Zaman et al., 2018; Ullah et al., 2021). In contrast, microbial technologies, particularly the application of plant growth-promoting bacteria (PGPB), provide a sustainable and eco-friendly alternative for alleviating the adverse effects of salt stress (Chinachanta et al., 2021; Gao et al., 2022; Kumawat et al., 2022; Sagar et al., 2022; Teo et al., 2022).
Plant growth-promoting bacteria enhance plant stress tolerance through multiple mechanisms, including the production of phytohormones such as indole-3-acetic acid (IAA) (Duca and Glick, 2020; Chinachanta et al., 2023), synthesis of osmolytes (Li et al., 2023), and solubilization of essential nutrients (Mohammadipanah and Zamanzadeh, 2019; Liang et al., 2024). Among PGPB, marine-derived actinobacteria have gained attention for their ability to mitigate salt stress in various crops, including tomato (Palaniyandi et al., 2014; Gong et al., 2020; Rangseekaew et al., 2021; Rangseekaew et al., 2022), soybean (Khan et al., 2021), and wheat (Sadeghi et al., 2011; Djebaili et al., 2021). Several marine actinobacterial strains, such as Dermacoccus abyssi MT1.1T (Rangseekaew et al., 2022), Glutamicibacter halophytocola KLBMP 5180 (Xiong et al., 2019) and Nocardiopsis yanglingensis (Suksaard et al., 2017), have demonstrated plant growth-promoting traits under saline conditions by producing IAA, siderophores, and phosphate-solubilizing compounds while also enhancing osmolyte accumulation.
Tomato (Solanum lycopersicum) is a globally important crop, valued for its nutritional and economic significance, and is widely cultivated in regions prone to salinity stress. Although wild tomato relatives exhibit considerable salt tolerance, domesticated varieties are more sensitive, often resulting in reduced growth and yield under saline conditions (Shrivastava and Kumar, 2015; Pailles et al., 2020). Its moderate salt sensitivity and well-characterized genetics make tomato an ideal model for evaluating strategies to enhance plant resilience, including the application of salt-tolerant plant growth-promoting bacteria (PGPB) as an eco-friendly approach to mitigate salt stress (Rothan et al., 2019; Rangseekaew et al., 2022).
Deep-sea ecosystems, covering more than 70% of Earth’s surface, harbor microorganisms adapted to high salinity, pressure, and nutrient limitation, making them a unique source of stress-tolerant microbes (Austin, 1988). Deep-sea actinobacteria, in particular, have recently been shown to confer salinity tolerance in tomato through compatible solute accumulation and antioxidative defense modulation (Rangseekaew et al., 2021, 2022). While certain deep-sea actinobacterial genera such as Dermacoccus have been investigated, the plant growth-promoting (PGP) mechanisms of other deep-sea actinobacterial genera remain poorly understood, including within the genus Tsukamurella. Tsukamurella sp. MT6.1, a deep-sea actinobacterium, is hypothesized to harbor unique traits for PGP mechanisms that differentiate it from known strains (e.g., in nutrient acquisition). While some non-genomic studies have demonstrated PGP potential in certain Tsukamurella strains such as Tsukamurella paurometabola strain C-924 (Marín et al., 2013), a comprehensive genome analysis of the plant growth promotion traits and the carbohydrate-active enzymes (CAZymes) has yet to be reported for the genus. Therefore, this study investigates the genomic basis of its plant growth-promoting potential, focusing on genes involved in nutrient acquisition and evaluates its ability to enhance tomato seedling growth and stress tolerance under saline conditions.
MATERIALS AND METHODS
Bacterial strain and growth condition
Tsukamurella sp. MT6.1, previously isolated from sediment collected from the Mariana Trench in the northwest Pacific Ocean, was utilized in this study (Pathom-aree et al., 2006). The strain was regularly cultivated and preserved on International Streptomyces Project 2 (ISP2) agar or Glucose Yeast Extract (GYE) medium for experimental purposes. Its genome, provided by Associate Professor Dr. Wasu Pathom-aree, was analyzed for genes related to plant growth-promoting traits.
Analysis of genome for plant growth-promoting properties of Tsukamurella sp. MT6.1
Bioinformatic tools such as the Rapid Annotation Subsystem Technology (RAST) (Aziz et al., 2008) server were employed to identify genes encoding proteins significant for plant growth promotion. Additionally, the eggNOG-mapper (Huerta-Cepas et al., 2018) was used for genes linked plant growth promotion. Carbohydrate-active enzymes of Tsukamurella sp. MT6.1 were annotated using the dbCAN2 meta server (Zheng et al., 2023).
Plant growth-promoting properties of Tsukamurella sp. MT6.1 under salt stress in vitro
Four plant growth-promoting properties (IAA production, siderophore production, phosphate solubilization, and ACC deaminase activity) of Tsukamurella sp. MT6.1 were determined under induced salinity stress at 4%, and 8% (w/v) NaCl. IAA production was quantified in GYE broth supplemented with 2 mg mL⁻¹ L-tryptophan, incubated for 7 days at 110 rpm in the dark, and determined colorimetrically using the Salkowski method as described by Rangseekaew et al. (2022). Siderophore production was qualitatively detected on chrome azurol S (CAS) agar (Schwyn and Neilands, 1987) after 7 days of incubation at 28°C in the dark. For quantitative assays, siderophores were produced in King’s B broth and hydroxamate- and catecholate-type siderophores were determined using the ferric perchlorate assay (Atkin et al., 1970) and the Arnow assay (Arnow, 1937), respectively. Phosphate solubilization was evaluated on Pikovskaya’s agar (PVK) containing 0.5% (w/v) tri-calcium phosphate and incubated at 28°C for 7 days (Nautiyal, 1999). Released phosphate was quantified in PVK broth supplemented with tri-calcium phosphate using the colorimetric method of Fiske and Subbarow (1925). ACC deaminase activity was assessed on DF minimal salt medium supplemented with three nitrogen sources: 3 mmol L⁻1 ACC, 3 mmol L⁻1 (NH4)2SO4 (positive control), and no nitrogen source (negative control), according to Palaniyandi et al. (2014).
Mitigation of salt stress in tomato by Tsukamurella sp. MT6.1
Preparation of Tsukamurella sp. MT6.1 inoculum
Tsukamurella sp. MT6.1 was initially grown on glucose yeast extract (GYE) agar at room temperature for three days. Then, five agar plugs of Tsukamurella sp. MT6.1 were inoculated in 100 ml GYE broth at ambient temperature on a shaker operating at 110 rpm for a duration of 7 days. After incubation, cells were harvested by centrifugation at 5,000 rpm for 15 minutes. The pellet was then resuspended in sterile distilled water, adjusted to a concentration of 107–108 CFU/mL, and used as the inoculum for plant experiments.
Enhancement of tomato seedlings growth under salt stress
Tomato seeds from Chia Tai brand were surface sterilized using the method described by Botta et al. (2013). In summary, the seeds were wrapped in cloth, washed with 70% ethanol for 3 minutes, and then immersed in a 5% sodium hypochlorite (NaClO) solution for 5 minutes. The seeds were then rinsed 6 times with sterile distilled water. To assess sterilization efficiency, five sterilized seeds were placed on nutrient agar and incubated at room temperature for 24 hours. The surface-sterilized seeds were inoculated with Tsukamurella sp. MT6.1 cell suspension (108 CFU/mL (OD600=1)), while control seeds were soaked in a sterile distilled water. The inoculated seeds were then planted in small pots (5 cm in diameter) containing 60 g of sterile planting material for 7 days and watered once a day under controlled laboratory conditions at room temperature and a 12-hour light/dark cycle was maintained to simulate natural photoperiod conditions. After that, salt stress was imposed on the uniformed tomato seedlings that have been grown for 7 days by applying NaCl solutions at the concentration of 200 mM, marking the start of the salt stress experiment (day 0). The experiment included the following treatments: (1) control (non-inoculated with stress) using sterile distilled water, and (2) inoculation with Tsukamurella sp. MT6.1 cell suspension (107–108 CFU/mL in sterile distilled water). Tsukamurella sp. MT6.1 cell suspension was applied to the soil near the stem area at 1 mL per pot. Additionally, a non-stressed treatment without bacterial inoculation was included for comparison. Each treatment consisted of three biological replicates (pots), with one seedling per pot.
Biochemical analysis in tomato plants
Proline accumulation in tomato leaves was determined following the colorimetric method of Bates et al. (1973). Leaf tissue (250 mg) was homogenized in 95% ethanol, centrifuged, and the supernatant reacted with acid ninhydrin reagent at 100°C. The chromophore was extracted in toluene, and absorbance was measured at 520 nm. Proline concentration was calculated from a standard.
Total soluble sugar (TSS) was quantified using the phenol–sulfuric acid method of DuBois et al. (1956). Leaf tissue (10 mg) was extracted in 80% ethanol, centrifuged, and the supernatant reacted with phenol and concentrated H2SO4. The solution was incubated at room temperature for color development, and absorbance was recorded at 490 nm. TSS concentration was determined from a standard curve.
Chlorophyll content was estimated using Arnon (1949). Fresh leaf tissue (100 mg) was extracted in 80% acetone, centrifuged, and the absorbance was measured at 480, 645, and 663 nm. Total chlorophyll was calculated as:
Total chlorophyll (mg/g) = 20.2 × OD645 + 8.02 × OD663 × V/(1000 × W)
Hydrogen peroxide was measured according to Velikova et al. (2000). Fresh leaf tissue (100 mg) was homogenized in 0.1% TCA, centrifuged, and the supernatant was mixed with potassium phosphate buffer and KI. Absorbance was recorded at 390 nm, and H2O2 concentration was determined from a standard curve.
Malondialdehyde (MDA) content was estimated by the TBA method of Hodges et al. (1999). Leaf tissue (2 g) was homogenized in 0.1% TCA, centrifuged, and the supernatant reacted with 20% TCA containing 0.5% TBA. After heating (98°C, 10 min) and cooling, absorbance was read at 532 and 600 nm. MDA concentration was calculated using the formula such as:
MDA content (nmol/g FW) = ((A532 - A600)/155) *1000.
Hydroxyl radical content was measured following a modified method of Hodges et al. (1999). Leaf tissue (2 g) was homogenized in phosphate buffer, incubated with 2-deoxy-D-ribose, and reacted with TBA–TCA. After heating at 98°C, absorbance was recorded at 532 nm. OH• concentration was calculated as:
OH• content ((nmol/g FW) = (A532/155) *1000.
Electrolyte leakage (EL), membrane stability was assessed by electrolyte leakage following Chan et al. (1985). Leaf discs (10 mm) were incubated in deionized water at 25°C, and initial conductivity (C1) was measured. After boiling (98°C, 15 min) and cooling, final conductivity (C2) was recorded. Electrolyte leakage was expressed as:
EL (%) = (C1 / C2) × 100.
Antioxidative enzyme activity in tomato plants
The methods used were adapted from Sunohara and Matsumoto (2004). One gram of finely chopped tomato leaves was homogenized in 10 mL of KP buffer (pH 7.8) containing 25 mM, with 0.4 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ascorbic acid (AsA) and 2x% polyvinylpolypyrrolidone (PVPP). The resulting mixture was centrifuged at 15,000 g at 4°C for 20 minutes. The supernatant was then filtered through No. 1 filter paper, and six milliliters of the enzyme extract were placed in a dialysis bag (cellulose tubular membrane Cellu•Sep®, USA) and immersed in 1 liter of 10 mM KP buffer (pH 7.8) at 4°C for 24 hours. After dialysis, the extract was centrifuged at 15,000 g at 4°C for 20 minutes, and only the clear supernatant was used for the analysis of SOD, CAT, APX and GPX enzyme activities.
Activity of superoxide dismutase (SOD)
Superoxide dismutase (SOD) activity was determined by the method of Sunohara and Matsumoto (2004) using a 2 mL reaction mixture composed of 0.2 mL of 500 mM potassium phosphate buffer (pH 7.8), 0.2 mL of 0.1 mM cytochrome c derived from horse heart, 0.2 mL of 1 mM xanthine dissolved in 10 mM NaOH, 0.04 mL of xanthine oxidase, 1.32 mL of distilled water, and 0.04 mL of enzyme extract. The reduction of cytochrome c was monitored spectrophotometrically by measuring the increase in absorbance at 550 nm. SOD activity was expressed as Units per milligram of protein, where one unit is defined as the amount of enzyme required to inhibit the rate of cytochrome c reduction by 50% under the assay conditions.
