
Production of High-Efficiency Organic Nitrogen Fertilizer from Liquid Digestate
Thiva Jamaree and Siriwat Sakhonwasee*Published Date : November 4, 2025
DOI : https://doi.org/10.12982/NLSC.2026.010
Journal Issues : Number 1, January-March 2026
Abstract Nitrogen is an essential macronutrient for plant growth and largely contributes to crop production. Typical organic fertilizers such as green manure and animal manure contain a low amount of plant-available nitrogen making it difficult to achieve high crop yields in organic farming. Therefore, this study aims to develop a process for production of high-efficiency nitrogen fertilizer which is eligible for use in organic farming. Liquid-digestate (LD) obtained from the anaerobic digestion of Napier grass and corn stalks in biogas plant was used as a source of nitrogen. Heating of LD obtained from the anaerobic digestion. Simultaneously, heating also promotes the release of CO2 and alkalinization of LD which support evaporation of NH3. Then, NH3, CO2 and IFOAM certified gypsum were allowed to react resulting in white crystalline powder consisting mainly of (NH4)2SO4. From 1.25 L of LD, 10.86 g of white crystalline powder was recovered (8.69 g/L). The powder was used as a nitrogen fertilizer in a trial with four different plant species. Results showed that the nitrogen fertilizer obtained from LD was either comparable to or superior to chemical nitrogen fertilizer in terms of supporting vegetative growth of different plants. Therefore, nitrogen- fertilizer obtained from LD through the process described in this study could be used for plant cultivation in organic farming.
Keywords: Organic fertilizer, Liquid digestate, Ammonium sulfate, Heating, Organic farming
Graphical Abstract:
Funding: The authors are grateful for the research funding provided by Faculty of Agricultural Production, Maejo University, Chiang Mai, Thailand.
Citation: Jamaree, T. and Sakhonwasee, S. 2026. Production of high-efficiency organic nitrogen fertilizer from liquid digestate. Natural and Life Sciences Communications. 25(1): e2026010.
INTRODUCTION
The area of organic farming is increasing every year making the current global market value of organic produce reach 134.8 billion euros (Willer et al., 2024). Parallelly, methods and inputs involving organic farming have been continuously improving to achieve higher quantity and quality of organic produce. One of the major factors affecting crop yield in organic farming is soil fertility. Generally, fertile soil should be enriched with plant nutrients in the form of ions, which can be readily absorbed by roots in order to obtain high crop performance. Natural and biological processes in soil are responsible for breaking down organic materials which in turn releases plant nutrients and improve soil fertility in organic farming systems. However, these processes are slow and uncertain and sometimes may lead to crop nutrient deficiency, especially nitrogen deficiency (Lazcano et al., 2021).
Nitrogen is a macronutrient required for plant growth and its amount in the soil is positively correlated with crop yield (Wang et al., 2018). Severe nitrogen deficiency can result in cessation of plant growth (Hsieh et al., 2018). Typically, plants absorbs nitrogen in forms of nitrate (NO3-), ammonium (NH4+) and urea (CO(NH2)2) through both roots and leaves (Garnica et al., 2010). Organic fertilizers such as green manure, animal manure, compost and biofertilizers may not supply sufficient nitrogen for crops in organic farms because they contain low amounts of plant available nitrogen and have a very slow rate of nitrogen mineralization. These are the major reasons making crop yield of organic farming lower than those of regular farming that uses chemical- fertilizer (De Jesus et al., 2024).
A source of nitrogen fertilizer that can be used in organic farming is liquid digestate (LD), a liquid solution derived from anaerobic digestion in biogas plants. In the process of anaerobic digestion, high-protein organic materials are digested until they become free amino-acids and enter tricarboxylic acid cycle (TCA cycle) of microorganisms which releases nitrogen in forms of ammonia (NH3) and ammonium (NH4+) contained in LD as in Equation (1) and (2) (Wu et al., 2006; Mikami et al., 2017). This process also releases hydrogen-carbonate (HCO3-) as shown in Equation (2). The typical LD contains NH3 and NH4+ at the concentration around 2,000-6,000 ppm (Möller and Müller, 2012; Sutaryo et al., 2014; Jahn et al., 2020; Kalamaras et al., 2021).
RCHNH2COOH + 2H2O→RCOOH + NH3 + CO2 + 2H2 (1)
NH3 + H2O + CO2 → NH4+ + HCO3- (2)
The main products from biogas production are methane (50-70%) and other gases such as carbon dioxide (CO2), hydrogen gas and ammonia (Naimi et al., 2017; Lin et al., 2018; Chinnadurai et al., 2019; Liu et al., 2019 and Jahn et al., 2020). The dissolution of ammonia in liquid digestate (LD) generates ammonium ions (NH4⁺) and hydroxide ions (OH⁻), which increase the alkalinity of LD, as shown in Equation (3) (Garcia-Gonzalez and Vanotti, 2015; Jahn et al., 2020).
NH3 + H2O ↔ NH4+ + OH- (3)
NH4+ + OH- (3)
The temperature in thermophilic anaerobic digestion process is approximately 50-60°C. This promotes changing of HCO3- to gaseous CO2, increasing the amount of OH- and therefore pH of LD solution. The basic condition of LD allows NH4+ to be easily changed to NH3 that can evaporate to the air (Wu et al., 2006; Al-Anezi et al., 2008; Guštin et al., 2011; Möller and Müller, 2012; Baldi et al., 2018; Oliveira Filho et al., 2018; Folino et al., 2020; Jahn et al., 2020; Qu and Zhang, 2021). Moreover, Podjanapon et al. (2023) report that drying of LD to reduce the cost of transportation results in losing almost all NH4+ from LD. Thus, it is difficult to use LD as a main source of nitrogen fertilizer for crop cultivation.
Ammonium sulfate is an important source of nitrogen fertilizer. It can be produced by reacting ammonia gas with water to form ammonium hydroxide, as shown in Equation (4). Subsequently, carbon dioxide is introduced into the reaction, generating ammonium carbonate, as described in Equation (5). Finally, ammonium carbonate reacts with gypsum (calcium sulfate) to yield ammonium sulfate, as shown in Equation (6). All of these reactions can take place under ambient conditions (Brienza et al., 2021).
NH3 + H2O → NH4OH (4)
2NH4OH + CO2 → (NH4)2CO3 + H2O (5)
(NH4)2CO3 + CaSO4 → (NH4)2SO4 + CaCO3 (6)
Presently, many biogas plants utilize sulfuric acid to capture ammonia gas from LD and produce ammonium sulfate fertilizer (Folino et al., 2020) as in Equation (7).
2NH3 + H2SO4 → (NH4)2SO4 (7)
In some cases, flue gas desulphurization gypsum (FGD-gypsum), a consequence of sulfur dioxide removal process in coal power plant, was used to capture ammonia (Sigurnjak et al., 2019; Brienza et al., 2021). Normally, the ammonia gas was separated from LD by adding basic compounds such as sodium hydroxide or potassium hydroxide to increase pH of LD solution (Baldi et al., 2018; Sigurnjak et al., 2019; Szymańska et al., 2019). Therefore, the fertilizer obtained from this process is regarded as a chemical fertilizer and cannot be used in organic farming.