Activity of catalase (CAT)
Catalase (CAT) activity was determined using a 2 mL reaction mixture consisting of 1.9 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 25 mM hydrogen peroxide (H2O2) and 0.1 mL of enzyme extract. The decomposition of H2O2 was monitored spectrophotometrically at 240 nm using an extinction coefficient of 0.0394 mM⁻1 cm⁻1. CAT activity was expressed as moles of H2O2 decomposed per gram of protein per minute (mol H2O2/g protein/min) (Chen and Zhang, 2016).
Activity of ascorbate peroxide (APX)
Ascorbate peroxidase (APX) activity was measured by the method of Das et al. (2015) using a 2 mL reaction mixture containing 0.5 mL of 100 mM potassium phosphate buffer (pH 7.0), 0.5 mL of 1 mM ascorbic acid, 0.5 mL of 0.4 mM EDTA, 0.02 mL of 10 mM hydrogen peroxide (H2O2), 0.38 mL of distilled water, and 0.1 mL of enzyme extract. The decrease in ascorbic acid concentration was monitored spectrophotometrically at 290 nm using an extinction coefficient of 2.8 mM⁻1 cm⁻1. APX activity was expressed as Units per milligram of protein. One unit (U) of ascorbate peroxidase (APX) activity is defined as the amount of enzyme that catalyzes the oxidation of 1 µmol of ascorbic acid (ASA) per minute per milligram of protein in a 1 mL reaction mixture at 25°C.
Activity of glutathione peroxidase (GPX)
Glutathione peroxidase (GPX) activity was measured in a 1 mL reaction mixture consisting of 0.16 M potassium phosphate buffer (pH 7.0), 1 mM sodium azide, 0.16 mM EDTA, 0.25 mM hydrogen peroxide (H2O2), 0.8 mM reduced glutathione (GSH), and 200 µL of crude enzyme extract. The mixture was incubated at 25°C for 10 minutes. Subsequently, 1 mL of the reaction mixture was combined with 3 mL of a solution containing 0.3 mM disodium hydrogen phosphate and 0.04% DTNB. The absorbance of the resulting solution was measured at 420 nm. GPX activity was expressed as millimoles of GSH oxidized per minute (Paglia and Valentine, 1967).
Statistical analysis
Data obtained from in vitro assays on plant growth-promoting traits, physiological characteristics of actinobacteria, and their role in alleviating salt stress in tomato under laboratory conditions were analyzed in triplicate. Results are expressed as means ± standard deviations (SD). A one-way analysis of variance (ANOVA) was applied based on a Completely Randomized Design (CRD), followed by Duncan’s multiple range test to determine significant differences among treatments at a 95% confidence interval. Statistical analyses were performed using IBM SPSS Statistics version 25, and data visualization was carried out using GraphPad Prism 9. Distinct letters were used to denote statistically significant differences between means.
RESULTS
Genome analysis of plant growth promoting properties and carbohydrate-active enzyme
The whole genome sequence of Tsukamurella sp. MT6.1 was annotated using the RAST server and plant growth-promoting related genes are summarized in Table S1. The genome of Tsukamurella sp. MT6.1 harbors genes for tryptophan biosynthesis, serving as the precursor for indole-3-acetic acid (IAA) production, including anthranilate phosphoribosyltransferase, tryptophan synthase, phosphoribosylanthranilate isomerase, and monoamine oxidase. Key IAA-related genes identified via KEGG and COG analyses include amidase (amiE) and aldehyde dehydrogenase (ALDH), while acyltransferase-related genes (Acetyltransf_1 and Acetyltransf_4) were also detected. The genome contains a complete set of iron acquisition genes, including ferrous (EfeUOB) and ferric transporters, as well as siderophore biosynthesis genes. Phosphate metabolism genes, including polyphosphate kinases, exophosphatases, and PHO regulon components, were present. Potassium transport and homeostasis genes, such as mechanosensitive channels, KefA, and potassium transporting ATPases (A, B, C subunits), were identified as shown in Table S1. Furthermore, genomic analysis of Tsukamurella sp. MT6.1, conducted through COG analysis, revealed several genes associated with the promotion of plant growth, as detailed in Table S2. Notably, nifU, involved in the nitrogen metabolism pathway, was identified in MT6.1. Additional regions related to nitrite transport and reduction (nirBD), nitrate transport and reduction (narBK), as well as an ammonium uptake transporter (amtB) and its regulatory elements, were also characterized in MT6.1.
Genome analysis of Tsukamurella sp. MT6.1 identified 102 carbohydrate-active enzymes (CAZymes), mainly glycosyltransferases (GTs), glycoside hydrolases (GHs), and carbohydrate esterases (CEs), with no polysaccharide lyases detected as shown in Figure 1. Among 47 GT genes spanning 17 families, GT4 was dominant (13 genes), followed by GT1, GT2, and GT83 (5 genes each), highlighting strong glycosidic bond–forming potential. The GH family comprised 23 genes, with GH13 (α-amylase; 5 genes) as the most abundant, while GH179 (4 genes) and GH3, GH23, GH183 (2 genes each) were also represented. Eighteen CE genes were identified, mainly CE5 (6 genes), CE1 (5 genes), and CE14 (4 genes), suggesting roles in plant polysaccharide deacetylation. Additional findings included one CBM gene (CBM48) and four auxiliary activity (AA) genes, primarily AA3 (9 genes). Moreover, the analysis of Tsukamurella sp. MT6.1 revealed a diverse array of carbohydrate-active enzymes (CAZymes), each associated with specific substrates, as summarized in Table S3. These substrates, which include glycogen, exopolysaccharides, sucrose, trehalose, fructan, xylan, starch, and β-glucan, suggest significant enzymatic versatility in carbohydrate metabolism.

Figure 1. Analysis of carbohydrate-active enzyme families in Tsukamurella sp. MT6.1.
Determination of in vitro plant growth promoting properties of Tsukamurella sp. MT6.1 under salt stress
Tsukamurella sp. MT6.1 exhibited the ability to produce indole-3-acetic acid (IAA) with and without the addition of L-tryptophan under various NaCl concentrations (0%, 4%, and 8% (w/v) NaCl). In the presence of L-tryptophan, the highest IAA production (159.17 ± 5.46 µg/mL) occurred without NaCl, with a significant decrease observed at higher NaCl concentrations. At 4% (w/v) NaCl, IAA levels dropped to around 106.94 ± 13.08 µg/mL, and further decreased to 59.17 ± 5.46 µg/mL at 8% (w/v) NaCl. Without L-tryptophan, IAA production by Tsukamurella sp. MT6.1 varied across different NaCl concentrations. At 0% NaCl, IAA production was 64.44 ± 4.28 µg/mL, but it significantly decreased to 46.39 ± 6.47 µg/mL at 4% (w/v) NaCl. However, at 8% (w/v) NaCl, IAA production increased to 61.39 ± 13.18 µg/mL as shown in Table 1.
The production of siderophores by Tsukamurella sp. MT6.1 was observed as a yellow zone around the agar plug on Chrome-azurol S (CAS) plate assay. The quantitative analysis of siderophore production in culture broth demonstrated significantly higher levels of hydroxamate-type siderophores (456.78 ± 31.68 µmol/mL) compared to catecholate-type siderophores (136.29 ± 9.61 µmol/mL) under non-saline conditions. Under salt stress (4% and 8% (w/v) NaCl), the production of hydroxamate siderophores decreased to 321.22 ± 41.94 µmol/mL and further dropped to 89 ± 14.53 µmol/mL at 8% (w/v) NaCl. Similarly, catecholate-type siderophore production declined from 136.29 ± 9.61 µmol/mL at 0% NaCl to 110.10 ± 11.35 µmol/mL at 4% NaCl and significantly reduced to 14.14 ± 2.58 µmol/mL at 8% (w/v) NaCl. The production of hydroxamate siderophores decreased significantly with increasing salinity, and the same trend was observed for catecholate-type siderophores, with a drastic reduction at 8% (w/v) NaCl as shown in Table 1.
Table 1. Plant growth promoting activity of Tsukamurella sp. MT6.1 under different level of salt stress in vitro.
|
NaCl Concentration (%) |
IAA Production (mg/ml) |
Siderophore production (mmol/ml) |
Released phosphate (mg/ml) |
||
|
with |
without L-tryptophan |
Hydroxamate |
Catecholate |
||
|
0% |
159.19a ± 5.46 |
64.44a ± 4.28 |
456.78a ± 31.68 |
136.29a ± 9.61 |
313.89c ± 15.12 |
|
4% |
106.94b ± 13.08 |
46.39a ± 6.47 |
321.22b ± 41.94 |
110.10b ± 11.35 |
556.11a ± 6.94 |
|
8% |
59.17c ± 5.46 |
61.39a ± 13.18 |
89.00c ± 14.53 |
14.14c ± 2.58 |
515.00b ± 27.84 |
Noted: All values are expressed as mean ± SD. Within each row, values followed by different letters (a, b, c) are significantly different at P<0.05 according to Duncan’s new multiple range test.
Tsukamurella sp. MT6.1 exhibited phosphate solubilization activity by forming a clear zone on PVK agar and released 319.83 ± 15.12 µg/mL of phosphorus in culture broth under non-saline conditions (0% NaCl). Notably, phosphate solubilization increased at elevated NaCl concentrations, with 556.11 ± 6.94 µg/mL of phosphorus released at 4% (w/v) NaCl, indicating a significant enhancement compared to the no-salt condition. However, at 8% (w/v) NaCl, phosphate solubilization slightly decreased to 515 ± 27.84 µg/mL, though it remained higher than the 0% NaCl condition as shown in Table 1. A change in the pH of the culture broth was recorded in both the presence and absence of NaCl, ranging from 6.39 to 5.1. Regarding ACC deaminase activity, Tsukamurella sp. MT6.1 exhibited limited growth on DF minimal salt medium, whereas more robust growth was observed on the minimal medium supplemented with ammonium sulfate (used as a positive control), as illustrated in the supplementary data (Figure S1).
Promotion of tomato growth by Tsukamurella sp. MT6.1 under salt stress condition
The growth-promoting effects of Tsukamurella sp. MT6.1 on tomato plants was evaluated under 0 and 200 mM NaCl based on fresh weight, dry weight, shoot length, and root length as shown in Figure 2. Fresh and dry weights were consistently higher in MT6.1-inoculated plants, with the most pronounced enhancements observed under non-stress (0 mM NaCl). At 0 mM NaCl, fresh weight increased from 6.57 ± 0.45 g in the control to 7.73 ± 0.28 g in the MT6.1-inoculated plants. At 200 mM NaCl, the difference was less pronounced, with fresh weights of 3.56 ± 0.07 g in the control and 4.04 ± 0.13 g in the MT6.1-inoculated group as shown in Figure 3A. Dry weight followed a similar trend. At 0 mM NaCl, dry weight increased from 0.83 ± 0.11 g in the control to 1.09 ± 0.03 g in MT6.1-inoculated plants. Under 200 mM NaCl, dry weights were 0.51 ± 0.05 g in the control and 0.56 ± 0.06 g in MT6.1-inoculated plants, indicating a reduced growth-promoting effect under severe salt stress as shown in Figure 3B. Similar positive effects were observed for shoot and root length. Inoculated plants exhibited longer shoots across all conditions. At 200 mM NaCl, shoot length increased modestly from 28.00 ± 0.00 cm in the control to 29.00 ± 1.00 cm in the MT6.1-inoculated group as shown in Figure 3C. Root length also increased in MT6.1-inoculated plants, particularly at 200 mM NaCl; root length increased from 14.00 ± 0.50 cm in the control to 19.67 ± 1.15 cm in the MT6.1-inoculated plants as shown in Figure 3D.

Figure 2. Growth of tomato seedlings inoculated and uninoculated with Tsukamurella sp. MT6.1 with and without salt stress.
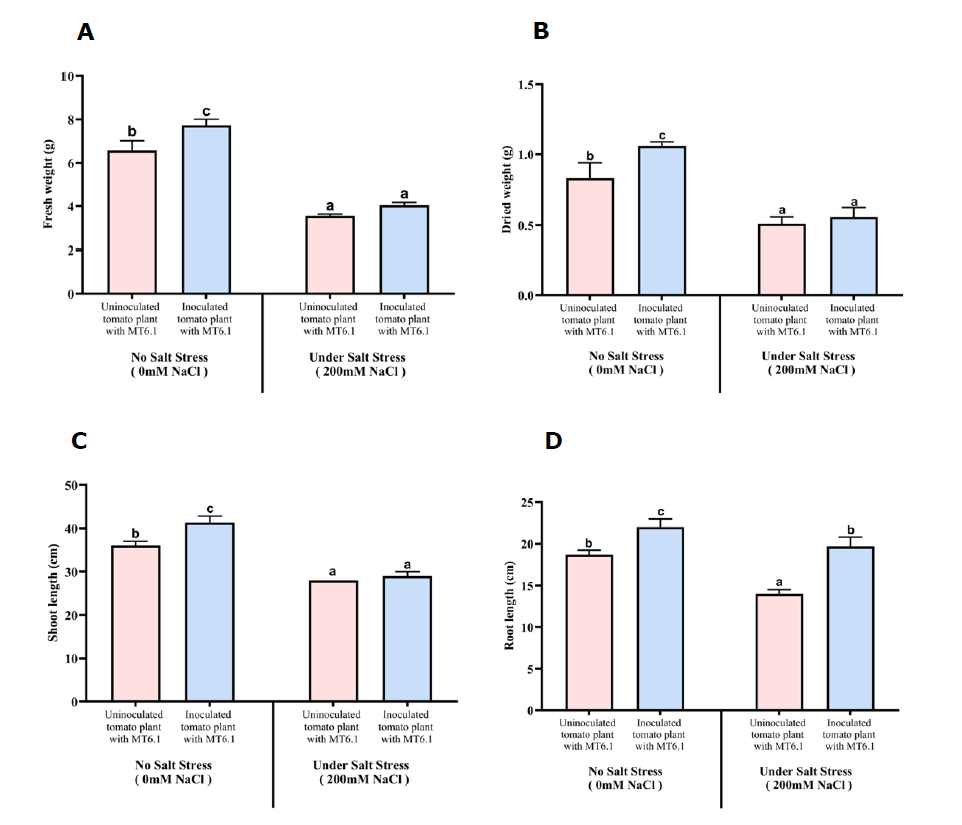
Figure 3. Morphological growth parameters of tomato seedlings inoculated and uninoculated with Tsukamurella sp. MT6.1 under salt stress. (A) fresh weight, (B) dry weight, (C) shoot length, (D) root length.
Biochemical analysis in tomato plants
Hydrogen peroxide content
Hydrogen peroxide content was consistently lower in inoculated plants under both conditions. At 0 mM NaCl, H2O2 levels decreased from 25.17 ± 3.26 mmol/g in the control group without MT6.1 to 16.13 ± 1.58 mmol/g in the inoculated group with MT6.1. Similarly, at 200 mM NaCl, H2O2 levels in inoculated plants were significantly lower than their respective control without MT6.1 (Figure 4A).
Total chlorophyll
Chlorophyll level was higher in plants with inoculation under both conditions, with the most pronounced improvement observed under salt stress which increased from 1.652 ± 0.077 mg/g without MT6.1 to 1.948 ± 0.133 mg/g with MT6.1 (Figure 4B).
Proline content
Proline content, an indicator of osmotic adjustment, was significantly elevated in inoculated plants under salt stress, rising from 2.33 ± 0.15 mg/g without MT6.1 to 3.12 ± 0.16 mg/g with MT6.1 (Figure 4C).
Total soluble sugar (TSS)
Total soluble sugar levels were higher in inoculated plants under non-stress conditions revealing 29.14 ± 0.41 mg/g without MT6.1 and 31.65 ± 1.36 mg/g with MT6.1. Under salt stress, similar total soluble sugar level was observed (Figure 4D).
Malonaldehyde content (MDA)
MDA content was lower in inoculated plants compared to uninoculated control especially under non-stress condition (0 mM NaCl). MDA content decreased slightly from 1.59 ± 0.18 µmol/kg in control plants without MT6.1 to 1.54 ± 0.03 µmol/kg in inoculated plants with MT6.1 at 0 mM NaCl. However, under severe salt stress (200 mM NaCl), the difference between inoculated and control plants were not significantly different, with MDA levels of 1.68 ± 0.14 µmol/kg with MT6.1 compared to 1.73 ± 0.18 µmol/kg without MT6.1 (Figure 4E).
Electrolyte leakage (EL)
Electrolyte leakage was consistently lower in inoculated plants. Under non-stress condition, leakage decreased from 15.38% in controls without MT6.1 to 14.10% in inoculated plants with MT6.1. At 200 mM NaCl, electrolyte leakage remained lower in inoculated plants with MT6.1 (17.89%) compared to controls without MT6.1 (19.88%) (Figure 4F).
Hydroxyl radical
Hydroxyl radical levels were lower in inoculated plants at 200 mM NaCl. Under non-stress condition, hydroxyl radical content slightly decreased from 3.57 ± 0.02 nmol/g FW in control plants without MT6.1 to 3.51 ± 0.10 nmol/g FW in inoculated plants with MT6.1. Under stress (200 mM NaCl), hydroxyl radical levels in inoculated plants with MT6.1 (3.54 ± 0.19 nmol/g FW) were significantly lower than in controls without MT6.1 (5.28 ± 0.41 nmol/g FW) (Figure 4G).
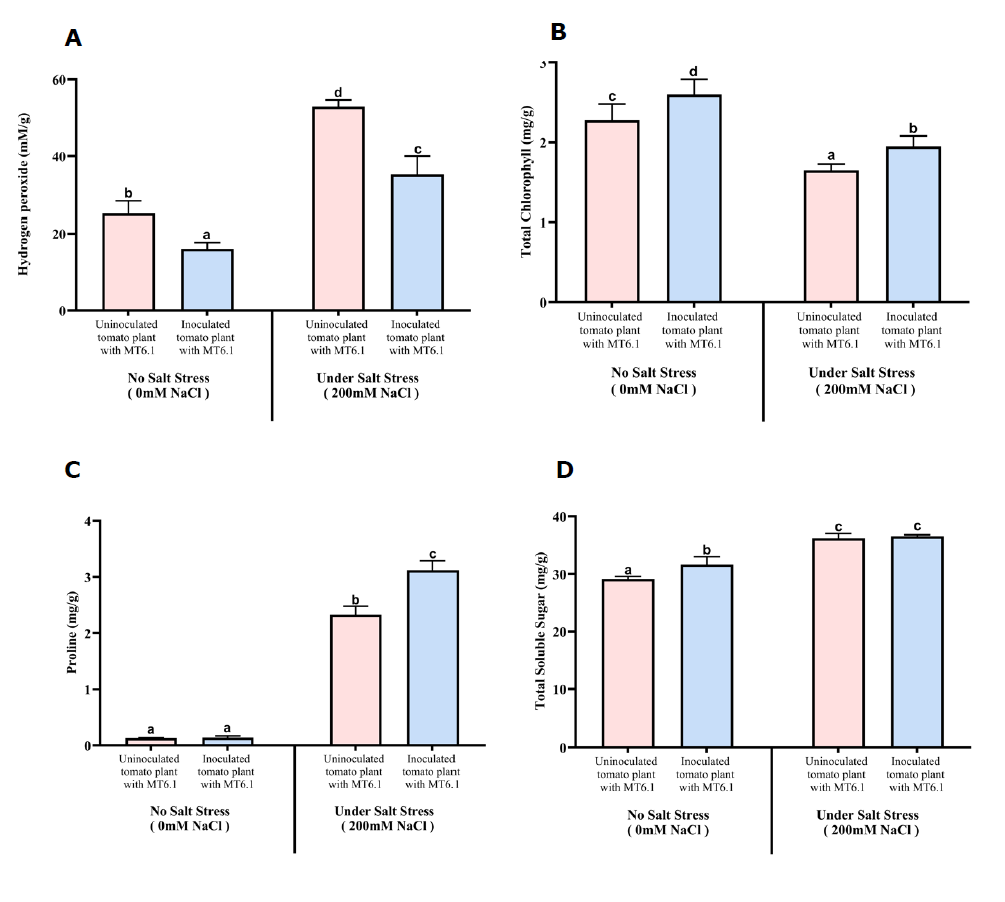
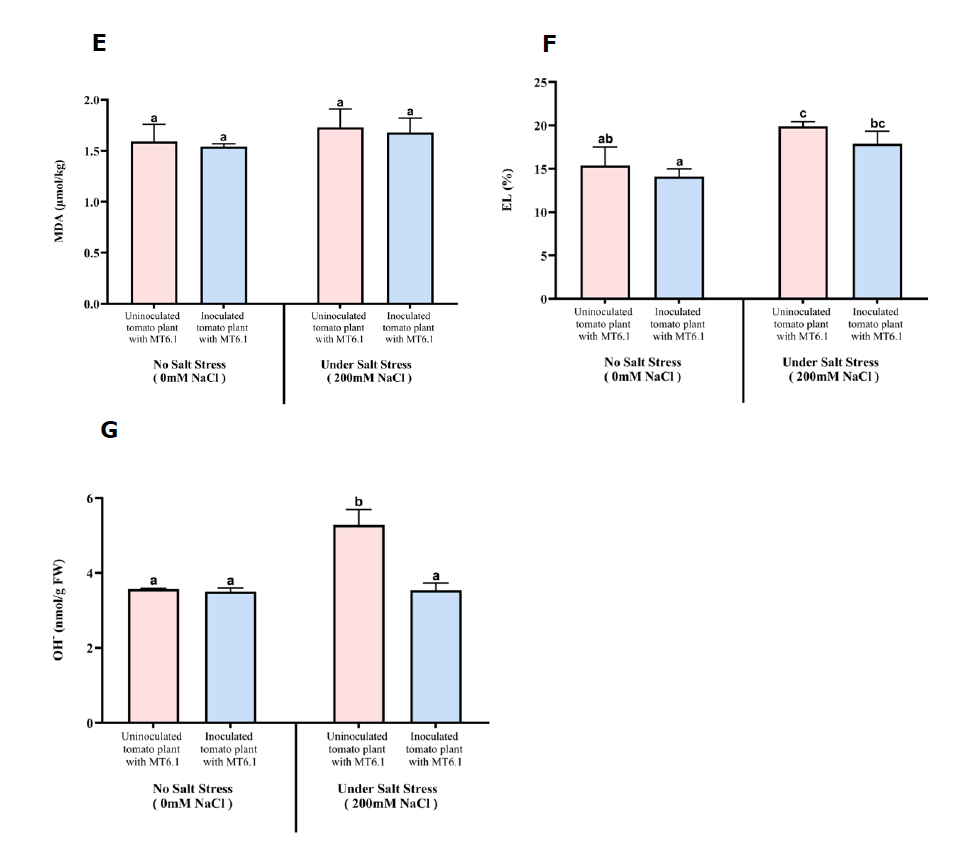
Figure 4. Oxidative stress markers in tomato seedlings inoculated and uninoculated with Tsukamurella sp. MT6.1 under salt stress (A) hydrogen peroxide, (B) total chlorophyll, (C) proline, (D) total soluble sugar, (E) Malondialdehyde (MDA), (F) Electrolyte leakage (EL), (G) Hydroxyl radicals (OH).
Antioxidative enzyme activities under salt stress
Activity of superoxide dismutase (SOD)
SOD activity was higher in uninoculated plants compared to those inoculated with Tsukamurella sp. MT6.1 both under non-stress and salt-stress conditions. At 0 mM NaCl, SOD activity was 14.19 U/mg protein in control plants without MT6.1, compared to 11.59 ± 2.73 U/mg protein in inoculated plants with MT6.1. However, under severe salt stress (200 mM NaCl), SOD activity remained lower in uninoculated control plants (10.20 ± 0.15 U/mg protein) compared to inoculated plants with MT6.1 (10.85 ± 2.18 U/mg protein) (Figure 5A).