In this study, the process of ammonium sulfate production from LD was modified in attempt to make the obtained fertilizer organic certifiable. NH3 and CO2 from LD were used as raw materials and International Federation of Organic Agriculture Movements (IFOAM) certified gypsum was used for capturing ammonia gas. The modified process was tested, and the resulting fertilizer was evaluated in a trial with four different plant species, which were selected to represent variations in physiological traits and nutrient requirements.
MATERIALS AND METHODS
Experiment 1: Effect of temperature on NH3 stripping and NH3 scrubbing processes
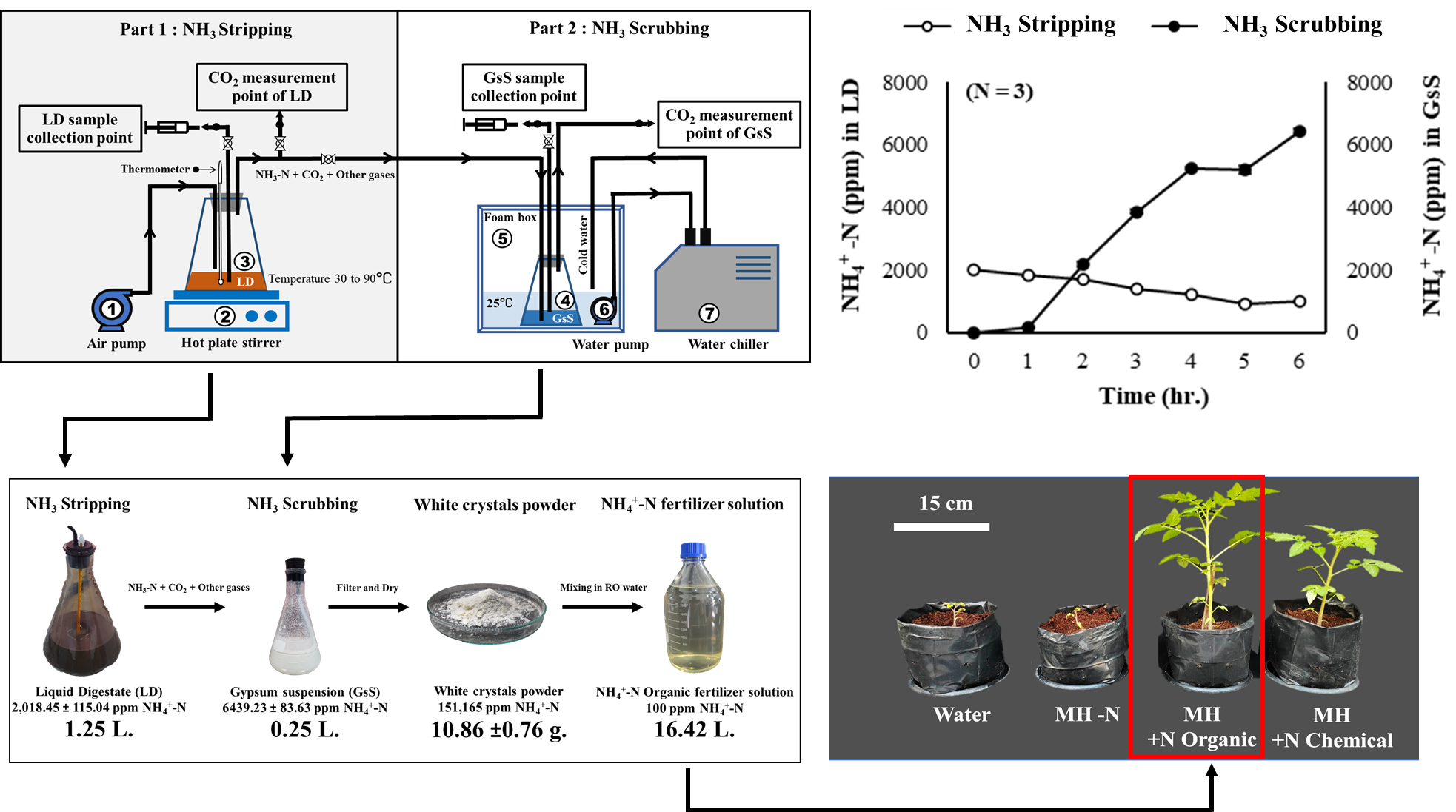
LD used in this study was obtained from anaerobic digestion of Napier grass and corn stalk in biogas plant of UAC Global Co., Ltd. located in Mae Taeng, Chiang Mai, Thailand. The warm LD samples (40-50 °C) were collected from releasing point of fermentation tank. At this point, the temperature was approximately 10°C lower than the temperature inside the fermentation tank. IFOAM-certified gypsum powder was purchased from B.K Plaster and Gypsum Corporation Co., Ltd. located in Nakhon Luang, Ayutthaya, Thailand. The LD, gypsum, growing media and white crystalline powder were chemically analyzed by plant nutrient analysis laboratory, Division of Soil Science, Faculty of Agricultural Production, Maejo University, Thailand and the analysis results were shown in Table 1, 2 and 3.
Experimental setup
There are two parts in this experiment which are NH3 stripping and NH3 scrubbing. In NH3 striping, 1,250 ml of LD was put into a flask and heated by hotplate to temperature of 30, 50, 70 and 90°C. An air pump was used to supply air to the flask at the rate of 60 L/min. LD samples and CO2 were collected and analyzed at each timepoint and temperature conditions.
Table 1. Characteristics and compositions of liquid digestate and gypsum powder used in this research.
|
Characteristics |
Raw materials |
|
|
Liquid digestate |
Gypsum powder |
|
|
Electrical conductivity(dS/m) |
18.7 |
- |
|
pH |
7.7 |
- |
|
Total N (ppm) |
4,145 |
280 |
|
NH4+ (ppm) |
2,044 |
0 |
|
NO3- (ppm) |
17.88 |
0 |
|
Total P (ppm) |
2,180 |
165 |
|
Total K (ppm) |
6,984 |
784 |
|
Total Ca (ppm) |
9,698 |
79,443 |
|
Total Mg (ppm) |
1,581 |
346 |
|
Total S (ppm) |
338 |
26,110 |
|
Total Zn (ppm) |
19 |
2 |
|
Total Mn (ppm) |
43 |
12 |
|
Total Fe (ppm) |
213 |
259 |
|
Total Cu (ppm) |
2 |
0.5 |
Ammonium nitrogen (NH4+-N) removal efficiency (RE) was calculated using Equation (8).
NH4+-N RE (%) = [(C0 – Ct)/ C0] x 100 (8)
C0 is a concentration of NH4+-N in LD before heating and Ct is a concentration of NH4+-N in LD after heating (Reza and Chen, 2021).
In NH3 scrubbing, ammonia gas from LD was captured by IFOAM-certified gypsum. The setup consisted of 250 ml of gypsum suspension (125 g of gypsum in 250 mL of water) in a flask (called GsS bottle). The whole setup was placed inside a box that was maintained the temperature at 25°C to avoid loss of NH3 gas from GsS bottle. Microtubes was used for collecting gypsum suspension sample and CO2 gas for further analysis. The volume ratio of LD bottle to GsS bottle was 5:1 (Figure 1).
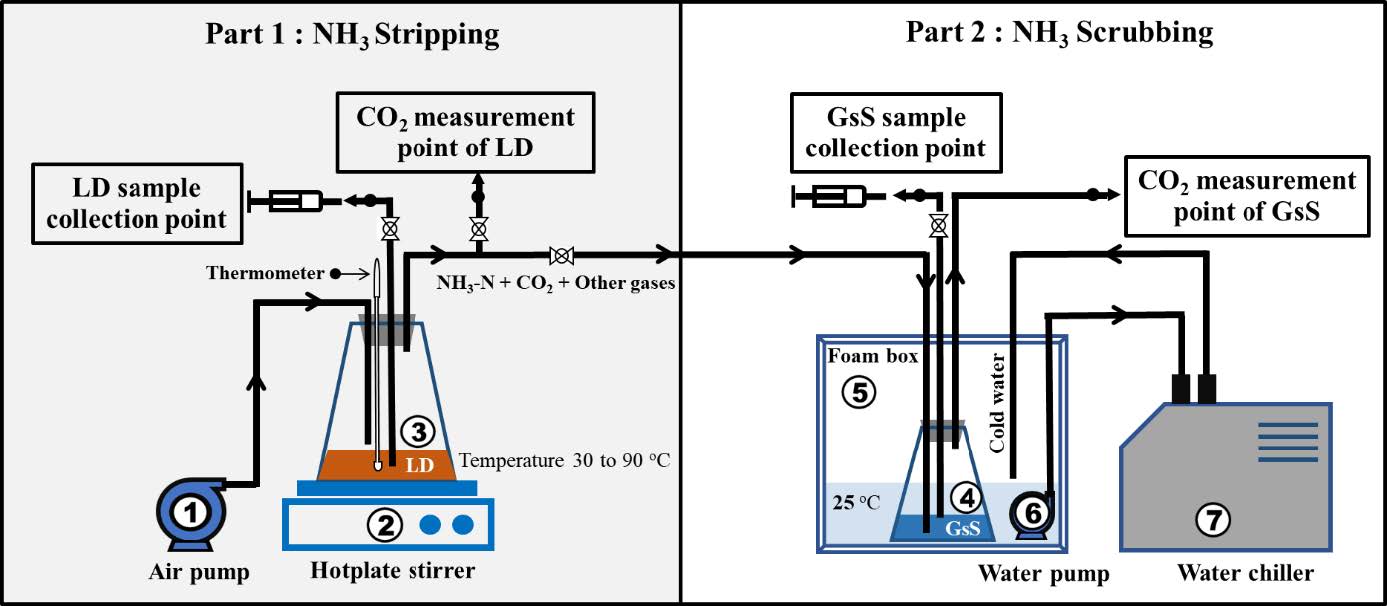
Figure 1. Schematic diagram of experimental apparatus for testing the production of high-efficiency organic nitrogen fertilizer from liquid digestate. (1) Air pump, (2) Hotplate stirrer, (3) LD bottle, (4) Gypsum suspension (GsS) bottle, (5) Foam box, (6) Constant flow pump and (7) Water chiller.
Measurements of CO2, electrical conductivity, pH and NH4+-N content
CO2 concentration was measured by data logger (HT-2000 CO2/Temp /RH DATA LOGGER, Dongguan Xintai Instrument Co., Ltd., China). Electrical conductivity (EC) of solution was measured by portable EC meter (LAQUA twin EC-11, HORIBA Co., Ltd., Japan). pH of solution was measured by portable pH meter (LAQUA twin pH-11, HORIBA Co., Ltd., Japan). NH4+ concentration was measured by ammonium test kit (v-color 9750, V Unique, Better Syndicate Co., Ltd., Thailand). Then, the concentration of ammonium nitrogen (NH4+-N) was calculated from NH4+ concentration, based on the ratio of the molar masses of nitrogen (N) and the ammonium ion (NH4+).
Experiment 2: Comparing the efficiency of NH4+-N fertilizer from LD and NH4+-N chemical fertilizer
Preparation of growing media, plant materials and fertilizer
Growing media was prepared by mixing coconut coir with coarse sand in the ratio of 3:1 (coconut coir: coarse sand). Before using, the coconut coir was washed thoroughly with tap water until the EC of washed solution was not increased. Plant nutrient in the growing media was analyzed by plant nutrient analysis laboratory, Division of Soil Science, Faculty of Agricultural Production, Maejo University, Thailand (Table 2). Lactuca sativa 'Frillice' (lettuce 'Frillice'), Brassica oleracea var. sabellica (kale), Capsicum annuum L. (Thai Chili Pepper) and Solanum lycopersicum L. 'Seeda' (tomato 'Seeda') (Chia Tai Seeds Co., Ltd., Thailand) were used for cultivation test. Seeds were germinated in growing media for 14 days and then transferred to pots (6 inches in diameter) filled with growing media. The pots were placed inside a plastic house at Division of Vegetable Technology, Maejo University, Chiang Mai, Thailand during November – December 2021. The experiments were conducted using a randomized complete block design (RCBD) with five replications. The pots were irrigated with four different treatment solutions as follows: (1) tap water (Water), (2) modified Hoagland solution (Epstein and Bloom, 2005) without nitrogen (MH–N), (3) modified Hoagland solution without nitrogen supplemented with ammonium-nitrogen obtained from LD at a concentration of 100 ppm (MH + Organic-N fertilizer), and (4) modified Hoagland solution without nitrogen supplemented with ammonium sulfate at a concentration of 100 ppm (MH + Chemical-N fertilizer). Each pot was irrigated with 300 mL of the corresponding solution twice per week. The modified Hoagland solution without nitrogen consists of FeDTPA (Fe 7%) 0.043 g/L, CaCl2.2H2O 0.0037 g/L, KH2PO4 0.35 g/L, MgSO4.7H2O 0.25 g/L, H3BO3 0.0015 g/L, MnSO4-H2O 0.0003 g/L, ZnSO4-7H2O 0.0006 g/L, CuSO4-5H2O 0.0001g/L and NaMoO4 0.0001 g/L. For the preparation of treatment 4 solution, (NH4)2SO4 (Chia Tai Co., Ltd., Thailand) was added to Modified Hoagland solution without nitrogen at the concentration of 0.47 g/L.
Table 2. Characteristics and composition of growing media consisting of coconut coir and coarse sand (3:1, v/v).
|
Characteristics |
Value |
|
Organic Matter (%) |
12.40 |
|
Electrical conductivity(dS/m) |
0.40 |
|
pH |
6 |
|
Total N (ppm) |
6,200 |
|
NH4+ (ppm) |
0 |
|
NO3- (ppm) |
5.43 |
|
Total P (ppm) |
12 |
|
Total K (ppm) |
824 |
|
Total Ca (ppm) |
586 |
|
Total Mg (ppm) |
175 |
|
Total S (ppm) |
435 |
|
Total Zn (ppm) |
2 |
|
Total Mn (ppm) |
32 |
|
Total Fe (ppm) |
4 |
|
Total Cu (ppm) |
0.4 |
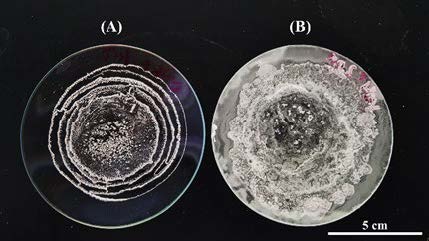
Figure 2. White crystalline powder obtained from 10 ml of filtered and dried GsS solution (A) before NH3 scrubbing (at 0 h) and (B) after NH3 scrubbing at 6 h.