Activity of glutathione peroxidase (GPX)
GPX activity showed similar values both with and without salt stress. In uninoculated plants, a value of 0.000274 ± 0.000062 mmol/mg protein/min was observed under non-stress condition. In contrast, MT6.1-inoculated plants exhibited a slightly lower activity under non-stress condition (0.000265 ± 0.000063 mmol/mg protein/min), but a modest increase under severe salt stress at 200 mM NaCl (0.000297 ± 0.000048 mmol/mg protein/min) (Figure 5B).
Activity of ascorbate peroxidase (APX)
APX activity significantly differed between control and inoculated plants. In uninoculated controls, activity decreased from 5.58 ± 0.39 U/mg protein at 0 mM NaCl to 4.55 ± 0.49 at 200 mM NaCl. In contrast, MT6.1-inoculated plants exhibited lower APX activity under non-stress condition (3.33 ± 0.58 U/mg protein) but more than double increase under severe salt stress, reaching 8.63 ± 1.01 U/mg protein (Figure 5C).
Activity of catalase (CAT)
CAT activity was significantly higher in inoculated plants under both non-stress and severe salt stress conditions. At 0 mM NaCl, CAT activity increased slightly from 0.049 ± 0.01 mol H2O2/g protein/min in uninoculated plants to 0.052 ± 0.02 mol H2O2/g protein/min in inoculated plants. Under 200 mM NaCl, inoculated plants maintained higher CAT activity (0.054 ± 0.01 mol H2O2/g protein/min) compared to uninoculated plants (0.026 ± 0.01 mol H2O2/g protein/min) (Figure 5D).
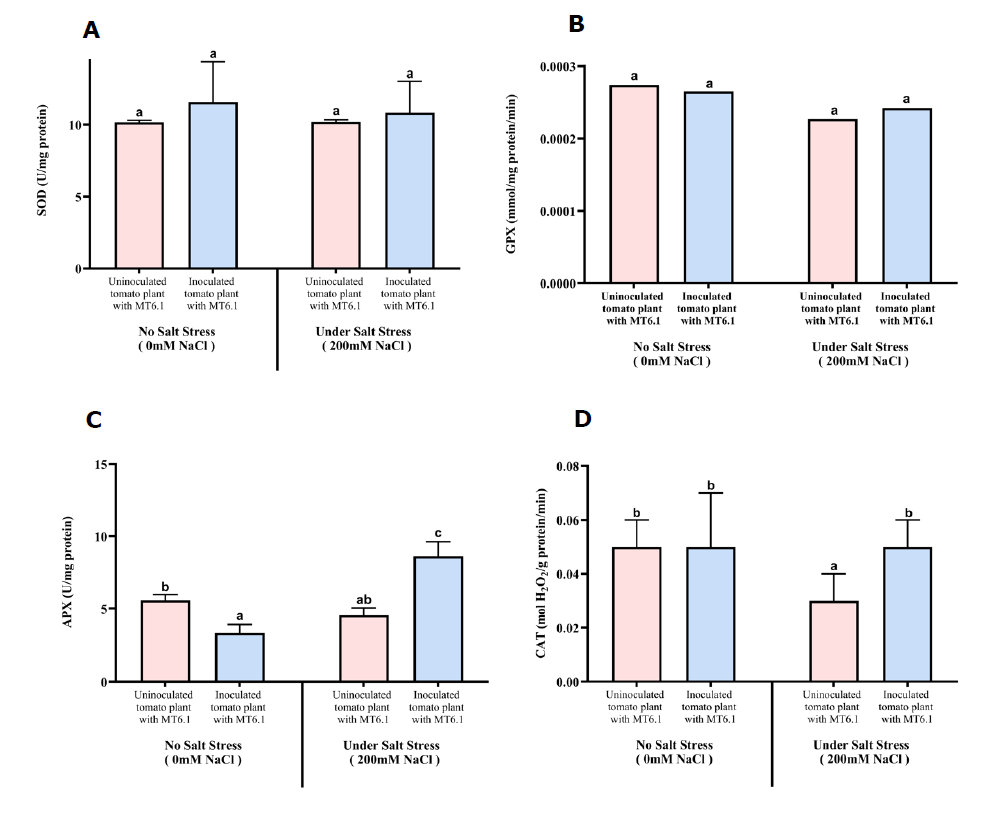
Figure 5. Antioxidative enzyme activities of tomato seedlings inoculated and uninoculated with Tsukamurella sp. MT6.1 under various salt stress (A) Superoxide dismutase (SOD), (B) Glutathione peroxidase (GPX), (C) Ascorbate peroxide (APX), (D) Catalase (CAT).
DISCUSSION
Plant growth promoting properties of Tsukamurella sp. MT6.1
The ability to produce IAA is a hallmark of plant growth-promoting actinobacteria (Mathew et al., 2020; Djebaili et al., 2021; Nozari et al., 2021; Saidi et al., 2021; Gao et al., 2022; Li et al., 2023). Many actinobacteria have been reported to synthesize IAA (Manulis et al., 1994; Battini et al., 2015; Lasudee et al., 2018), and this has been commonly reported among marine actinobacteria. In this study, Tsukamurella sp. MT6.1 demonstrated robust IAA production under salinity conditions, with or without L-tryptophan supplementation, confirming its capacity to utilize both tryptophan-dependent and -independent pathways for auxin synthesis (Tang et al., 2023). Genome analysis also identified genes encoding essential enzymes for tryptophan biosynthesis, such as anthranilate synthase, phosphoribosyl anthranilate isomerase, indole-3-glycerol phosphate synthase and tryptophan synthase (alpha and beta chains), supporting its IAA biosynthetic versatility. Quantitatively, the peak IAA production by MT6.1 (159.17 ± 5.46 μg/mL with L-tryptophan at 0% NaCl) significantly surpasses the range reported for many other actinobacteria, such as Streptomyces strains from coastal areas (4.12–49.7 μg/mL) (Rashad et al., 2015) and Saccharomonospora sp. LNS-1 (49.46μg/mL) (Nafis et al., 2019). This high level of IAA production, maintained at 59.17μg/mL even at 8% NaCl, suggests a superior ability to overcome severe salt stress. Similar salinity-dependent trends in IAA production have been noted in other deep-sea strains like Dermacoccus abyssi MT1.1T and D. nishinomiyaensis DSM20448T, indicating a shared adaptive mechanism within deep-sea actinobacteria (Rangseekaew et al., 2021, 2022). The high IAA levels of Tsukamurella sp. MT6.1, particularly in L-tryptophan-supplemented media, also surpass previously reported strain, such as Bacillus megaterium strains (Bessai et al., 2023). These findings underscore the strain’s potential as a potent IAA producer, with genes supporting auxin biosynthesis even in saline environments. Similar genetic capabilities have been reported in other actinobacteria, including D. abyssi MT1.1ᵀ (Rangseekaew et al., 2022), Micromonospora chalcea CMU55-4 (Insuk et al., 2020) and Streptomyces adelaidensis CAP261T (Kaewkla et al., 2021) which also harbor genes critical for IAA production, such as indole-3-glycerol phosphate synthase and tryptophan synthase. Functionally, the high levels of bioavailable IAA secreted by Tsukamurella sp. MT6.1 were also reflected in the growth response of inoculated tomato plants. The increase in shoot length, fresh weight, and particularly root length observed under 200 mM NaCl stress can be directly attributed to bacterial IAA because auxins, particularly indole-3-acetic acid (IAA), are essential plant growth regulators that influence numerous physiological and developmental processes, including root elongation, cell division, and root hair proliferation (Radwan et al., 2002; Ahmad et al., 2005; Goudjal et al., 2013; Olanrewaju et al., 2017; Wahyudi et al., 2019).
Siderophores are essential for bacterial iron acquisition, particularly under limiting conditions, and contribute to plant growth promotion through both direct and indirect mechanisms, such as enhancing iron uptake, and indirect effects, including the inhibition of phytopathogens (Sayyed et al., 2012; Gu et al., 2020). These molecules are classified into carboxylate, hydroxamate and catecholate types, with hydroxamate and catecholate being the most prevalent among actinobacteria (Sadeghi et al., 2011; Acquah et al., 2020; Timofeeva et al., 2022). In this study, Tsukamurella sp. MT6.1 produced both hydroxamate and catecholate-type siderophores, as confirmed by the Chrome-azurol S (CAS) assay, which revealed a yellow zone around the agar plug. Quantitative analysis showed that under non-saline conditions (0% NaCl), Tsukamurella sp. MT6.1 produced higher levels of hydroxamate-type siderophores (456.78 µmol/mL) than catecholate-type (136.29 µmol/mL). However, siderophore production decreased with increasing salinity, with hydroxamate levels dropping to 321.22 µmol/mL at 4% NaCl and 89 µmol/mL at 8% NaCl, while catecholate production declined from 136.29 µmol/mL at 0% NaCl to 14.14 µmol/mL at 8% NaCl. These results are consistent with findings in other marine actinobacteria, although Tsukamurella sp. MT6.1 displayed lower adaptability to salt stress compared to some strains. For example, Dermacoccus abyssi MT1.1ᵀ showed a significant increase in hydroxamate production from 46.67 µmol/mL to 170.83–189.17 µmol/mL under moderate salinity (Rangseekaew et al., 2022), while D. barathri MT2.1ᵀ produced 240.00 µmol/mL. These differential siderophore production profiles suggest that actinobacteria possess distinct strategies for coping with environmental stress, particularly in relation to iron acquisition. The genome of Tsukamurella sp. MT6.1 also revealed the presence of genes related to iron transport systems, including ferrous iron transport periplasmic protein (EfeO), ferrous iron transport permease (EfeU), and iron transport peroxidase (EfeB) which are crucial for iron uptake in iron-limited and oxygen-depleted environments, such as marine sediments. Similar iron-acquisition strategies have been observed in other deep-sea actinobacteria, including D. abyssi MT1.1ᵀ, D. barathri MT2.1ᵀ, and D. profundi MT2.2ᵀ, which also produce hydroxamate and catecholate-type siderophores (Rangseekaew et al., 2022). These findings suggest that deep-sea actinobacteria, including Tsukamurella sp. MT6.1, may produce iron-chelating compounds with unique structures that could be further explored for their potential in biotechnological applications.
Phosphorus is an essential macronutrient for plant growth, yet its availability in the soil is often limited due to its presence in insoluble forms. Phosphate-solubilizing microorganisms play a critical role in converting insoluble phosphate into forms accessible to plants, thereby promoting nutrient cycling and enhancing soil fertility (Illmer and Schinner, 1995; H. Li et al., 2023). In this study, Tsukamurella sp. MT6.1 exhibited phosphate solubilization on PVK agar, forming a distinct clear zone and releasing 319.83 µg/mL of phosphorus under non-saline conditions (0% NaCl). Remarkably, another distinctive metabolic feature of Tsukamurella sp. MT6.1 is its salt-enhanced phosphate solubilization strategy, activity increased significantly under moderate salinity (4% (w/v) NaCl), reaching 556.11 μg/mL, an enhancement over the non-saline condition. Although a slight decline was observed at 8% (w/v) NaCl (515 μg/mL), Tsukamurella sp. MT6.1 maintained high solubilization even under elevated salt stress. This salt-optimizing P-solubilization is crucial for maintaining nutrient availability under osmotic stress, providing a metabolic advantage over other deep-sea actinobacteria. In comparison, Dermacoccus profundi MT2.2T and D. barathri MT2.1T showed lower solubilization (165.47 μg/mL and 169.27 μg/mL, respectively) under non-saline conditions (Rangseekaew et al., 2022), while D. abyssi MT1.1T exhibited only 71.62 μg/mL with minimal variation under salinity. These findings highlight Tsukamurella sp. MT6.1’s superior ability to maintain and even enhance phosphate solubilization in saline environments and solidifies the role of Tsukamurella sp. MT6.1 in enhanced nutrient cycling and biomass increase in tomato plant under 200mM salt stress. High phosphate-solubilizing activity was also reported from members of the genera Streptomyces, Microbacterium, Angustibacter, Kocuria, Isoptericola, and Agromyces isolated from marine sediment in Chorao Island, India (Dastager and Damare, 2013), supporting the phosphate-solubilizing potential of diverse marine actinobacteria. The acidification of MT6.1 culture medium (pH drop from 6.39 to 5.1) across all salinity levels points to the likely involvement of organic acids in phosphate solubilization, a well-documented mechanism in phosphate-solubilizing microorganisms (Pérez et al., 2007; Boubekri et al., 2021; Wang et al., 2022). Genomic evidence further supports this functional versatility, MT6.1 harbors genes encoding alkaline phosphatase, exopolyphosphatase, and protein kinases, which enable the utilization of both organic and inorganic phosphate sources. Although no direct genes for organic acid biosynthesis were detected, the presence of high-affinity phosphate uptake regulators such as PhoR (SphS), PhoU, and PhoB (SphR) indicates a robust capacity for phosphate acquisition and regulation under stress (Santos-Beneit, 2015).