Preparation of fertilizer solution with NH4+-N obtained from LD
NH3 evaporated from LD at 90°C was captured by GsS. Then, undissolved gypsum was filtered out using filter paper No.1 (Whatman, England). The remaining solution was dried in an oven at 105°C. The obtained white crystalline powder (Figure 2) was analyzed by plant nutrient analysis laboratory, Division of Soil Science, Faculty of Agricultural Production, Maejo University, Thailand (Table 3). After knowing the concentration of NH4+, the powder was mixed with modified Hoagland solution without nitrogen to make the concentration of NH4+-N become 100 ppm (0.66 g/L) (Figure 3).
Table 3. The elemental composition of white crystalline powder obtained from GsS solution after 6 h of NH3 scrubbing process.
|
Characteristics |
Value |
|
Total N (%) |
19.77 |
|
NH4+ (%) |
15.12 |
|
NO3- (ppm) |
684 |
|
Total P (ppm) |
0 |
|
Total K (ppm) |
3,457 |
|
Total Ca (%) |
3.68 |
|
Total Mg (ppm) |
716 |
|
Total S (%) |
10.89 |
|
Total Zn (ppm) |
3 |
|
Total Mn (ppm) |
1 |
|
Total Fe (ppm) |
22 |
|
Total Cu (ppm) |
0 |

Figure 3. Preparation of fertilizer solution with NH4+-N obtained from liquid digestate.
Nitrogen use efficiency (NUE)
Nitrogen use efficiency was calculated using Equation (10).
NUE = (Ytreatment - Ycontrol) / Ndose (10)
Ytreatment is a biomass of plant (g·DM·pot-1) received nitrogen in the experiment whereas Ycontrol is a biomass of those grown without nitrogen. Ndose is the amount of nitrogen that the plant received (g/pot) (Szymańska et al., 2019).
Other measurements, experimental design and statistical analysis
Dry weights of shoots and roots of 'Frillice' lettuce, kale, Thai pepper and tomato plants were analyzed 35, 21, 28 and 28 days after beginning the experiment respectively. Fresh samples were dried in a hot air oven at 95°C for 3 days. A complete randomized design was used for the experiment. Data are reported as mean ± standard error (SE). Significant differences among means were determined by Duncan’s post hoc test at P<0.05. All statistical analyses were performed by SPSS V.22 (SPSS Inc., Chicago, IL, USA).
RESULTS
Experiment 1: Effect of temperature on NH3 stripping and NH3 scrubbing processes
LD solution contained NH4+-N at concentration of 2,044 ppm and had EC of 18.7 dS/m and pH of 7.7 (Table 1). When the solution was heated at different temperature, it was found that temperature of 90°C resulted in the fastest reduction of NH4+-N. At 3 hours after heating, NH4+-N concentration was reduced to 143.78 ppm and NH4+-N RE was 91.94%. After 6 hours of heating, the RE value increased to 99.03%. Whereas the LD solution heated at 70°C has RE values at 3 and 6 hours of 59.76 and 96.89%, respectively (Figure 4A, B). When analyzing the relationship between NH4+-N RE and heating temperature, it was found that both values were highly associated as indicated by the coefficient of determination (R2) of 0.97 (Figure 4C). At 3 hours after heating, EC values of LD solution was found to have negative correlation with heating temperature whereas pH values showed positive relationship (Figure 4D).
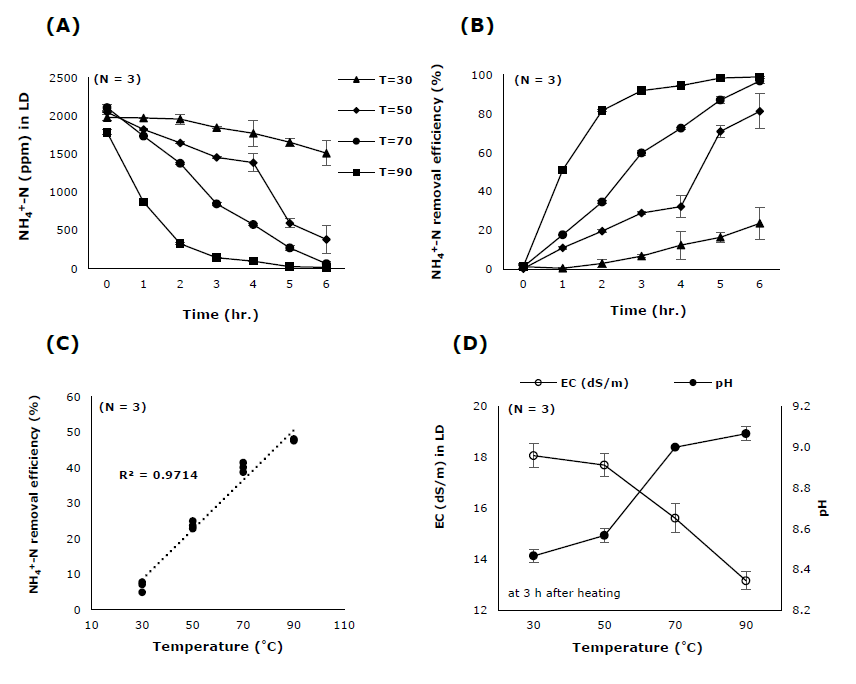
Figure 4. The effect of temperature on (A) NH4+-N concentration, (B) NH4+-N removal efficiency, (C) Relationship between NH4+-N removal efficiency and heating temperature and (D) Electrical conductivity (EC) and pH of LD solution in LD bottle.
During the first 3 hours of the 90°C heating treatment, the concentrations of CO2 and NH4+-N in LD bottle decreased sharply whereas the pH of LD solution increased. Both CO2 and NH4+-N concentrations in LD bottle became relatively constant after 4 hours of heating at 90°C (Figure 5A, B).
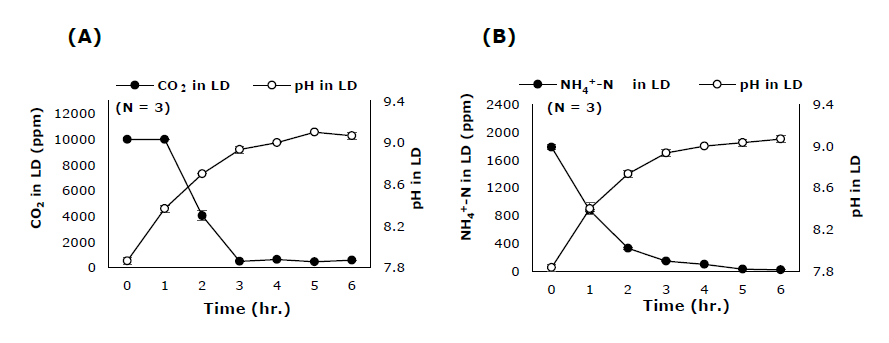
Figure 5. The relationship of pH and the concentrations of (A) CO2 and (B) NH4+-N of LD solution in LD bottle heated at 90°C through time.
Table 4. Correlation analysis on time, electrical conductivity (EC), pH, NH4+-N concentration, and CO2 of LD solution that was boiled at 90°C in LD bottle for 6 hours.
|
Correlation |
Time |
EC |
pH |
[NH4+-N] |
CO2 |
|
Time |
1.00 |
|
|
|
|
|
EC |
-0.94** |
1.00 |
|
|
|
|
pH |
0.90** |
-0.98** |
1.00 |
|
|
|
[NH4+-N] |
-0.90** |
0.93** |
-0.91** |
1.00 |
|
|
CO2 |
-0.89** |
0.96** |
-0.93** |
0.96** |
1.00 |
Note: **is significant difference at 0.01
The EC values and NH4+-N concentrations of NH3 Stripping (LD bottle) and NH3 Scrubbing (GsS bottle) bottles were changed in an opposite manner to each other under 90°C heating treatment. The EC values and NH4+-N concentrations of LD bottle declined at relatively constant rate until they became stable at 13 dS/m and 935.55 ppm after 5 hours of heating (Figure 6A, B). The CO2 concentration of LD bottle was 10,000 ppm at 1 hour after heating and then decreased rapidly until the value was stable at about 503.42 ppm at 3 hours after heating (Figure 6C). Changings of all parameters in LD bottle were significantly correlated with each other (Table 4).
In contrast to the LD bottle, EC values and NH4+-N concentrations of GsS bottle were found to increase continuously after heating. The EC value was 33 dS/cm at 6 hours and the NH4+-N concentration was 5,264.92 ppm at 4 hours after heating (Figure 6A, B). CO2 concentration in GsS bottle also increased rapidly to 10,000 ppm at 1 hour after heating (Figure 6C). Then, the value decreased and was stable at about 1,304 ppm during 3 - 6 hours after heating (Figure 6C).
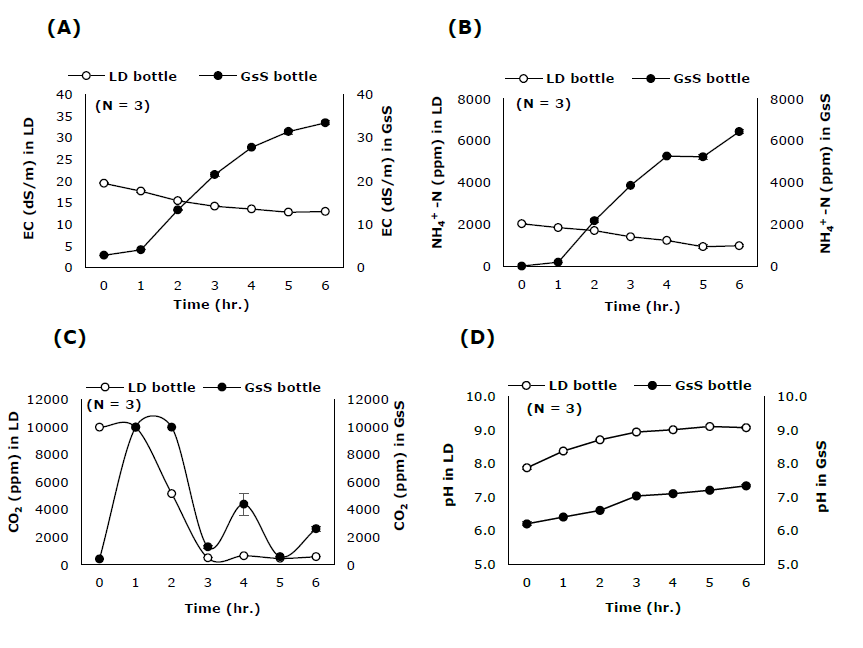
Figure 6. Changing of (A) Electrical conductivity (EC), (B) NH4+-N concentration, (C) CO2 concentration and (D) pH in LD bottle heated at 90°C and GsS bottle at 25°C through time.
The pH of both LD and GsS bottles increased continuously and reached 9.1 and 7.3 at 6 hours after heating (Figure 6D). In GsS bottle, the values of EC, pH, NH4+-N concentration and time were significantly correlated whereas CO2 concentration did not show significant correlation with any other parameters (Table 5).
Table 5. Correlation analysis on time, electrical conductivity (EC), pH, NH4+-N concentration, and CO2 of the solution at 25°C in GsS bottle from the beginning of reaction until 6 hours.
|
Correlation |
Time |
EC |
pH |
[NH4+-N] |
CO2 |
|
Time |
1.00 |
|
|
|
|
|
EC |
-0.94** |
1.00 |
|
|
|
|
pH |
0.90** |
-0.98** |
1.00 |
|
|
|
[NH4+-N] |
-0.90** |
0.93** |
-0.91** |
1.00 |
|
|
CO2 |
-0.89** |
0.96** |
-0.93** |
0.96** |
1.00 |
Note: ** is significant difference at 0.01
Experiment 2: Comparing the efficiency of NH4+-N fertilizer from LD and NH4+-N chemical fertilizer
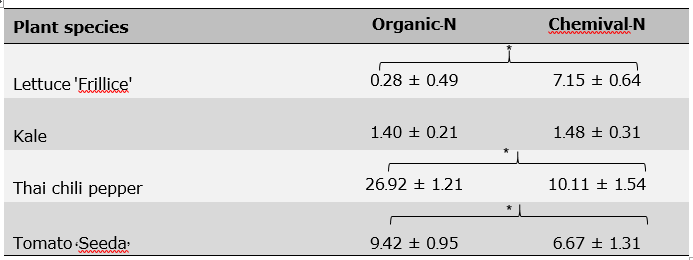
It was found that shoot and root dry weights of lettuce 'Frillice' and kale received MH + N organic fertilizer were not significant different with those received MH + N chemical fertilizer (Figure 7A, B). In contrast, Thai chili pepper and tomato 'Seeda' plants received MH + N organic fertilizer had higher shoot dry weight than those received MH + N chemical fertilizer (Figure 7C, D). All plants received water and MH – N had no growth after transferred to growing media (Figure 7, 8). Based on two-way ANOVA results, there were significant interactions between plant species and nitrogen source on shoot dry weight and NUE (Table 6). Moreover, it was found that lettuce 'Frillice', Thai chili pepper and tomato ‘Seeda’ plants received MH + N organic fertilizer had higher NUE than those received MH + N chemical fertilizer (Table 7).