Plants under stress often increase ethylene production, a hormone that can inhibit growth under adverse conditions, and plant growth-promoting bacteria (PGPB) with ACC deaminase (ACCD) activity mitigate this effect by degrading its direct precursor, 1-aminocyclopropane-1-carboxylate (ACC), into α-ketobutyrate and ammonia, thereby reducing ethylene levels and alleviating plant stress (Del Carmen Orozco-Mosqueda et al., 2020; Mathew et al., 2020). In this study, no detectable ACC deaminase activity of Tsukamurella sp. MT6.1 was observed, indicating that alternative mechanisms are likely employed to alleviate salinity stress in tomato seedlings.
The genomic analysis of Tsukamurella sp. MT6.1 represents the first genome analysis of carbohydrate-active enzymes (CAZymes) in this genus, revealing a total of 102 CAZymes. This remarkable diversity of carbohydrate-active enzymes (CAZymes) underscores the strain's adaptability to various environmental niches and its ability to utilize complex carbohydrates as nutrient sources (Hibbing et al., 2010). The presence of strain specific CAZymes not only enhances the strain's metabolic versatility but also suggests a potential role in biocontrol by inhibiting phytopathogens. The substrate analysis indicates that these CAZymes can act on a wide range of substrates, including glycogen, exopolysaccharides, and plant polysaccharides, which may facilitate the hydrolysis of phytopathogen cell walls (Chen et al., 2019). The predominance of glycosyltransferases (GTs) in the genome, particularly from the GT4 family, highlights the strain's significant capacity for synthesizing complex carbohydrates, such as disaccharides and oligosaccharides. This ability is crucial for energy storage and may enhance plant growth by providing readily available sugars. Additionally, the substantial presence of glycoside hydrolases (GHs), especially those from the GH13 family, indicates a robust capability for starch and sucrose metabolism, were identified as the most abundant CAZyme class, further supporting the strain's role in carbohydrate breakdown and nutrient availability for plants (Tashkandi and Baz, 2023). Moreover, the identification of carbohydrate esterases (CEs) and their specific families suggests that Tsukamurella sp. MT6.1 can effectively degrade plant polysaccharides, thereby enhancing the overall nutrient cycling in the rhizosphere. The enzymatic versatility of Tsukamurella sp. MT6.1, as evidenced by its diverse CAZyme repertoire, not only contributes to its metabolic adaptability but also positions it as a potential candidate for agricultural applications. The ability to produce these enzymes could lead to industrial applications, such as biocontrol agents or biofertilizers, thereby promoting sustainable agricultural practices. We speculate that, based on these findings, the diverse CAZyme repertoire of Tsukamurella sp. MT6.1 significantly enhances its survival, nutrient acquisition, and potential biocontrol capabilities, making it a valuable asset for tomato plant growth promotion under salt stress.
Mitigation of salt stress in tomato by Tsukamurella sp. MT6.1
Mitigation of salt stress in tomato plant via compatible solute production by Tsukamurella sp. MT6.1
The inoculation of Tsukamurella sp. MT6.1 significantly enhanced tomato growth under both non-stress (0 mM NaCl) and salt-stress (200 mM NaCl) conditions, highlighting its potential as a plant growth-promoting agent. Under non-saline conditions, inoculated plants exhibited higher fresh weight, dry weight, shoot length, and root length compared to uninoculated controls. This observation is consistent with findings from Salinispora arenicola–treated tomato plants, which also showed notable growth enhancement in salt-free environments (Becerril-Espinosa et al., 2022). Similarly, Glutamicibacter halophytocola KLBMP 5180 increased tomato seedling growth in terms of fresh weight, height, root development, and chlorophyll content (Xiong et al., 2019). These improvements under non-stress conditions suggest that Tsukamurella sp. MT6.1 enhances nutrient uptake while solubilizing phosphate, thereby promoting overall plant vigor because excessive salt accumulation in soil lowers water availability, reduces water and nutrient uptake, resulting in poor growth and low productivity of plants (Zhao et al., 2021; Ondrasek et al., 2022).
Under severe salinity (200 mM NaCl), Tsukamurella sp. MT6.1 still exerted positive effects, though the magnitude was reduced. Fresh and dry weights were slightly higher in inoculated plants compared to controls, while root length showed a more pronounced increase, suggesting that root system development is a key adaptive trait supported by the production of IAA in Tsukamurella sp. MT6.1. These findings are consistent with previous reports where actinobacterial inoculants, such as Dermacoccus abyssi MT1.1T and Streptomyces paradoxus D2-8, alleviated salt stress and improved growth parameters in tomato (Gao et al., 2022; Rangseekaew et al., 2022).
Biochemical analyses further confirmed the positive impact of MT6.1 on tomato physiology under salinity. In this study, proline content in tomato leaves increased under 200 mM NaCl in both inoculated and non-inoculated plants; especially, a significantly higher accumulation was observed in MT6.1-inoculated plants. Because proline accumulation is a well-documented adaptive response of both bacteria and plants under salinity stress, where it functions as a compatible solute and antioxidant to protect cellular structures and maintain osmotic balance (Rangseekaew et al., 2021; Saidi et al., 2021; Potestio et al., 2024). This enhanced proline accumulation reflects improved osmotic adjustment and is consistent with reports of halotolerant PGPR promoting proline biosynthesis in tomato under saline conditions (Rangseekaew et al., 2021; Potestio et al., 2024). Similar effects have been described in other host–microbe systems, such as mungbean inoculated with Bacillus cereus Pb25, where elevated proline content correlated with improved salt tolerance (Islam et al., 2015). However, total soluble sugar (TSS) levels remained stable in inoculated plants, showing slightly increases under non-stress conditions with minimum difference under salt stress. This suggests that MT6.1 supports osmotic homeostasis without excessive sugar accumulation, consistent with reports on species-specific osmolyte regulation in PGPR-treated plants (Rangseekaew et al., 2021; Saidi et al., 2021). These results indicate that Tsukamurella sp. MT6.1 mitigates salt stress in tomato by enhancing compatible solute accumulation, particularly proline while stabilizing TSS levels to maintain photosynthetic performance and osmotic balance.
Mitigation of salt stress in tomato plant by reducing oxidative stress indicators and enhancing antioxidative defense mechanisms by Tsukamurella sp. MT6.1
Salt stress is known to disrupt cellular redox homeostasis by promoting the excessive accumulation of reactive oxygen species (ROS), including superoxide anion (O2⁻), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH), which collectively trigger oxidative damage to lipids, proteins, and nucleic acids (Rahman et al., 2017; Parvin et al., 2019). These ROS perturbations compromise membrane integrity, enzymatic functions, and cellular viability by overwhelming the plant’s antioxidant defense system. Under such conditions, superoxide dismutase (SOD) provides the first line of defense by catalyzing the dismutation of O2⁻ to H2O2, which is subsequently detoxified to water and oxygen by catalase (CAT) and ascorbate peroxidase (APX). Glutathione peroxidase (GPX) and glutathione S-transferase (GST) further scavenge peroxides using glutathione as an electron donor (Hasanuzzaman et al., 2019).
In the present study, inoculation with Tsukamurella sp. MT6.1 significantly modulated antioxidant defense mechanisms in tomato plants under both non-stress and saline conditions. Inoculated plants consistently exhibited lower H2O2 and hydroxyl radical levels than controls, indicating efficient ROS scavenging. Consequently, these plants maintained higher chlorophyll content, consistent with the protective role of plant growth-promoting rhizobacteria (PGPR) in sustaining photosynthetic pigments under stress (Saidi et al., 2021; Xu et al., 2022), as elevated H2O2 levels have been reported to reduce chlorophyll in tomato (Rangseekaew et al., 2022). Similarly, lipid peroxidation, measured as malondialdehyde (MDA), was reduced in MT6.1-treated plants, particularly under non-stress conditions, while electrolyte leakage (EL) was also lower, confirming improved membrane stability, which likely contributed to an enhanced overall growth performance, including shoot and root length as well as fresh and dry weight, in MT6.1-treated plants. However, these changes were not statistically significant and may not represent the primary mechanism by which MT6.1 mitigates salt stress in tomato seedlings.
Plants also utilize antioxidant enzymes to mitigate stress-induced oxidative damage, which is a key strategy to improve tolerance to environmental stresses. In this study, SOD and GPX activities in MT6.1-inoculated plants remained relatively unchanged, suggesting a supplementary rather than primary defense role. In contrast, catalase activity (CAT) was consistently higher in MT6.1-inoculated plants under both 0 and 200 mM NaCl compared to uninoculated controls, highlighting its central role in H2O2 detoxification. Similarly, enhanced CAT activity has been reported in PGPR-inoculated plants such as Streptomyces dioscori SF1 and Bacillus cereus Pb25 (Islam et al., 2015; Li et al., 2023). CAT can directly eliminate excess hydrogen peroxide (Xie et al., 2019) contribute to an improved H₂O₂ detoxification, which may help maintain photosynthetic pigments and support overall plant growth under saline conditions. APX activity exhibited a distinct pattern, being lower in inoculated plants under non-stress conditions but markedly higher under 200 mM NaCl, suggesting an inducible defense mechanism activated under elevated oxidative pressure. Similar trends have been observed in tomato inoculated with PGPR such as Streptomyces dioscori SF1 (Potestio et al., 2024). Overall, MT6.1 inoculation enhanced CAT activity and induced APX under salt stress, providing an effective antioxidant defense that minimized ROS accumulation, lipid peroxidation, and membrane damage. This multi-tiered enzymatic response highlights the potential of Tsukamurella sp. MT6.1 as a promising bioinoculant for mitigating salt-induced oxidative stress in tomato.
CONCLUSION
Tsukamurella sp. MT6.1 harbors diverse genomic traits that support plant growth, including IAA biosynthesis, siderophore production, phosphate solubilization, and nutrient transport. Inoculation of Tsukamurella sp. MT6.1 enhanced tomato growth under both non-stress and saline conditions by improving biomass, root and shoot development, osmotic adjustment, and oxidative stress mitigation. The strain’s enzymatic versatility and stress-responsive features highlight its potential as a bioinoculant for improving crop productivity in saline environments.
ACKNOWLEDGEMENTS
This project is funded by National Research Council of Thailand (NRCT) and Chiang Mai University (Grant Number N42A670709). May Tharaphu Thein Win is grateful for the opportunity to pursue a Master’s Degree in Applied Microbiology at the Faculty of Science, Chiang Mai University, under the CMU Presidential Scholarship.
REFERENCES
Acquah, K.S., Beukes, D.R., Warner, D.F., Meyers, P.R., Sunassee, S.N., Maglangit, F., Deng, H., Jaspars, M., and Gammon, D.W. 2020. Novel South African rare Actinomycete Kribbella speibonae strain SK5: A prolific producer of hydroxamate siderophores including new dehydroxylated congeners. Molecules. 25(13): 2979. https://doi.org/10.3390/molecules25132979
Ahmad, F., Ahmad, I., and Khan, M.S. 2005. Indole acetic acid production by the indigenous isolates of azotobacter and fluorescent pseudomonas in the presence and absence of tryptophan. Turkish Journal of Biology. 29(1): 29–34. https://journals.tubitak.gov.tr/biology/issues/biy-05-29-1/biy-29-1-5-0410-1.pdf
Arnon, D.I. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgarus. Plant Physiology. 24(1): 1–15. https://doi.org/10.1104/pp.24.1.1
Arnow, L.E. 1937. Colorimetric determination of the components of 3,4-dihydroxyphenylalaninetyrosine mixtures. Journal of Biological Chemistry. 118(2): 531–537. https://doi.org/10.1016/s0021-9258(18)74509-2
Atkin, C.L., Neilands, J.B., and Phaff, H.J. 1970. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. Journal of Bacteriology. 103(3): 722–733. https://doi.org/10.1128/jb.103.3.722-733.1970
Austin, B. 1988. Marine Microbiology. Cambridge University Press, Cambridge.