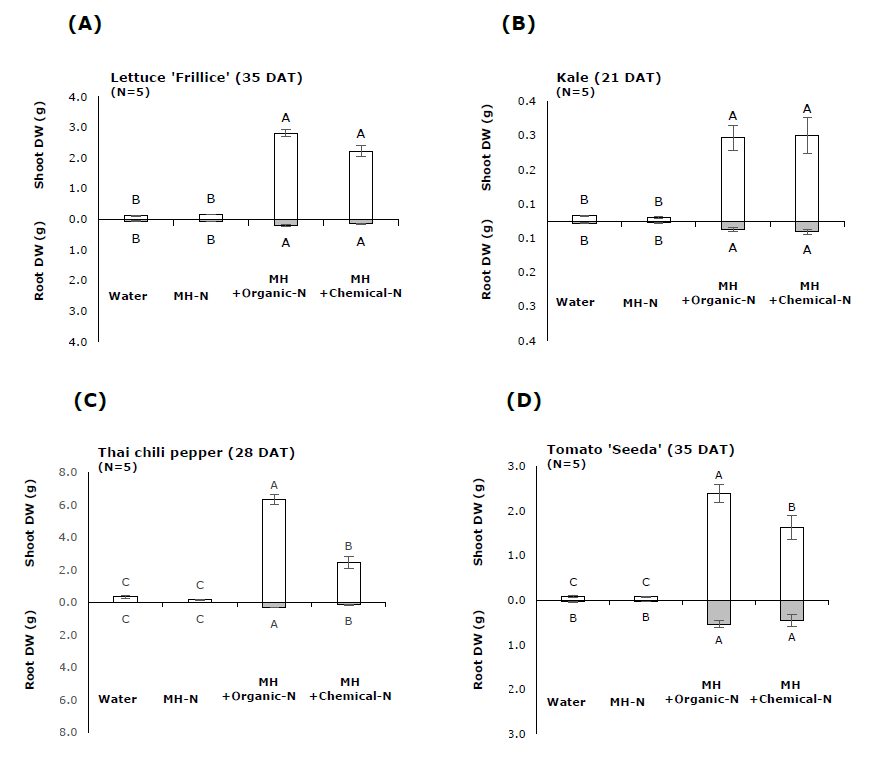
Figure 7. The effect of organic and chemical nitrogen fertilizers on shoot and root dry weights of lettuce ‘Frillice’, kale, Thai chili pepper and tomato ‘Seeda’ in experiment 2. Water = Tap water, MH - N = Modified Hoagland solution without nitrogen, MH + Organic-N = Modified Hoagland solution without nitrogen added with ammonium nitrogen obtained from LD (NH4+-N at concentration of 100 ppm) and MH + Chemical-N = Modified Hoagland solution without nitrogen added with ammonium sulfate (NH4+-N at concentration of 100 ppm).
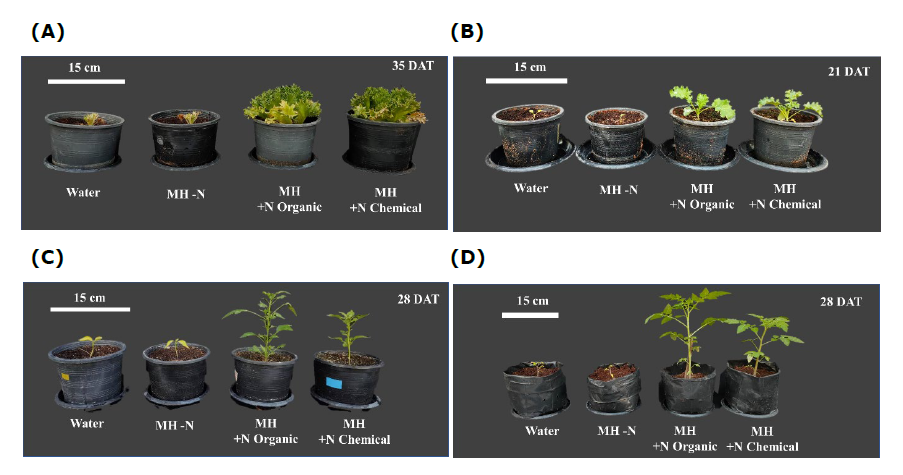
Figure 8. Images of representative plants of 'Frillice' lettuce, kale, Thai chili pepper and tomato ‘Seeda’ from all treatments in Experiment 2. Water = Tap water, MH-N = Modified Hoagland solution without nitrogen, MH + N Organic = Modified Hoagland solution without nitrogen added with ammonium nitrogen obtained from LD (NH4+-N at concentration of 100 ppm) and MH + N Chemical = Modified Hoagland solution without nitrogen added with ammonium sulfate (NH4+-N at concentration of 100 ppm).
Table 6. The significance level, calculated by two-way analysis of variance (ANOVA) of the effect of Plant species (A) and Nitrogen source (B) on Shoot DW, Root DW, Shoot/Root ratio and NUE of plants.
|
Dependent variables |
Independent variables |
||
|
Plant species (A) |
Nitrogen source (B) |
A x B |
|
|
Shoot DW |
>0.01** |
>0.01** |
>0.01** |
|
Root DW |
>0.01** |
0.10 |
0.53 |
|
Shoot/Root ratio |
>0.01** |
0.39 |
0.95 |
|
NUE |
>0.01** |
>0.01** |
>0.01** |
Table 7. Independent sample T-Test of the Nitrogen use efficiency (NUE) of nitrogen fertilizer from LD (Organic-N) and chemical nitrogen fertilizer (Chemical-N) on dry mass of 'Frillice' lettuce, kale, Thai pepper and tomato.
DISCUSSION
The NH4+ present in LD is a source of organic nitrogen fertilizer which is widely used for crop cultivation. However, NH4+ in LD is unstable as it can be readily changed to NH3 and evaporated under basic conditions and high temperature. Direct application of LD to plant in organic farming is therefore not effective. Extraction of NH4+ from LD and capturing it into a soluble form will increase fertilizer stability and make it become more effective for agricultural use. Therefore, an IFOAM-certified gypsum, consisting of calcium and sulfate, was used for capturing ammonia evaporated from LD to form (NH4)2SO4, a stable ionic compound.
In experiment 1, NH3 was evaporated from LD under four different levels of heating temperature. The temperature of 90°C resulted in the fastest evaporation rate and had highest NH4+-N RE after 3 hours of heating (Figure 4A, B). There was a strong positive correlation between NH4+-N RE and heating temperature suggesting that high temperature condition stimulates changing of NH4+ to NH3 in LD. This is in accordance with results of Mohammed et al. (2019) that increasing of LD solution temperature from 70°C to 100°C under pH ranging between 7-12 increased NH4+-N RE from LD.
Fast releasing of CO2 from LD at 90°C resulted in LD solution that had lower EC and higher pH values than those under temperature of 30 50 and 70°C at 3 hours after heating (Figure 4D). This is consistent with previous results of Baldi et. al. (2018) showing that increasing the temperature of manure digestate solution from 45 to 55°C resulted in increasing of pH from 9.2 to 9.5. Moreover, Jamaludin et al. (2018) showed that heating of sewage sludge to 70°C caused an increasing of pH from 7.6 to 10.2 in 1 hour. Our results suggested that increasing temperature of LD solution likely reduces HCO3- solubility resulting in releasing of CO2 and enrichment of OH- as described in Equation (11). This leads to increasing of pH of LD solution (Al-Anezi et al., 2008, Oliveira Filho et al., 2018).
HCO3- → CO2 + OH- (11)
Alkalization of wastewater has been shown to facilitate releasing of NH3. Addition of NaOH to artificial wastewater at 20°C caused changing of pH from 8.9 to 10.8 and increasing of NH4+-N RE from 13.9% to 72.6% in 2.5 hours (Kim et al., 2021). Moreover, an increasing of pH from 8.5 to 10.5 of LD (swine wastewater) resulted in increasing of NH4+-N RE from 27.4% to 92.8% in 2 hours (Guštin et al., 2011). Accordingly, our results showed that increasing of pH is accompanied with decreasing of NH4+-N concentration in LD during 1-3 hours (Figure 5b).