Aziz, R.K., Bartels, D., Best, A.A., DeJongh, M., Disz, T., Edwards, R.A., Formsma, K., Gerdes, S., Glass, E.M., Kubal, M., et al. 2008. The RAST Server: Rapid annotations using subsystems technology. BMC Genomics. 9(1). https://doi.org/10.1186/1471-2164-9-75
Bates, L.S., Waldren, R.P., and Teare, I.D. 1973. Rapid determination of free proline for water-stress studies. Plant and Soil. 39(1): 205–207. https://doi.org/10.1007/bf00018060
Battini, F., Cristani, C., Giovannetti, M., and Agnolucci, M. 2015. Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiological Research. 183: 68–79. https://doi.org/10.1016/j.micres.2015.11.012
Becerril-Espinosa, A., Hernández-Herrera, R.M., Meza-Canales, I.D., Perez-Ramirez, R., Rodríguez-Zaragoza, F.A., Méndez-Morán, L., Sánchez-Hernández, C.V., Palmeros-Suárez, P.A., Palacios, O.A., Choix, F.J., et al. 2022. Habitat-adapted heterologous symbiont Salinispora arenicola promotes growth and alleviates salt stress in tomato crop plants. Frontiers in Plant Science. 13. https://doi.org/10.3389/fpls.2022.920881
Bessai, S.A., Cruz, J., Carril, P., Melo, J., Santana, M.M., Mouazen, A.M., Cruz, C., Yadav, A.N., Dias, T., and Nabti, E. 2023. The plant growth-promoting potential of halotolerant bacteria is not phylogenetically determined: Evidence from two Bacillus megaterium strains isolated from saline soils used to grow wheat. Microorganisms. 11(7): 1687. https://doi.org/10.3390/microorganisms11071687
Botta, A.L., Santacecilia, A., Ercole, C., Cacchio, P., and Del Gallo, M. 2013. In vitro and in vivo inoculation of four endophytic bacteria on Lycopersicon esculentum. New Biotechnology. 30(6): 666–674. https://doi.org/10.1016/j.nbt.2013.01.001
Boubekri, K., Soumare, A., Mardad, I., Lyamlouli, K., Hafidi, M., Ouhdouch, Y., and Kouisni, L. 2021. The screening of potassium- and phosphate-solubilizing actinobacteria and the assessment of their ability to promote wheat growth parameters. Microorganisms. 9(3): 470. https://doi.org/10.3390/microorganisms9030470
Chan, H.T., Sanxter, S., and Couey, H.M. 1985. Electrolyte leakage and ethylene production induced by chilling injury of papayas. HortScience. 20(6): 1070–1072. https://doi.org/10.21273/hortsci.20.6.1070
Chen, J., Chen, J., Wang, S., Zhou, G., Chen, D., Zhang, H., and Wang, H. 2019. Development and validation of polar RP-HPLC method for screening for ectoine high-yield strains in marine bacteria with green chemistry. Natural Product Research. 33(8): 1122-1126
Chen, T. and Zhang, B. 2016. Measurements of proline and malondialdehyde content and antioxidant enzyme activities in leaves of drought stressed cotton. Bio-Protocol. 6(17). https://doi.org/10.21769/bioprotoc.1913
Chinachanta, K., Shutsrirung, A., Herrmann L., Lesueur, D., and Pathom-aree, W. 2021. Enhancement of the aroma compound 2-Acetyl-1-pyrroline in Thai jasmine rice (Oryza sativa) by rhizobacteria under salt stress. Biology. 10: 1065. https://doi.org/10.3390/ biology10101065.
Chinachanta, K., Shutsrirung, A., Santasup, C., Pathom-Aree, W., Luu, D.T., Herrmann, L., Lesueur, D., and Prom-u-thai, C. 2023. Rhizoactinobacteria enhance growth and antioxidant activity in Thai jasmine rice (Oryza sativa) KDML105 seedlings under salt stress. Plants. 12: 3441. https://doi.org/10.3390/plants12193441.
Das, P., Lakra, N., Nutan, K., Singla-Pareek, S., and Pareek, A. 2015. Pot level drought stress tolerance assay in tobacco through plant phenotyping and antioxidant assay. Bio-Protocol. 5(19). https://doi.org/10.21769/bioprotoc.1605
Dastager, S.G. and Damare, S. 2013. Marine Actinobacteria showing phosphate-solubilizing efficiency in Chorao Island, Goa, India. Current Microbiology. 66(5): 421–427. https://doi.org/10.1007/s00284-012-0288-z
Del Carmen Orozco-Mosqueda, M., Glick, B.R., and Santoyo, G. 2020. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiological Research. 235: 126439. https://doi.org/10.1016/j.micres.2020.126439
Duca, D.R. and Glick, B.R. 2020. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Applied Microbiology and Biotechnology. 104(20): 8607–8619. https://doi.org/10.1007/s00253-020-10869-5
DuBois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., and Smith, F. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 28(3): 350–356. https://doi.org/10.1021/ac60111a017
Djebaili, R., Pellegrini, M., Rossi, M., Forni, C., Smati, M., Del Gallo, M., and Kitouni, M. 2021. Characterization of plant growth-promoting traits and inoculation effects on Triticum durum of Actinomycetes isolates under salt stress conditions. Soil Systems. 5(2): 26. https://doi.org/10.3390/soilsystems5020026
FAO launches first major global assessment of salt-affected soils in 50 years. 2024 (November 12). Newsroom. https://www.fao.org/newsroom/detail/fao-launches-first-major-global-assessment-of-salt-affected-soils-in-50-years/en
Fiske, C.H. and Subbarow, Y. 1925. The colorimetric determination of phosphorus. Journal of Biological Chemistry. 66(2): 375–400. https://doi.org/10.1016/s0021-9258(18)84756-1
Gao, Y., Han, Y., Li, X., Li, M., Wang, C., Li, Z., Wang, Y., and Wang, W. 2022. A salt-tolerant Streptomyces paradoxus D2-8 from rhizosphere soil of Phragmites communis augments soybean tolerance to soda saline-alkali stress. Polish Journal of Microbiology. 71(1): 43–53. https://doi.org/10.33073/pjm-2022-006
Gong, Y., Chen, L., Pan, S., Li, X., Xu, M., Zhang, C., Xing, K., and Qin, S. 2020. Antifungal potential evaluation and alleviation of salt stress in tomato seedlings by a halotolerant plant growth-promoting actinomycete Streptomyces sp. KLBMP5084. Rhizosphere. 16: 100262. https://doi.org/10.1016/j.rhisph.2020.100262
Goudjal, Y., Toumatia, O., Yekkour, A., Sabaou, N., Mathieu, F., and Zitouni, A. 2013. Biocontrol of Rhizoctonia solani damping-off and promotion of tomato plant growth by endophytic actinomycetes isolated from native plants of Algerian Sahara. Microbiological Research. 169(1): 59–65. https://doi.org/10.1016/j.micres.2013.06.014
Gu, S., Wei, Z., Shao, Z., Friman, V., Cao, K., Yang, T., Kramer, J., Wang, X., Li, M., Mei, X., et al. 2020. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nature Microbiology. 5(8): 1002–1010. https://doi.org/10.1038/s41564-020-0719-8
Hasanuzzaman, M., Bhuyan, M.H.M.B., Anee, T.I., Parvin, K., Nahar, K., Mahmud, J.A., and Fujita, M. 2019. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 8(9): 384. https://doi.org/10.3390/antiox8090384
Hibbing, M.E., Fuqua, C., Parsek, M.R., and Peterson, S.B. 2010. Bacterial competition: Surviving and thriving in the microbial jungle. Nature Reviews Microbiology. 8(1): 15–25. https://doi.org/10.1038/nrmicro2259
Hodges, D.M., DeLong, J.M., Forney, C.F., and Prange, R.K. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 207(4): 604–611. https://doi.org/10.1007/s004250050524
Huerta-Cepas, J., Szklarczyk, D., Heller, D., Hernández-Plaza, A., Forslund, S.K., Cook, H., Mende, D.R., Letunic, I., Rattei, T., Jensen, L.J., et al. 2018. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Research. 47(D1): D309–D314. https://doi.org/10.1093/nar/gky1085
Illmer, P. and Schinner, F. 1995. Solubilization of inorganic calcium phosphates—solubilization mechanisms. Soil Biology and Biochemistry. 27(3): 257–263. https://doi.org/10.1016/0038-0717(94)00190-c
Insuk, C., Kuncharoen, N., Cheeptham, N., Tanasupawat, S., and Pathom-Aree, W. 2020. Bryophytes harbor cultivable actinobacteria with plant growth promoting potential. Frontiers in Microbiology. 11. https://doi.org/10.3389/fmicb.2020.563047
Islam, F., Yasmeen, T., Arif, M.S., Ali, S., Ali, B., Hameed, S., and Zhou, W. 2015. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regulation. 80(1): 23–36. https://doi.org/10.1007/s10725-015-0142-y
Kaewkla, O., Suriyachadkun, C., and Franco, C.M.M. 2021. Streptomyces adelaidensis sp. nov., an actinobacterium isolated from the root of Callitris preissii with potential for plant growth-promoting properties. Archives of Microbiology. 203(6): 3341–3352. https://doi.org/10.1007/s00203-021-02308-4
Khan, M.A., Sahile, A.A., Jan, R., Asaf, S., Hamayun, M., Imran, M., Adhikari, A., Kang, S., Kim, K., and Lee, I. 2021. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biology. 21(1). https://doi.org/10.1186/s12870-021-02937-3
Kumawat, K.C., Nagpal, S., and Sharma, P. 2021. Potential of plant growth-promoting rhizobacteria-plant interactions in mitigating salt stress for sustainable agriculture: A review. Pedosphere. 32(2): 223–245. https://doi.org/10.1016/s1002-0160(21)60070-x
Lasudee, K., Tokuyama, S., Lumyong, S., and Pathom-Aree, W. 2018. Actinobacteria associated with Arbuscular Mycorrhizal Funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: Evidence obtained from mung bean (Vigna radiata) and Thai jasmine rice (Oryza sativa). Frontiers in Microbiology. 9. https://doi.org/10.3389/fmicb.2018.01247
Li, H., Han, Q., Liu, Q., Gan, Y., Rensing, C., Rivera, W.L., Zhao, Q., and Zhang, J. 2023. Roles of phosphate-solubilizing bacteria in mediating soil legacy phosphorus availability. Microbiological Research. 272: 127375. https://doi.org/10.1016/j.micres.2023.127375
Li, X., Lang, D., Wang, J., Zhang, W., and Zhang, X. 2023. Plant-beneficial Streptomyces dioscori SF1 potential biocontrol and plant growth promotion in saline soil within the arid and semi-arid areas. Environmental Science and Pollution Research. 30(27): 70194–70212. https://doi.org/10.1007/s11356-023-27362-x
Liang, X., Ishfaq, S., Liu, Y., Jijakli, M.H., Zhou, X., Yang, X., and Guo, W. 2024. Identification and genomic insights into a strain of Bacillus velezensis with phytopathogen-inhibiting and plant growth-promoting properties. Microbiological Research. 285: 127745. https://doi.org/10.1016/j.micres.2024.127745
Manulis, S., Shafrir, H., Epstein, E., Lichter, A., and Barash, I. 1994. Biosynthesis of indole-3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology. 140(5): 1045–1050. https://doi.org/10.1099/13500872-140-5-1045
Marín, M., Wong, I., Mena, J., Morán, R., Pimentel, E., Sánchez, I., Basulto, R., and Moreira, A. 2013. Zea mays L. plant growth promotion by Tsukamurella paurometabola strain C-924. Biotecnologia Aplicada. 30: 105-110.