Changing of NH4+ to NH3 is supported by increasing of pH and temperature (Jamaludin et al., 2018). Therefore, many studies utilize these strategies to extract NH4+-N from LD (Oliveira Filho et al., 2018; Folino et al., 2020; Jahn et al., 2020). Since our study focuses on production of nitrogen fertilizer that can be used in organic farming, synthetic chemical is not an option for alkalization of LD. Instead, increasing pH of LD solution in our study was achieved by heating. However, it is also possible to use organic material for alkalinizing LD. For example, Wang et al. (2023) applied plant ash, an alkaline organic- material, to LD solution in the ratio of 2:10 (w/v) at 30°C and found that pH of the solution was increased from 8.43 to 9.93. This resulted in NH4+-N RE of 85.27% in 2 hours after the application.
Although concentration of NH4+-N in LD bottle declined continuously during 1-5 hours of heating, the concentration became relatively stable at about 935 ppm after that (Figure 6B). Similarly, the pH in LD bottle increased during 1-5 hours and became stable at about 9 after that (Figure 6C). It is conceivable that cessation of pH increase was due to the depletion of HCO3- which could be a major source of CO2 and OH- in LD bottle as explained in Equation (11). Accordingly, the result showing that CO2 level in LD bottle increased and declined sharply during 1-3 hours of heating (Figure 6D). Thus, it is likely that the depletion of HCO3- causes cessation of pH increase and then limits change of NH4+ to NH3 resulting in moderately high amount of NH4+-N remaining in LD bottle.
Production of (NH4)2SO4 requires water, NH3, CO2 and CaSO4 as explained in Equation (4), (5) and (6). In our study, NH3 and CO2 released from the LD bottle were delivered to GsS bottle where the three reactions occurred. The results show that concentration of NH4+-N and EC value of the solution inside GsS bottle increased rapidly after the experiment started whereas those in LD bottle decreased accordingly. The solution in GsS bottle was filtered and dried in an oven until the white crystalline powder was obtained. The amount of white crystalline powder obtained from solution in GsS after reaction is obviously more than those obtained from the solution before the reaction (Figure 3). The crystalline powder was analyzed for its composition and result showed that NH4+-N and S were the major constituents (Table 3). A total of 10.86 g of white crystalline powder was obtained from 1.25 L of LD, equivalent to 8.69 g/L. The findings of this study suggest that NH3 released from the LD solution can be sequestered as a chemical compound, potentially in accordance with Equation (6), resulting in a concentrated nitrogen solid that is both water-soluble and stable, thereby enhancing its suitability for application in organic agriculture. However, the total nitrogen content was 19.78%, whereas the quantified inorganic nitrogen accounted for less: NH4+-N was 15.12% and NO3- was measured at 684 ppm. This indicates that a portion of nitrogen remains in other forms that could not be identified in this study, and further investigation is required to clarify these fractions.
The effects of nitrogen fertilizer obtained from LD and chemical nitrogen fertilizer on growth of 4 different plant species were compared. The results showed that nitrogen fertilizer from LD was either comparable or superior to chemical nitrogen fertilizer in terms of supporting vegetative growth of plants (Figure 7A-D). This could occur because nitrogen-fertilizer from LD also contain nitrogen in the forms other than NH4+ such as NO3-. There was 15.12 % of NH4+-N in white crystal powder obtained from LD whereas the total nitrogen in the powder was 19.78 %. As both nitrogen fertilizer solutions used in this study were prepared based only on NH4+-N concentration, it is likely that plants received the solution of nitrogen fertilizer from LD would obtain more total nitrogen than those received solution of chemical nitrogen fertilizer. Moreover, there was other plant essential elements, such as calcium and potassium, presented in the white crystalline powder. These could explain the higher NUE of nitrogen fertilizer from LD (Table 7). Notably, the two-way ANOVA results indicate that Notably, two-way ANOVA results indicated that the effect of nitrogen fertilizer on shoot growth depended on plant species that different plant species may exhibit different growth response to nitrogen fertilizer from LD (Table 7).
CONCLUSION
NH4+-N can be effectively extracted from LD without using synthetic chemical. The extraction process involves heating of LD to directly stimulate change of NH4+ to NH3. Moreover, heating reduces HCO3- solubility in LD leading to releasing of CO2 and alkalinization of LD which support evaporation of NH3. Then, NH3, CO2 and IFOAM certified gypsum were allowed to react and produce white crystalline powder consisting mainly of (NH4)2SO4 which can be used as nitrogen fertilizer. The efficiency of nitrogen fertilizer obtained from LD was either comparable or superior to chemical nitrogen fertilizer in terms of supporting vegetative growth of different plant species. Results from this study suggest that the nitrogen fertilizer obtained from LD is suitable for plant is suitable for use in organic agriculture.
ACKNOWLEDGEMENTS
Authors thank Faculty of Agricultural Production, Maejo University, Chiang Mai, Thailand for providing facilities for conducting experiments.
AUTHOR CONTRIBUTIONS
Thiva Jamaree conducted all the experiments, performed the statistical analysis and data visualization and wrote the manuscript in Thai language. Siriwat Sakhonwasee gave advices throughout the experiments and wrote the manuscript in English language. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Al-Anezi, K., Somerfield, C., Mee, D., and Hilal, N. 2008. Parameters affecting the of solubility carbon dioxide in seawater at the conditions encountered in MSF desalination plants. Desalination. 222(1-3): 548-571. https://doi.org/10.1016/j.desal.2007.01.128
Baldi, M., Collivignarelli, M.C., Abbà, A., and Benigna, I. 2018. The valorization of ammonia in manure digestate by means of alternative stripping reactors. Sustainability. 10(9): 3073. https://doi.org/10.3390/su10093073
Brienza, C., Sigurnjak, I., Meier, T., Michels, E., Adani, F., Schoumans, O., and Meers, E. 2021. Techno-economic assessment at full scale of a biogas refinery plant receiving nitrogen rich feedstock and producing renewable energy and biobased fertilisers. Journal of Cleaner Production. 308: 127408. https://doi.org/10.1016/j.jclepro.2021.127408
Chinnadurai, S., Muruganantham, B., Pradeep, A.K., Kinjal, P.P., Himanshu, P.B., Ganesh, S.P., and Paulchamy, C. 2019. Evaluation of the biomethanation potential of enriched methanogenic cultures on gelatin. Bioresources and Bioprocessing. 6: 1-8. https://doi.org/10.1186/s40643-019-0247-7
De Jesus, H.I., Cassity-Duffey, K., Dutta, B., da Silva, A.L.B.R., and Coolong, T. 2024. Influence of soil type and temperature on nitrogen mineralization from organic fertilizers. Nitrogen. 5(1): 47-61. https://doi.org/10.3390/nitrogen5010004
Epstein, E. and Bloom, A.J. 2005. Mineral nutrition of plants: Principles and perspectives. Sinauer Associates. Sunderland.