Mathew, B.T., Torky, Y., Amin, A., Mourad, A.I., Ayyash, M.M., El-Keblawy, A., Hilal-Alnaqbi, A., AbuQamar, S.F., and El-Tarabily, K.A. 2020. Halotolerant marine rhizosphere-competent actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Frontiers in Microbiology. 11. https://doi.org/10.3389/fmicb.2020.00552
Mohammadipanah, F. and Zamanzadeh, M. 2019. Bacterial mechanisms promoting the tolerance to drought stress in plants. In Springer eBooks (pp. 185–224). https://doi.org/10.1007/978-981-13-5862-3_10
Mukhopadhyay, R., Sarkar, B., Jat, H.S., Sharma, P.C., and Bolan, N.S. 2020. Soil salinity under climate change: Challenges for sustainable agriculture and food security. Journal of Environmental Management. 280: 111736. https://doi.org/10.1016/j.jenvman.2020.111736
Nafis, A., Raklami, A., Bechtaoui, N., Khalloufi, F.E., Alaoui, A.E., Glick, B.R., Hafidi, M., Kouisni, L., Ouhdouch, Y., and Hassani, L. 2019. Actinobacteria from extreme niches in Morocco and their plant growth-promoting potentials. Diversity. 11(8): 139. https://doi.org/10.3390/d11080139
Nautiyal, C. 1999. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters. 170(1): 265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
Nozari, R.M., Ortolan, F., Astarita, L.V., and Santarém, E.R. 2021. Streptomyces spp. enhance vegetative growth of maize plants under saline stress. Brazilian Journal of Microbiology. 52(3): 1371–1383. https://doi.org/10.1007/s42770-021-00480-9
Olanrewaju, O.S., Glick, B.R., and Babalola, O.O. 2017. Mechanisms of action of plant growth promoting bacteria. World Journal of Microbiology and Biotechnology. 33(11). https://doi.org/10.1007/s11274-017-2364-9
Ondrasek, G., Rathod, S., Manohara, K.K., Gireesh, C., Anantha, M.S., Sakhare, A.S., Parmar, B., Yadav, B.K., Bandumula, N., Raihan, F., et al. 2022. Salt stress in plants and mitigation approaches. Plants. 11(6): 717. https://doi.org/10.3390/plants11060717
Paglia, D.E. and Valentine, W.N. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. PubMed. 70(1): 158–169. https://pubmed.ncbi.nlm.nih.gov/6066618
Pailles, Y., Awlia, M., Julkowska, M., Passone, L., Zemmouri, K., Negrão, S., Schmöckel, S.M., and Tester, M. 2020. Diverse traits contribute to salinity tolerance of wild tomato seedlings from the Galapagos islands. Plant Physiology. 182(1): 534–546. https://doi.org/10.1104/pp.19.00700
Parihar, P., Singh, S., Singh, R., Singh, V.P., and Prasad, S.M. 2014. Effect of salinity stress on plants and its tolerance strategies: A review. Environmental Science and Pollution Research. 22(6): 4056–4075. https://doi.org/10.1007/s11356-014-3739-1
Parvin, K., Hasanuzzaman, M., Bhuyan, M.H.M.B., Mohsin, S.M., and Fujita, M. 2019. Quercetin mediated salt tolerance in tomato through the enhancement of pant antioxidant defense and glyoxalase systems. Plants. 8(8): 247. https://doi.org/10.3390/plants8080247
Palaniyandi, S., Damodharan, K., Yang, S., and Suh, J. 2014. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. Journal of Applied Microbiology. 117(3): 766–773. https://doi.org/10.1111/jam.12563
Pathom-Aree, W., Stach, J.E.M., Ward, A.C., Horikoshi, K., Bull, A.T., and Goodfellow, M. 2006. Diversity of actinomycetes isolated from Challenger Deep sediment (10,898 m) from the Mariana Trench. Extremophiles. 10(3): 181–189. https://doi.org/10.1007/s00792-005-0482-z
Pérez, E., Sulbarán, M., Ball, M.M., and Yarzábal, L.A. 2007. Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biology and Biochemistry. 39(11): 2905–2914. https://doi.org/10.1016/j.soilbio.2007.06.017
Potestio, S., Giannelli, G., Degola, F., Vamerali, T., Fragni, R., Cocconi, E., Sandei, L., and Visioli, G. 2024. Salt stress mitigation and improvement in fruit nutritional characteristics of tomato plants: New opportunities from the exploitation of a halotorelant Agrobacterium strain. Plant Stress. 13: 100558. https://doi.org/10.1016/j.stress.2024.100558
Radwan, T.E.E., Mohamed, Z.K., and Reis, V.M. 2002. Production of indole-3-acetic acid by different strains of Azospirillum and Herbaspirillum spp. Symbiosis. 32(1): 39–53. https://dalspace.library.dal.ca/handle/10222/77878
Rahman, A., Nahar, K., Mahmud, J.A., Hasanuzzaman, M., Hossain, M.S., and Fujita, M. 2017. Salt stress tolerance in rice: Emerging role of exogenous phytoprotectants. In InTech eBooks. https://doi.org/10.5772/67098
Rangseekaew, P., Barros-Rodríguez, A., Pathom-Aree, W., and Manzanera, M. 2022. Plant beneficial deep-sea Actinobacterium, Dermacoccus abyssi MT1.1T promote growth of tomato (Solanum lycopersicum) under salinity stress. Biology. 11(2): 191. https://doi.org/10.3390/biology11020191
Rangseekaew, P., Barros-Rodríguez, A., Pathom-Aree, W., and Manzanera, M. 2021. Deep-sea actinobacteria mitigate salinity stress in tomato seedlings and their biosafety testing. Plants. 10(8): 1687. https://doi.org/10.3390/plants10081687
Rashad, F.M., Fathy, H.M., El-Zayat, A.S., and Elghonaimy, A.M. 2015. Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments in Egypt. Microbiological Research. 175: 34–47. https://doi.org/10.1016/j.micres.2015.03.002
Rothan, C., Diouf, I., and Causse, M. 2019. Trait discovery and editing in tomato. The Plant Journal. 97(1): 73–90. https://doi.org/10.1111/tpj.14152
Sadeghi, A., Karimi, E., Dahaji, P.A., Javid, M.G., Dalvand, Y., and Askari, H. 2011. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World Journal of Microbiology and Biotechnology. 28(4): 1503–1509. https://doi.org/10.1007/s11274-011-0952-7
Sagar, A., Rai, S., Ilyas, N., Sayyed, R.Z., Al-Turki, A.I., Enshasy, H.a.E., and Simarmata, T. 2022. Halotolerant rhizobacteria for salinity-stress mitigation: Diversity, mechanisms and molecular approaches. Sustainability. 14(1): 490. https://doi.org/10.3390/su14010490
Saidi, S., Cherif-Silini, H., Bouket, A.C., Silini, A., Eshelli, M., Luptakova, L., Alenezi, F.N., and Belbahri, L. 2021. Improvement of Medicago sativa crops productivity by the co-inoculation of Sinorhizobium meliloti–actinobacteria under salt stress. Current Microbiology. 78(4): 1344–1357. https://doi.org/10.1007/s00284-021-02394-z
Santos-Beneit, F. 2015. The Pho regulon: A huge regulatory network in bacteria. Frontiers in Microbiology. 6. https://doi.org/10.3389/fmicb.2015.00402
Sayyed, R.Z., Chincholkar, S.B., Reddy, M.S., Gangurde, N.S., and Patel, P.R. 2012. Siderophore producing PGPR for crop nutrition and phytopathogen suppression. In Springer eBooks (pp. 449–471). https://doi.org/10.1007/978-3-642-33639-3_17
Schwyn, B. and Neilands, J. 1987. Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry. 160(1): 47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Shrivastava, P. and Kumar, R. 2015. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences. 22(2): 123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
Suksaard, P., Pathom-aree, W., and Duangmal, K. 2017. Diversity and plant growth promoting activities of actinomycetes from mangroves. Chiang Mai Journal of Science. 44: 1210–1223.
Sunohara, Y. and Matsumoto, H. 2004. Oxidative injury induced by the herbicide quinclorac on Echinochloa oryzicola Vasing. and the involvement of antioxidative ability in its highly selective action in grass species. Plant Science. 167(3): 597–606. https://doi.org/10.1016/j.plantsci.2004.05.005
Tang, J., Li, Y., Zhang, L., Mu, J., Jiang, Y., Fu, H., Zhang, Y., Cui, H., Yu, X., and Ye, Z. 2023. Biosynthetic pathways and functions of indole-3-acetic acid in microorganisms. Microorganisms. 11(8): 2077. https://doi.org/10.3390/microorganisms11082077
Tashkandi, M. and Baz, L. 2023. Function of CAZymes encoded by highly abundant genes in rhizosphere microbiome of Moringa oleifera. Saudi Journal of Biological Sciences. 30(3): 103578. https://doi.org/10.1016/j.sjbs.2023.103578
Teo, H.M., Aziz A., Wahizatul, A.A., Bhubalan, K., Siti Nordahliawate, M.S., Muhamad Syazlie, C.I., and Lee Chuen, Ng. 2022. Setting a plausible route for saline soil-based crop cultivations by application of beneficial halophyte-associated bacteria: A review. Microorganisms. 10(3): 657. https://doi.org/10.3390/microorganisms10030657
Timofeeva, A.M., Galyamova, M.R., and Sedykh, S.E. 2022. Bacterial siderophores: Classification, biosynthesis, perspectives of use in agriculture. Plants. 11: 3065. https://doi.org/10.3390/plants11223065
Ullah, A. and Bano, A. 2021. Modulation of secondary metabolites: A halotolerance strategy of plant growth promoting rhizobacteria against sodium chloride stress. Current Microbiology. 78(12): 4050–4059. https://doi.org/10.1007/s00284-021-02647-x
Velikova, V., Yordanov, I., and Edreva, A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Science. 151(1): 59–66. https://doi.org/10.1016/s0168-9452(99)00197-1
Wahyudi, A.T., Priyanto, J.A., Afrista, R., Kurniati, D., Astuti, R.I., and Akhdiya, A. 2019. Plant growth promoting activity of actinomycetes isolated from soybean rhizosphere. OnLine Journal of Biological Sciences. 19(1): 1–8. https://doi.org/10.3844/ojbsci.2019.1.8
Wang, Z., Zhang, H., Liu, L., Li, S., Xie, J., Xue, X., and Jiang, Y. 2022. Screening of phosphate-solubilizing bacteria and their abilities of phosphorus solubilization and wheat growth promotion. BMC Microbiology. 22(1): 296. https://doi.org/10.1186/s12866-022-02715-7
Xie, Z., Chu, Y., Zhang, W., Lang, D., and Zhang, X. 2019. Bacillus pumilus alleviates drought stress and increases metabolite accumulation in Glycyrrhiza uralensis Fisch. Environmental and Experimental Botany. 158: 99–106. https://doi.org/10.1016/j.envexpbot.2018.11.021
Xiong, Y., Gong, Y., Li, X., Chen, P., Ju, X., Zhang, C., Yuan, B., Lv, Z., Xing, K., and Qin, S. 2019. Enhancement of growth and salt tolerance of tomato seedlings by a natural halotolerant actinobacterium Glutamicibacter halophytocola KLBMP 5180 isolated from a coastal halophyte. Plant and Soil. 445(1–2): 307–322. https://doi.org/10.1007/s11104-019-04310-8
Xu, Y., Li, Y., Long, C., and Han, L. 2022. Alleviation of salt stress and promotion of growth in peanut by Tsukamurella tyrosinosolvens and Burkholderia pyrrocinia. Biologia. 77(9): 2423–2433. https://doi.org/10.1007/s11756-022-01073-z
Zaman, M., Shahid, S.A., and Heng, L. 2018. Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. In Springer eBooks. https://doi.org/10.1007/978-3-319-96190-3
Zhao, S., Zhang, Q., Liu, M., Zhou, H., Ma, C., and Wang, P. 2021. Regulation of plant responses to salt stress. International Journal of Molecular Sciences. 22(9): 4609. https://doi.org/10.3390/ijms22094609
Zheng, J., Ge, Q., Yan, Y., Zhang, X., Huang, L., and Yin, Y. 2023. dbCAN3: Automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Research. 51(W1): W115–W121. https://doi.org/10.1093/nar/gkad328
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Supplementary

Figure S1. (A) Minimal medium+(NH4)2SO4, (B) Minimal medium+ACC, (C) Minimal Medium.
Table S1. Protein coding sequences related with plant growth promoting traits of Tsukamurella sp. MT6.1.
|
Plant growth promoting properties |
Protein coding sequences |
Ec number |
|
Amino acids and derivatives |
Proline Synthesis |
|
|
|
Pyrroline-5-carboxylate reductase |
(EC 1.5.1.2) |
|
|
Gamma-glutamyl phosphate reductase |
(EC 1.2.1.41) |
|
|
Glutamate 5-kinase |
(EC 2.7.2.11) |
|
|
RNA-binding C-terminal domain PUA |
|
|
|
NADP-specific glutamate dehydrogenase |
(EC 1.4.1.4) |
|
|
Proline, 4-hydroxyproline uptake and utilization |
|
|
|
D-amino-acid oxidase |
(EC 1.4.3.3) |
|
|
Delta-1-pyrroline-5-carboxylate dehydrogenase |
(EC 1.2.1.88) |
|
|
Proline/sodium symporter PutP |
(TC 2.A.21.2.1) |
|
|
Proline iminopeptidase |
(EC 3.4.11.5) |
|
|
Tryptophan synthesis |
|
|
|
Tryptophan synthase beta chain |
(EC 4.2.1.20) |
|
|
Anthranilate synthase, aminase component |
(EC 4.1.3.27) |
|
|
Aminodeoxychorismate lyase |
(EC 4.1.3.38) |
|
|
Anthranilate synthase, amidotransferase component |
(EC 4.1.3.27) |
|
|
Para-aminobenzoate synthase, aminase component |
(EC 2.6.1.85) |
|
|
Phosphoribosylanthranilate isomerase |
(EC 5.3.1.24) |
|
|
Tryptophan synthase alpha chain |
(EC 4.2.1.20) |
|
|
Indole-3-glycerol phosphate synthase |
(EC 4.1.1.48) |
|
|
Acting phosphoribosylanthranilate isomerase |
(EC 5.3.1.24) |
|
|
Anthranilate phosphoribosyltransferase |
(EC 2.4.2.18) |
|
|
Para-aminobenzoate synthase, amidotransferase component |
(EC 2.6.1.85) |
|
Iron acquisition and metabolism |
Iron acquisition in Streptococcus |
|
|
|
Ferric iron ABC transporter, iron-binding protein |
|
|
|
Ferric iron ABC transporter, ATP-binding protein |
|
|
|
Ferric iron ABC transporter, permease protein |
|
|
|
Ferrous iron transporter EfeUOB, low-pH-induced |
|
|
|
Ferrous iron transport periplasmic protein EfeO, contains peptidase-M75 domain and (frequently) cupredoxin-like domain |
|
|
|
Ferrous iron transport permease EfeU |
|
|
|
Ferrous iron transport peroxidase EfeB |
|
|
|
Encapsulating protein for DyP-type peroxidase and ferritin-like protein oligomers |
|
|
|
Predicted dye-decolorizing peroxidase (DyP), encapsulated subgroup |
|
|
|
Encapsulating protein for a DyP-type peroxidase or ferritin-like protein oligomers |
|
|
Nitrogen metabolism |
Allantoin Utilization |
|
|
|
Glycerate kinase |
(EC 2.7.1.31) |
|
|
Glyoxylate carboligase |
(EC 4.1.1.47) |
|
|
2-hydroxy-3-oxopropionate reductase |
(EC 1.1.1.60) |
|
|
Allantoinase |
(EC 3.5.2.5) |
|
|
Ammonia assimilation |
|
|
|
Nitrogen regulatory protein P-II |
|
|
|
Glutamate synthase [NADPH] large chain |
(EC 1.4.1.13) |
|
|
Glutamate-ammonia-ligase adenylyltransferase |
(EC 2.7.7.42) |
|
|
Glutamine synthetase type I |
(EC 6.3.1.2) |
|
|
Glutamate synthase [NADPH] small chain |
(EC 1.4.1.13) |
|
|
Ammonium transporter |
|
|
|
[Protein-PII] uridylyltransferase |
(EC 2.7.7.59) |
|
Phosphorus metabolism |
High affinity phosphate transporter and control of PHO regulon |
|
|
|
Phosphate regulon transcriptional regulatory protein PhoB (SphR) |
|
|
|
Phosphate transport system regulatory protein PhoU |
|
|
|
Phosphate regulon sensor protein PhoR (SphS) |
(EC 2.7.13.3) |
|
|
Polyphosphate kinase |
(EC 2.7.4.1) |
|
|
Phosphate metabolism |
|
|
|
Exopolyphosphatase |
(EC 3.6.1.11) |
|
|
Alkaline phosphatase |
(EC 3.1.3.1) |
|
|
Predicted ATPase related to phosphate starvation-inducible protein PhoH |
|
|
|
NAD(P) transhydrogenase subunit beta |
(EC 1.6.1.2) |
|
|
Probable low-affinity inorganic phosphate transporter |
|
|
|
Phosphate transport system regulatory protein PhoU |
|
|
|
Phosphate starvation-inducible protein PhoH, predicted ATPase |
|
|
|
Inorganic pyrophosphatase |
(EC 3.6.1.1) |
|
|
Phosphate regulon transcriptional regulatory protein PhoB (SphR) |
|
|
|
Phosphate regulon sensor protein PhoR (SphS) |
(EC 2.7.13.3) |
|
|
Polyphosphate kinase |
(EC 2.7.4.1) |
|
|
Polyphosphate |
|
|
|
Polyphosphate glucokinase |
(EC 2.7.1.63) |
|
|
Polyphosphate kinase 2 |
(EC 2.7.4.1) |
|
|
Exopolyphosphatase |
(EC 3.6.1.11) |
|
|
Polyphosphate kinase |
(EC 2.7.4.1) |
|
Potassium metabolism |
Potassium homeostasis |
|
|
|
Potassium efflux system KefA protein |
|
|
|
Potassium-transporting ATPase C chain |
(EC 3.6.3.12) (TC 3.A.3.7.1) |
|
|
Potassium-transporting ATPase B chain |
(EC 3.6.3.12) (TC 3.A.3.7.1) |
|
|
Potassium-transporting ATPase A chain |
(EC 3.6.3.12) (TC 3.A.3.7.1) |
|
|
Potassium channel protein |
|
|
|
Large-conductance mechanosensitive channel |
|
|
Secondary metabolism |
Auxin biosynthesis |
|
|
|
Anthranilate phosphoribosyltransferase |
(EC 2.4.2.18) |
|
|
Tryptophan synthase alpha chain |
(EC 4.2.1.20) |
|
|
Phosphoribosylanthranilate isomerase |
(EC 5.3.1.24) |
|
|
Monoamine oxidase |
(1.4.3.4) |
|
|
Tryptophan synthase beta chain |
(EC 4.2.1.20) |
Table S2. Genes related to the promotion of plant growth and plant's defense mechanism in Tsukamurella sp. MT6.1 Pathway ID.
|
KEGG Pathway id |
Annotation information |
Functions |
|
K00128, K00129 |
Aldedh; aldehyde dehydrogenase family |
Detoxification of reactive aldehyde, stress tolerance |
|
K01426 |
Amidase; amidase family |
Auxin biosynthesis, stress adaptation |
|
K04488 |
NifU N; nitrogen fixation protein |
Nitrogen fixation |
|
K00362 |
nirB; nitrite and sulfite reductase 4Fe-4S domain family |
Nitrogen assimilation, sulfur assimilation |
|
K00363 |
nirD; nitrite reductase |
Nitrate assimilation, nutrient uptake, and utilization |
|
K00380, K02567, K02575 |
narB; narK; molybdopterin-containing oxidoreductase family |
Nitrate assimilation, sulfur, and carbon metabolism |
|
K03320 |
Amt, AmtB Ammonium transporter |
Soil ammonium uptake |
Table S3. Targeted substrates predicted for CAZymes genes clusters in Tsukamurella sp. MT6.1.
|
dbCAN Subfam |
Substrate |
E Value |
Coverage |
|
AA1_e46 |
lignin, lignin |
1.50E-46 |
0.52742616 |
|
CBM48_e0 |
Glycogen |
1.10E-24 |
0.72815534 |
|
CBM48_e2 |
Glycogen |
7.70E-23 |
0.86 |
|
CBM48_e3 |
Glycogen |
1.40E-29 |
0.91089109 |
|
CBM50_e773 |
chitin, peptidoglycan |
1.90E-26 |
0.95744681 |
|
CE14_e1 |
Chitin |
5.80E-70 |
0.99259259 |
|
CE14_e2 |
exo-polysaccharide |
1.20E-56 |
0.99285714 |
|
CE1_e32 |
trehalose, polyphenol, xylan |
1.20E-81 |
0.97637795 |
|
CE1_e32 |
trehalose, polyphenol, xylan |
5.70E-82 |
0.97244094 |
|
GH10_e26 |
Xylan |
2.00E-186 |
0.99401198 |
|
GH13_e105 |
sucrose, starch |
2.00E-106 |
0.98026316 |
|
GH13_e200 |
sucrose, alpha-glucan |
4.50E-160 |
0.99335548 |
|
GH13_e234 |
sucrose, starch |
1.50E-165 |
0.98005698 |
|
GH13_e234 |
sucrose, starch |
4.70E-161 |
0.98290598 |
|
GH13_e41 |
Sucrose |
2.30E-94 |
0.99078341 |
|
GH13_e48 |
sucrose, starch, starch |
1.50E-151 |
0.98603352 |
|
GH13_e92 |
sucrose, starch |
3.50E-148 |
0.99684543 |
|
GH15_e22 |
Trehalose |
4.00E-161 |
0.99178082 |
|
GH32_e28 |
Fructan |
4.00E-164 |
0.99342105 |
|
GH38_e38 |
host glycan |
3.00E-131 |
0.98046875 |
|
GH3_e197 |
beta-glucan, beta-glucan, xylan, arabinan, host glycan |
9.70E-105 |
0.97285068 |
|
GH5_e221 |
arabinogalactan protein |
2.00E-116 |
0.99270073 |
Table S4. CAZyme classes and functional relevance in plant stress mitigation.
|
CAZy Class |
Primary activity |
Relevance to salt stress mitigation |
|
Glycoside Hydrolases (GHs) |
Hydrolysis of glycosidic bonds |
Nutrient acquisition, mobilization of osmolyte precursors, plant defense (biocontrol). |
|
Polysaccharide Lyases (PLs) |
Non-hydrolytic cleavage of glycosidic bonds |
Degradation of pectic substances (e.g., alginate), supporting nutrient cycling and carbon access. |
|
Carbohydrate Esterases (CEs) |
Hydrolysis of carbohydrate esters |
De-esterification of complex plant polysaccharides (e.g., hemicellulose), enhancing overall biomass degradation. |
|
Auxiliary Activities (AAs) |
Redox enzymes |
Cooperate with GHs/PLs in the deconstruction of complex, recalcitrant plant cell wall components. |
|
GlycosylTransferases (GTs) |
Formation of glycosidic bonds (biosynthesis) |
Critical for Exopolysaccharide (EPS) and biofilm synthesis; ionic and osmotic protection. |
May Tharaphu Thein Win1, Inthira Wongchomphu1, Kawiporn Chinachanta2, Pharada Rangseekaew3, and Wasu Pathom-aree3, *
1 Master of Science Program in Applied Microbiology (International Program), Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Department of Soil Science, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand.
3 Center of Excellence in Microbial Diversity and Sustainable Utilization, Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Wasu Pathom-aree, E-mail: wasu.p@cmu.ac.th
ORCID iD:
Wasu Pathom-aree: https://orcid.org/0000-0003-0299-3731
Pharada Rangseekaew: https://orcid.org/0000-0002-8934-3880
Kawiporn Chinachanta: https://orcid.org/0000-0002-9785-1412
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: September 22, 2025;
Revised: October 8, 2025;
Accepted: October 9, 2025;
Online First: November 11, 2025