Folino, A., Calabrò, P.S., and Zema, D.A. 2020. Effects of ammonia stripping and other physico-chemical pretreatments on anaerobic digestion of swine wastewater. Energies. 13(13): 3413. https://doi.org/10.3390/en13133413
Garcia-González, M.C. and Vanotti, M.B. 2015. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Management. 38: 455-461. https://doi.org/10.1016/j.wasman.2015.01.021
Garnica, M., Houdusse, F., Zamarreño, A.M., and Garcia-Mina, J.M. 2010. The signal effect of nitrate supply enhances active forms of cytokinins and indole acetic content and reduces abscisic acid in wheat plants grown with ammonium. Journal of Plant Physiology. 167(15): 1264-1272. https://doi.org/10.1016/j.jplph.2010.04.013
Guštin, S. and Marinšek-Logar, R. 2011. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Safety and Environmental Protection. 89(1): 61-66. https://doi.org/10.1016/j.psep.2010.11.001
Hsieh, P.H., Kan, C.C., Wu, H.Y., Yang, H.C., and Hsieh, M.H. 2018. Early molecular events associated with nitrogen deficiency in rice seedling roots. Scientific Reports. 8(1): 1-23. https://doi:10.1038/s41598-018-30632-1
Jamaludin, Z., Rollings-Scattergood, S., Lutes, K., and Vaneeckhaute, C. 2018. Evaluation of sustainable scrubbing agents for ammonia recovery from anaerobic digestate. Bioresource Technology. 270: 596-602. https://doi.org/10.1016/j.biortech.2018.09.007
Jahn, L., Baumgartner, T., Krampe, J., and Svardal, K. 2020. Effect of NH3 and organic loading on the inhibition of mesophilic high‐solid digestion. Journal of Chemical & Technology Biotechnology. 95(3): 702-709. https://doi10.1002/jctb.6252
Kalamaras, S.D., Vitoulis, G., Christou, M.L., Sfetsas, T., Tziakas, S., Fragos, V., and Kotsopoulos, T.A. 2021. The effect of ammonia toxicity on methane production of a full-scale biogas plant—an estimation method. Energies. 14(16): 5031. https://doi.org/10.3390/en14165031
Kim, E.J., Kim, H., and Lee, E. 2021. Influence of ammonia stripping parameters on the efficiency and mass transfer rate of ammonia removal. Applied Sciences. 11(1): 441. https://doi.org/10.3390/app11010441
Lazcano, C., Zhu-Barker, X., and Decock, C. 2021. Effects of organic fertilizers on the soi microorganisms responsible for N2O emissions: A review. Microorganisms. 9(5): 983. https://doi.org/10.3390/microorganisms9050983
Lin, R., Deng, C., Cheng, J., Xia, A., Lens, P.N., Jackson, S.A., and Murphy, J.D. 2018. Graphene facilitates biomethane production from protein-derived glycine in anaerobic digestion. Iscience. 10: 158-170. https://doi.org/10.1016/j.isci.2018.11.030
Liu, Y., Ngo, H.H., Guo, W., Peng, L., Wang, D., and Ni, B. 2019. The roles of free ammonia (FA) in biological wastewater treatment processes: A review. Environment international. 123: 10-19. https://doi.org/10.1016/j.envint.2018.11.039
Mikami, Y., Yoneda, H., Tatsukami, Y., Aoki, W., and Ueda, M. 2017. Ammonia production from amino acid-based biomass-like sources by engineered Escherichia coli. AMB Express. 7(1): 1-7. https://doi.org/10.1016/B978-0-323-88516-4.00014-7
Mohammed-Nour, A., Al-Sewailem, M., and El-Naggar, A.H. 2019. The influence of alkalization and temperature on ammonia recovery from cow manure and the chemical properties of the effluents. Sustainability. 11(8): 2441. https://doi.org/10.3390/su11082441
Möller, K. and Müller, T. 2012. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Engineering in Life Sciences. 12(3): 242-257. https://doi.org/10.1002/elsc.201100085
Naimi, Y., Saghir, M., Cherqaoui, A., and Chatre, B. 2017. Energetic recovery of biomass in the region of Rabat, Morocco. International Journal of Hydrogen Energy. 42(2): 1396-1402. https://doi.org/10.1016/j.ijhydene.2016.07.055
Oliveira Filho, J.D.S., Daguerre-Martini, S., Vanotti, M.B., Saez-Tovar, J., Rosal, A., Perez-Murcia, M.D., and Moral, R. 2018. Recovery of ammonia in raw and co-digested swine manure using gas-permeable membrane technology. Frontiers in Sustainable Food Systems. 2: 30. https://doi.org/10.3389/fsufs.2018.00030
Podjanapon, P., Jamaree, T., And Sakhonwasee, S. 2023. Optimization of liquid digestate fertigation and light intensity for lettuce cultivation in closed plant production system. Environmental Control in Biology. 61(2): 9-16. https://doi.org/10.2525/ecb.61.9
Qu, Q. and Zhang, K. 2021. Effects of pH, total solids, temperature and storage duration on gas emissions from slurry storage: A systematic review. Atmosphere. 12(9): 1156. https://doi.org/10.3390/atmos12091156
Reza, A. and Chen, L. 2021. Optimization and modeling of ammonia nitrogen removal from high strength synthetic wastewater using vacuum thermal stripping. Processes. 9(11): 2059. https://doi.org/10.3390/pr9112059
Sigurnjak, I., Brienza, C., Snauwaert, E., De Dobbelaere, A., De Mey, J., Vaneeckhaute, C., and Meers, E. 2019. Production and performance of bio-based mineral fertilizers from agricultural waste using ammonia (stripping-) scrubbing technology. Waste Management. 89: 265-274. https://doi.org/10.1016/j.wasman.2019.03.043
Sutaryo, S., Ward, A.J., and Moller, H.B. 2014. Ammonia inhibition in thermophilic anaerobic digestion of dairy cattle manure. Journal of the Indonesian Tropical Animal Agriculture. 39(2): 83-90. https://doi.org/10.14710/jitaa.39.2.83-90
Szymańska, M., Sosulski, T., Szara, E., Wąs, A., Sulewski, P., Van Pruissen, G.W., and Cornelissen, R.L. 2019. Ammonium sulphate from a bio-refinery system as a fertilizer Agronomic and economic effectiveness on the farm scale. Energies. 12(24): 4721. https://doi.org/10.3390/en12244721
Wang, C., Azeem, M., Zhang, H., Qu, W., Qiao, C., and Yang, S. 2023. Ammonia stripping with plant ash enhanced removal and recovery rate of ammonia nitrogen from biogas slurry. Polish Journal of Environmental Studies. 32(1): 843-852. https://doi.org/10.15244/pjoes/154735
Wang, G., Luo, Z., Wang, E., and Zhang, W. 2018. Reducing greenhouse gas emissions while maintaining yield in the croplands of Huang-Huai-Hai Plain, China. Agricultural and Forest Meteorology. 260: 80-94. https://doi.org/10.1016/j.agrformet.2018.06.003
Wu, M.C., Sun, K.W., and Zhang, Y. 2006. Influence of temperature fluctuation on thermophilic anaerobic digestion of municipal organic solid waste. Journal of Zhejiang University Science B. 7(3): 180-185. https://doi.org/10.1631/jzus.2006.B0180
Willer, H., Trávníček, J., and Schlatter, S. 2024. The world of organic agriculture statistics and emerging trends 2024. Druckerei Hachenburg PMS GmbH. Germany.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Thiva Jamaree and Siriwat Sakhonwasee*
Program in Interdisciplinary Agriculture, Faculty of Agricultural Production, Maejo University, Chiang Mai 50290, Thailand.
Corresponding author: Siriwat Sakhonwasee, E-mail: siriwat@mju.ac.th
ORCID iD: Siriwat Sakhonwasee: https://orcid.org/0000-0002-3630-903X
Total Article Views
Editor: Tonapha Pusadee,
Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: June 6, 2025;
Revised: September 29, 2025;
Accepted: October 2, 2025;
Online First: November 4, 2025

